Abstract
Benfotiamine, a lipid-soluble analogue of vitamin B1, is a potent anti-oxidant that is used as a food supplement for the treatment of diabetic complications. Our recent study indicates a novel role of benfotiamine in the prevention of bacterial endotoxin, lipopolysaccharide (LPS)-induced cytotoxicity and inflammatory response in murine macrophages. Nevertheless, it remains unclear how benfotiamine mediates anti-inflammatory effects. In this study, we investigated the anti-inflammatory role of benfotiamine in regulating the arachidonic acid (AA) pathway generated inflammatory lipid mediators in RAW 264.7 macrophages. Benfotiamine prevented the LPS-induced activation of cPLA2 and release of AA metabolites such as leukotrienes (LTB4), prostaglandin E2 (PGE2), thromboxanes 2 (TXB2) and prostacyclin (PGI2) in macrophages. Further, LPS-induced expressions of AA metabolizing enzymes such as COX-2, LOX-5, TXB synthase and PGI2 synthase were significantly blocked by benfotiamine. Furthermore, benfotiamine prevented the LPS-induced phosphorylation of ERK1/2 and expression of transcription factors NF-kB, and Egr-1. Benfotiamine also prevented the LPS-induced oxidative stress and protein-HNE adducts formation. Most importantly, as compared to specific COX-2 and LOX-5 inhibitors, benfotiamine significantly prevented the LPS-induced macrophage death and monocytes adhesion to endothelial cells. Thus, our studies indicate that the dual regulation of COX and LOX pathways in AA metabolism could be a novel mechanism by which benfotiamine exhibits its potential anti-inflammatory response.
Keywords: Vitamin B1, inflammation, arachidonic acid, Cox and Lox
Introduction
Overwhelming inflammatory responses accompanied by deregulated immunological and coagulation functions results in multiple organ failure leading to septic shock a major cause of mortality [1]. Although, treatment with the antibiotics kills bacteria and impedes the infection, the bacterial debris which contains large amounts of endotoxins could trigger the inflammatory responses [2]. Bacterial lipopolysaccharide (LPS), the structural component of the Gram-negative bacterial outer cell wall, is a potent initiator of inflammatory response during most commonly seen bacterial infections [3]. Binding of LPS to its cognate CD14 receptor on the monocyte/macrophage cell membranes induces the release of various pro-inflammatory cytokines and chemokines [4-6], which are implicated in the pathogenesis of the major inflammatory complications such as sepsis [7]. Although a number of non-steroidal anti-inflammatory drugs (NSAID) and antioxidants recently emerged as potential therapeutic targets for intervention of sepsis complications [8], their success rate at the translational studies is low [9-11]. Therefore, identification of novel therapeutic compounds with both anti-oxidative and anti-inflammatory properties could yield potential therapeutic approaches to sepsis and other related inflammatory complications.
Thiamine (vitamin B1) has been used for decades for the treatment of several disorders such as neurological, diabetic, and cardiovascular complications [12-15]. Since, thiamine is water-soluble, it cannot be stored in the body for so long and is rapidly excreted in the urine. The bioavailability of orally administered thiamine salt is comparatively low when compared with fat-soluble analogs [16-18]. In 1954, variety of lipid-soluble thiamine derivatives were discovered, subsequently they were named as allithiamines since they belong to Allium family of vegetables such as crushed garlic, onions and leeks etc [19]. These lipid-soluble thiamine derivatives have much higher absorption and bioavailability than water-soluble thiamine salts [20, 21]. Benfotiamine, one of the lipid-soluble derivatives of vitamin B1 was found to be the most effective antioxidant and was developed to improve bioavailability for pharmacological administration [22, 23]. Benfotiamine contains an open thiazole ring, which allows it to pass through the cell membrane, and the open ring is closed by undergoing reduction reaction making biologically active thiamine [21]. Oral administration of benfotiamine raises thiamine levels in blood and tissues to a much higher degree than the water-soluble salts [20, 21]. We have recently shown that benfotiamine prevents LPS-induced NF-kB –dependent expression of inflammatory cytokines and chemokines in macrophages. We have also shown that benfotiamine prevents LPS-induced generation of reactive oxygen species (ROS) and macrophage death in vitro [24]. Further, we have also demonstrated that benfotiamine prevents LPS-induced ocular inflammatory complication, uveitis, in rats [25]. Thus, our previous studies identified benfotiamine as a novel anti-inflammatory compound. However, the molecular mechanisms through which benfotiamine exert its anti-inflammatory effect is not clear.
Recent studies indicate that LPS-stimulated macrophages lead to the activation of phospholipase A2 (cPLA2) which causes the release of AA from the cell membrane [26-28]. AA (20:4, n-6) is esterified in glycerol in the lipid bilayer of cell membrane at sn-2 position [26]. Once AA is released it is further metabolized via COX-2 and LOX-5 pathways. COX-2 catalyzed AA pathway leads the generation of prostanoids such as prostaglandins (PGs), prostacyclins (PGI2), and thromboxanes (TXBs) which have been shown to be most potent inflammatory mediators. LOX-5 catalyzed AA pathway result in the generation of leukotrienes (LTBs) involved in several inflammatory diseases such as asthma pathogenesis [29, 30]. Even though, the specific inhibitors of COX-2 and LOX-5 have been shown to be anti-inflammatory, they are not much effective, since they only stunt either COX or LOX pathway moving AA metabolism towards the other pathway [31]. Therefore, in order to identify a possible mechanism by which benfotiamine acts as an anti-inflammatory agent, in this study, we have examined the efficacy of benfotiamine in regulating the AA pathway generated inflammatory lipid mediators in RAW264.7 macrophages. Our results suggest that benfotiamine drastically inhibited the release of AA metabolites by inhibiting the activation of cPLA2. Benfotiamine also prevented the LPS-induced COX-2 and LOX-5 enzymes and generation of PGE2, TXB and LTB4. Further, benfotiamine also prevented the LPS-induced protein-HNE adducts and lipid hydroperoxides formation in macrophages. Thus, our results indicate that benfotiamine could be a novel anti-inflammatory agent which can ameliorate endotoxin induced inflammation by preventing both COX and LOX pathways in the AA metabolism.
Materials and Methods
Materials
Dulbecco’s modified Eagle’s medium with 4 mM l-glutamine and 4.5 g/l glucose, phosphate-buffered saline (PBS), penicillin/streptomycin solution, trypsin, and fetal bovine serum (FBS) were purchased from Invitrogen Gibco (Grand Island, NY). [5, 6, 8, 9, 11, 12, 14, 15-3H (N)] arachidonic acid was purchased from NEN Life Science (Boston, MA). RIPA cell lysis buffer, antibodies against LOX-5, PGI2 synthase and β-actin were purchased from Santa Cruz biotechnology, (Santa Cruz, CA) USA. Antibodies against cPLA2 were obtained from Cell Signaling Inc. (Beverly, MA). Antibodies against Thromboxane synthase and COX-2 were obtained from Abcam Inc. (Cambridge, MA). Thromboxane B2 and leukotriene B4 EIA kits and REV-590 were obtained from Cayman chemical Inc. (Ann Arbor, MI). Prostaglandin E2 (PGE2) and prostacyclin (PGI2) assay kits were obtained from Assay Designs, Inc (Ann Arbor, MI). Benfotiamine, lipopolysaccharide (Escherichia coli 0111:B4), indomethacin and the reagents used in Western blot analysis were obtained from Sigma (St. Louis, MO). All other reagents used were of analytical grade.
Cell Culture
RAW264.7 macrophages obtained from American Type Culture Collection (Manassas, VA) were grown in DMEM containing 10% FBS, 1% penicillin/streptomycin in a 95% air 5% CO2-humidified atmosphere at 37°C. Macrophages were pretreated with benfotiamine with either 50 or 100 μM or carrier for overnight in serum-free medium and subsequently stimulated with 1 μg/ml LPS for indicated time periods.
Release of arachidonic acid and its metabolites in RAW 264.7 cells culture medium
RAW264.7 cells were seeded in 12-well plate at the density of ~0.35 ×106 cells/well, without or with benfotiamine (100 μM) in the complete growth media. Media were removed and replaced with 1 ml of serum-free DMEM containing 0.1 μCi/ml 3H-arachidonic acid and incubated for 16 h. The cells were washed twice with phosphate-buffered saline (PBS) containing 0.1% bovine serum albumin to remove unadsorbed arachidonic acid and stimulated with 1 μg/ml LPS for 1 h. Subsequently, the cells were incubated with fresh medium containing benfotiamine (100 μM) for additional 18 h. The culture medium was collected and centrifuged for 15 min at 10,000 rpm and supernatant was used to measure the radioactivity using a Beckman liquid scintillation counter (Beckman Coulter, Fullerton, CA).
Western Blot Analysis
The confluent macrophages were incubated without or with benfotiamine (50 or 100 μM), followed by treatment with 1 μg/ml LPS for 18 h. The cells were washed twice with PBS and lysed in an ice-cold RIPA lysis buffer. The crude lysates were cleared by centrifugation at 12,000g for 10 min at 4°C. Equal amounts of cell lysates (30-50 μg) were separated on 10 % SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Immobilon; Millipore, Bedford, MA). The membranes were incubated in blocking solution containing 5% w/v dried fat-free milk and 0.1% v/v Tween-20 in Tris-buffered saline. Subsequently, the membranes were incubated with specific antibodies against cPLA2, COX-2, LOX-5, TXB synthase and PGI2 synthase. The blots were then washed, exposed to HRP-conjugated secondary antibodies (1:5,000 dilution) for 1 h, and the antigen-antibody complex was detected by enhanced chemiluminescence (Pierce, Rockford, IL). The membranes were stripped and probed with antibodies against glyceraldehyde-3-phosphate dehydrogenase (GAPDH) to ensure equal protein loading. Fold changes in the band intensities were quantified by densitometry analysis by using Kodak Image Station software.
Determination of PGE2, 6k-PGF1α (PGI2), TXB2, LTB4 and cPLA2 levels
The RAW cells (~0.3×106 cells/well in 6-well plates) were growth-arrested in the serum-free medium without or with benfotiamine (100 μM) followed by incubation with 1 μg LPS/ml for another 18 h. The medium was collected from each well and cleared by centrifugation (5000 rpm; 5 min). The levels of PGE2 and PGI2 were determined using respective assay kits according to the manufacturer’s instructions (Assay Designs, Inc.). For determining the levels of TXB2, LTB4 and cPLA2 specific ELISA kits were used according to the manufacturer’s instructions (Cayman chemical).
Reverse transcription-PCR
The RAW264.7 cells were pre-incubated without and with benfotiamine for overnight followed by incubation with 1 μg/ml LPS for additional 6 h. Total RNA was isolated by using RNeasy® Mini Kit from Qiagen, USA. Equal amount of RNA was used for RT-PCR using Qiagen® one step RT-PCR kit. The gene specific primer (purchased from sigma; Sigma Genosys, USA) sequences were; COX-2, sense: 5′-GGT TAC AAA AGC TGG GAA GC-3′, anti-sense: 5′-GGG GGT TCC GAG TTT ATA CT-3′. LOX-5, sence: 5′-GGC ACC GAC GAC TAC ATC TAC-3′, anti-sense: 5′-CAA TTT TGC ACG TCC ATC CC-3′; TXB synthase, sense: 5′-ATC AGC CAA GCC TGT GAA CT-3′, anti-sense: 5′-CCT CTC TTC TGC TGC TTG CT-3′ and GAPDH, sense: 5- TCC ACT CAC GGC AAA TTC AAC G-3′, anti-sense 5′- TAG ACT CCA CGA CAT ACT CAG C-3′. The PCR reaction was carried out in a GeneAmp 2700 thermocycler (Applied Biosystems, Foster City, CA) under the following conditions: initial denaturation at 95°C for 15 min; 30 cycles of 94°C 30 s, 62°C 30 s, 72°C 1 min, and then 72°C 5 min for final extension. PCR products were electrophoresed with 1 % agarose-1xTAE gels containing 0.5 μg/ml ethidium bromide. The band densities were analyzed using Kodak image station.
Determination of Lipid hydroperoxides
The lipid hydroperoxides were determined colorimetrically by using a lipid hydroperoxides assay kit obtained from Cayman chemicals as per the supplier’s instructions. Briefly, lipid hydroperoxides kit measures the hydroperoxides directly utilizing the redox reactions with ferrous ions. Lipid hydroperoxides were extracted from the samples into chloroform. To 450μl of chloroform-methanol solvent and 50μl of chromogen was added, kept at room temperature for 5 min, and absorbance was read at 500 nm using a 96 well plate reader.
Protein-HNE Adducts formation
RAW264.7 cells were seeded on glass chamber slides without or with benfotiamine (100 μM) followed by incubation with 1 μg LPS/ml for 18 h. The slides were fixed with 4% paraformaldehyde for 30 min. After the slides were thoroughly washed with Tris-buffered saline (TBS) containing 0.1% Triton X-100, they were incubated with anti-HNE antibodies for overnight at 4° C followed by washing and incubation with 1:160 diluted secondary antibodies (goat anti-rabbit IgG conjugated with FITC) for 1 h. The slides were mounted and photographs were taken with a Nikon fluorescence microscope.
Macrophage viability and U-937 cell adhesion to HUVECs
The RAW cells (0.3×106 cells/well in 6-well plates) were growth arrested in the serum-free medium without or with benfotiamine (100 μM), indomethacin (COX-2 inhibitor, 10 μM) and REV 5901 (LOX-5 inhibitor, 10 μM) for overnight followed by incubation with 1 μg/ml LPS for another 24 h. Cell viability was determined using Millipore cell counter. For cell adhesion HUVECs were plated in 96-well plate, grown to 70% confluence, and treated without or with benfotiamine, indomethacin and REV 5901 for overnight in the absence and presence of 1 ug/ml of LPS. Cells were rinsed twice with serum-free medium and U-937 cells were added to each well followed by incubation with 1 μg/ml LPS for additional 12 h. MTT assay was performed to determine cell adhesion at recoding the absorbance at 562 nm.
Statistical analysis
Data presented as mean ± SE (n=6) and p-values were determined by unpaired Student’s t test using Microsoft Office Excel 2007 software. P<0.05 was considered as statistically significant.
Results
Benfotiamine prevents release of arachidonic acid metabolism in LPS-induced macrophages
To investigate the anti-inflammatory effect of benfotiamine against LPS-induced toxicity; at first, we have examined metabolism of AA in macrophages. Macrophages were incubated with 3H-AA followed by stimulation with LPS with or without benfotiamine. Results shown in the Fig. 1 indicate that in the LPS treated macrophages the release of pre-labeled AA and its metabolites were significantly increased to ~5-fold as compared to un-stimulated macrophages. Benfotiamine significantly (~90%) prevented the LPS-induced formation of AA metabolites. Thus, our studies indicate that benfotiamine prevents LPS-induced AA metabolism in macrophages.
Figure 1.
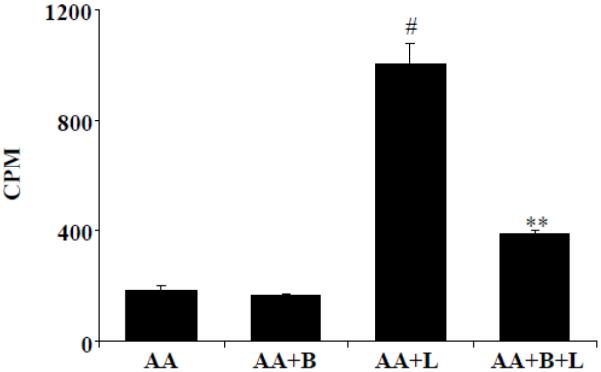
Benfotiamine prevents the release of arachidonic acid (AA) metabolites from LPS-stimulated RAW264.7 cells. The RAW254.7 cells were incubated with 3H-AA (0.1 μCi/ml) as described in methods and stimulated with 1 μg/ml LPS for 1 h. The cells were washed and incubated for 18 h in a fresh medium containing benfotiamine (100 μM). The release of metabolites in the medium was determined by measuring the radioactivity in the culture medium. Data represent mean ± S.E. (n=6). #p <0.0001 as compared with AA alone, **p<0.0001 as compared with AA+LPS-treated cells; AA; Arachidonic acid, L; LPS, B; Benfotiamine.
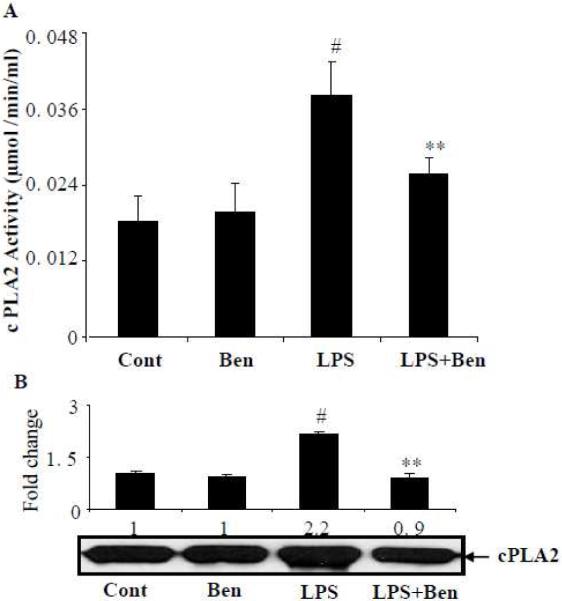
Benfotiamine prevents LPS-induced activation of cPLA2 in macrophages
Phospholipase A2, especially cPLA2 is well known to mediate the release of AA from membrane phospholipids; we next examined the potential of benfotiamine in preventing the activation of cPLA2 in LPS-induced macrophages. Stimulation of macrophages with LPS caused an approximately 2-fold increase in the cPLA2 activity and pre-incubation of macrophages with benfotiamine significantly (>70%) prevented the LPS-induced activity of cPLA2 (Fig 2A). Further, the results shown in Fig 2B indicate that LPS-induced approximately 2-fold increase in cPLA2 expression. The LPS-induced increase in the expression of cPLA2 was significantly (>90%) prevented by benfotiamine. Thus, our results suggest that benfotiamine prevents LPS-induced AA metabolism by preventing the expression of cPLA2.
Figure 2.
Benfotiamine prevents LPS-induced generation of cPLA2 in RAW264.7 macrophages. The RAW cells were growth-arrested in a medium containing 0.1% serum without or with benfotiamine (100 μM) and challenged with 1 μg/ml LPS. The cPLA2 levels and expression were determined in cell homogenate using the monoclonal enzyme immunoassay kit and western blotting, respectively. Data represent mean ± S.E. (n=6). #p<0.0001 Vs control; **p<0.0001 Vs LPS treated cells. Cont; Control, Ben; benfotiamine.
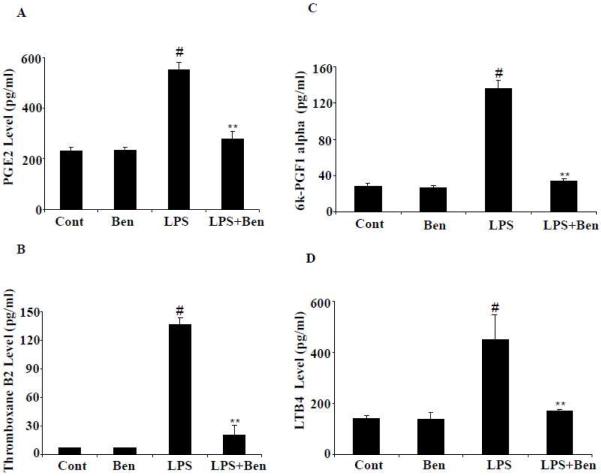
Effect of benfotiamine on LPS-induced production of AA pathway- generated lipid mediators in RAW264.7 macrophages
Since PGs, TXBs, LTBs and 6k-PGF1α are AA pathway-generated lipid mediators which play a major role in inflammatory complications, we next examined the effect of benfotiamine on LPS-induced generation of PGE2, TXB2, 6k-PGF1α and LTB4 in cell culture media. LPS caused >3-fold and >20-fold increase in the levels of PGE2 and TXB2, respectively, in the macrophages as compared to untreated macrophages. The increases in PGE2 and TXB2 levels were significantly brought down by benfotiamine (Fig. 3A and B). Since, PGI2 has a very short half-life and is readily converted to 6k-PGF1α, we next determined 6 k-PGF1α levels in RAW264.7 macrophages. Macrophages treated with LPS showed a significant (>4 fold) increase in the levels of 6k-PGF1α which was significantly prevented by benfotiamine (Fig. 3 C). Further, we have also measured the effect of benfotiamine on LPS-induced LTB4 levels in macrophages. As shown in Fig. 3D, RAW264.7 cells stimulated with LPS showed a >3 fold increase in the level of LTB4 which was significantly (>60%) reduced in cells treated with benfotiamine. These results suggest that benfotiamine could efficiently prevent LPS-induced production of AA pathway- generated inflammatory metabolites in RAW264.7 macrophages.
Figure 3.
Effect of benfotiamine on LPS-induced production of PGE2, LTB4, TXB2 and 6k-PGF1α and in RAW264.7 macrophages. A-D) The RAW cells were growth-arrested in Dulbecco’s modified Eagle’s medium containing 0.1% serum with or without benfotiamine (100 μM) for overnight and challenged with 1 μg/ml LPS for 18 h. The levels of PGE2, LTB4, TXB2 and 6k-PGF1α were determined in the culture medium by using the monoclonal enzyme immune assay kits as per manufacturer’s instructions. Data represent mean ± S.E. (n=6). #p<0.0001 Vs control cells; ** p <0.0001 Vs LPS-treated cells. Cont; control, Ben; Benfotiamine.
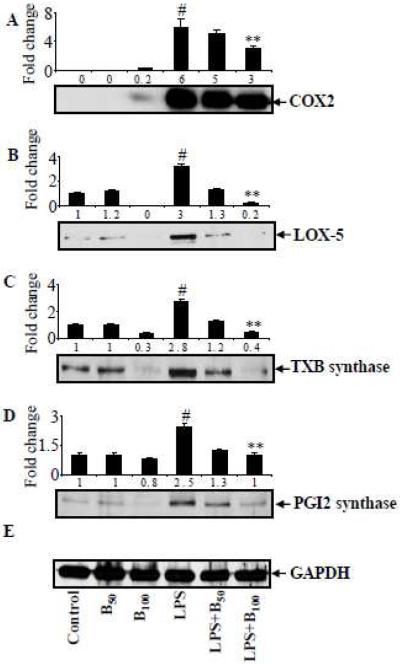
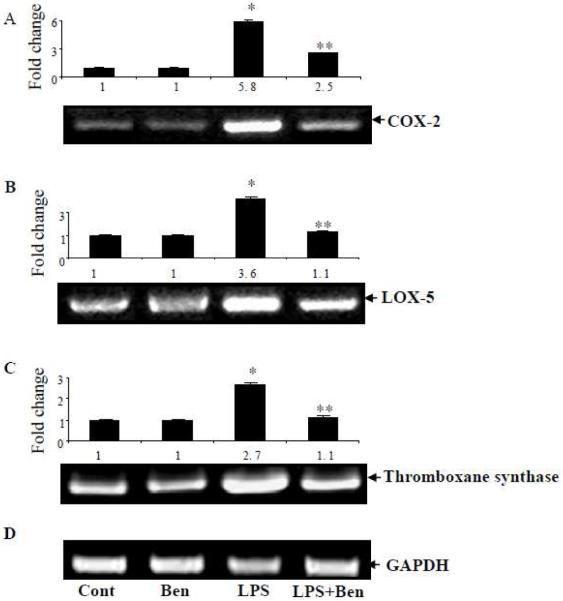
Benfotiamine prevents LPS-induced activation of AA pathway enzymes in macrophages
Since PGs, TXs, LTBs and 6k-PGF1α are catalyzed by COX-2, TXB synthase, PGI2 synthase and LOX-5, respectively in the AA metabolism pathway, we next examined the effect of benfotiamine on LPS-induced expression of these proteins in macrophages. Our results shown in Fig 4 A-D indicate that LPS-induced approximately 6-fold increase in COX-2, >3 fold in LOX-5, 3-fold in TXB synthase and 2.5 fold in PGI2 synthase expressions in macrophages. The increases in COX-2, LOX-5, TXB synthase and PGI2 synthase were significantly abolished in the presence of benfotiamine by 50%, 95%, 95% and 90%, respectively. Further, to examine the effect of benfotiamine on LPS-induced AA metabolism enzymes at the transcriptional levels, we next measured the effect of benfotiamine on the LPS-induced mRNA levels of AA pathway enzymes. The results shown in the figure 5A-C indicate that LPS caused a significant 6-, 3.6- and 3-folds increase in the levels of COX-2, LOX-5 and TXB synthase mRNA levels, respectively. However, in the macrophages pretreated with benfotiamine followed by LPS treatment, the increase in the mRNA levels of COX-2, LOX-5 and TXB synthase were significantly (60 to 90%) decreased. Thus, our studies indicate that benfotiamine prevents the expression of AA pathway enzymes at their transcriptional level.
Figure 4.
Benfotiamine prevents LPS-induced expression of COX2, LOX-5, TXB synthase and PGI2 synthase in RAW cells. The RAW cells were growth-arrested by incubating in 0.1% FBS medium without or with benfotiamine (100 μM), and stimulated with 1 μg/ml LPS for 18 h. Cytosolic extracts were prepared and equal amounts of cytosolic proteins were subjected to Western blot analysis using antibodies against (A) COX-2, (B) LOX-5 (C) TXB synthase (D) PGI2 synthase and (E) GAPDH. A representative blot from each group is shown. Data represent mean ± S.E. (n=3). #p<0.0001 Vs control; ** p <0.0001 Vs LPS treated cells. B-50 and 100; Benfotiamine 50 and 100μM.
Figure 5.
Effect of benfotiamine on LPS-induced COX2, LOX-5 and TXB synthase at transcriptional level in RAW macrophages. Macrophages were growth-arrested in Dulbecco’s modified Eagle’s medium containing 0.1% serum with or without benfotiamine (100 μM) overnight and challenged with 1 μg/ml LPS for 6 h. The total RNA was isolated and reverse transcriptase-PCR analysis was carried out using specific primers for COX-2, LOX-5 and TXB synthase. Equal amount of PCR products were electrophoresed with 1% agarose-TAE gels containing ethidium bromide. GADPH served as control in reverse transcriptase-PCR analysis. A representative blot from each group is shown. Data represent mean ± S.E. (n=3).* p<0.0001 Vs control; ** p <0.0001 Vs LPS treated cells. Cont; control, Ben; Benfotiamine.
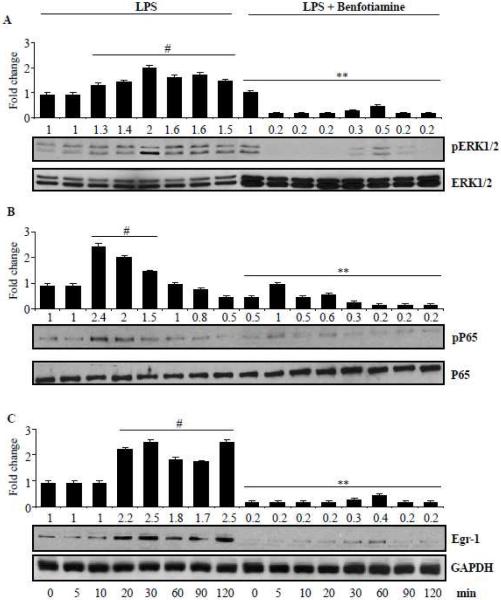
Effect of benfotiamine on phosphorylation of ERK1/2 and expression of transcription factors NF-kB and Egr-1
Since, transcription factors such as NF-kB and Egr-1 are known to regulate the transcriptional regulation of AA pathway enzymes; we next examined the effect of benfotiamine on LPS-induced activation of these transcription factors. Our results shown in the Fig 6 A-C indicate that benfotiamine significantly prevented the LPS-induced activation ERK1/2 and the transcription factors NF-kB and Egr-1 which transcribe gene for COX-2 and mPGES-1 [32]. Thus, our results suggest that benfotiamine prevents LPS-induced expression of AA pathway enzymes by preventing their transcriptional activation.
Figure 6.
Effect of benfotiamine on ERK1/2 phosphorylation and avtivation of p65 and Egr-1. Growth-arrested murine macrophages were treated with LPS without and with benfotiamine (100 μM) for indicated times at 37°C. Cell lysates were prepared and equal amounts of protein were subjected to western blot analysis using antibodies against A) ERK1/2, B) p65 and C) Egr-1. A representative blot from each group is shown. Data represent mean ± S.E. (n=3). # p<0.0001 Vs control; **p <0.0001 Vs LPS treated cells.
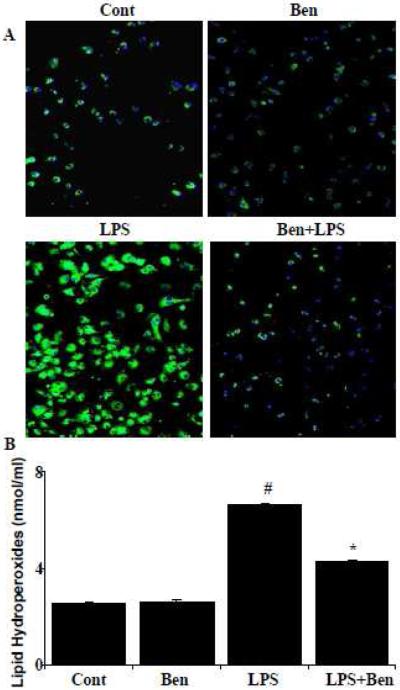
Effect of benfotiamine on LPS-induced protein-HNE adduct and lipid hydroperoxides formation
Since transcription factors such as NF-kB, Egr-1 are known to be activated under oxidative stress generated reactive oxygen species, we next examined the effect of benfotiamine on LPS-induced oxidative stress. The levels of protein-HNE and lipid hydroperoxides were used as markers for increased oxidative stress. Our results shown in fig. 7A indicate that the benfotiamine ameliorated protein-HNE adduct formation in LPS-induced macrophages. Further, macrophages challenged with LPS showed significantly (>2.5 fold) increased lipid hydroperoxides levels which were significantly (60%) decreased in the macrophages pretreated with benfotiamine (Fig.7B), suggesting that benfotiamine prevents LPS-induced activation of redox sensitive transcription factors by decreasing cellular oxidative stress in macrophages.
Figure 7.
Effect of benfotiamine on LPS-induced protein-HNE adduct and lipid hydroperoxides formation. Macrophages were growth arrested in 0.1 % FBS and with or without benfotiamine (100μM) subsequently, the cells were exposed to LPS (1μg/ml) for 24 h. (A) Protein-HNE adduct formation was evaluated using rabbit anti-protein-HNE antibodies and FITC-labeled anti-rabbit IgG in goat. A representative blot is shown (200 X magnification). (B) Lipid hydroperoxides were measured using a microplate assay kit according to the manufacturer’s instructions. Data represent mean ± S.E. (n=6). #p<0.0001 Vs control cells; **p <0.0001 Vs LPS treated cells. Cont; control, Ben; Benfotiamine.
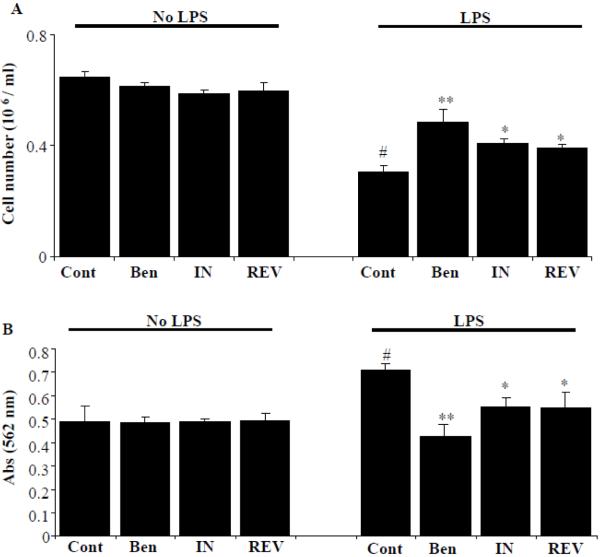
Effect of benfotiamine and specific inhibitors of COX-2 and LOX-5 on LPS-induced macrophage viability and U-937 cell adhesion to HUVECs
Since, our previous results indicate that benfotiamine prevents both COX-2 and LOX-5 enzymes; we next examined the dual inhibitory activity of benfotiamine on LPS-induced macrophage death and compared with the specific inhibitors of COX and LOX enzymes. Benfotiamine, indomethacin (COX-2 inhibitor) and REV 5901 (LOX-5 inhibitor) were pre-incubated with macrophages followed by stimulation with LPS for another 24 h and cell viability was measured by using an automatic electronic cell counter (Scepter, Millipore). LPS-caused a significant cell death and all three inhibitors prevented the LPS-induced cell death (Fig 8A). However, out of all three agents, benfotiamine significantly prevented (~70%) LPS-induced cell death followed by COX-2 inhibitor (40%) and LOX-5 inhibitor (30%). Further, benfotiamine significantly prevented the LPS-induced monocyte adhesion (~70%) to endothelial cells as compared to COX-2 or LOX-5 inhibitors (Fig 8B). Thus, our results indicate that benfotiamine could be a novel anti-inflammatory agent as compared to specific COX-2 and LOX-5 inhibitors, since it could efficiently prevent LPS-induced generation of inflammatory lipid mediators by inhibiting both the COX and LOX enzymes.
Figure 8.
Effect of benfotiamine and specific inhibitors of COX-2 and LOX-5 on LPS-induced macrophage viability and monocyte adhesion to endothelial cells. A) Growth-arrested macrophages were pre-incubated in the presence or absence of benfotiamine (100μM), COX-2 inhibitor indomethacin (10μM) and LOX-5 inhibitor REV 5901 (10μM) for overnight at 37°C. (A) Cell viability was determined by trypan blue exclusion method. (B) Serum-starved HUVECs cells were pretreated with benfotiamine (100μM), COX-2 inhibitor indomethacin (10μM) and LOX-5 inhibitor REV 5901 (10μM) for 12 h in the absence and presence of LPS (1 ug/ml). The cells were washed and U-937 monocytes were added to each well and the incubation continued for another 12 h. Cell adhesion assays were performed by MTT assay. Data represent mean ± S.E. (n=4). #p<0.001 Vs control; *p<0.01 and **p <0.001 Vs LPS-treated cells.
Discussion
During Gram-negative bacterial infections, the pathological condition of inflammation is initiated bacterial outer cell wall components, especially endotoxins such as LPS [33]. LPS can directly activate macrophages by triggering the production of pro-inflammatory cytokines such as TNF [34], IL-1, IL-6, IFN, chemokines such as MCP-1, MIP1 [35], leukotrienes [36] and others such as nitric oxide and prostaglandins [37]. Overproduction of these inflammatory markers amplifies the host immune response and leads to inflammatory complications such as sepsis [38]. Therefore, the pharmacological interventions of LPS-induced production of inflammatory mediators by macrophages are essential events to control a variety of inflammatory related disorders. In this study, we have for the first time shown that benfotiamine, a fat-soluble analogue of vitamin B1, modulates the production of AA pathway generated inflammatory lipid mediators.
Several studies indicate that the use of natural anti-inflammatory products provides an attractive and safe alternative to modulate various inflammatory disorders [39-41]. Due to its therapeutic action in some frequently observed clinical syndromes, thiamine hydrochloride has been advised and used over a long period of time [42-45]. There are no reports of adverse effects of oral thiamine, even at dosages of several hundred milligrams a day [44]. Benfotiamine, a unique derivative of thiamine is the most potent of the allithiamines, a group of lipid-soluble forms of thiamine [46]. Compared to water-soluble thiamine salts, benfotiamine is absorbed better in the intestine reaching maximum plasma levels of thiamine about 5 times higher and increases active metabolite thiamine diphosphate (ThDP) to 120 folds. The bioavailability is at maximum about 4 times as high as that of thiamine hydrochloride and other lipophilic thiamine derivates [47]. In fact, a recent randomized, double blind, placebo-controlled clinical study in Germany indicated that benfotiamine at a dose of 600 mg/day and higher had no side effects [48]. A number of studies have revealed its potential to alleviate diabetic microangiopathy, neuropathy and other oxidative stress-induced pathological conditions in various experimental animal models [49-53]. However, no reports are available that show benfotiamine supplementation prevents sepsis complications in animal or human studies. We have recently reported that the anti-oxidative and anti-inflammatory properties of benfotiamine in preventing the LPS-induced cytotoxicity in macrophages as well as preventing the ocular inflammation in rats [24, 25]. The present study investigates the potential efficacy of benfotiamine in preventing AA pathway dependent inflammatory signals in macrophages. This is an important advance for the use of benfotiamine as an anti-inflammatory food supplement for treating inflammatory disease conditions.
COX-2 and LOX-5 are the critical enzymes of AA metabolism which catalyzes AA to PGs and LKBs, major lipid mediators involved in various inflammatory diseases [32]. In the present study, we have investigated the effect of benfotiamine on LPS-induced AA pathway generated inflammatory lipid mediators. Our results demonstrate that benfotiamine significantly prevented the AA pathway generated inflammatory lipid mediators as well as the expression of enzymes such as COX-2 and LOX-5, which catalyze the formation of lipid mediators. Thus, inhibition of COX-2 and LOX-5 appears to be responsible for the decreased biosynthesis of pro-inflammatory PGs and LTB4. Septic patients and animals treated with LPS have increased expression of pro-inflammatory cytokines and PGI2 synthase [54]. Since, PGI2 once formed rapidly gets converted into 6-ketoPGF1, and is involved in vasodilation resulting in reduced blood pressure, and can ultimately lead to sepsis [55]. In this study, benfotiamine significantly prevented 6-ketoPGF1 levels and PGI2 expression suggesting that benfotiamine could efficiently prevent PGI2 -induced vasculopathy upon bacterial infections.
Lipoxygenases catalyze the oxygenation of AA [56]. It is known that leukotrienes are important regulators of cancer cell proliferation and cause apoptosis in non-cancer cells [57-61]. Therefore, we examined whether benfotiamine could regulate the expression and synthesis of LOX-5. Our results demonstrate that LPS-induced expression and synthesis of LOX-5 in RAW264.7 cells were significantly prevented by benfotiamine (fig. 4 and 5B). Our results thus indicate that benfotiamine prevents the expression of two key regulatory enzymes of AA metabolism i.e. COX-2 and LOX-5. Indeed, we observed that as compared to specific COX and LOX inhibitors, benfotiamine is more potent in preventing the LPS-induced macrophage death as well as monocytes adhesion to endothelial cells. During bacterial infections and systemic inflammatory pathologies such as severe sepsis, increased production of ROS causes oxidative stress leading to alterations in protein functions and increased susceptibility to protein degradation [58]. In addition to proteins, polyunsaturated fatty acids such as AA, present in membrane lipids are highly susceptible to ROS-mediated damage [59]. Peroxidation of membrane lipids results in the fragmentation of polyunsaturated fatty acids resulting in the production of various cytotoxic and highly reactive aldehydes, such as malonaldehyde and 4-hydroxy-2-nonenal (HNE) [60]. Since benfotiamine prevents the formation of AA acid metabolites by preventing the activation of cPLA2, we next examined if benfotiamine prevents AA induced generation of HNE. Our results indicate that benfotiamine prevents lipid peroxidation as well as subsequent protein-HNE adducts formation. These results suggest that anti-oxidative properties of benfotiamine could prevent LPS-induced lipid peroxidation and HNE formation.
LPS-induced inflammatory signals are known to mediate through ROS, resulting in the activation of protein kinase C (PKC) and mitogen activated protein kinase (MAPK) [60, 62] which can cause inflammation by activating transcription factor such as NF-kB. It has been shown earlier that various antioxidants such as vitamin C and vitamin E inhibit ROS-induced inflammatory responses by preventing the activation of NF-kB [63,64]. Our current studies indicate that benfotiamine prevents activation of NF-kB as well as Egr-1 and phosphorylation of ERK1/2. Thus prevention of LPS-induced AA metabolizing enzymes and formation of inflammatory lipid mediators by benfotiamine could be mediated regulating redox-sensitive transcription factors.
In summary, this study shows that AA pathway generated inflammatory lipid mediators in response to endotoxin exposure could be prevented by a dietary supplement, benfotiamine. Further, benfotiamine inhibited LPS-induced NF-kB and Egr-1 and expression of key regulatory enzymes of AA metabolism such as cPLA2, COX-2 and LOX-5 in murine macrophages. Our studies indicate that benfotiamine is a more potent anti-inflammatory compound as compared to specific LOX and COX inhibitor. Thus, dual regulation of COX and LOX pathways by benfotiamine could be a novel mechanism by which benfotiamine exhibits its anti-inflammatory response and benfotiamine supplementation could be a potential therapeutic approach to prevent inflammatory complications such as sepsis.
Highlights.
Vitamin B1 analogue benfotiamine regulates the arachidonic acid metabolism.
Benfotiamine prevents LPS-induced TXBs, PGs and LTBs.
Benfotiamine prevents LPS-induced expression of Cox-2 and Lox-5.
Benfotiamine prevents NF-kB and EGr-1 in macrophages.
Benfotiamine could be novel anti-inflammatory agent in the prevention of sepsis.
Acknowledgements
This work was supported by National Institutes of Health (NIH) Grant GM071036 to KVR.
Abbreviations
- AA
Arachidonic acid
- LPS
Lipopolysaccharide
- PGE2
Prostaglandin E2
- PGG2
Prostaglandin G2
- PGH2
Prostaglandin H2
- PGI2
Prostacyclin
- TXB
Thromboxane
- LTB
Leukotriene
- cPLA2
Cytosolic phospholipase A2
- sPLA2
soluble phospholipase A2
- COX-2
Cyclooxygenase-2
- LOX 5
Lipoxygenase-5
- MAPK
Mitogen-activated protein kinase
- ERK
Extracellular signal-regulated kinase
- NF-kB
Nuclear Factor-KappaB
- Egr-1
Early growth response factor 1
- ROS
Reactive oxygen species
- NSAID
Nonsteroidal anti-inflammatory drugs
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pastores SM, Papadopoulos E, van den Brink M, Alicea M, Halpern NA. Septic shock and multiple organ failure after hematopoietic stem cell transplantation: treatment with recombinant human activated protein C. Bone Marrow Transplantation. 2002;30:131–134. doi: 10.1038/sj.bmt.1703618. [DOI] [PubMed] [Google Scholar]
- [2].Ren W, Zhang R, Hawkins M, Shi T, Markel DC. Efficacy of periprosthetic erythromycin delivery for wear debris-induced inflammation and osteolysis. Inflamm Res. 2010;59:1091–7. doi: 10.1007/s00011-010-0229-x. [DOI] [PubMed] [Google Scholar]
- [3].Dermott JM, Reddy MR, Onesime D, Reddy EP, Dhanasekaran N. Oncogenic mutant of Galpha12 stimulates cell proliferation through cycloxygenase-2 signaling pathway. Oncogene. 1999;18:7185–7189. doi: 10.1038/sj.onc.1203345. [DOI] [PubMed] [Google Scholar]
- [4].Blatteis CM, Li S, Li Z, Feleder C, Perlik V. Cytokines, PGE2 and endotoxic fever: a re-assessment. Prostaglandins Other Lipid Mediat. 2005;76:1–18. doi: 10.1016/j.prostaglandins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- [5].Ramana KV, Reddy AB, Tammali R, Srivastava SK. Aldose reductase mediates endotoxin-induced production of nitric oxide and cytotoxicity in murine macrophages. Free Radic Biol Med. 2007;42:1290–302. doi: 10.1016/j.freeradbiomed.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kalariya NM, Shoeb M, Reddy AB, Zhang M, van Kuijk FJ, Ramana KV. Prevention of endotoxin-induced uveitis in rats by plant sterol guggulsterone. Invest Ophthalmol Vis Sci. 2010;51:5105–13. doi: 10.1167/iovs.09-4873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;17:1838–46. doi: 10.1161/CIRCULATIONAHA.106.630830. [DOI] [PubMed] [Google Scholar]
- [8].William ML;, Charles LS. Corticosteroids, nonsteroidal anti-inflammatory drugs, and naloxone in the sepsis syndrome. World Journal of Surgery Volume. 1987;11:218–225. doi: 10.1007/BF01656405. [DOI] [PubMed] [Google Scholar]
- [9].Bertolini A, Ottani A, Sandrini M. Dual acting anti-inflammatory drugs: a reappraisal. Pharmacol Res. 2001;44:437–450. doi: 10.1006/phrs.2001.0872. [DOI] [PubMed] [Google Scholar]
- [10].Meyer-Kirchrath J, Schrör K. Cyclooxygenase-2 inhibition and side-effects of non-steroidal anti-inflammatory drugs in the gastrointestinal tract. Curr Med Chem. 2000;7:1121–1129. doi: 10.2174/0929867003374219. [DOI] [PubMed] [Google Scholar]
- [11].Capone ML, Tacconelli S, Rodriguez LG, Patrignani P. NSAIDs and cardiovascular disease: transducing human pharmacology results into clinical read-outs in the general population. Pharmacol Rep. 2010;62:530–535. doi: 10.1016/s1734-1140(10)70310-8. [DOI] [PubMed] [Google Scholar]
- [12].Ang CD, Alviar MJ, Dans AL, Bautista-Velez GG, Villaruz-Sulit MV, Tan JJ, Co HU, Bautista MR, Roxas AA. Vitamin B for treating peripheral neuropathy. Cochrane Database Syst Rev. 2008;16:CD004573. doi: 10.1002/14651858.CD004573.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Beltramo E, Nizheradze K, Berrone E, Tarallo S, Porta M. Thiamine and benfotiamine prevent apoptosis induced by high glucose-conditioned extracellular matrix in human retinal pericytes. Diabetes Metab Res Rev. 2009;25:647–56. doi: 10.1002/dmrr.1008. [DOI] [PubMed] [Google Scholar]
- [14].Pomero F, Molinar Min A, La Selva M, Allione A, Molinatti GM, Porta M. Benfotiamine is similar to thiamine in correcting endothelial cell defects induced by high glucose. Acta Diabetol. 2001;38:135–138. doi: 10.1007/s005920170010. [DOI] [PubMed] [Google Scholar]
- [15].Beltramo E, Berrone E, Buttiglieri S, Porta M. Thiamine and benfotiamine prevent increased apoptosis in endothelial cells and pericytes cultured in high glucose. Diab Metab Res Rev. 2004;20:330–336. doi: 10.1002/dmrr.470. [DOI] [PubMed] [Google Scholar]
- [16].Greb A, Bitsch R. Comparative bioavailability of various thiamine derivatives after oral administration. Int J Clin Pharmacol Ther. 1998;36:216–221. [PubMed] [Google Scholar]
- [17].Fujimara M. Allithiamine and its properties. J Nutr Sci Vitaminol. 1976;22:57–62. doi: 10.3177/jnsv.22.supplement_57. [DOI] [PubMed] [Google Scholar]
- [18].Baker H, Frank O. Absorption, utilization and clinical effectiveness of allithiamines compared to water-soluble thiamines. J Nutr Sci Vitaminol. 1976;22:63–68. doi: 10.3177/jnsv.22.supplement_63. [DOI] [PubMed] [Google Scholar]
- [19].Fujimara M, Sasakawa S, Itokawa Y, Ikeda K. Affinity of thiamine propyl disulfide-S35 to organs. J Vitaminol. 1964;10:79–87. doi: 10.5925/jnsv1954.10.79. [DOI] [PubMed] [Google Scholar]
- [20].Balakumar P, Rohilla A, Krishan P, Solairaj P, Thangathirupathi A. The multifaceted therapeutic potential of benfotiamine. Pharmacol Res. 2010;61:482–488. doi: 10.1016/j.phrs.2010.02.008. [DOI] [PubMed] [Google Scholar]
- [21].Baker H, Frank O. Absorption, utilization and clinical effectiveness of allithiamines compared to water-soluble thiamines. J Nutr Sci Vitaminol (Tokyo) 1976;22:63–68. doi: 10.3177/jnsv.22.supplement_63. [DOI] [PubMed] [Google Scholar]
- [22].Greb A, Bitsch R. Comparative bioavailability of various thiamine derivatives after oral administration. Int J Clin Pharmacol Ther. 1998;36:216–221. [PubMed] [Google Scholar]
- [23].Schreeb KH, Freudenthaler S, Vormfelde SV, Gundert-Remy U, Gleiter CH. Comparative bioavailability of two vitamin B1 preparations: benfotiamine and thiamine mononitrate. Eur J Pharmacol. 1997;52:319–320. [PubMed] [Google Scholar]
- [24].Yadav UC, Kalariya NM, Srivastava SK, Ramana KV. Protective role of benfotiamine, a fat-soluble vitamin B1 analogue, in lipopolysaccharide-induced cytotoxic signals in murine macrophages. Free Radic Biol Med. 2010;48:1423–34. doi: 10.1016/j.freeradbiomed.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yadav UC, Subramanyam S, Ramana KV. Prevention of endotoxin-induced uveitis in rats by benfotiamine, a lipophilic analogue of vitamin B1. Invest Ophthalmol Vis Sci. 2009;50:2276–82. doi: 10.1167/iovs.08-2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Leslie CC. Properties and regulation of cytosolic phospholipase A2. J. Biol.Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- [27].Okamoto F, Saeki K, Sumimoto H, Yamasaki S, Yokomizo T. Leukotriene B4 augments and restores Fc gammaRs-dependent phagocytosis in macrophages. J. Biol. Chem. 2010;285:41113–41121. doi: 10.1074/jbc.M110.175497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Goldsmith M, Daka A, Lamour NF, Mashiach R, Glucksam Y, Meijler MM, Chalfant CE, Zor T. A ceramide analog inhibits cPLA(2) activity and consequent PGE(2) formation in LPS-stimulated macrophages. Immunol Lett. 2011;135:136–43. doi: 10.1016/j.imlet.2010.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jeon SG, Moon HG, Kim YS, Choi JP, Shin TS, Hong SW, Tae YM, Kim SH, Zhu Z, Gho YS, Kim YK. 15-lipoxygenase metabolites play an important role in the development of a T-helper type 1 allergic inflammation induced by double-stranded RNA. Clin. Exp. Allergy. 2009;39:908–917. doi: 10.1111/j.1365-2222.2009.03211.x. [DOI] [PubMed] [Google Scholar]
- [30].Rossi A, Pergola C, Koeberle A, Hoffmann M, Dehm F, Bramanti P, Cuzzocrea S, Werz O, Sautebin L. The 5-lipoxygenase inhibitor, zileuton, suppresses prostaglandin biosynthesis by inhibition of arachidonic acid release in macrophages. Br J Pharmacol. 2010;161:555–70. doi: 10.1111/j.1476-5381.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Moraes J, Assreuy J, Canetti C, Barja-Fidalgo C. Leukotriene B4 mediates vascular smooth muscle cell migration through v 3 integrin transactivation. Atherosclerosis. 2010;212:406–413. doi: 10.1016/j.atherosclerosis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- [32].Shoeb M, Yadav UC, Srivastava SK, Ramana KV. Inhibition of aldose reductase prevents endotoxin-induced inflammation by regulating arachidonic acid pathway in murine macrophages. Free Radical Biology and Medicine. 2011;51:1686–96. doi: 10.1016/j.freeradbiomed.2011.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Schumann RR. Old and new findings on lipopolysaccharide-binding protein: a soluble pattern-recognition molecule. Biochem Soc Trans. 2011;39:989–93. doi: 10.1042/BST0390989. [DOI] [PubMed] [Google Scholar]
- [34].Wall EA, Zavzavadjian JR, Chang MS, Randhawa B, Zhu X, Hsueh RC, Liu J, Driver A, Bao XR, Sternweis PC, Simon MI, Fraser ID. Suppression of LPS-induced TNF-alpha production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Sci Signal. 2009;2:ra28. doi: 10.1126/scisignal.2000202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu S, Zhang K, Lv C, Wang H, Cheng B, Jin Y, Chen Q, Lian Q, Fang X. Nuclear factor-kB mediated lipopolysaccharide-induced mRNA expression of hepcidin in human peripheral blood leukocytes. Innate Immun. 2011 doi: 10.1177/1753425911405087. Ahead of print. [DOI] [PubMed] [Google Scholar]
- [36].Sato R, Yamaga S, Watanabe K, Hishiyama S, Kawabata K, Kobayashi T, Fujioka D, Saito Y, Yano T, Watanabe K, Watanabe Y, Ishihara H, Kugiyama K. Inhibition of secretory phospholipase A2 activity attenuates lipopolysaccharide-induced acute lung injury in a mouse model. Exp Lung Res. 2010;36:191–200. doi: 10.3109/01902140903288026. [DOI] [PubMed] [Google Scholar]
- [37].Ramana KV, Srivastava SK. Mediation of aldose reductase in lipopolysaccharide-induced inflammatory signals in mouse peritoneal macrophages. Cytokine. 2006;36:115–22. doi: 10.1016/j.cyto.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ, Srivastava S, Ramana KV. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact. 2011;191:330–8. doi: 10.1016/j.cbi.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gosslau A, Li S, Ho CT, Chen KY, Rawson NE. The importance of natural product characterization in studies of their anti-inflammatory activity. Mol Nutr Food Res. 2011;55:74–82. doi: 10.1002/mnfr.201000455. [DOI] [PubMed] [Google Scholar]
- [40].Venkatesha SH, Berman BM, Moudgil KD. Herbal medicinal products target defined biochemical and molecular mediators of inflammatory autoimmune arthritis. Bioorg Med Chem. 2011;19:21–9. doi: 10.1016/j.bmc.2010.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Choi DK, Koppula S, Suk K. Inhibitors of microglial neurotoxicity: focus on natural products. Molecules. 2011;16:1021–43. doi: 10.3390/molecules16021021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Thornalley PJ. The potential role of thiamine (vitamin B1) in diabetic complications. Curr. Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- [43].Malecka SA, Poprawski K, Bilski B. Prophylactic and therapeutic application of thiamine (vitamin B1)—a new point of view. Wiad. Lek. 2006;59:383–387. [PubMed] [Google Scholar]
- [44].Hinze-Selch D, Weber MM, Zimmermann U, Pollmächer T. Thiamine treatment in psychiatry and neurology. Fortschr. Neurol. Psychiatr. 2000;68:113–120. doi: 10.1055/s-2000-11622. [DOI] [PubMed] [Google Scholar]
- [45].Wooley JA. Characteristics of thiamin and its relevance to the management of heart failure. Nutr. Clin. Pract. 2008;23:487–493. doi: 10.1177/0884533608323430. [DOI] [PubMed] [Google Scholar]
- [46].Monograph. Altern. Med. Rev. Vol. 11. 2006. Benfotiamine; pp. 238–242. [PubMed] [Google Scholar]
- [47].Loew D. Pharmacokinetics of thiamine derivatives especially of benfotiamine. Int. J. Clin. Pharmacol. Ther. 1996;34:47–50. [PubMed] [Google Scholar]
- [48].Stracke H, Gaus W, Achenbach U, Federlin K, Bretzel RG. Benfotiamine in diabetic polyneuropathy (BENDIP): results of a randomised, double blind, placebo-controlled clinical study. Exp Clin Endocrinol Diabetes. 2008;116:600–605. doi: 10.1055/s-2008-1065351. [DOI] [PubMed] [Google Scholar]
- [49].Babaei-Jadidi R, Karachalias N, Ahmed N, Battah S, Thornalley PJ. Prevention of incipient diabetic nephropathy by high-dose thiamine and benfotiamine. Diabetes. 2003;52:2110–2120. doi: 10.2337/diabetes.52.8.2110. [DOI] [PubMed] [Google Scholar]
- [50].Hammes HP, Du X, Edelstein D. Benfotiamine blocks three major pathways of ACCEPTED MANUSCRIPT hyperglycemic damage and prevents experimental diabetic retinopathy. Nat Med. 2003;9:294–299. doi: 10.1038/nm834. [DOI] [PubMed] [Google Scholar]
- [51].Schupp N, Dette EM, Schmid U. Benfotiamine reduces genomic damage in peripheral lymphocytes of hemodialysis patients. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:283–291. doi: 10.1007/s00210-008-0310-y. [DOI] [PubMed] [Google Scholar]
- [52].Schmid U, Stopper H, Heidland A, Schupp N. Benfotiamine exhibits direct antioxidative capacity and prevents induction of DNA damage in vitro. Diabetes Metab Res Rev. 2008;24:371–377. doi: 10.1002/dmrr.860. [DOI] [PubMed] [Google Scholar]
- [53].Stracke H, Hammes HP, Werkmann D. Efficacy of benfotiamine versus thiamine on function and glycation products of peripheral nerves in diabetic rats. Exp Clin Endocrinol Diabetes. 2001;109:330–336. doi: 10.1055/s-2001-17399. [DOI] [PubMed] [Google Scholar]
- [54].Takakuwa T, Endo S, Nakae H, Kikichi M, Inada K, Yoshida M. PAF acetylhydrolase and arachidonic acid metabolite levels in patients with sepsis. Res. Commun. Chem. Pathol. Pharmacol. 1994;84:283–290. [PubMed] [Google Scholar]
- [55].Wang P, Zhou M, Cioffi WG, Bland KI, Ba ZF, Chaudry IH. Is prostacyclin responsible for producing the hyperdynamic response during early sepsis? Crit. Care Med. 2000;28:1534–1539. doi: 10.1097/00003246-200005000-00046. [DOI] [PubMed] [Google Scholar]
- [56].Rådmark O, Samuelsson B. 5-Lipoxygenase: mechanisms of regulation. J Lipid Res. 2009;50:S40–S45. doi: 10.1194/jlr.R800062-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Steele VE, Holmes CA, Hawk ET, Kopelovich L, Lubet RA, Crowell JA, Sigman CC, Kelloff GJ. Lipoxygenase inhibitors as potential cancer chemopreventives. Cancer Epidemiol. Biomarkers Prev. 1999;8:467–483. [PubMed] [Google Scholar]
- [58].Russell ST, Michael JT. Mechanism of attenuation by β-hydroxy-β-methylbutyrate of muscle protein degradation induced by lipopolysaccharide. Molecular and Cellular Biochemistry. 2009;330:171–179. doi: 10.1007/s11010-009-0130-5. [DOI] [PubMed] [Google Scholar]
- [59].Nigam S, Schewe T. Phospholipase A2s and lipid peroxidation. Biochim. Biophys. Acta. 2000;1488:167–181. doi: 10.1016/s1388-1981(00)00119-0. [DOI] [PubMed] [Google Scholar]
- [60].Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- [61].Kramer RM, Roberts EF, Um SL, Borsch-Haubold AG, Watson SP, Fisher MJ, Jakubowski JA. p38 mitogen-activated protein kinase phosphorylates cytosolic phospholipase A2 (cPLA2) in thrombin-stimulated platelets. Evidence that proline-directed phosphorylation is not required for mobilization of arachidonic acid by cPLA2. J. Biol. Chem. 1996;271:27723–27729. doi: 10.1074/jbc.271.44.27723. [DOI] [PubMed] [Google Scholar]
- [62].Fouda SI, Molski TF, Ashour MS, Sha’afi RI. Effect of lipopolysaccharide on mitogen-activated protein kinases and cytosolic phospholipase A2. Biochem. J. 1995;308:815–822. doi: 10.1042/bj3080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Reiter E, Jiang Q, Christen S. Anti-inflammatory properties of alpha- and gamma-tocopherol. Mol. Aspects Med. 2007;28:668–691. doi: 10.1016/j.mam.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kusmic C, Basta G, Lazzerini G, Vesentini N, Barsacchi R. The effect of Ginkgo biloba in isolated ischemic/reperfused rat heart: a link between vitamin E preservation and prostaglandin biosynthesis. J Cardiovasc Pharmacol. 2004;44:356–62. doi: 10.1097/01.fjc.0000137164.99487.42. [DOI] [PubMed] [Google Scholar]