Abstract
Dynein is a large cytoskeletal motor protein that belongs to the AAA+ (ATPases associated with diverse cellular activities) superfamily. While dynein has had a rich history of cellular research, its molecular mechanism of motility remains poorly understood. Here we describe recent x-ray crystallographic studies that reveal the architecture of dynein’s catalytic ring, mechanical linker element, and microtubule binding domain. This structural information has given rise to new hypotheses on how the dynein motor domain might change its conformation in order to produce motility along microtubules.
1. Introduction
Dynein is a molecular motor that uses the chemical energy of ATP to produce mechanical work on microtubules. Initially discovered as the driving force of flagellar motility in Tetrahymena cilia [1], dynein has been found in many different organisms and cellular locations. This large family of molecular motors consists of two major classes: axonemal and cytoplasmic dyneins.
Axonemal dyneins power the beating movements of cilia and flagella, and can be further subdivided into inner arm and outer arm dyneins based upon their position in the axoneme. Cytoplasmic dyneins, which are involved in the transport of various intracellular cargoes, are subdivided into into two major groups. Cytoplasmic dynein 1 is responsible for most microtubule minus-end-directed motility in animal and fungi, including organelle transport, mitotic spindle positioning, and nuclear segregration (see [2] for review). Cytoplasmic dynein 2 (also known as intraflagellar transport (IFT) dynein or dynein 1B) appears to function exclusively within the flagellum, where it transports IFT particles along the axoneme towards the cell body.
Despite their functional diversity, all dyneins have a similar molecular organization consisting of several heavy chains, intermediate chains, and light chains. The heavy chains contain catalytic motor activity and bind the intermediate chains. The intermediate chains, in turn, bind to light chains, and also interact with cargoes. In addition to this core complex of heavy, intermediate, and light chains, dynein interacts with several adaptor proteins such as dynactin, NudE, and Lis1, which are essential for the proper regulation and localization of dynein (see [3] for review).
The well-conserved heavy chain consists of three major parts: the AAA ring, the tail, and the stalk (Figure 1A and B). The AAA ring, which is structurally related to hexameric ATPases in the AAA+ superfamily [4-6], serves as the catalytic engine of the motor; it consists of six AAA (AAA1-6) domains, four of which (AAA1-4) contain nucleotide binding Walker A (P-loop) motifs. AAA1 is the primary ATPase site and essential for motility [7], whereas ATP binding and/or hydrolysis at AAA2-4 play supporting roles in regulating the motor [8-10]. AAA5 and AAA6 do not contain any known nucleotide binding motifs and are thought to serve structural roles. Lying N-terminal to the AAA ring is the “linker”, which has been proposed to serve as a mechanical element [11]. Emerging from the AAA ring near AAA4 is the stalk, a ~15 nm anti-parallel coiled-coil that has a globular microtubule-binding domain (MTBD) at its tip [12].
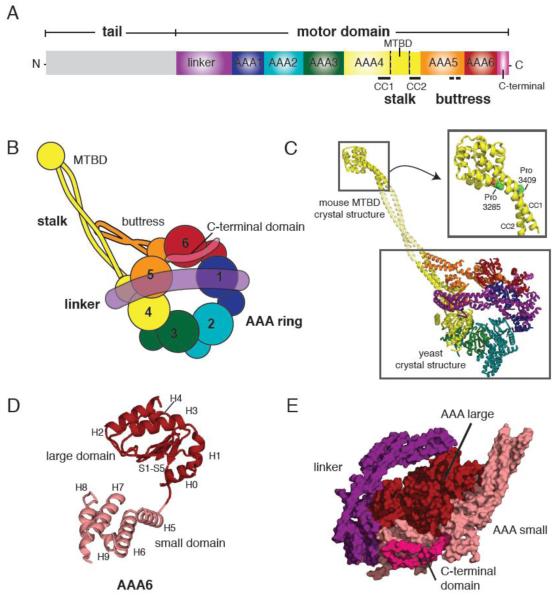
Figure 1.
(A) Primary structure of the dynein heavy chain showing the functional domains of dynein. (MTBD = microtubule binding domain, CC=coiled coil) (B) Cartoon depiction of the dynein motor domain as revealed by the crystal structures of yeast and Dictyostelium cytoplasmic dynein. (C) A crystallographic model of yeast cytoplasmic dynein based on the 6 Å crystal structure of the motor domain (lower box, PDB ID: 3QMZ) and the 2.3 Å resolution structure of the mouse cytoplasmic dynein MTBD (upper box, PDB ID: 3ERR). Domains are color-coded as in (B). Inset shows the MTBD structure in detail, highlighting the proline residues in CC1 and CC2 that induce a kink in the stalk. The middle portion of the stalk coiled coil is modeled from a typical anti-parallel coiled coil. (D) Architecture of the yeast dynein AAA6 domain. The large domain (H0-H4 and S1-S5) is colored in red, and the small domain (H5-H9) in pink. A flexible peptide connects the large and small domains. (E) A side view of the AAA ring, linker, and C-terminal domain. Large domains (red) form one level near the linker (purple), while the small domains (pink) form another level near the C-terminal domain (magenta).
Dynein’s molecular mechanism remains poorly understood compared to other cytoskeletal motors such as kinesin or myosin. One reason why research on dynein has lagged behind is due to the biochemical challenges posed by its large size (the heavy chain polypeptide is ~500 kilodalton and the entire dynein holoenzyme with its associated chains is ~2 megadalton). Despite these technical challenges, several groups have now succeeded in obtaining dynein from yeast, Dictyostelium, and Chlamydomonas in quantities sufficient for structural analyses. These advances have recently culminated in the solution of two crystal structures of the cytoplasmic dynein motor domain, one from yeast [13], and the other from Dictyostelium [14] (Figure 1C and 2A). The resolution of these structures (4.5-6 Å) is not sufficient for visualizing side chains and accurately tracing the polypeptide chain. However, they reveal the secondary structure of the motor domain, which provides valuable new insights into its organization and allosteric communication mechanism. Here we will review these advances, compare the yeast and Dictyostelium dynein motor domain structures, and discuss the implications for dynein motility.
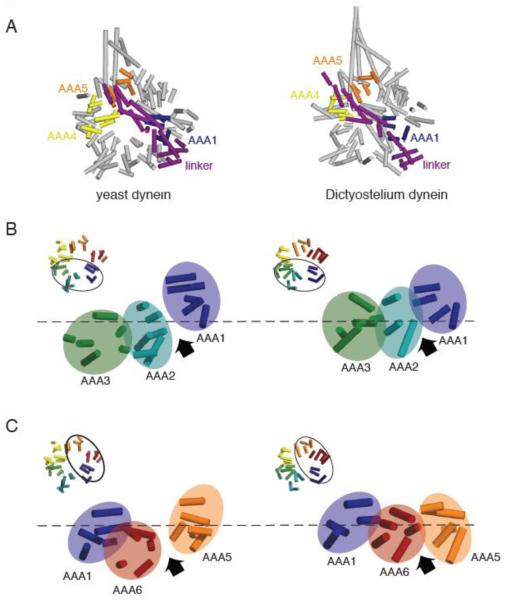
Figure 2.
(A) Comparison of the yeast (PDB ID: 3QMZ) and Dictyostelium dynein motor domain structures (PDB ID: 3AY1). The motor domains were superimposed by aligning the helices in the linker and AAA1, which superimpose well in the yeast and Dictyostelium structures. In the yeast dynein structure, the ring is more open and asymmetric than in the Dictyostelium dynein structure. Also, AAA5 is more shifted towards AAA4 in the yeast structure, resulting in a slight difference in the position where the N-terminus of the linker sits relative to the ring. (B) Comparison of yeast and Dictyostelium dynein AAA1-3. Asymmetry is more prominent and the AAA1-2 gap is wider in the yeast structure due to the different heights of AAA domains; specifically AAA2 is shifted further down with respect to AAA1. (C) Comparison of yeast and Dicytostelium dynein AAA1, 5, and 6. The AAA ring is more planar and the AAA5-6 gap smaller in the Dictyostelium structure. As discussed in the text, we speculate that the differences between yeast and Dictyostelium reflect distinct nucleotide occupancy in AAA1 (empty and ADP for yeast and Dictyostelium respectively). Note: the model for Dictyostelium dynein lacks several AAA helices that are present in the yeast model.
2. Structural organization of the dynein heavy chain
The C-terminal ~350 kDa fragment of the dynein heavy chain is sufficient for motor activity in vitro [15-17]. In this section, we describe the structure of the three essential components of this motor domain: the AAA ring, the linker, and the stalk/microtubule binding domain.
2.1 AAA ring
Dynein is a unique AAA ATPase that has 6 distinct AAA domains (AAA1-6) linked in tandem on a single polypeptide chain. Midasin/Rea1 is the only other AAA ATPase known to have 6 tandem AAA domains encoded on a single gene [18, 19]. Early electron microscopy studies first observed the asymmetric ring structure of dynein [20, 21]. Further work mapped the position of AAA1-6 on the ring and also confirmed that no other domain besides the AAA domains were required to form a closed ring [22]. The new crystal structures reveal the secondary structure of individual AAA domains and how these domains are organized within the ring.
Dynein’s AAA domains contain the signature structural fold of AAA ATPases: a large α/β domain (five helices (H0-H4) that flank a five-stranded beta sheet (S1-S5)); the large domain is connected to a small α-helical domain (H5-H9) by a short, flexible peptide (Figure 1D). Each AAA large domain has unique inserts that protrude upwards from the face of the ring (Figure 4B). For example, AAA2 has hairpin inserts in H2 and H3-S4, while AAA4 has a helix-loop-helix insert in H3-S4. The small domains all have one additional helix (H9) compared with most other AAA proteins; this C-terminal helix enables the polypeptide chain exiting the small domain of one AAA domain to connect to the large domain of the neighboring AAA domain.
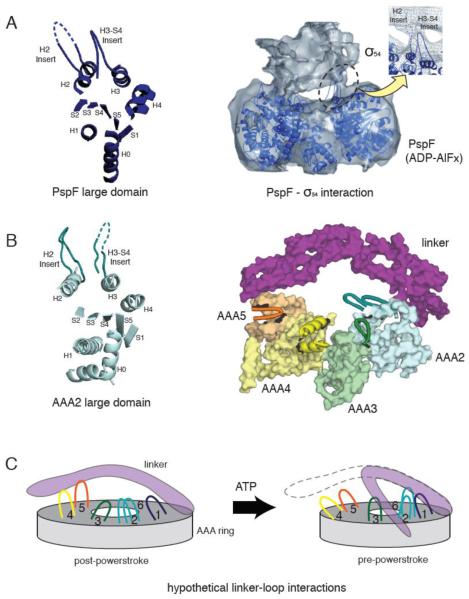
Figure 4.
(A) The crystal structure of a PspF large domain showing the position of the H2 and H3-S4 insert loops (left). A 20 Å resolution cryo-electron microscopy reconstruction of the PspF hexamer in complex with its binding partner, σ54 in the presence of ADP-AlFx (right). The PspF hexamer crystal structure is docked into the electron microscopy structure, showing the H2 and H3-S4 insert loops at the site of PspF - σ54 interaction (reprinted from [46] with permission). (B) The crystal structure of AAA2 large domain in yeast cytoplasmic dynein, showing a similar position of the H2 and H3-S4 insert loops compared with PspF (left). The position of the unique loop inserts in the large domains of dynein AAA2-5, with respect to the linker (right). AAA1 and AAA6 are not shown for clarity. Insert loops lying at the top surface of the ring are depicted in cartoon format. The loops provide potential docking sites for the linker or dynein regulatory proteins. (C) A speculative model of how the loop insertions could act as docking sites for the linker at different stages of the ATPase cycle.
Although the structural fold of each AAA domain is similar, the angle between the large and small domains differs for each AAA domain. This angular variation is achieved through the flexibility of a short peptide that connects the final beta strand (S5) of the large domain to the first helix (H5) of the small domain (Figure 1D). Interestingly, all of the small domains pack against the neighboring large domains in a similar manner, thus creating six rigid units in the dynein motor domain (see Fig. 3C,D from [13]). For example, AAA1 small-AAA2 large and AAA2 small-AAA3 large constitute two such rigid units. Similar packing of small domains against neighboring large domains has been noted for the bacterial protease AAA proteins ClpX [23] and HslU [24], both of which self-assemble into rings from monomers. Thus, building rings from rigid units (small domain-neighboring large domain) separated by flexible linkers may be a common organizational principle of many AAA ATPases.
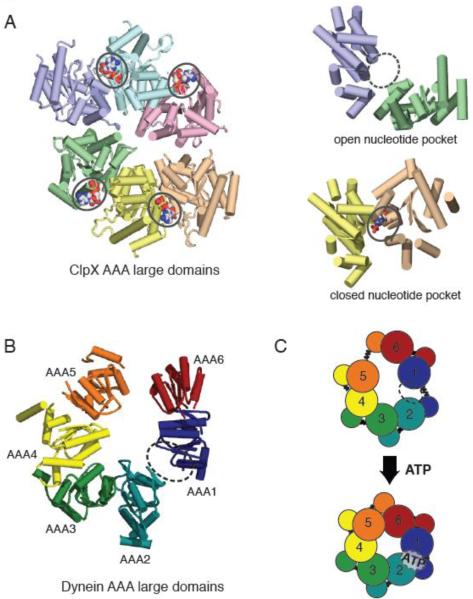
Figure 3.
(A) X-ray crystal structure of the large domains of the ClpX hexamer (PDB ID: 3HWS), color coded by chain. The nucleotide-free domain interfaces (purple-green) show an open conformation, while the nucleotide-bound (ADP) domain interfaces (yellow-peach) show a closed conformation. (B) Asymmetric structure of the yeast cytoplasmic dynein AAA ring (only large domains shown). When viewed from the linker-face, the ring shows prominent openings between AAA1-AAA2 and AAA5-AAA6 large domains. The AAA1-AAA2 interface is the main ATP hydrolysis site for dynein. (C) A model for AAA ring communication in dynein, based upon the nucleotide-free and bound forms of ClpX. Upon ATP binding at the AAA1 nucleotide binding pocket, the AAA1-AAA2 switches from an open to closed conformation, triggering an overall shift in AAA domains, and ultimately an iris-like contraction of the AAA ring.
The dynein AAA ring has several asymmetric features. First, it is a two-tiered structure with the α/β large domains on one level near the linker, and the small domains positioned below the large domains away from the linker (Figure 1E). However, these levels are not uniform, as the positions of each of the six large and small domains vary with respect to the plane of the ring. When the ring is viewed from above (from the linker face), the AAA domains also display uneven spacing around the ring. This is particularly pronounced in the yeast motor domain structure, where prominent gaps exist between adjacent AAA large domains (AAA1-AAA2 and AAA5-AAA6), as will be discussed later.
Following the AAA6 domain, a helical C-terminal domain packs on the bottom of the small helical domains. In yeast, this structure is small compared to other dyneins, and stretches from the AAA6 small domain to the bottom of AAA5 small. In Dictyostelium, however, the C-terminal domain is longer and has an additional segment that wraps back from AAA5 small and ends underneath AAA1 small. The C-terminal domain may help to interconnect and perhaps rigidify this portion of the ring.
2.2 Linker
The linker was originally identified by negative stain electron microscopy as a proximal region of the tail that could dock and undock from the AAA ring [11]. Based on different orientations of the linker in the apo and ADP-Vi states, Burgess and co-workers [11] proposed that the linker is a mechanical element that undergoes a nucleotide-dependent power stroke. Subsequent studies further supported this model by placing fluorescent probes at different positions on the linker and AAA domains. Based on negative stain electron microscopy [22] and fluorescent resonance energy transfer (FRET) [25], the N-terminus of the linker was proposed to move from a pre-powerstroke state near AAA2 to a post-powerstroke state near AAA4.
The two crystal structures of yeast [13] and Dictyostelium [14] dynein, now reveal that the linker is a predominantly α-helical structure that arches over the large domain face of the AAA ring like a basket handle (Figure 4B). Although there is still some ambiguity in the overall connectivity of the helices that compose the bulk of the linker, it appears to be composed of four subdomains. Subdomains 1 and 2 contain antiparallel triple helical bundles that resemble spectrin repeats [26]; spectrin repeats generally exist as modular units within long structures of high elasticity. Subdomain 3 is a less defined parallel helical structure, which has particularly weak electron density. This suggests that the middle region of the linker, particularly the juncture of subdomain 2 and 3, might serve as a hinge where the linker can bend. Subdomain 4 at the C-terminus is a five-helix bundle that contains two unique helices that lie perpendicular to the main axis of the linker.
Contrary to earlier suggestions that the linker stacks on top of the ring, the crystal structures show that the linker makes limited contacts with the ring only at its terminal portions. At its C-terminus, subdomain 4 of the linker has extensive interactions with both the large and small domains of AAA1 as well as part of the small domain of AAA6. This extensive interface is suggestive of a relatively stable interaction. At the N-terminus, the interactions of linker subdomain 1 with the ring are less clear at the present resolution of these crystal structures. In Dictyostelium dynein, some of the linker helices sit between AAA4 and 5 large domains while they sit above AAA5 in yeast dynein (Figure 2A). The interactions appear more tenuous compared to the other end of the linker, which is consistent with electron microscopy studies indicating that tail-AAA ring contacts are broken during the ATPase cycle to enable the linker to adopt a pre-power stroke state.
2.3 Stalk and Microtubule Binding Domain (MTBD)
The stalk is a ~15 nm long anti-parallel coiled coil that protrudes out of the AAA ring and has a microtubule binding domain at its tip [27]. Sequence prediction studies [28] showed that the helix returning from the microtubule binding domain to the ring (CC2) has a clear heptad repeat along its length that is typical of coiled coils, with the first and fourth residues (“a” and “d” positions) being hydrophobic residues that can pack in the interior of the coiled coil. In contrast, the partner helix (CC1) tends to have only one repeating hydrophobic residue per seven residues along its length, creating a less well-packed coiled coil. The single hydrophobic in CC1 can be aligned potentially with either of the two hydrophobic residues in CC2, creating two potential registries. Interestingly, the distinct hydrophobic repeat patterns in CC1 and CC2 appear to be evolutionarily conserved among all dyneins. Based upon these features, it was proposed that CC1 might slide relative to CC2 during the ATPase cycle and that the shift from one registry to the other might confer different binding affinities to the distal microtubule binding domain. In support of this idea, fusions of an isolated microtubule binding domain to a stalk in the “α-registry” displayed weak microtubule binding while fusions in the “β+registry” displayed ~10-fold tighter binding [28]. A second and more direct demonstration of the coiled coil sliding model came from a study of disulfide crosslinking in the stalk of an active dynein motor [29]. When CC1 and CC2 were locked into the α and β+ registries by crosslinking cysteines, dynein showed an order of magnitude difference in microtubule binding affinity, as well as changes in ATPase activity.
An atomic resolution structure of the mouse cytoplasmic dynein microtubule binding domain (Figure 1C) revealed the details of the microtubule binding region as well as intriguing structural features of the distal stalk (in the α-registry) [30]. In this structure, CC2 makes extensive contacts with several helices (H2, H4, H5, and H6) in the microtubule binding domain, while CC1 only packs against a single helix (H4) with limited contacts. This difference agrees with the model that CC2 is fixed in position and that CC1 could slide relative to CC2. The structure also revealed a disruption in the coiled coil between a highly conserved pair of staggered prolines, one in CC1 and the other in CC2 (Figure 1C). This region might help to facilitate or propagate a shift in the registry of the two coiled coil helices.
Further work on the structure of the whole motor domain [13, 14] provide a refined view of the structural relationship between the stalk and the AAA ring, as well as revealing novel interactions of the stalk. The stalk was originally modeled as a structure exiting the AAA ring by coiled coil helix 1 (CC1) at AAA4, and re-entering via its partner coiled coil helix 2 (CC2) at AAA5 [12, 27, 31]. Now the crystal structures reveal that the stalk is an integral part of the AAA4 small domain. CC1 and 2 are long extensions of the H7 and H8 helices (see Fig. 5C of reference [13]), somewhat analogous to the ~8 nm long anti-parallel coiled coil in the small domain of ClpB [32]. Thus, the base of the CC1 and CC2 helices are likely to be highly constrained by interactions with the other small domain helices.
Figure 5.
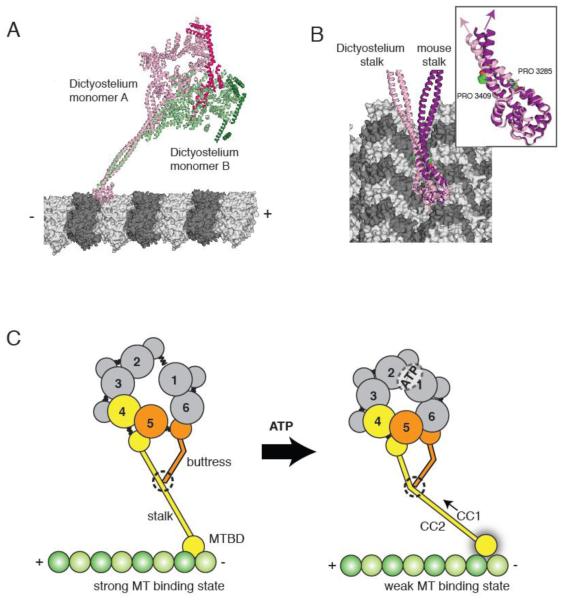
(A) The two Dictyostelium dynein monomers (monomer A in pink monomer B in green; linkers highlighted in darker hues) modeled onto microtubules based on docking of the mouse microtubule binding domain as in [30]. The different stalk angles of monomer A and B results in a different orientation of the dynein heads with respect to the microtubule longitudinal axis. (B) The stalks of the Dictyostelium stalk (pink) and mouse stalk (purple) viewed from the microtubule minus end. The inset shows the difference in stalk angle, which might be due to differences in the vicinity of the proline kinks in CC1 and CC2. (C) A model for stalk communication. Upon ATP binding, movements of AAA4 and AAA5 small domains are relayed to the stalk and buttress coiled coil extensions, respectively. Due to its interaction between the stalk, the buttress can push or pull on CC1, causing relative sliding motions between CC1 and CC2 and ultimately changing the microtubule binding affinity at the tip of the stalk.
An important revelation of the crystal structures is the existence of an additional antiparallel coiled coil near the base of the stalk (see Figure 5 of [13]). This second coiled coil, which is an extension of the H5 and H6 helices of the AAA5 small domain, has a sharp kink in its middle and its tip interacts with the side of the stalk. Based on its appearance, it has been named the “buttress” [13] or the “strut” [14], suggesting that it supports the base of the stalk (we use the term “buttress” in this review). In addition to serving a structural role, we think it likely that the buttress regulates the conformation of stalk during the ATPase cycle, given the apparent interaction of the end of the buttress with the stalk, and a proposed mechanism for regulation will be discussed in section 4.
3. Comparison of the Yeast and Dictyostelium Dynein Motor Domain Structures
The crystal structures of the yeast and Dictyostelium dynein motor domains have a similar overall organization, as well as many common features. However, several differences exist between the two structures, which could be due to either species differences, crystal contacts, or the truncations used in the constructs. However, we believe that it is likely that the distinct conformations reflect different nucleotide states in AAA1 (the main hydrolytic site), since the Dictyostelium motor domain was crystallized with ADP and the yeast motor domain was crystallized in nucleotide-free conditions.
Both the yeast and Dictyostelium dynein crystal structures contain two monomers in the crystallographic asymmetric unit. In yeast, the monomers are joined at the N-terminus via GST, forming a dimeric conformation that is compatible with processive motility [15]. The two monomers of the yeast dimer have virtually identical structures. In contrast, the two Dictyostelium monomers in the unit cell (monomer A and B), which pack together through a back-to-back interaction of the C-terminal face, show pronounced structural differences, even though both are presumably in the same nucleotide state. Differences are notable in AAA3, 4 and 5 and changes in orientations of these domains result in a prominent change in the angle (~37°) of the distal region of the stalks in the two monomers. The cause of this difference is unclear, although these differences might be due to different crystal contacts. Regardless, these results reveal considerable conformational flexibility of dynein, which might be important for its mechanism.
Several notable differences are found in the AAA ring of yeast and Dictyostelium dynein. The yeast AAA ring is much more asymmetric and slightly expanded compared to the Dictyostelium AAA ring (Figure 2A). This gross difference results from larger gaps between AAA domains in yeast dynein and differences in the positions of the AAA domains with respect to the plane of the ring (Figure 2B and C). More specifically, AAA2 is positioned higher up in the plane of the ring in Dictyostelium, and AAA5 is shifted farther towards the linker and above AAA4 in yeast. We speculate that this difference in the shape of the ring might be due to different nucleotide states (nucleotide-free for yeast and ADP for Dictyostelium), as will be discussed later.
The linker structures are fairly similar in Dictyostelium and yeast as well as the manner in which the linker packs against AAA1. However, the position of the linker N-terminus relative to the ring differs slightly; in yeast, it is located above AAA5, while in Dictyostelium, it is closer to AAA4 (Figure 2A). This variation seems to be a consequence of the above mentioned differences in the position of AAA5 in yeast and Dictyostelium, rather than a change in the linker structure.
The tip of the stalk of Dictyostelium dynein structure also differs from the crystal structure of the isolated mouse stalk/microtubule binding domain structure. In the mouse microtubule binding domain structure, the stalk is locked into a “weak-binding” α-registry of the coiled coil [30]. In this conformation, the conserved pair of staggered prolines near the microtubule binding domain introduces a kink in the stalk. However, in the Dictyostelium dynein structure, a kink is less obvious and the stalk smoothly continues into the microtubule binding domain (Figure 5B). Higher resolutions structures will be needed to ascertain the registry of the stalk coiled coil in this motor domain structure.
Finally, the C-terminal domains of yeast and Dictystelium dynein differ not only in size, but have considerably distinct structures. The smaller yeast C-terminus has an elongated structure consisting mainly of one long helix that bridges AAA6 small and AAA5 small. The larger Dictyostelium dynein C-terminus, however, has two-lobes which appear to consist of helical bundles. The first lobe assumes a position similar to the yeast C-terminus, while the second lobe packs underneath AAA5small and AAA1small. In between the two lobes seems to be a flexible hinge region that can affect motor processivity [33]. The difference in how the C-terminal structure crossbridges AAA domains is intriguing, and might be related to the different motile properties of yeast and Dictyostelium dynein.
4. Intramolecular communication of dynein
A fundamental question regarding the dynein mechanism is how allosteric communication can occur across long distances within the motor domain. The affinity of the MTBD for microtubules is known to be regulated by the nucleotide binding state of AAA1 [34]. Furthermore, binding of the microtubules can stimulate the ATP turnover cycle of dynein by 10-20-fold [8, 9]. This transfer of information is particularly remarkable given that the distance from the ATP binding site of AAA1 to the microtubule binding interface is ~25 nm. Furthermore, hydrolysis of ATP at AAA1 must relay a signal to the opposite side of the ring to cause the detachment and mechanical swing of the linker. With the structural information available from two crystal structures and electron microscopy reconstructions, we can begin to speculate how these communication pathways might work.
4.1 Communication within the AAA ring
The nucleotide binding pockets of AAA domains are formed at the interface of two domains, a feature that likely facilitates interdomain crosstalk as indicated in other AAA proteins [35, 36]. Surprisingly, there is a very large gap between AAA1 and AAA2 in yeast dynein (Figure 3B), which constitutes the primary site of nucleotide hydroysis by dynein. Based upon structural information from other AAA proteins, the extent of this gap would be too large to enable ATP hydrolysis and potentially even ATP binding. The gap, however, is consistent with no nucleotide being bound at AAA1, and indeed the yeast crystal was obtained under nucleotide-free conditions; however, confirmation of this important point must await a higher resolution structure in which the presence or absence of nucleotide can be clearly discerned in the electron density map.
From our yeast structure, we proposed that the gap between AAA1-AAA2 must close upon ATP binding [13]. Akin to a switch, nucleotide binding or release could trigger an open vs. closed conformation of the AAA domain interface. This type of mechanism is supported by a structure of a ClpX hexamer [23], which has two nucleotide-free and four nucleotide-bound subunits (Figure 3A). In this structure, the nucleotide-free interfaces are more open (large domains separated further apart) than the nucleotide-bound interfaces (Figure 3A). This hypothesis also is consistent with differences between the nucleotide-free yeast motor domain and the Dictyostelium motor domain (crystallized with ADP). In Dictyostelium, the AAA2 large domain is displaced higher in the ring and thus closer to the AAA1 large domain (Figure 2B). However, there is still a significant gap between the two, suggesting that a further lateral closure may occur in the ATP state, which brings the domains closer to enable the hydrolysis of the β–γ phosphate bond.
Because the packing interactions between neighboring AAA domains are relatively conserved, closing of the AAA1-AAA2 cleft would probably drive a global conformational change such as contraction of the whole AAA ring (Figure 3C). Considering that AAA2-4 have been suggested to play regulatory roles in dynein motility [37] [9], we initially proposed that such change might be primarily transmitted around the ring from AAA1 to AAA2-4 via sequential nucleotide changes [13]. Alternatively, Kon et al. [14] suggested a conformational propagation around the other side of the ring, from AAA1 to AAA6 and 5, which is mediated by the C-terminal domain. Distinguishing between these two distinct allosteric mechanisms of dynein will require new structures and/or experimental probes of different nucleotide states. In any case, however, changes in the AAA ring would be expected to drive downstream allosteric movement in the linker and stalk as discussed below.
4.2 Communication between the linker and the AAA ring
The current crystal and electron microscopy structures of the nucleotide-free and ADP state show the distal end of the linker extending across the ring and perhaps docked near AAA4/AAA5 in the post-stroke conformation (also referred to as the “unprimed conformation [22]”). In order to reset after a mechanical stroke, ATP hydrolysis at AAA1 must be relayed to the linker so that it can undock and move from this post-powerstroke to pre-powerstroke state. How might such a change be communicated? Carter et al. proposed that a “domino effect”, which is triggered by the aforementioned AAA ring contraction after ATP binding at AAA1, causes the distal linker to detach from the ring and perhaps adopt a more disordered state with its position centered around near AAA2 (as suggested by EM data of Roberts et al. [22]).
Besides the overall closure of the ring, it is also possible that local structural remodeling of AAA domains near the linker contact sites plays an important role in the process of linker movement. A good candidate for such conformational changes might be the hairpin and helix insert sequences of the AAA large domains in H2 and H3-S4 (Figure 4B). The protrusion of these loops towards the linker makes them attractive candidates for a docking surface. In NtrC and PspF, two bacterial transcriptional activating proteins of the AAA family, H2 and H3-S4 hairpin inserts mediate intermolecular interactions between these AAA ATPases and their substrate (σ54 factor of bacterial RNA polymerase) and also undergo nucleotide-dependent structural changes [38, 39] (Figure 4A). One intriguing possibility is that dynein has evolved a similar mechanism, but in this case uses its loops to interact with the linker, which sits on top of the ring in a similar manner to the way that σ54 factor sits on top of the NtrC/PspF rings (Figure 4C). It is particularly intriguing that dynein’s AAA2 large domain has two inserts loops in the same positions as NtrC/PspF. This is an unusual feature among crystal structures of AAA proteins; to our knowledge, the H2 and H3-S4 hairpin inserts are only found in dynein AAA2, NtrC/PspF, and the magnesium chelatase BchI. It is also possible that a subset of dynein’s six insert loops interacts with regulatory molecules that interact with the motor domain, such as Lis1/NudE ([40]).
The above model assumes a more passive role for the linker, responding to rather than instigating allosteric communication. However, a more active role for the linker is certainly possible. For example, ATP-dependent rearrangements in subdomain 4 of the linker through its interaction with AAA1 could be propagated towards the N-terminus to facilitate conformational change and possible bending of the linker. In this model, a conformational change in the linker could play an active role in swinging the linker in a particular direction, rather than passively being pried off its post-powerstroke position by AAA ring movements.
4.3 Communication between the microtubule binding domain (MTBD) and the AAA ring
Since the discovery of the dynein stalk as an anti-parallel coiled coil [27], it has been recognized that the stalk must somehow relay information bidirectionally along its length to modulate microtubule binding affinity and ATP turnover in the AAA ring. Various mechanisms of communication along the stalk have been proposed including stalk tilting [41] or coiled coil melting [42], but more recent studies favor a model where coiled coil 1 (CC1) slides with respect to coiled coil 2 (CC2), as discussed in section 2.3.
The discovery of the buttress coiled coil [13, 14] has prompted a new model for how sliding of the two helices in the coiled coil might be initiated (Figure 5C). The original coiled coil sliding model postulated that CC1 was pulled relative to CC2 at the base of the stalk, thus propagating a sliding motion of the entire helix. However, since CC1 and CC2 are tightly packed helices in the AAA4 small domain, it seems unlikely that they could slide relative to one another at their base. As an alternative possibility, sliding might be propagated at the stalk-buttress contact site. In such a model, relative movements of the rigid body units, AAA3small-AAA4large and AAA4small-AAA5large would move the stalk and buttress relative to one another. If the two coiled coils remain in contact, then this ATP-generated motion within the ring could be translated into a distortion of the stalk coiled coil that could propagate as a registry shift to the proline region near the microtubule binding domain. It also could control the angle in which the stalk emerges from the ring, as suggested by the different stalk angles seen in the two monomers in the Dictyostelium crystal structure (Figure 5A).
5. Implications of the dynein structure for processive movement
The new crystal structures also have generated information that is relevant for understanding the orientation of the two motor domains when dynein moves processively along a microtubule. From studies of kinesin and myosin V (two well studied dimeric, processive motors), it is widely believed that both motor domains must be polymer-bound for at least some phase of their ATPase cycle, so that they can walk without falling off. How might dynein achieve such a two-head-bound intermediate?
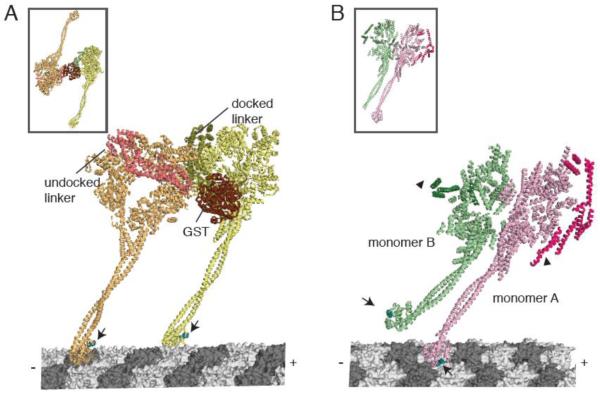
In the yeast dynein structure, the two motor domains are dimerized by the fusion of gluthione S-transferase (GST) N-terminal to where the linker interacts with the ring. This GST-dynein fusion moves processively with a similar run length and velocity to wildtype yeast dynein [15]. In the crystal structure, the two motor domains point away from one another in a pseudo-two-fold symmetry (Figure 6A inset). However, for processive motion, both microtubule binding domains must dock onto the same microtubule. Carter et al. modeled this two-head-bound intermediate with both rings in the same orientation (linker faces pointing to the left as one looks down the microtubule axis towards its minus end) (Figure 6A). However, because of the short tether connecting the linkers to the GST, this state required the N-terminus of the linker in the front head to detach from the ring and move ~8 nm, so that it could point backward to its partner motor domain. It is also possible that the linker completely detaches from the ring to achieve larger step sizes that have been observed for dynein. Based upon the power stroke model of the linker proposed by Roberts et al. [22], this would create a pre-powerstroke state in the leading motor domain and a post-powerstroke state in the rear head. In addition to this dramatic reorientation in the linker, one of the stalks must bend or twist to enable docking of both microtubule binding domains onto the polymer. Another interesting observation that emerged from this modeling is that the two motor domains are unlikely to be bound to the same protofilament due to the thickness of the AAA ring [13]. Therefore, it seems likely that dynein walks on microtubules with its two microtubule binding domains attached to different protofilaments.
Figure 6.
(A) A model for dimeric yeast dynein docked onto microtubules. (Inset shows the dimeric conformation in the crystal structure). The front head (orange) has an undocked linker (salmon) and a small kink in the stalk to accommodate a docked microtubule binding domain conformation. The rear head (yellow) has a docked linker and a straight stalk. The linkers are connected at the N-terminus by a GST dimerization domain. Arrows indicate equivalent positions in the microtubule binding domain (cyan). The linker face of the rear head is facing the C-terminal face of the front head. (B) The Dictyostelium dynein crystal structure (inset), showing the stacked conformation of monomer A (pink) and B (green). Using this crystal structure, monomer A was docked onto the microtubule. The putative conformation of the microtubule binding domain in monomer B was modeled based on the conformation of the distal stalk in monomer A. Arrows indicate equivalent positions in the microtubule binding domain (cyan), which are pointing in different directions. Arrowheads indicate the N-terminus of the linkers that must connect to a dimerization domain. In this configuration, the linkers are on the outside and the C-termini are on the inside of the stacked AAA rings.
The Dictyostelium crystal structure shows a very different arrangement of the two motor domains in the unit cell. The two monomers (which are separate polypeptide chains without a dimerizing domain) are interacting back-to-back (C-terminal domains facing one another); the two rings are essentially stacking on one another through interactions between the C-terminal domain of one head and the small domain face of AAA2-4 of the other head (Figure 6B inset). This interaction might simply reflect the way that the monomers pack in the crystal.
However, Kon et al. [14] suggested that the rings might interact in this conformation during processive movement and that this interaction could allow for communication/coordination between the two motor domains. Indeed, a compact form of the dynein dimer would be expected during processive motility, as dynein mostly takes small steps (8 nm, the minimal subunit spacing along the microtubule), and ring stacking has been observed in axonemal dyneins by electron microscopy [43]. Furthermore, truncation studies show that the processivity of Dictyostelium dynein is enhanced by the presence of its distal C-terminal domain [33], which might be related to a role in a stacking interaction between the two heads. However, in this back-to-back orientation of the rings, the microtubule binding domains would be oriented in opposite directions, thus requiring the stalk in one motor domain to have considerable rotational flexibility in order for its distal microtubule binding domain to interact with the microtubule (Figure 6B).
Thus, in conclusion, the Carter et al. and Kon et al. models propose distinct orientations of the rings (back-to-front and back-to-back) in a processively moving dynein. However, both models have structural challenges for achieving a two-head bound state. The first challenge lies in the position of the linkers; they must have sufficient space to swing with respect to the ring, while still being close enough to be adjoined by a dimerization domain. In the Carter et al. back-to-front orientation of the rings, one of the linkers is sandwiched between the rings, creating possible steric clashes. In the Kon et al. back-to-back stacking of rings, the linkers are pointing in opposite directions on the outside of a double stacked ring and thus must cross a long distance around the side of the rings to form a connected dimer. In addition to joining the linkers into a dimer, a second challenge lies in the simultaneous binding of two microtubule binding domains on the microtubule lattice. For two microtubule binding domains to bind 8 nm apart, the stalks must be rotated in both structures, although the magnitude of rotation is significantly smaller in the Carter et al. model. This implies that there might be considerable flexibility in the stalk, which is also suggested by the variable conformations that the stalk assumes in different crystal structures (Figure 5B). To distinguish between these models derived from crystal structures, it will be important to establish the orientation of the rings and the stalks in a processively moving dynein molecule.
6. Future perspectives
Although recent structural studies have added to our understanding of dynein, many missing pieces of information remain to be elucidated in order to emerge with a comprehensive understanding of dynein motility. In addition to obtaining better diffracting crystals that provide atomic resolution, a crystal structure of an “ATP” and/or “ADP-Pi” state will be needed to reveal the key “pre-stroke” state of the cycle. The current ADP (Dictyostelium) and nucleotide-free (yeast) structures are “post-stroke” states, where the N-terminus of the linker is docked near AAA4/5 and the microtubule binding domain is in a presumed strong binding conformation. Information on both the pre- and post-powerstroke states will be essential for understanding the motility cycle of dynein. But there are likely to be many more important conformations to investigate, since dynein has three additional nucleotide binding sites (AAA2-4) in addition to the main hydrolytic site. Variations in the nucleotide state of these sites are likely to change the conformation of the AAA ring.
The function of the interacting two coiled-coils (the stalk and buttress) and the conformational changes in the stalk are important subjects for further investigation. The current reigning hypothesis of a half-heptad shift in the stalk helices is intriguing, but a long range conformational change in a coiled coil is without clear precedence in the literature and the energy barrier for the proposed half heptad sliding of the helices is not known. Thus, this model requires more direct data to determine whether this or other conformational changes occur during dynein’s ATPase cycle. Such investigations of the dynein stalk will likely provide broader insight into how coiled coils might be used in biological systems. Computational studies as well as designing probes that can measure the conformational changes of the stalk and buttress will be important next steps in this investigation. It also will be interesting to ascertain whether angular changes in the stalk contribute to dynein movement by facilitating a diffusional search of the microtubule binding domain.
Further down the road, it will also be important to understand not only the properties and structure of a minimal dynein motor domain, but also the dynein holoenzyme as it exists in the cell. Several studies have already started to dissect how adaptor proteins such as Lis1 or dynactin regulate dynein ATPase activity [44], processivity [45], and force persistence [40]. Thus, an important frontier lies in reconstituting and obtaining structures of the dynein holoenzyme and complexes with its associated regulatory proteins.
Highlights.
-
➢
An overview and comparison of recent dynein crystal structures.
-
➢
Proposal of allosteric communication mechanisms of dynein based on other AAA proteins.
-
➢
Discussion of models of processive dynein motility on microtubules based on structural evidence.
Acknowledgements
Figures were prepared based on PDB structures 3QMZ and 3AY1. We thank Andrew Carter for helpful discussions that contributed to many of the ideas in this review. This work is supported by an American Heart Association Predoctoral Fellowship to CC, and by an NIH grant to RDV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carol Cho, Department of Cellular and Molecular Pharmacology, University of California, San Francisco.
Ronald D. Vale, The Howard Hughes Medical Institute, University of California, San Francisco.
References
- [1].Gibbons IR, Rowe AJ. Dynein: A Protein with Adenosine Triphosphatase Activity from Cilia. Science. 1965;149:424–426. doi: 10.1126/science.149.3682.424. [DOI] [PubMed] [Google Scholar]
- [2].Vallee RB, Williams JC, Varma D, Barnhart LE. Dynein: An ancient motor protein involved in multiple modes of transport. J Neurobiol. 2004;58:189–200. doi: 10.1002/neu.10314. [DOI] [PubMed] [Google Scholar]
- [3].Kardon JR, Vale RD. Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–865. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neuwald AF, Aravind L, Spouge JL, Koonin EV. AAA+: A class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 1999;9:27–43. [PubMed] [Google Scholar]
- [5].Vale RD. AAA proteins. Lords of the ring. J Cell Biol. 2000;150:F13–19. doi: 10.1083/jcb.150.1.f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Erzberger JP, Berger JM. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu Rev Biophys Biomol Struct. 2006;35:93–114. doi: 10.1146/annurev.biophys.35.040405.101933. [DOI] [PubMed] [Google Scholar]
- [7].Gibbons BH, Gibbons IR. Vanadate-sensitized cleavage of dynein heavy chains by 365-nm irradiation of demembranated sperm flagella and its effect on the flagellar motility. J Biol Chem. 1987;262:8354–8359. [PubMed] [Google Scholar]
- [8].Kon T, Nishiura M, Ohkura R, Toyoshima YY, Sutoh K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry. 2004;43:11266–11274. doi: 10.1021/bi048985a. [DOI] [PubMed] [Google Scholar]
- [9].Cho C, Reck-Peterson SL, Vale RD. Regulatory ATPase sites of cytoplasmic dynein affect processivity and force generation. J Biol Chem. 2008;283:25839–25845. doi: 10.1074/jbc.M802951200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Silvanovich A, Li MG, Serr M, Mische S, Hays TS. The third P-loop domain in cytoplasmic dynein heavy chain is essential for dynein motor function and ATP-sensitive microtubule binding. Mol Biol Cell. 2003;14:1355–1365. doi: 10.1091/mbc.E02-10-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burgess SA, Walker ML, Sakakibara H, Knight PJ, Oiwa K. Dynein structure and power stroke. Nature. 2003;421:715–718. doi: 10.1038/nature01377. [DOI] [PubMed] [Google Scholar]
- [12].Koonce MP. Identification of a microtubule-binding domain in a cytoplasmic dynein heavy chain. J Biol Chem. 1997;272:19714–19718. doi: 10.1074/jbc.272.32.19714. [DOI] [PubMed] [Google Scholar]
- [13].Carter AP, Cho C, Jin L, Vale RD. Crystal structure of the dynein motor domain. Science. 2011;331:1159–1165. doi: 10.1126/science.1202393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kon T, Sutoh K, Kurisu G. X-ray structure of a functional full-length dynein motor domain. Nature structural & molecular biology. 2011;18:638–642. doi: 10.1038/nsmb.2074. [DOI] [PubMed] [Google Scholar]
- [15].Reck-Peterson SL, Yildiz A, Carter AP, Gennerich A, Zhang N, Vale RD. Single-molecule analysis of dynein processivity and stepping behavior. Cell. 2006;126:335–348. doi: 10.1016/j.cell.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hook P, Mikami A, Shafer B, Chait BT, Rosenfeld SS, Vallee RB. Long-range allosteric control of cytoplasmic dynein ATPase activity by the stalk and C-terminal domains. J Biol Chem. 2005;18:18. doi: 10.1074/jbc.M504693200. [DOI] [PubMed] [Google Scholar]
- [17].Nishiura M, Kon T, Shiroguchi K, Ohkura R, Shima T, Toyoshima YY, Sutoh K. A single-headed recombinant fragment of Dictyostelium cytoplasmic dynein can drive the robust sliding of microtubules. J Biol Chem. 2004;279:22799–22802. doi: 10.1074/jbc.M313362200. [DOI] [PubMed] [Google Scholar]
- [18].Garbarino JE, Gibbons IR. Expression and genomic analysis of midasin, a novel and highly conserved AAA protein distantly related to dynein. BMC Genomics. 2002;3:18. doi: 10.1186/1471-2164-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ulbrich C, Diepholz M, Bassler J, Kressler D, Pertschy B, Galani K, Bottcher B, Hurt E. Mechanochemical removal of ribosome biogenesis factors from nascent 60S ribosomal subunits. Cell. 2009;138:911–922. doi: 10.1016/j.cell.2009.06.045. [DOI] [PubMed] [Google Scholar]
- [20].Samso M, Radermacher M, Frank J, Koonce MP. Structural characterization of a dynein motor domain. J Mol Biol. 1998;276:927–937. doi: 10.1006/jmbi.1997.1584. [DOI] [PubMed] [Google Scholar]
- [21].Burgess SA, Walker ML, Sakakibara H, Oiwa K, Knight PJ. The structure of dynein-c by negative stain electron microscopy. J Struct Biol. 2004;146:205–216. doi: 10.1016/j.jsb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- [22].Roberts AJ, Numata N, Walker ML, Kato YS, Malkova B, Kon T, Ohkura R, Arisaka F, Knight PJ, Sutoh K, Burgess SA. AAA+ Ring and linker swing mechanism in the dynein motor. Cell. 2009;136:485–495. doi: 10.1016/j.cell.2008.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Glynn SE, Martin A, Nager AR, Baker TA, Sauer RT. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell. 2009;139:744–756. doi: 10.1016/j.cell.2009.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bochtler M, Hartmann C, Song HK, Bourenkov GP, Bartunik HD, Huber R. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature. 2000;403:800–805. doi: 10.1038/35001629. [DOI] [PubMed] [Google Scholar]
- [25].Kon T, Mogami T, Ohkura R, Nishiura M, Sutoh K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nat Struct Mol Biol. 2005;12:513–519. doi: 10.1038/nsmb930. [DOI] [PubMed] [Google Scholar]
- [26].Grum VL, Li D, MacDonald RI, Mondragon A. Structures of two repeats of spectrin suggest models of flexibility. Cell. 1999;98:523–535. doi: 10.1016/s0092-8674(00)81980-7. [DOI] [PubMed] [Google Scholar]
- [27].Gee MA, Heuser JE, Vallee RB. An extended microtubule-binding structure within the dynein motor domain. Nature. 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- [28].Gibbons IR, Garbarino JE, Tan CE, Reck-Peterson SL, Vale RD, Carter AP. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J Biol Chem. 2005;280:23960–23965. doi: 10.1074/jbc.M501636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kon T, Imamula K, Roberts AJ, Ohkura R, Knight PJ, Gibbons IR, Burgess SA, Sutoh K. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nat Struct Mol Biol. 2009;16:325–333. doi: 10.1038/nsmb.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carter AP, Garbarino JE, Wilson-Kubalek EM, Shipley WE, Cho C, Milligan RA, Vale RD, Gibbons IR. Structure and functional role of dynein’s microtubule-binding domain. Science. 2008;322:1691–1695. doi: 10.1126/science.1164424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].King SM. AAA domains and organization of the dynein motor unit. J Cell Sci. 2000;113:2521–2526. doi: 10.1242/jcs.113.14.2521. [DOI] [PubMed] [Google Scholar]
- [32].Lee S, Sowa ME, Watanabe YH, Sigler PB, Chiu W, Yoshida M, Tsai FT. The structure of ClpB: a molecular chaperone that rescues proteins from an aggregated state. Cell. 2003;115:229–240. doi: 10.1016/s0092-8674(03)00807-9. [DOI] [PubMed] [Google Scholar]
- [33].Numata N, Shima T, Ohkura R, Kon T, Sutoh K. C-sequence of the Dictyostelium cytoplasmic dynein participates in processivity modulation. FEBS letters. 2011;585:1185–1190. doi: 10.1016/j.febslet.2011.03.036. [DOI] [PubMed] [Google Scholar]
- [34].Imamula K, Kon T, Ohkura R, Sutoh K. The coordination of cyclic microtubule association/dissociation and tail swing of cytoplasmic dynein. PNAS. 2007;104:16134–16139. doi: 10.1073/pnas.0702370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bowman GD, O’Donnell M, Kuriyan J. Structural analysis of a eukaryotic sliding DNA clamp-clamp loader complex. Nature. 2004;429:724–730. doi: 10.1038/nature02585. [DOI] [PubMed] [Google Scholar]
- [36].Yu RC, Hanson PI, Jahn R, Brunger AT. Structure of the ATP-dependent oligomerization domain of N-ethylmaleimide sensitive factor complexed with ATP. Nat Struct Mol Biol. 1998;5:803–811. doi: 10.1038/1843. [DOI] [PubMed] [Google Scholar]
- [37].Kikushima K, Yagi T, Kamiya R. Slow ADP-dependent acceleration of microtubule translocation produced by an axonemal dynein. FEBS Lett. 2004;563:119–122. doi: 10.1016/S0014-5793(04)00278-9. [DOI] [PubMed] [Google Scholar]
- [38].Chen B, Doucleff M, Wemmer DE, De Carlo S, Huang HH, Nogales E, Hoover TR, Kondrashkina E, Guo L, Nixon BT. ATP ground- and transition states of bacterial enhancer binding AAA+ ATPases support complex formation with their target protein, sigma54. Structure. 2007;15:429–440. doi: 10.1016/j.str.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Burrows PC, Joly N, Cannon WV, Camara BP, Rappas M, Zhang X, Dawes K, Nixon BT, Wigneshweraraj SR, Buck M. Coupling sigma factor conformation to RNA polymerase reorganisation for DNA melting. J Mol Biol. 2009;387:306–319. doi: 10.1016/j.jmb.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].McKenney RJ, Vershinin M, Kunwar A, Vallee RB, Gross SP. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–314. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xie P, Dou SX, Wang PY. Model for unidirectional movement of axonemal and cytoplasmic dynein molecules. Acta Biochim Biophys Sin (Shanghai) 2006;38:711–724. doi: 10.1111/j.1745-7270.2006.00223.x. [DOI] [PubMed] [Google Scholar]
- [42].Carter AP, Vale RD. Communication between the AAA+ ring and microtubule-binding domain of dynein. Biochem Cell Biol. 2010;88:15–21. doi: 10.1139/o09-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nicastro D, Schwartz C, Pierson J, Gaudette R, Porter ME, McIntosh JR. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- [44].Mesngon MT, Tarricone C, Hebbar S, Guillotte AM, Schmitt EW, Lanier L, Musacchio A, King SJ, Smith DS. Regulation of cytoplasmic dynein ATPase by Lis1. J Neurosci. 2006;26:2132–2139. doi: 10.1523/JNEUROSCI.5095-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kardon JR, Reck-Peterson SL, Vale RD. Regulation of the processivity and intracellular localization of Saccharomyces cerevisiae dynein by dynactin. PNAS. 2009;106:5669–5674. doi: 10.1073/pnas.0900976106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Rappas M, Schumacher J, Beuron F, Niwa H, Bordes P, Wigneshweraraj S, Keetch CA, Robinson CV, Buck M, Zhang X. Structural insights into the activity of enhancer-binding proteins. Science. 2005;307:1972–1975. doi: 10.1126/science.1105932. [DOI] [PMC free article] [PubMed] [Google Scholar]