Abstract
Gap junctional intercellular communication (GJIC) regulates cellular homeostasis by propagating signaling molecules, exchanging cellular metabolites, and coupling electrical signals. In cancer, cells exhibit altered rates of GJIC which may play a role in neoplastic progression. KATP channels help maintain membrane polarity; and, linkages between KATP channel activity and rates of GJIC have been established. The mechanistic relationship has not been fully elucidated. We report the effects of treatment with multiple KATP antagonist compounds on GJIC in metastatic cell lines demonstrating an increase in communication rates following treatment with compounds possessing specificities towards the SUR2 subunit of KATP. These effects remained consistent using cell lines with different expression levels of SUR1 and SUR2, suggesting possible off target effects on GJIC by these compounds.
Keywords: Sulfonylurea, KATP, gap junction, glibenclamide
1. Introduction
Gap junctional intercellular communication (GJIC) is a process by which cells communicate soluble factors and electrical signals through physical interactions at the plasma membrane via regulated channels known as connexons (1). Connexons are hexameric structures comprised of a family of monomeric proteins, connexins. More than 20 connexin proteins have been reported in mammalian cells, each which varying specificities of regulation. Once assembled in the plasma membrane connexon channels can be opened or closed through a variety of signals including cellular pH, ion concentrations, and ATP levels (2–4). From a metabolic perspective, exchange of small molecules (<1.5kDa) between these channels allows cells to share nutrients and secondary signaling molecules (e.g. IP3), in addition to regulating multiple cell types (e.g. cardiac, neuronal, epithelial) through electrical communication which contributes to the coordination of tissue function.
While GJIC is a means for normal cellular homeostasis, in cancer cells this communication is often dysregulated. Initially, reports demonstrated that during neoplastic progression, GJIC between cancer cells was often reduced compared to non-transformed cells of the same origin (5–7). More recently however, it is appreciated that GJIC may also be increased in cancer cells, or between cancer cells and stromal cells at secondary metastatic sites, highlighting cell and tissue context specific events (8–10). In any case, alterations of the level of GJIC between cancer cells is commonly observed.
The ATP sensitive potassium channel (KATP) regulates K+ conductance in cells and is closed by increasing concentrations of ATP. Closure of these channels results in depolarization of the plasma membrane due to reduced potassium conductance. The structure of the KATP channel is composed of an inwardly rectifying potassium channel KIR (KIR6.1, KIR6.2), and a sulfonylurea subunit (SUR1, SUR2A and SUR2B) which regulates the activity of KIR through sensitivity to ATP levels, as well as other metabolites (e.g. PIP2) (11). Growing evidence has supported a role for KATP in the regulation of GJIC activity. Vera et al. initially demonstrated that reduction of endogenous ATP levels (which would relieve KATP inhibition) decreased GJIC in astrocytes and that this effect was reversible (12). Further work showed that closure of KATP channels by the sulfonylurea receptor inhibitors tolbutamide and glibenclamide lead to increases in GJIC, suggesting that KATP may play a regulatory role in opening of connexon channels, possibly through mechanisms related to membrane depolarization (13,14). Collectively these studies proposed evidence that inhibition of KATP channels leads to greater rates of GJIC between cells, while opening of the same channels decreases GJIC. These data provide a link to the metabolic regulation of gap junctions through ATP. Additionally, development of tolbutamide as a therapeutic agent for cancer treatment through its effects on connexin regulation and gap junction modulation remains promising (15–18). Interestingly however, an increased risk of cancer mortality in Type 2 diabetes patients administered sulfonylureas has been reported (19,20), implicating context-dependent mechanisms and a possible role for modulation of KATP conductance in the progression of cancer.
In the present study we examined the effect of treatment of highly metastatic cancer cell lines (MDA-MB-231, MDA-MB-435, C8161.9), which exhibit low baseline gap junction activity, with KATP inhibitory compounds. Treatment of cells with the sufonylurea receptor inhibitor glibenclamide produced a robust and consistent increase in calcein dye transfer indicative of GJIC between cancer cells expressing detectable protein levels of SUR2 with little to no detection of SUR1. Upon further examination of additional KATP inhibitors with the MDA-MB-231 breast carcinoma cell line, we found that inhibitors with dual specificities to both the SUR1 and SUR2 subunits increased GJIC while those with primary specificities to SUR1 had little to no effect on GJIC suggesting that inhibition of SUR2 KATP channels was responsible for the increase in GJIC. To evaluate this hypothesis, we screened additional breast cancer cell lines and identified the SUM159 as expressing inverse levels of SUR1 and SUR2 compared to MDA-MB-231, MDA-MB-435 and C8161.9. Treatment of SUM159 with the KATP inhibitors resulted in the same pattern of GJIC. These data suggest the possibility of novel effects on GJIC by KATP inhibitors that is independent of their KATP specificity and will be important for future studies involving these compounds.
2. Materials and methods
2.1 Cell lines
MDA-MB-231, MDA-MB-435 and C8161.9 were grown in Dulbecco’s-modified Eagle's medium mixed 1:1 (v:v) with Ham's F-12 medium (DMEM/F12, Invitrogen #11330) supplemented with 2 mmol/ L of L-glutamine, 0.2 mmol/L of nonessential amino acids with 5% fetal bovine serum (FBS). MDA-MB-231 and MDA-MB-435 are human breast carcinoma–derived cell lines. For the origin of MDA-MB-435 the reader is referred to Chambers, Can Res, 2009 (21). The C8161.9 is a clone derived from the C8161 human melanoma. The SUM159 cell line was a generous gift provided by the laboratory of David Salomon, National Cancer Institute. SUM159 were maintained in Ham’s F12 media (Invitrogen #11765) supplemented with 5 µg/ml insulin, 0.1 µg/ml epidermal growth factor, 10 mM HEPES and 10% fetal bovine serum. All cell lines were tested for Mycoplasma spp. contamination using PCR (#302108; Aligent Technologies, Santa Clara, CA).
2.2 Chemicals
The following chemicals were obtained from Sigma (chlorpropamide #C1290, glibenclamide #G0639, gliclazide #G6127, glimepiride #G2295, repaglinide #R9028, tolbutamide #T0891). Calcein-AM #C1430 and 1,1'-dilinoleyl-3,3,3',3'-tetramethylindocarbocyanine (DiI) #C7001 were purchased from Invitrogen.
All compounds were dissolved in DMSO, aliquoted and frozen at −20°C. At time of experiments aliquots were thawed and working concentrations made fresh. DMSO alone (NT, non-treated) had no effect on dye transfer. No morphological changes or signs of toxicity were observed for each compound/dose reported in this study.
2.3 GJIC assay
Gap junction assays were performed as previously described (22). "Donor" cells were loaded with Calcein-AM and DiI, a lipophilic dye that does not transfer between cells used to mark donor cells. After washing 3 times with Dulbecco's phosphate buffered saline (DPBS), donor cells were plated with nonlabeled "acceptor" cells for 6 hours. Calcein spread from donor to acceptor cells was indicative of GJIC. All experiments reported herein were conducted in serum-free media in order to observe the effects of KATP inhibitors in the absence of additional growth factors. Flow cytometry with a BD LSRII Cell analytic flow cytometer using BD FACS Diva software was used to calculate the average number of cells that received calcein per donor cell and represented as fold change.
2.4 Immunoblot assay and antibodies
Cells were lysed in buffer containing 25 mmol/L Tris (tris(hydroxymethyl)aminomethane), 1% Triton-X100, 500 mmol/L β-glycerolphosphate, 0.5 mM EDTA and 5% glycerol on ice followed by sonication to disrupt cell membranes. Lysates were resolved by 12% SDS-PAGE and transferred to PVDF membranes. Antibodies to SUR1 (Abcam #ab32844) and SUR2 (BD Pharmigen # 550429) were incubated at 1:1000 in 5% bovine serum albumin (BSA) in Tris-buffered saline Tween-20 (TBST) overnight at 4°C followed by incubation with horse radish peroxidase (HRP) conjugated anti-mouse (GE Healthcare #NXA931) or conjugated anti-rabbit (GE Healthcare #NA934) at 1:2500 in 5% non-fat dry milk in TBST for 3 hours at room temperature. Membranes were developed with ECL (Thermo Scientific #32209). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) HRP-conjugated antibody (AbCam #ab9385) and Alpha Actin (Millipore #1501) were used for equal loading control.
2.5 Statistical analysis
Data for GJIC are presented as mean ± standard deviation and represented as fold change compared to non-treated (NT) group. Student t-test was used for statistical analysis between groups.
3. Results and Discussion
Dysregulation of gap junction coupling is a phenotypic alteration commonly observed in neoplastic cells. We and others have previously demonstrated a specific loss of homotypic and heterotypic GJIC in metastatic cells (22–24). While the dysregulation of GJIC in neoplastic cells is apparent, the specific signaling events and the mediators of those signaling events between malignant cells or between malignant cells and the surrounding stromal compartment appear to be largely context dependent (24,25). Further, restoration of GJIC appears to reduce metastatic ability of cancer cells in some cases (26,27). The ability of clinically used pharmacologic agents to alter GJIC in cultured astrocytes was the first indication to topically relate the pharmacology of sulfonylureas with specific alterations in GJIC (13).
Increased risk for cancer in patients with Type-2 diabetes is thought to be mediated through the development of metabolic syndrome which encompasses hyperinsulinemia and insulin resistance (28,29). Sulfonylureas are used as a treatment option for Type-2 diabetes and recent reports show epidemiological evidence for increased cancer-related mortality in patients treated with sulfonylureas rather than biguanides (19). In this report, we demonstrate that treatment of cancer cells in vitro with sulfonamides results in a consistent increase in GJIC, a phenomenon that has been associated with a reduction in metastatic potential (6,9,23,24,26,27). Our results highlight a germane paradox where a supposed reduction in metastatic potential due to increased GJIC conflicts with clinical data showing increased mortality from the use of sulfonamide agents. Importantly, our results appear to indicate that further investigation needs to address specific mediators and context dependency rather than the generic process of GJIC itself between cancer cells.
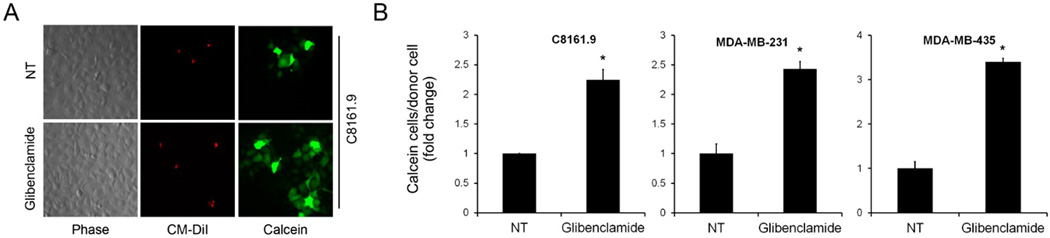
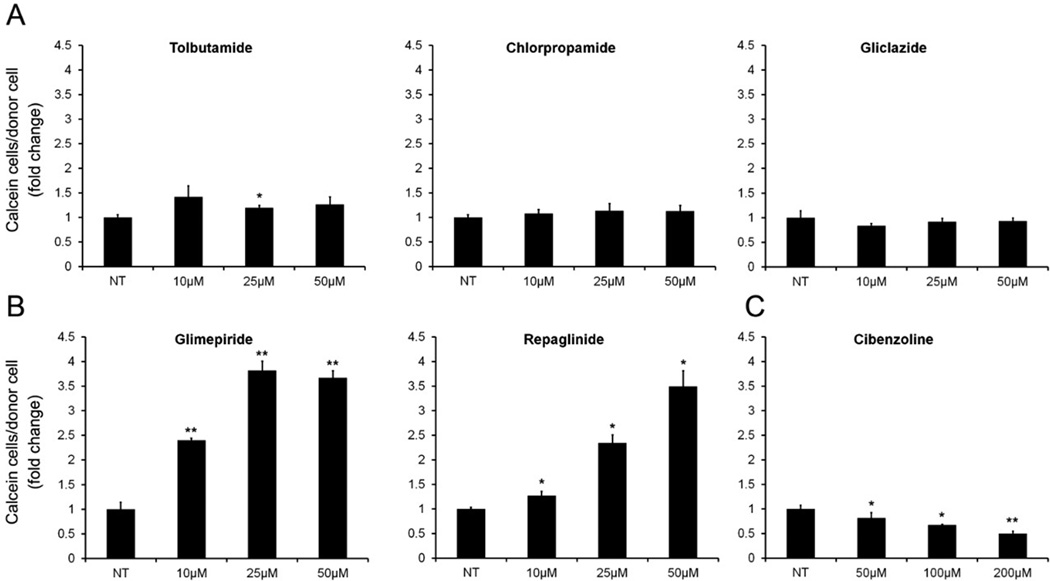
For our experiments, we utilized first generation sulfonylureas tolbutamide and chlorpropamide and second generation agents glibenclamide and gliclazide. To initially evaluate the effect of sulfonamides on GJIC, metastatic cancer cell lines of breast and melanocytic origin which exhibit basally low gap junction coupling were treated with 10 µmol/L of glibenclamide. A consistent increase in GJIC as measured by the passage of fluorescent calcein dye passing from labeled donor cells to co-cultured acceptor cells over 6 h was observed visually (Figure 1A) and quantified by flow cytometry (Figure 1B). Since glibenclamide consistently increased GJIC following KATP channel inhibitor treatment, the metastatic breast cancer cell line MDA-MB-231 was utilized for further investigation. Similar to the addition of glibenclamide, treatment of MDA-MB-231 cells with glimepiride (a sulfonylurea KATP channel inhibitor) and repaglinide, an unrelated meglitinide class KATP channel inhibitor resulted in a dose-dependent increase in the passage of calcein from labeled donor cells to acceptor cells indicative of increased GJIC (Figure 2B). These results taken together initially indicated that an increase in GJIC may be dependent on inhibition of KATP channels and independent of structure and therefore class of these inhibitors.
Figure 1.
Treatment of cancer cells with glibenclamide increases gap junction communication. A) Representative images of metastatic C8161.9 donor cells were labeled with Calcein (green) and CM-DiI (red) and co-cultured with non-labeled acceptor cells in the absence or presence of 10µmol/L glibenclamide. Calcein can be visualized spreading from donor cells to acceptor cells. B) Quantification of calcein/DiI assays measuring dye transfer from donor to acceptor cells using flow cytometry in MDA-MB-231, MDA-MB-435 and C8161.9 cancer cell lines. Results are represented as fold change between non-treated (NT) and glibenclamide treated groups measuring the number of acceptor cells receiving calcein per donor cell. (* P < 0.05, error bars represent mean ± standard deviation)
Figure 2.
KATP inhibitors with SUR2 specificity increase GJIC in MDA-MB-231. Flow cytometry quantification of Calcein/CM-DiI assays measuring dye transfer from donor to acceptor cells after 6 hr co-culture. Results are represented as fold change between non-treated (NT) or increasing concentrations of KATP inhibitors. (A) KATP inhibitors with SUR1 specificity did not increase GJIC in MDA-MB-231, experiments were performed as described in Figure 1 with increasing concentrations of tolbutamide, gliclazide and chlorpropamide (10, 25, 50 µmol/L). (B) treatment with glimperide and repaglinide (10, 25, 50 µmol/L) significantly increased GJIC. (C) KATP inhibitor cibenzoline succinate does not increase GJIC in MDA-MB-231. Cells were treated with increasing concentrations of cibenzoline succinate (50, 100, 200 µmol/L) and GJIC assays were performed as described. Minor decreases in GJIC were observed in a dose dependent manner. NT, non-treated. (* P < 0.05, ** P < 0.01, error bars represent mean ± standard deviation)
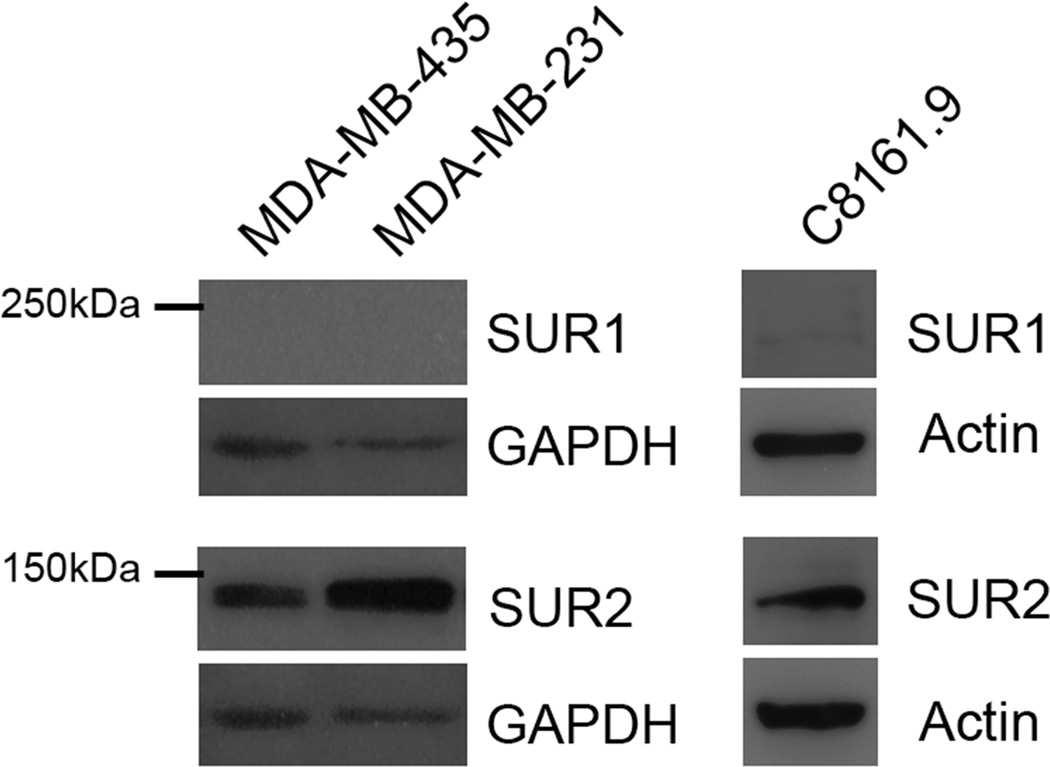
Treatment of MDA-MB-231 cells with tolbutamide, gliclazide, and chlorpropamide failed to cause a consistent increase in GJIC (Figure 2A). Glibenclamide, glimepiride and repaglinide show comparable efficacy in the inhibition of SUR1 and SUR2, while tolbutamide, gliclazide, and chlorpropamide are selective for SUR1 inhibition at low doses, suggesting a possible differentiation of effects through these different agents (references for specificities of compounds 30–33, summarized in 34). To determine the expression of SUR1 and SUR2, whole cell lysates from MDA-MB-231,MDA-MB-435, and C8161.9 cells were probed with antibodies directed towards SUR1 and SUR2. SUR2 was evident in each cell line, while expression of SUR1 could not be detected in MDA-MB-231 and MDA-MB-435 with low levels of detection in C8161.9 (Figure 3). These results suggested a mechanistic explanation for the pattern of GJIC changes observed following treatment with KATP inhibitors specific for SUR1 and SUR2, and why we did not observe a consistent increase in GJIC with tolbutamide (although used at lower concentrations in our studies (i.e., 50 µM vs. 400 µM)). KATP channels of the SUR2 subtype (SUR2A, SUR2B) are most commonly paired with KIR6.2 channels as in cardiac, skeletal, and smooth muscle. However, although expression analysis of KIR subunits was not performed in this study, treatment of MDA-MB-231 with cibenzoline succinate (50 – 200 µmol/L), a KATP inhibitor that binds directly to the KIR subunit (35), did not induce GJIC, but rather slightly decreased GJIC levels (Figure 2C), suggesting possible secondary effects on GJIC of glibenclamide, glimepiride and repaglinide that are not related to the inhibition of KATP channels.
Figure 3.
Immunoblot analysis of SUR1 and SUR2 in lysates from MDA-MB-231, MDA-MB-435 and C8161.9. Whole cell lysates were collected from indicated cancer cell lines and probed for the expression of SUR1 and SUR2. SUR2 expression was readily detected in each cell line, however SUR1 protein could not be detected in MDA-MB-231 and MDA-MB-435 with minimal detection in C8161.9.
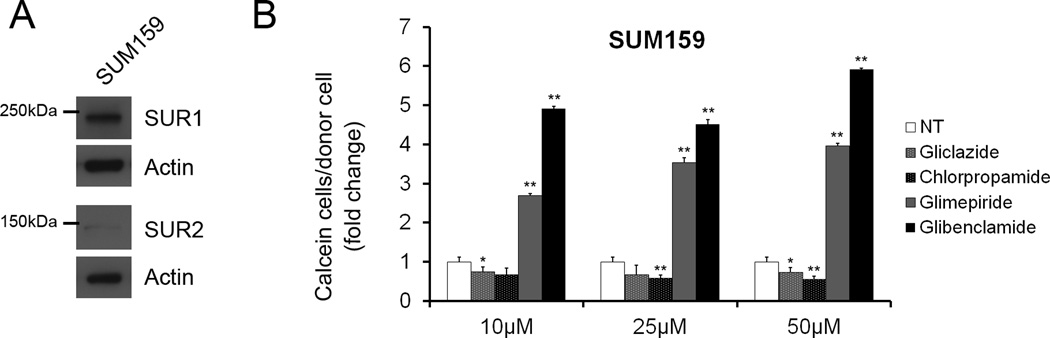
To further explore this possibility, we used the SUM159 breast cancer cell line which expresses inverse levels of SUR1 and SUR2 by comparison to MDA-MB-231, MDA-MB-435 and C8161.9 (Figure 4A). Interestingly, treatment of SUM159 with the SUR1 specific KATP channel inhibitors chlorpropamide and gliclazide (10, 25, 50µM) failed to induce GJIC while glibenclamide and glimepiride (10, 25, 50µM) significantly increased GJIC similar to treatment in MDA-MB-231, MDA-MB-435 and C8161.9 (Figure 4B). Although an explanation for the latter result could indicate that inhibition of SUR1 KATP in SUM159 by glibenclamide and gimepiride was responsible for GJIC, failure of chlorpropamide and gliclazide to increase GJIC contradicts these results. The data propose the possibility of novel KATP independent effects on GJIC by glibenclamide, repaglinide and glimepiride. A second explanation would be that inhibition of the levels of SUR2 expressed in SUM159 is sufficient to increase GJIC but that this effect is not shared by inhibition of SUR1, although the SUR1 specific inhibitor tolbutamide has been shown to increase GJIC in other cell types (15–18), suggesting possible cell context specificities.
Figure 4.
SUR1 and SUR2 protein expression in SUM159. (A) Whole cell lysates from the SUM159 cell line were analyzed via immunoblot analysis for SUR1 and SUR2. SUR1 protein levels were readily detected while SUR2 expression was low by comparison to MDA-MB-231, MDA-MB-435 and C8161.9 probed under the same conditions. (B) Quantified data from GJIC assays demonstrating changes in GJIC with increasing concentrations (10, 25, 50µM) of gliclazide, chlorpropamide, glimepiride and glibenclamide. Significant increases in GJIC were observed with glimepiride and glibenclamide treatments with no increases during treatment with gliclazide and chlorpropamide, similar to results obtained from MDA-MB-231, MDA-MB-435 and C8161.9.
In addition to the complexity of KATP channel composition, the roles of KATP channels located other than the cell membrane in the mitochondria, sarcolemma, and nucleus beg further investigation, although our preliminary results involving the treatment of cells with 5-hydroxydecanoate (30 – 300 µmol/L), a compound with specificity towards the mitochondrial-KATP channel produced no effect on GJIC in our experiments (data not shown). Although much work remains to further understand the role of KATP channels with GJIC and importantly in identifying the key players that define a paradoxical relationship between pharmacological KATP channel inhibition and patient outcome, especially in cancer, we report that KATP inhibitors with SUR2 specificity increase GJIC independently of differences in SUR1 and SUR2 expression between cell lines and warrant further molecular investigation. Our results provoke thought in the role of conventional therapy not only for the treatment of Type-2 diabetes, but also cancer and eventual metastasis.
Highlights.
Inhibition of KATP channels in metastatic breast and melanoma cells increases gap junction communication
Evaluation of seven KATP inhibiting compounds on GJIC showed that only compounds inhibiting the SUR2 subunit of KATP increased GJIC
Cibenzoline, which acts by binding KIR, fails to increase GJIC, suggesting alternative mechanisms of action for the KATP channels and their association with GJIC
Novel characterization of KATP subunits in breast and melanoma cell lines
Acknowledgments
The authors thank Drs. Janet Price (University of Texas M. D. Anderson Cancer Center) for providing the MDA-MB-231 and -435 cell lines and Frank Meyskens for initially providing the C8161 cell line. We also gratefully acknowledge support from the following agencies: National Foundation for Cancer Research (DRW), National Institutes of Health RO1-CA134981 (DRW); RO1-CA87728-09 (DRW) and Susan G. Komen for the Cure SAC110037 (DRW).
Abbreviations
- KATP
ATP-sensitive K+ channel
- BSA
bovine serum albumin
- EDTA
ethylenediaminetetraacetic acid
- FBS
fetal bovine serum
- GJIC
gap junction intercellular communication
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide electrophoresis
- NFDM
non-fat dry milk
- TBST
TRIS-buffered saline Tween-20
- TRIS
tris(hydroxymethyl)aminomethane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mese G, Richard G, White TW. Gap junctions: basic structure and function. J Invest Dermatol. 2007;127:2516–2524. doi: 10.1038/sj.jid.5700770. [DOI] [PubMed] [Google Scholar]
- 2.Revel JP, Hoh JH, John SA, Laird DW, Puranam K, Yancey SB. Aspects of gap junction structure and assembly. Semin Cell Biol. 1992 Feb;3(1):21–28. doi: 10.1016/s1043-4682(10)80005-4. [DOI] [PubMed] [Google Scholar]
- 3.Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc Res. 2004 May 1;62(2):233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation of connexins. Arch Biochem Biophys. 2000 Dec 15;384(2):205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- 5.Carystinos GD, Bier A, Batist G. The role of connexin-mediated cell– cell communication in breast cancer metastasis. J Mamm Gland Biol Neopl. 2001;6:431–440. doi: 10.1023/a:1014787014851. [DOI] [PubMed] [Google Scholar]
- 6.Mehta PP, Bertram JS, Loewenstein WR. Growth-inhibition of transformed-cells correlates with their junctional communication with normal-cells. Cell. 1986;44:187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- 7.Mesnil M, Crespin S, Avanzo JL, Zaidan-Dagli ML. Defective gap junctional intercellular communication in the carcinogenic process. Biochim Biophys Acta Biomemb. 2005;1719:125–145. doi: 10.1016/j.bbamem.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson GL, Gallick GE, Dulski KM, Spohn WH, Lembo TM, Tainsky MA. Lack of correlation between intercellular junctional communication, p21rasEJ expression, and spontaneous metastatic properties of rat mammary cells after transfection with c-H-rasEJ or neo genes. Oncogene. 1990;5:747–753. [PubMed] [Google Scholar]
- 9.Naus CC, Laird DW. Implications and challenges of connexin connections to cancer. Nat Rev Cancer. 2010;10:435–441. doi: 10.1038/nrc2841. [DOI] [PubMed] [Google Scholar]
- 10.Czyz J. The stage-specific function of gap junctions during tumourigenesis. Cell Mol BioLett. 2008;13:92–102. doi: 10.2478/s11658-007-0039-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Babenko AP, Aguilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–687. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- 12.Vera B, Sánchez-Abarca LI, Bolaños JP, Medina JM. Inhibition of astrocyte gap junctional communication by ATP depletion is reversed by calcium sequestration. FEBS Lett. 1996;392(3):225–228. doi: 10.1016/0014-5793(96)00794-6. [DOI] [PubMed] [Google Scholar]
- 13.Granda B, Tabernero A, Sánchez-Abarca LI, Medina JM. The K-ATP channel regulates the effect of Ca2+ on gap junction permeability in cultured astrocytes. FEBS Lett. 1998;1; 427(1):41–45. doi: 10.1016/s0014-5793(98)00390-1. [DOI] [PubMed] [Google Scholar]
- 14.Velasco A, Tabernero A, Granda B, Medina JM. ATP-sensitive potassium channel regulates astrocytic gap junction permeability by a Ca2+-independent mechanism. J Neurochem. 2000;74(3):1249–1256. doi: 10.1046/j.1471-4159.2000.741249.x. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Alvarez R, Tabernero A, Sánchez-Abarca LI, Orfao A, Giaume C, Medina JM. Proliferation of C6 glioma cells is blunted by the increase in gap junction communication caused by tolbutamide. FEBS Lett. 2001;509(2):202–206. doi: 10.1016/s0014-5793(01)03181-7. [DOI] [PubMed] [Google Scholar]
- 16.Sánchez-Alvarez R, Tabernero A, Medina JM. The increase in gap junctional communication decreases the rate of glucose uptake in C6 glioma cells by releasing hexokinase from mitochondria. Brain Res. 2005;1039(1–2):189–198. doi: 10.1016/j.brainres.2005.01.079. [DOI] [PubMed] [Google Scholar]
- 17.Sánchez-Alvarez R, Paíno T, Herrero-González S, Medina JM, Tabernero A. Tolbutamide reduces glioma cell proliferation by increasing connexin43, which promotes the up-regulation of p21 and p27 and subsequent changes in retinoblastoma phosphorylation. Glia. 2006;54(2):125–134. doi: 10.1002/glia.20363. [DOI] [PubMed] [Google Scholar]
- 18.Paíno T, Gangoso E, Medina JM, Tabernero A. Inhibition of ATP-sensitive potassium channels increases HSV-tk/GCV bystander effect in U373 human glioma cells by enhancing gap junctional intercellular communication. Neuropharmacology. 2010;59(6):480–491. doi: 10.1016/j.neuropharm.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin: Response to Farooki and Schneider. Diabetes Care. 2006;(2):254–258. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 20.Gray TC, Siegel E, Govindarajan R. Impact of antidiabetic agents on the survival of subjects with colorectal cancer (CRC) J Clin Oncol. 2010;28:15s. (suppl; abstr 2586). [Google Scholar]
- 21.Chambers AF. MDA-MB-435 and M14 cell lines: identical but not M14 melanoma? Cancer Res. 2009;69:5292–5293. doi: 10.1158/0008-5472.CAN-09-1528. [DOI] [PubMed] [Google Scholar]
- 22.Bodenstine TM, Vaidya KS, Ismail A, Beck BH, Cook LM, Diers AR, Landar A, Welch DR. Homotypic gap junctional communication associated with metastasis suppression increases with PKA activity and is unaffected by PI3K inhibition. Cancer Res. 20101;70(23):10002–10011. doi: 10.1158/0008-5472.CAN-10-2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders MM, Seraj MJ, Li Z, Zhou Z, Winter CR, Welch DR, Donahue HJ. Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res. 2001;61(5):1765–1767. [PubMed] [Google Scholar]
- 24.Kapoor P, Saunders MM, Li Z, Zhou Z, Sheaffer N, Kunze EL, Samant RS, Welch DR, Donahue HJ. Breast cancer metastatic potential: correlation with increased heterotypic gap junctional intercellular communication between breast cancer cells and osteoblastic cells. Int J Cancer. 2004;111(5):693–697. doi: 10.1002/ijc.20318. [DOI] [PubMed] [Google Scholar]
- 25.Li Z, Zhou Z, Welch DR, Donahue HJ. Expressing connexin 43 in breast cancer cells reduces their metastasis to lungs. Clin Exp Metastasis. 2008;25(8):893–901. doi: 10.1007/s10585-008-9208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu D, Caveney S, Kidder GM, Naus CC. Transfection of C6 glioma cells with connexin 43 cDNA: analysis of expression, intercellular coupling, and cell proliferation. Proc Natl Acad Sci U S A. 1991;88(5):1883–1887. doi: 10.1073/pnas.88.5.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin W, Zempel G, Hülser D, Willecke K. Growth inhibition of oncogene-transformed rat fibroblasts by cocultured normal cells: relevance of metabolic cooperation mediated by gap junctions. Cancer Res. 1991;51(19):5348–5351. [PubMed] [Google Scholar]
- 28.Legakis I, Syrigos K. Obesity modulation - the role in carcinogenesis. Anticancer Agents Med Chem. 2010;10(6):481–490. doi: 10.2174/1871520611009060481. [DOI] [PubMed] [Google Scholar]
- 29.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28(26):4058–4065. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissue specificity of sulphonylureas: studies on cloned cardiac and beta cell KATP channels. Diabetes. 1998;47:1412–1418. doi: 10.2337/diabetes.47.9.1412. (1998) [DOI] [PubMed] [Google Scholar]
- 31.Gribble FM, Ashcroft FM. Differential sensitivity of β-cell and extrapancreatic KATP channels to gliclazide. Diabetologia. 1999;42:845–848. doi: 10.1007/s001250051236. (1999) [DOI] [PubMed] [Google Scholar]
- 32.Song DK, Ashcroft FM. Glimepiride block of cloned β-cell, cardiac and smooth muscle K-ATP channels. Brit J Pharmacol. 2001;133:193–199. doi: 10.1038/sj.bjp.0704062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dabrowski M, Wahl P, Holmes WE, Ashcroft FM. Effect of repaglinide on cloned beta cell, cardiac and smooth muscle types of ATP-sensitive potassium channel. Diabetologia. 2001;44:747–756. doi: 10.1007/s001250051684. [DOI] [PubMed] [Google Scholar]
- 34.Gribble FM, Reimann F. Sulphonylurea action revisited: the post-cloning era. Diabetologia. 2003;46:875–891. doi: 10.1007/s00125-003-1143-3. [DOI] [PubMed] [Google Scholar]
- 35.Horie M, Watanuki M, Tsuji K, Ishida H, Ishida-Takahashi A, Yuzuki Y, Seino Y, Sasayama S. Blockade of cardiac ATP-sensitive K+ channel by cibenzoline targets its pore-forming subunit. J Cardiovasc Pharmacol. 2000 Mar;35(3):434–442. doi: 10.1097/00005344-200003000-00014. [DOI] [PubMed] [Google Scholar]