Abstract
α-Crystallin is a major protein in the human lens that is perceived to help to maintain the transparency of the lens through its chaperone function. In this study, we demonstrate that many lens proteins including αA-crystallin are acetylated in vivo. We found that K70 and K99 in αA-crystallin and, K92 and K166 in αB-crystallin are acetylated in the human lens.To determine the effect of acetylation on the chaperone function and structural changes, αA-crystallin was acetylated using acetic anhydride. The resulting protein showed strong immunoreactivity against a Nε-acetyllysine antibody, which was directly related to the degree of acetylation. When compared to the unmodified protein, the chaperone function of the in vitro acetylated αA-crystallin was higher against three of the four different client proteins tested. Because a lysine (residue 70; K70) in αA-crystallin is acetylated in vivo, we generated a protein with an acetylation mimic, replacing Lys70 with glutamine (K70Q). The K70Q mutant protein showed increased chaperone function against three client proteins compared to the Wt protein but decreased chaperone function against γ-crystallin. The acetylated protein displayed higher surface hydrophobicity and tryptophan fluorescence, had altered secondary and tertiary structures and displayed decreased thermodynamic stability. Together, our data suggest that acetylation of αA-crystallin occurs in the human lens and that it could affect the chaperone function of αA-crystallin.
Keywords: Lysine, acetylation, α-crystallin, chaperone, human lens, aging
1. Introduction
The acetylation and deacetylation of histones in the cell nucleus regulates gene expression [1–3]. Recent studies show that a large number of cytosolic proteins are acetylated, suggesting an important role for acetylation in the function of cellular proteins [4–7]. The acetylation of lysine residues is mediated by lysine acetyltransferases (formerly called histone acetyltransferases), which transfer the acetyl group from acetyl-CoA to the epsilon amino group of lysine residues in proteins [8]. The reverse reaction is catalyzed by protein deacetylases, which include NAD-dependent sirtuins (SIRT) [9]. Although there are no reported studies on lysine acetyltransferases in the human lens, a recent study showed that the expression of SIRT1 is increased in age-related cataracts in humans and suggested that SIRT1 might protect lens epithelial cells against apoptosis [10].
The human lens contains high concentrations of proteins, up to 400 mg/mL. The tight orderly packing of lens proteins, known as crystallins, gives the lens the required transparency to focus images onto the retina. Among lens crystallins, α-crystallin is a major type. It is made up of two subunits, αA and αB-crystallin, with 60% sequence homology between the subunits. Both subunits can function as molecular chaperones [11]. They inhibit the aggregation of unfolded proteins to limit their denaturation, in an ATP-independent manner. It is widely believed that the chaperone function of α-crystallin plays a critical role in maintaining lens transparency during aging. Mutations of α-crystallin that reduce its chaperone function have been known to result in cataract formation, emphasizing the importance of the chaperone function for lens transparency [12–15].
α-Crystallin is also a robust anti-apoptotic protein. It inhibits apoptosis of cells through a number of apoptotic stimuli: by binding to procaspase-3, binding to Bax, inhibiting cytochrome-C release from the mitochondria, by activating PI3 kinase, promoting the activation of PDK-1 and AKT and inhibiting PTEN [16–19]. For some of these functions, the chaperone function appears to be necessary.
α-Crystallin is present in other tissues as well, where it plays an important role in tissue homeostasis. For example, in retinal pigment epithelial cells, it prevents oxidative stress-induced apoptosis [20]. In breast cancer cells, it promotes proliferation [21]. In retinal angiogenesis, it helps proper folding and secretion of VEGF-A [22].
Crystallin acetylation occurs in the lens. There are several reports on N-terminal acetylation [23–25]; however, reports on acetylation at lysine residues are sparse [26–28]. Other studies have shown that lysine residues in α-crystallin can be readily acetylated and acetylation leads to alteration in protein structure [29, 30]. Previous studies have also shown that treatment with aspirin acetylates lens proteins and inhibits glycation in vivo and in vitro [31, 32]. One report indicates that up to 5% of αA-crystallin in the human lens is acetylated at K70 [26]. K70 lies in the crystallin-core domain, and it is one of the identified sites for the chaperone function. Furthermore, one of the “mini-chaperones” of α-crystallin identified in Sharma’s laboratory resides in close proximity to the K70 residue [33], which could have an impact on the chaperone function. Considering that the chaperone and anti-apoptotic functions are directly related, it is likely that in vivo acetylation of αA-crystallin would have effects on both functions. In this study, we have investigated the effects on the chaperone function through the in vitro acetylation of αA-crystallin and by using an acetylation mimic of αA-crystallin.
2. Methods
Dithiothreitol (DTT), bovine insulin, citrate synthase (CS), acetic anhydride and lysozyme were obtained from Sigma-Aldrich Chemical Co. LLC (St. Louis, MO, USA). CS was dialyzed against 40 mM HEPES buffer, pH 7.4, for 24 h before use. 2-(p-toluidinyl) naphthalene-6-sulfonate (TNS) was obtained from Molecular Probes (Invitrogen, Carlsbad, CA, USA). Bovine γ-crystallin was purified from calf lenses as previously described [34]. All other chemicals were of analytical grade.
2.1. Identification of Nε-acetyllysine in α-crystallin of the human lens
This study adhered to the tenets of the Declaration of Helsinki. Water-soluble protein from a 59-year old lens (isolated as described below) at 1.0 mg in 200 µl of cell lysis buffer (Cell Signaling Technologies, Danvers, MA) was pre-cleaned by treating with 25 µl of Protein A/G Sepharose (Thermo Scientific) for 30 min at 4°C. The suspension was centrifuged at 3,000g for 2 min. To the supernatant, 24 µl of a monoclonal antibody against Nε-acetyllysine (Cell Signaling Technologies, Danvers, MA) was added and incubated for 2 hrs at 37°C. To this suspension, 20 µl of ProteinA/G Sepharose was added and incubated for 16 hrs at 4°C. The gel suspension was then centrifuged at 1,000g for 2 min and the gel was separated. The gel was then washed three times with ice-cold cell lysis buffer by centrifugation as above. The washed gel was incubated for 5 min at 95°C in the sample buffer and subjected SDS-PAGE on a 12% gel. Gel bands between 20–25 kDa were excised from the gel and were fist destained with 50% acetonitrile in 100 mM ammonium bicarbonate, and 100% acetonitrile. Then, the protein was reduced by 20 mM DTT at room temperature for 60 min followed by the alkylation using 50 mM idoacetamide for 30 min in the dark. The reaction reagents were removed and the gel pieces were washed with 100 mM ammonium bicarbonate and dehydrated in acetonitrile. Sequencing grade modified trypsin (Promega, Madison, WI) in 50 mM ammonium bicarbonate was added to the dried gel pieces and incubated at 37°C for overnight. Proteolytic peptides extracted from the gel with 50% acetonitrile in 5% formic acid were then dried and dissolved in 0.1% formic acid. Liquid chromatography-tandem mass spectrometry analysis of the resulting peptides was performed on a LTQ Orbitrap XL linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) equipped with an Ultimate 3000 HPLC system (Dionex, Sunnyvale, CA). The spectra were acquired by data dependent methods consisting of a full scan and MS/MS on the five most abundant precursor ions at the normalized collision energy of 30%. The obtained data were submitted to Mascot Daemon (Matrix Science, Boston, MA) by searching the acetylation on Lys residues. The modification sites were then verified by manual interpretation of the obtained MS/MS spectra.
2.2. Detection of Nε-acetyllysine in human lens proteins by ELISA
Human lenses were obtained from Heartland Lions Eye Bank, Columbia, MO. Lenses were stored at −80°C until use. Each lens was decapsulated and homogenized in 1.0 ml of PBS containing 0.2 mM EDTA (PBS-EDTA) and centrifuged at 20,000 × g for 30 min. The resulting supernatant was separated from the pellet. A volume of 0.5 ml of the above buffer was added to the pellet, which was re-suspended and centrifuged as before. The two supernatant fractions were pooled and designated the water-soluble fraction (WS). The pellet was subjected to sonication (amplitude=30%, three 40 second cycles) in 1.0 ml PBS-EDTA. The sonicated sample was centrifuged at 20,000 × g for 30 min. The resulting supernatant fraction was designated as the sonicated water insoluble (SWI) fraction. Unless otherwise mentioned, protein concentrations in samples were determined using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL) and BSA as the standard.
Microplate wells were coated with 5 µg protein/well of the WS and SWI fractions in 50 µl of 50 mM sodium carbonate buffer, pH 9.6, overnight at 4°C followed by washing three times with PBST. The wells were blocked with 5% non-fat dry milk (NFDM) in PBST for 2 h. Following washing, a 1:5,000 diluted mouse monoclonal antibody (in PBST-NFDM) against Nε-acetyllysine was added and incubated for 1 h at 37°C. The wells were then washed three times with PBST and incubated with 1:5,000-diluted goat anti-mouse antibody conjugated to horseradish peroxidase (Promega, Madison, WI). Following incubation for 1 h and washing three times with PBST, the wells were incubated with 100 µl of 3,3’,5,5’-tetramethylbenzidine dihydrochloride (Sigma-Aldrich) substrate for 45 min at 37°C. The enzymatic reaction was stopped at 45 min by the addition of 50 µl of 2N H2SO4, and the optical density of the wells was read at 450 nm in a microplate reader.
2.3. Cloning and purification of wild-type (Wt) and a K70Q mutant of human αA-crystallin
Wild type αA-crystallin was amplified using the following forward and reverse primers:
F: 5-GGCCATATGGACGTGACCATCCAGCAC
R: 3-CCCAAGCTTGGACGAGGGAGCCGAGGTG
The amplified PCR product was cloned into pET23a vector using Nde I and HindII restriction sites. To generate the K70Q mutant protein, αA-crystallin in a pET23a (+) vector was amplified with the forward primer 5’- GAC CGG GAC CAA TTC GTC ATC- 3’ and a complementary reverse primer. The resulting PCR product was digested with DpnI and transformed into E.coli DH5alpha cells. Plasmids from the resulting colonies were sequenced to confirm the presence of the mutation. This plasmid was then transformed into E. coli BL21 (DE3) cells for protein purification.
When the culture reached its target density of OD ~0.6 (OD600 nm), the recombinant proteins were overexpressed in E. coli BL21(DE3) cells by induction with 250 µM IPTG. The bacterial pellet obtained after centrifugation at 5,000 × g was suspended in 50 mM Tris, pH 8.0, containing 50 mM NaCl, 2 mM EDTA and 10 µl/ml of a protease inhibitor cocktail (Sigma, Cat# P8849). Lysozyme was added to the cell suspension at 0.3 mg/ml and incubated for 10 min at 37°C followed by sonication on ice at 30% amplitude and duty cycle=40. To the resulting cell lysate, 1.0 µl of benzonase nuclease (Sigma-Aldrich, Cat# E1014) was then added and incubated at 37°C in a shaker for 20 min, which was followed by the addition of sodium deoxycholate at 1.0 mg/ml and another incubation for 10 min at 37°C. DTT was then added to the lysate at a 5 mM concentration and incubated for 10 min at 37°C. The cell lysate was centrifuged at 20,000 × g for 30 min at 4°C. DNA in the lysate was precipitated by adding 0.2% polyethyleneimine followed by centrifugation at 20,000 × g for 15 min. Ammonium sulfate was added to the lysate to reach 70% saturation; the protein solution was then left at 4°C overnight and centrifuged at 20,000 × g for 5 min. The resulting pellet was suspended in 50 mM sodium phosphate buffer, pH 7.4, containing 150 mM NaCl and 5 mM DTT, and it was centrifuged at 20,000 × g for 5 min. The supernatant was filtered through a 0.45-µm filter and loaded onto a Superdex-200 prep-grade gel filtration column (GE Healthcare, WI) that was pre-equilibrated with 50 mM sodium phosphate buffer, pH 7.4, containing 5 mM DTT. Fractions of 3.0 ml were collected, and their OD280 nm was recorded. The protein peak fractions were pooled and dialyzed overnight at 4°C against 20 mM Tris, pH 8.0, containing 0.1 mM EDTA. The dialyzed sample was applied onto a Q-Sepharose anion exchange column (GE Healthcare, NJ), equilibrated with 20 mM Tris, pH 8.0, containing 0.1 mM EDTA. The bound protein was eluted with a 0–1 M NaCl gradient in the same buffer. The protein peak fractions were pooled and dialyzed against PBS containing 0.1 mM EDTA.
2.4. Acetylation of α A-crystallin
Acetic anhydride (Ac2O) in dioxane was added to 550 µg of αA-crystallin in 150 µl of PBS drop-wise over a period of 1 h to obtain lysine (in αA-crystallin) to Ac2O molar ratios of 1:0, 1:2, 1:5, 1:10, 1:25 and 1:100 (pH was maintained at 7.4 with diluted NH4OH). The volume of the reaction mixture was adjusted to 500 µl with PBS and dialyzed overnight against 1 L of PBS.
2.5. Mass spectral identification of Nε-acetyllysine in acetylated human α A-crystallin
In gel digestion was performed as described in methods 2.1, except that 0.2 µg sequencing grade modified endopeptidase Asp-N (Reche Diagnostics Corporation, Indianapolis, IN) was used instead of trypsin. Other details are same as in 2.1.
2.6. Determination of amino groups in acetylated α A-crystallin
The amino group content in the control and the acetylated αA-crystallin was measured using fluorescamine as previously described [35].
2.7. Chaperone assays
The chaperone assays were carried out as previously described [36]. The following ratios of αA-crystallin to client proteins (w/w) were used: αA-crystallin:citrate synthase-1:14; αA-crystallin:βL-crystallin- 1:30; αA-crystallin:γ-crystallin- 1:12; αA-crystallin:lysozyme-1:4.
2.8. Western blotting for Nε-acetyllysine in proteins
Proteins were separated on a 12% denaturing gel, transferred to nitrocellulose and probed with a monoclonal antibody to Nε-acetyllysine (1:50,000 diluted) and an HRP-conjugated goat anti-mouse IgG (1:5,000 diluted). The immunoreactivity was detected using a Pierce Enhanced Chemiluminescence Detection Kit.
2.9. Surface hydrophobicity and tryptophan fluorescence
The surface hydrophobicity of the protein was measured using TNS (emission: 350–520 nm, excitation: 320 nm) as previously described [37]. Tryptophan fluorescence of the protein was recorded at emission wavelengths of 300–400 nm and an excitation wavelength of 290 nm [38]. Protein samples (0.1 mg/ml) in 50 mM phosphate buffer, pH 7.2, were used in both of the experiments.
2.10. CD spectra of acetylated αA-crystallin
Far-UV CD spectra were measured at 25°C using a Jasco 810 spectropolarimeter (Jasco, Inc., Japan). Spectra were collected from 250 to 200 nm using a cylindrical quartz cell with a 1.0 mm path length. Proteins (0.2 mg/ml) were dissolved in 10 mM phosphate buffer, pH 7.2. The resultant spectra after five scans were analyzed for secondary structure by the CONTINLL curve-fitting program [38].
Near-UV CD spectra were measured at 25°C using the same spectropolarimeter. Spectra were measured with 0.5 mg/ml protein solutions in 50 mM phosphate buffer, pH 7.2. The reported spectra were the average of 5 scans.
2.11. Multiangle light scattering studies
We analyzed Wt, K70Q and acetylated αA-crystallin (2 molar excess of Ac2O) by MLS conditions as previously described [39]. Briefly, the samples were incubated at 37°C for 30 min before injecting (75 µg) into a TSKG5000PWXL column connected to MLS detectors. The data was analyzed using the ASTRA software (5.3.4.20) developed by Wyatt Technology Corp., Santa Barbara, CA.
2.12. Structural stability of acetylated α A-crystallin
The effect of acetylation on the stability of wild-type αA-crystallin was determined by an equilibrium chemical denaturation experiment. αA-crystallin (0.05 mg/ml in 50 mM phosphate buffer, pH 7.5) was incubated with various urea concentrations (0–6 M) for 18 h at 25°C. The tryptophan fluorescence spectra of all samples were recorded in the 300 – 400 nm region using an excitation wavelength of 295 nm. The equilibrium unfolding profile was fitted according to a three-state model [33].
3. Results
3.1. Nε-acetyllysine in human lens proteins
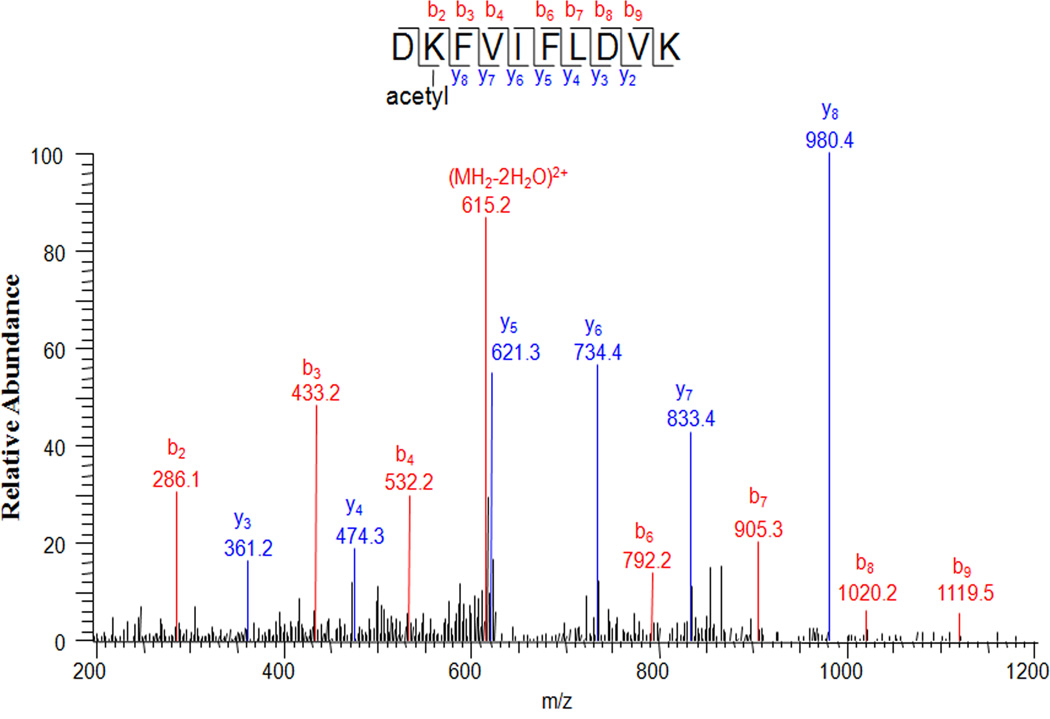
To determine the in vivo acetylation sites in human lens α-crystallin, we used the SDS-PAGE protein band corresponding to α-crystallin from a 59 year old normal lens. Mass spectrometric analyses indicated that K70 and K99 are acetylated in αA-crystallin and K92 and K166 are acetylated in αB-crystallin (Table 1). Fig. 1 shows an example of tandem mass spectrum of an acetylated peptide. Comparing with unmodified peptide DKFVIFLDVK of αA-crystallin, the mass shift of 42.013 Da was observed on parent ion and through b2 to b9 ions, while all y2 to y8 ions remained unchanged. This strongly suggested that K70 was acetylated.
Table 1.
Identification Nε-acetyllysine human lens α-crystallin
| Proteins | Peptides | Acetyl site |
Obs. Mass | Cal. Mass | Mass error (ppm) |
|---|---|---|---|---|---|
| αB-crystallin | VKVLGDVIEVHGK | K92 | 1433.8262 | 1433.8242 | 1 |
| EEKPAVTAAPK | K166 | 1181.6314 | 1181.6292 | 2 | |
| αA-crystallin | SDRDKFVIFLDVK | K70 | 1622.8711 | 1622.8668 | 3 |
| DKFVIFLDVK | K70 | 1264.7096 | 1264.7067 | 2 | |
| VQDDFVEIHGKHNER | K99 | 1863.8860 | 1863.8864 | 0 |
Fig. 1. Mass spectrometric detection of Nε-acetyllysine at K70 in human lens αA-crystallin.
Tandem mass spectrum of DKFVIFLDVK of αA-crystallin with m/z=633.3621(2+). The mass shift of 42.013 Da on the parent ion and b series ions suggested acetylation of K70.
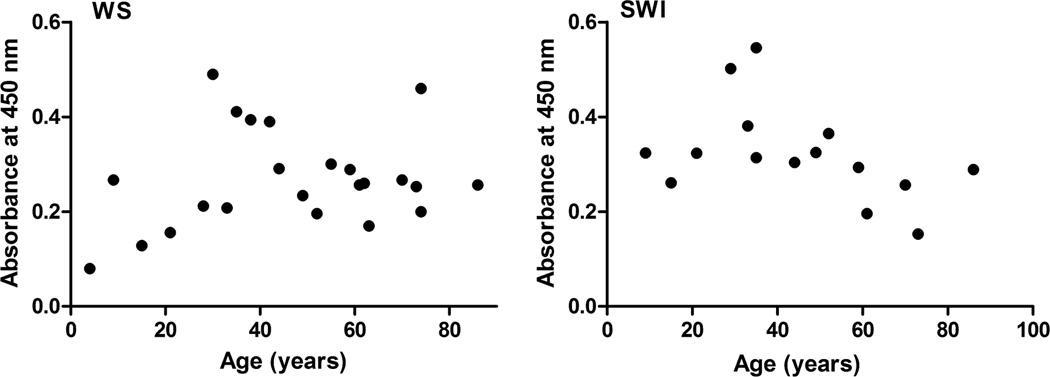
The ELISA results showed that both the WS and SWI fractions react with the Nε-acetyllysine antibody. We sought to determine the effect of age on the total Nε-acetyllysine content in the lens. The results indicated that a slow progressive accumulation of Nε-acetyllysine occurs up to ~40 years in both the WS and SWI fractions, peaking between 30 and 40 years and then gradually declining with age (Fig. 2). The immunoreactivity was completely abolished by prior incubation of the antibody with acetylated BSA, confirming that the reaction was specific for Nε-acetyllysine in αA-crystallin (data not shown).
Fig. 2. Effect of age on the Nε-acetyllysine content in human lens proteins.
Human lens proteins were separated into water soluble (WS) and insoluble fractions by homogenization and centrifugation. The water-insoluble fraction was sonicated and centrifuged to obtain a soluble fraction (SWI). The two fractions were tested in a direct ELISA for Nε-acetyllysine content.
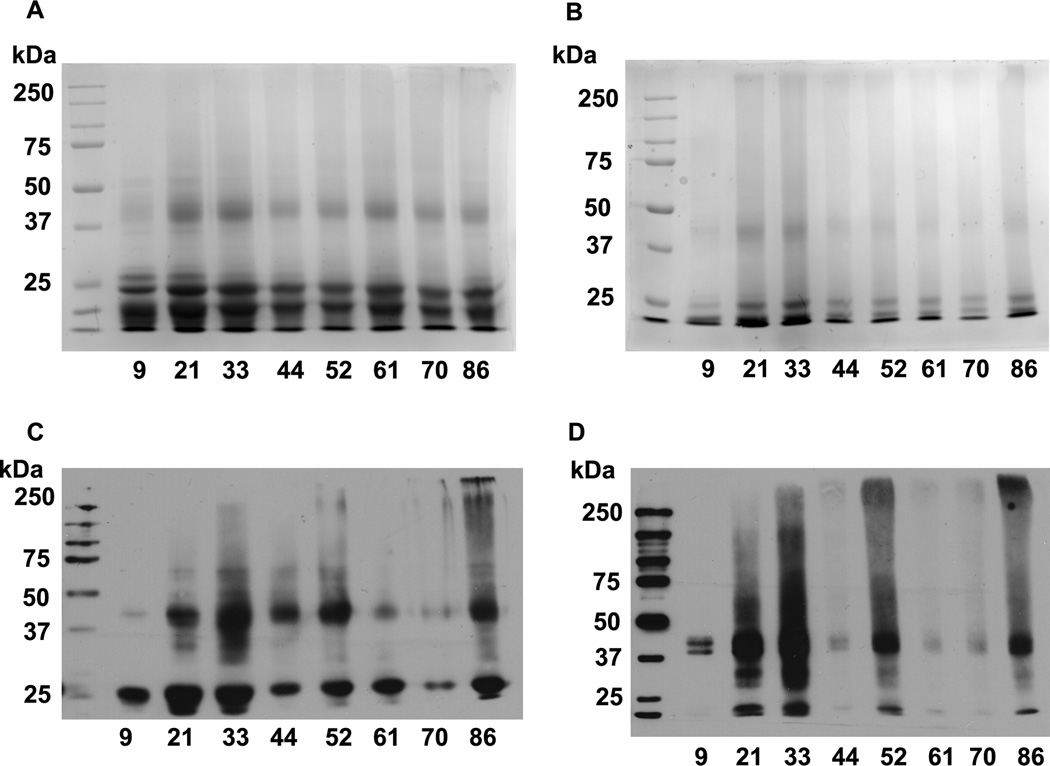
SDS-PAGE separation of WS and SWI fractions are shown in Fig. 3A and B. Western blotting showed multiple immunoreactive protein bands both in WS and SWI fractions (Fig. 3C and D) with a strong reaction in the 20–25 and 40–45 kDa regions. Some immunoreactivity was also detected in highly cross-linked high molecular weight proteins.
Fig. 3. Detection of Nε-acetyllysine in human lens proteins by western blotting.
WS and SWI fractions were subjected to SDS-PAGE (A and B) under reducing conditions, transferred to a nitrocellulose membrane and probed with a monoclonal antibody to Nε-acetyllysine (C and D). Representative lenses from every decade of life were chosen. The numbers below the lanes are the age of the lens.
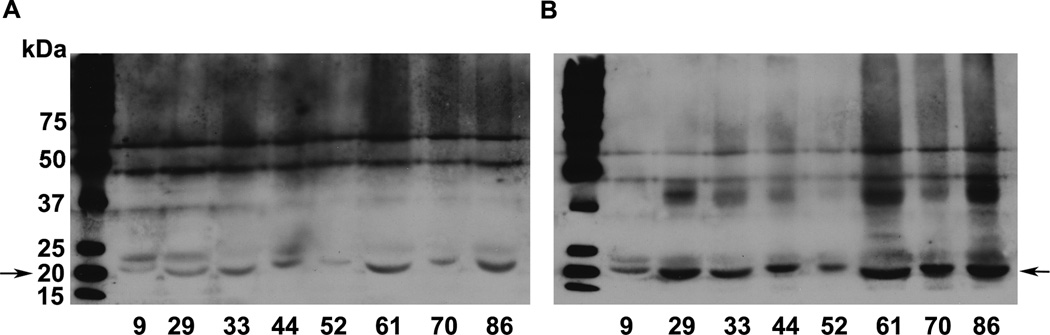
Next, we wanted to demonstrate by immunoprecipitation and western blotting that αA-crystallin is acetylated in vivo. Our attempts to immunoprecipitate lens proteins using either an antibody for Nε-acetyllysine or an antibody to αA-crystallin failed because of a non-specific interaction between α-crystallin with protein A/G Sepharose. However, western blotting for αA-crystallin and Nε-acetyllysine showed the same protein reacting with two antibodies (Fig. 4), indicating that αA-crystallin is acetylated in human lens proteins.
Fig. 4. Acetylation of αA-crystallin in the human lens.
Water-soluble lens proteins were western blotted using an antibody for αA-crystallin (A) and for Nε-acetyllysine (B). The arrow indicates the location of αA-crystallin. The numbers below the lanes indicate the age of the lens.
3.2. Acetylation of αA-crystallin
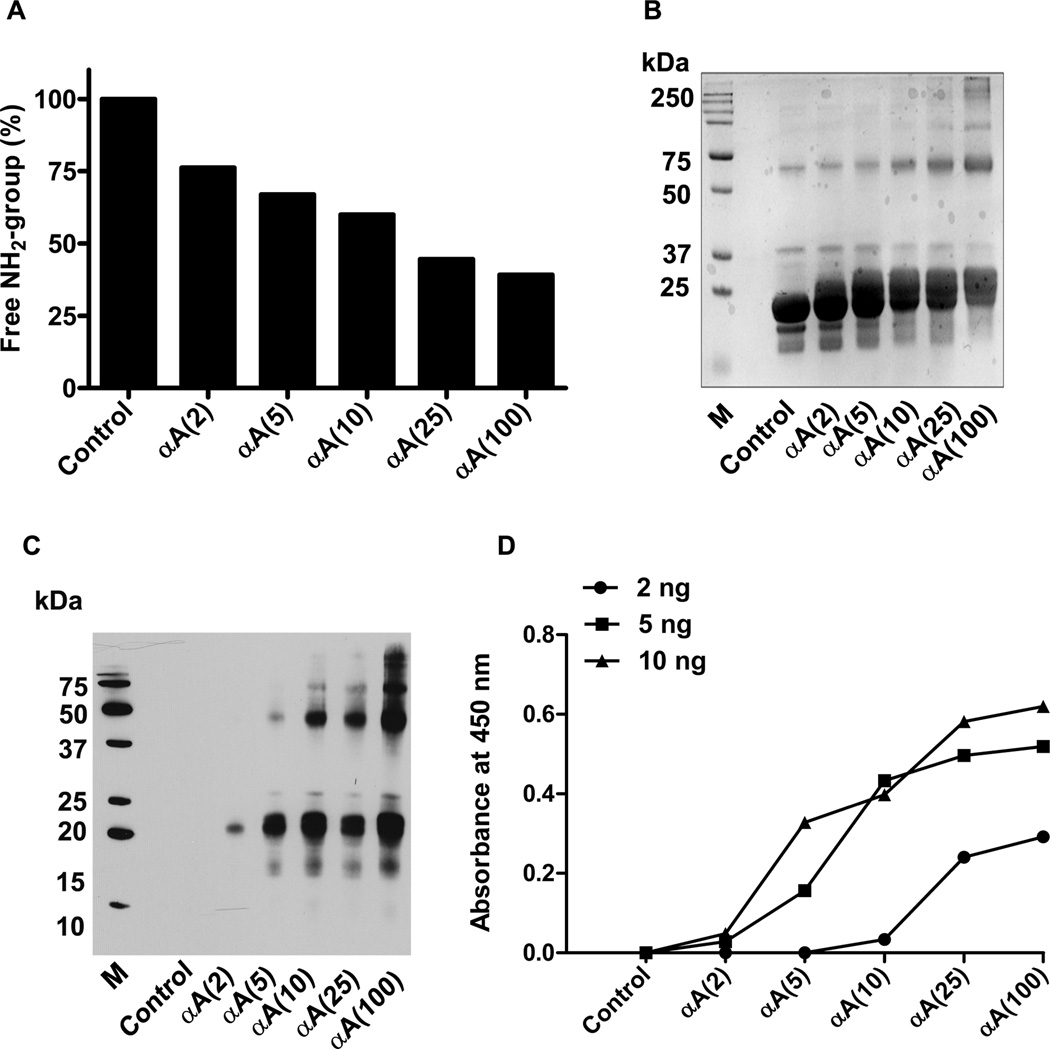
To determine the effect of acetylation on the chaperone function of αA-crystallin, recombinant αA-crystallin was acetylated using Ac2O. We used 2, 5, 10, 25 and 100 molar excesses of Ac2O versus lysine (considering 7 lysine residues per molecule of αA-crystallin) for the acetylation studies. The amino group measurement using fluorescamine showed a progressive decline in the free amino group content with increased concentrations of Ac2O (Fig. 5A). A two molar excess of Ac2O reduced the free amino group by 25%, and a 100 molar excess reduced it by ~60%. SDS-PAGE profile showed some crosslinking in acetylated proteins (Fig. 5B). Western blotting using an Nε-acetyllysine antibody showed a strong reaction with monomeric αA-crystallin (Fig. 5C), and the intensity of the reaction increased with increasing concentrations of acetic anhydride. It is noteworthy that a 40-kDa protein, possibly arising from the cross-linking of two αA-crystallin molecules, was present in all samples treated with Ac2O. It is not clear how acetylation produced cross-linked αA-crystallin. In addition, many high molecular weight cross-linked proteins were also seen with acetylated αA-crystallin. The ELISA results with three concentrations of protein (2, 5 and 10 ng/well) used for coating the wells also showed a direct relationship between acetylation and the Nε-acetyllysine content αA-crystallin (Fig. 5D).
Fig. 5. In vitro acetylation of αA-crystallin.
Human αA-crystallin was incubated with acetic anhydride (Ac2O) at lysine:Ac2O molar ratios of 1:0, 1:2, 1:5, 1:10, 1:25 or 1:100 and are shown as control, αA(2), αA(5), αA(10), αA(25) and αA(100), respectively, in this and subsequent figures. The amino group content in the modified protein was assessed using fluorescamine (A). The SDS-PAGE profile of acetylated αA-crystallin is shown in panel (B). Western blotting of the acetylated αA-crystallin showed strong immunoreactivity against the Nε-acetyllysine antibody (C). The immunoreactivity increased with increasing concentrations of acetic anhydride. In addition to the monomeric protein, cross-linked protein also reacted. A direct ELISA was used to determine the Nε-acetyllysine content in acetylated αA-crystallin. M=molecular weight markers (D). The microplate well was coated with 2, 5 or 10 ng of acetylated αA-crystallin in these assays. Other details of the ELISA have been described in Methods.
Mass spectrometry of the acetylated sample (10 molar excess of Ac2O) showed acetylation of all lysine residues (Table 2). However, we have not determined the extent of the acetylation of individual lysine residues.
Table 2.
Identification of Nε-acetyllysine in acetylated αA-crystallin
| Peptides | No. | Obs. Mass | Acetylation Sites |
|---|---|---|---|
| MDVTIQHPWFKRTLGPFYPSRLF | 1–23 | 2893.4740 | Protein N-term |
| 2935.4845 | Protein N-term, K11 | ||
| DVTIQHPWFKRTLGPFYPSRLF | 2–23 | 2746.4368 | K11 |
| DKFVIFL | 69–75 | 922.5157 | K70 |
| DVKHFSPE | 76–83 | 999.4656 | K78 |
| DVKHFSPEDLTVKVQ | 76–90 | 1782.9132 | K88 |
| 1824.9238 | K78, K88 | ||
| DLTVKVQ | 84–90 | 843.4706 | K88 |
| DDFVEIHGKHNERQ | 91–104 | 1764.8180 | K99 |
| DFVEIHGKHNERQ | 92–104 | 1649.7907 | K99 |
| DGMLTFCGPKIQTGL | 136–150 | 1678.8052 | K145 |
| DATHAERAIPVSREEKPTSAPSS | 151–173 | 2477.2147 | K166 |
αA-crystallin was acetylated with 10 molar excess of Ac2O (compared to the lysine content)
3.3. Chaperone function of acetylated αA-crystallin
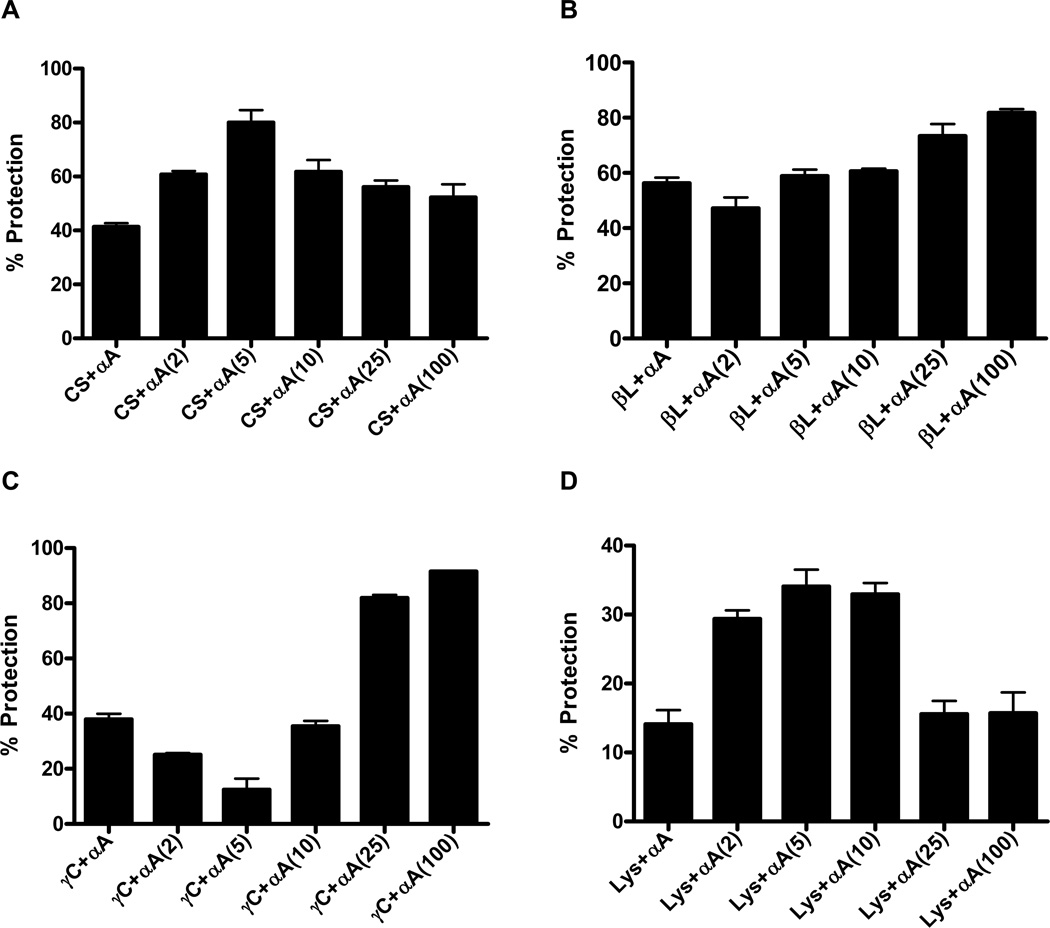
The chaperone function of the acetylated protein was evaluated using four different client proteins. With CS as the client protein, the chaperone function of acetylated αA-crystallin increased by 20% and 40% when acetylated with a 2 and 5 molar excess of Ac2O over the control (unmodified αA-crystallin), respectively (Fig. 6A). Such an increase in the chaperone function was also noted at lower degrees of acetylation with 0.01 and 0.05 molar excess of Ac2O (Supplemental Data, Fig. 1S). Lesser improvement in the chaperone function was noticed with a higher Ac2O concentration (>10 molar excess), but the improvement was still greater than with the unmodified protein (10–20% higher). With βL-crystallin as the client protein, the chaperone function progressively improved with increased concentrations of Ac2O (Fig. 6B) except for a 2 molar excess of Ac2O, where a slight reduction was noticed. The improvement in the chaperone function reached 25% with a 100 molar excess of Ac2O. With γ-crystallin as the client protein, at low concentrations of Ac2O (2–10 molar excess), there was a decrease in chaperone function (Fig. 6C). However, with higher concentrations of Ac2O (>25 molar excess), there was a 50–60% increase in chaperone function. The three assays above are based on the thermal aggregation of client proteins. To determine if acetylation affected the chemical aggregation of proteins, we used lysozyme as the client protein. The aggregation of lysozyme was induced by DTT. Like CS, at low concentrations of Ac2O (<25 molar excess), the chaperone function of αA-crystallin improved by 15–20% with acetylation (Fig. 6D). However, at higher concentrations of Ac2O, the chaperone function was similar to that of the unmodified protein. Taken together, these data suggest that the acetylation of lysine residues in αA-crystallin alters its chaperone function and that there is a discordant effect with regard to the client protein. The improved chaperone function at low concentrations of Ac2O (<10 molar excess) with three out of the four client proteins tested suggests that acetylation could be a protective mechanism to preserve the chaperone function.
Fig. 6. Effect of acetylation on the chaperone function of αA-crystallin.
The chaperone function of αA-crystallin was assessed using four client proteins, as described in Methods. (A) Citrate synthase (CS); (B) βL-crystallin; (C) γ-crystallin and (D) lysozyme. Assays were done in triplicate and the bars represent the mean ± SD.
3.4. Chaperone function of K70 acetylation mimic
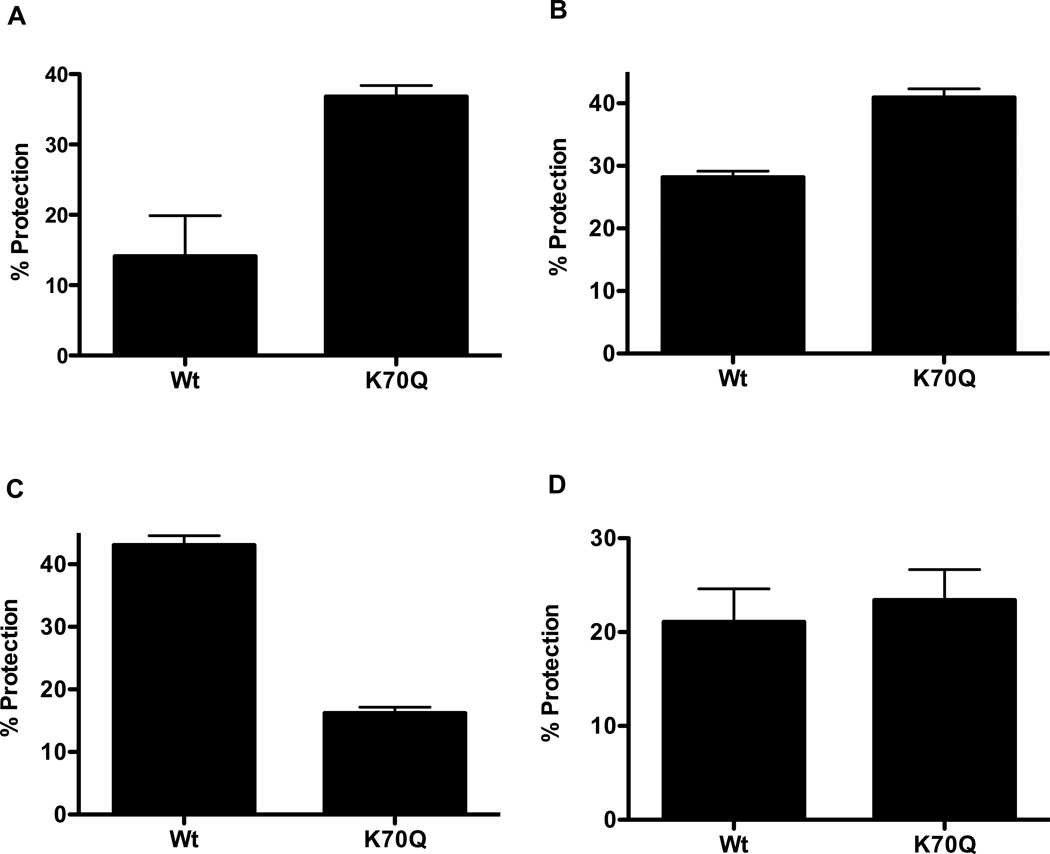
Because it has been reported that K70 in αA-crystallin is the major site of acetylation in the human lens [26], which is corroborated by our mass spectrometry data, we generated an acetylation mimic at this site by replacing lysine with glutamine (K70Q). Many studies have used this strategy to study the effects of acetylation on specific lysine residues in proteins [7, 40, 41]. The K70Q was purified by the same procedure as the Wt protein. SDS-PAGE showed a single protein band for both proteins (Supplemental Data, Fig. 2S). The chaperone function of the mutant protein was compared with the Wt protein using the same four client proteins that were used to test the effect of acetylation on the chaperone function and adhering to the same ratios between αA-crystallin and the client proteins. With CS, βL-crystallin and lysozyme, the mutant protein showed 26, 15 and 5% increases in the chaperone function compared to the Wt protein, respectively (Fig. 7A to C). However, with γ-crystallin as the client protein, the mutant protein’s chaperone function was reduced by 30% compared to the Wt protein (Fig. 7D), which was similar to the mildly acetylated protein (Fig. 7C). Taken together, similar to acetylated protein, the acetylation mimic in general showed an increase in the chaperone function.
Fig. 7. Effect of the introduction of an acetylation mimic at K70 on the chaperone function of αA-crystallin.
The K70 residue in αA-crystallin was replaced with glutamine (K70Q) to mimic acetylation. The chaperone function was evaluated using four client proteins. (A) Citrate synthase (CS); (B) βL-crystallin; (C) γ-crystallin and (D) lysozyme. Assays were done in triplicate and the bars represent the mean ± SD.
3.5. Tryptophan fluorescence
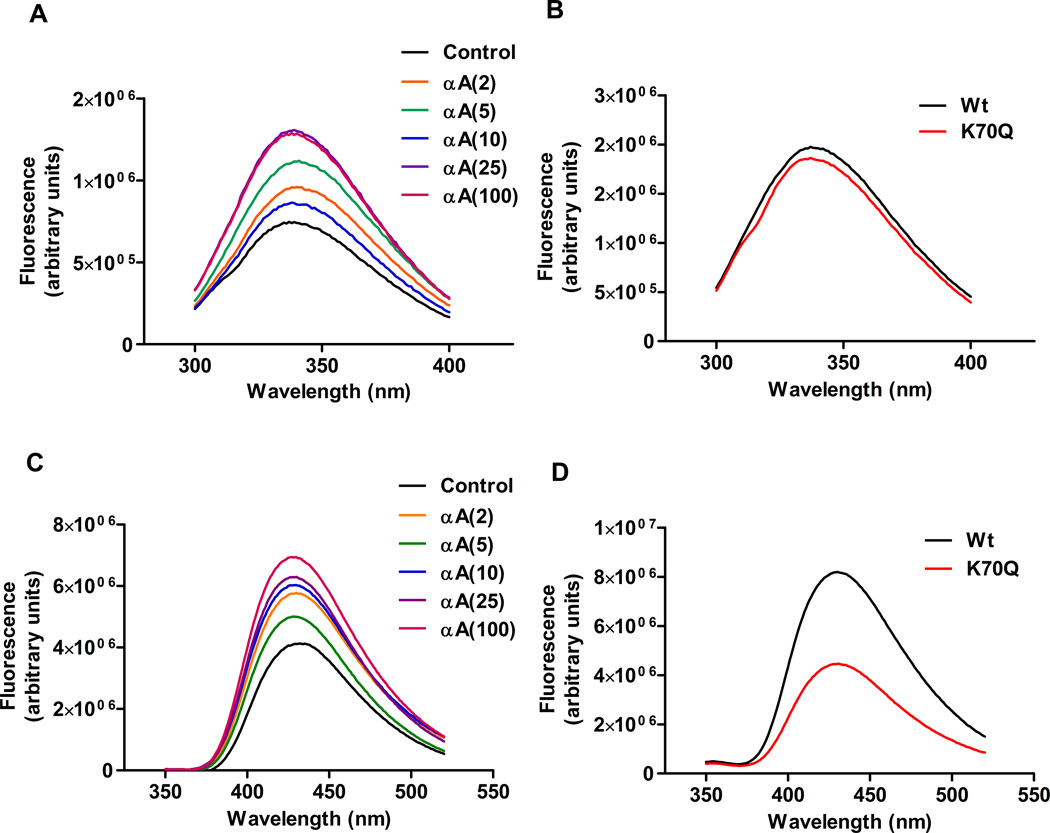
Tryptophan fluorescence of the acetylated proteins was in general increased, although there was no strict relationship between tryptophan fluorescence and the extent of acetylation (Fig. 8A). Proteins modified with 25 and 100 molar excesses of Ac2O showed the highest fluorescence, which was two times that of the unmodified protein at the excitation maximum of 340 nm. αA-crystallin has one tryptophan residue per molecule. The increased tryptophan fluorescence indicated that as a result of acetylation, the protein’s tertiary structure was altered to expose tryptophan. The tryptophan fluorescence was slightly reduced in the K70Q mutant protein when compared to the Wt protein (Fig. 8B), indicating that acetylation at K70 mildly alters the tertiary structure of the protein.
Fig. 8. Effect of acetylation on tryptophan fluorescence and surface hydrophobicity in αA-crystallin.
Tryptophan fluorescence was recorded at an excitation wavelength of 290 nm and emission wavelengths of 300–400 nm for in vitro acetylated αA-crystallin (A) and the acetylation mimic (B). The surface hydrophobicity of the acetylated (C) and the K70Q mutant protein (D) was assessed using TNS. TNS fluorescence was recorded at an excitation wavelength of 320 nm and emission wavelengths 350–520 nm.
3.6. Surface hydrophobicity
We found the surface hydrophobicity of the protein increased with acetylation; the increase was directly proportional to the extent of acetylation (Fig. 8C) except for the protein modified with a 2 molar excess of Ac2O, which was more hydrophobic than the protein modified with a 5 molar excess of Ac2O. The increase in surface hydrophobicity was almost 2-fold higher in the protein modified with a 100 molar excess of Ac2O compared to the unmodified protein. The surface hydrophobicity of the K70Q mutant protein was nearly half that of the Wt protein (Fig. 8D), which suggested that the increased chaperone function of the mutant protein was unrelated to the surface hydrophobicity.
3.7. Multi-angle light scattering analysis
We found that the average molar mass of Wt and K70Q to be comparable and they were, 1.325 ± 0.012e+6 g/mol and 1.308 ± 0.014e+6 g/mol respectively. However, the average molar mass for acetylated αA-crystallin (2 molar excess of Ac2O) was reduced; it was 1.068 ± 0.010e+6 g/mol. The chromatograms are shown in Supplemental Figure 3S.
3.8. UV-CD spectroscopy
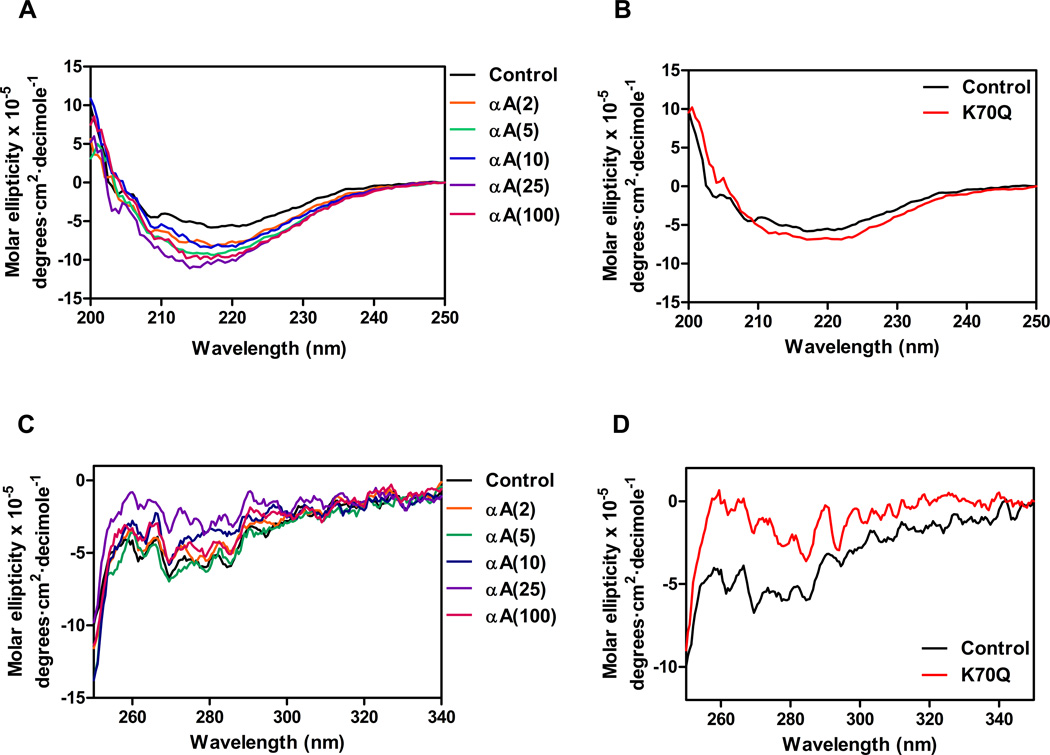
Far-UV CD spectra indicated that in vitro acetylation of αA-crystallin perturbed its secondary structure (Fig. 9A). We found that unmodified/wild-type αA-crystallin is a major β-sheet protein (39.2% β-sheet and only 6% α-helix). We also found that the extent of the increase in β-sheet content (39.2% – 51%) with Ac2O treatment directly related to the degree of acetylation modification in αA-crystallin: the higher the Ac2O concentration, the greater the perturbation in the secondary structure of αA-crystallin. However, the K70Q mutant had little effect on the secondary structural packing of αA-crystallin (Fig. 9B).
Fig. 9. Effect of acetylation on the secondary and tertiary structure of αA-crystallin.
The secondary structure of the acetylated (A) and K70Q mutant (B) was assessed by far-UV CD spectroscopy, and the tertiary structure of the acetylated (C) and K70Q mutant protein (D) was assessed by near-UVCD spectroscopy.
Near-UV CD data (Fig. 9C) supports our intrinsic tryptophan fluorescence results (Fig. 8). The signal for phenylalanine (250–270 nm region) was considerably altered in the acetylated and K70Q proteins compared to the Wt protein (Fig. 9D). Moreover, peaks beyond 270 nm for the acetylated and K70Q proteins were also found to differ both in intensity and in position from those of the Wt protein. These changes in spectral characteristics of the acetylated and mutant proteins suggest that the microenvironments of the amino acid residues (Phe, Tyr and Trp) were significantly perturbed by acetylation.
3.9. Thermodynamic stability of acetylated αA-crystallin
We compared the thermodynamic stability of acetylated and mutant proteins by equilibrium urea unfolding measurements. Tryptophan fluorescence intensities were recorded at 337 and 350 nm at various urea concentrations. The data were plotted as the ratio of the intensities at 337 and 350 nm as a function of the urea concentration (Fig. 10). A crude estimate of the transition midpoint (C1/2) from a sigmoidal analysis of the denaturation profiles revealed that the C1/2 value decreased from 2.34 M for the wild-type/unmodified control protein to 1.7 M urea for the acetylated αA-crystallin (Fig. 10A, Table 3). This decreasing trend in the C1/2 values clearly indicated that acetylation destabilized the oligomeric assembly of αA-crystallin. On the other hand, the transition midpoint (C1/2) value increased slightly in the K70Q mutant (Fig. 10B; Table 3). To quantify the stability against chemical denaturation, all of the profiles were analyzed by a global three-state fitting procedure, according to the equation:
where F0, F1 and F∞ are the signal intensities for the 100% native, 100% intermediate and 100% unfolded form, respectively. ΔG1 0 refers to the standard free energy change between the native and the intermediate form, and ΔG2 0 refers to the standard free energy change between the intermediate and unfolded form. ΔG0, being the sum of ΔG1 0 and ΔG2 0, refers to the standard free energy change of unfolding (between the native and unfolded form) at a zero urea concentration. The standard free energy change (N→U) of the unfolding of the wild-type αA-crystallin, obtained according to a three-state model, was 11.89 kcal/mol (Table 3). This standard free energy change magnitude decreased gradually upon treatment with higher concentrations of Ac2O. On the other hand, the K70Q mutant had very minute effect on the structural stability of human αA-crystallin.
Fig. 10. Effect of acetylation on the equilibrium urea unfolding profile of αA-crystallin.
Proteins (0.05 mg/ml) were suspended in 50 mM phosphate buffer (pH 7.2) containing 0–6 M urea at 25°C. The equilibrium urea unfolding profiles of acetylated (A) and K70Q (B) αA-crystallin have been normalized to a scale of 0–1. The values were calculated as per the three state model. The magnitudes of C1/2 and ΔG0 have also been plotted for acetylated (C) and K70Q (D) αA-crystallin
Table 3.
C1/2 and ΔG0 values of acetylated and K70Q αA-crystallin at 25°C.
| Sample | C1/2 (M) | ΔG0 (kcal/mole) |
|---|---|---|
| Unacetylated/control | 2.34 | 11.89 ± 0.7* |
| αA(2) | 2.34 | 11.06 ± 0.6 |
| αA(5) | 2.18 | 7.06 ± 1.2 |
| αA(10) | 1.73 | 5.44 ± 0.9 |
| αA(25) | 2.16 | 8.61 ± 1.1 |
| αA(100) | 2.05 | 6.35 ± 0.5 |
| K70Q | 2.61 | 12.62 ± 0.8 |
mean ± SD
4. Discussion
The present study was conducted in light of the discoveries in Jean Smith’s laboratory that α-crystallin is acetylated in vivo [26, 27]. The availability of a monoclonal antibody against Nε-acetyllysine allowed us to develop an ELISA to determine the effect of aging on the Nε-acetyllysine content in the human lens. Acetylated lysine residues were observed in both the WS and SWI fractions. We found that the acetylation of lens proteins occurs very early in age and stays somewhat constant throughout life, with the exception of a peak in Nε-acetyllysine content between ages 30–40 in several lenses in both the WS and SWI fractions. The trend toward a decrease in Nε-acetyllysine content after ~40 years of age could be due to masking of the antigen in proteins because of increased age-associated crosslinking and aggregation.
Jean Smith’s laboratory also reported that nearly 5% of the αA-crystallin in the human lens is acetylated at K70 [26]. The present study confirmed that acetylation can occur at this lysine residue. The immediate question was: how does acetylation affect the function of αA-crystallin? One major function of αA-crystallin is its ability to chaperone structurally perturbed proteins and prevent their denaturation [11]. Because lens proteins have a negligible turnover rate during life, they can accumulate post-synthetic modifications during aging. Such modifications could result in lens protein aggregation and lead to cataract formation. The chaperone function of α-crystallin has been perceived to be important for preventing such protein aggregation and to maintain lens transparency during aging.
Our study showed that at low levels of acetylation, αA-crystallin’s chaperone function is generally increased. However, not all client proteins behaved the same way; for example, with γ-crystallin as the client protein, there was a loss of chaperone function with low levels of acetylation. This suggests the possibility that not all client proteins bind to the same chaperoning site in αA-crystallin, which has been previously proposed [42, 43]. Some chaperone sites may be negatively affected and others may be positively affected by acetylation. Similar observations were made with the K70Q mutant acetylation mimic. It showed better chaperone function against CS, βL-crystallin and lysozyme but showed weaker chaperone function against γ-crystallin compared to the Wt protein, reiterating the notion that the chaperone sites for client proteins could be different in αA-crystallin. Whether or not the altered chaperone function of αA-crystallin from acetylation results in increased interactions with client proteins and consequently their ubiquitination and degradation, akin to Hsp70, needs to be verified [44]. Similarly, whether acetylation of αA-crystallin is beneficial or detrimental to the lens is not clear, because of the discordant effects on the aggregation of γ- and βL-crystallins.
Acetylation increased the intensity of tryptophan fluorescence, suggesting a structural perturbation leading to protein unfolding and the exposure of the buried tryptophan to the surface in αA-crystallin. Such unfolding of the protein by acetylation could have led to higher surface hydrophobicity, as measured by TNS binding. Whether or not higher surface hydrophobicity was the reason for the higher chaperone function was not clear, but it seems unlikely given the reduced chaperone function against γ-crystallin when the surface hydrophobicity was already increased. Previous studies also have observed that there is no strict relationship between surface hydrophobicity and the chaperone function in α-crystallin [45, 46]. Supporting this argument is another observation from this study that the K70Q mutant protein, which displayed higher chaperone function against three of the four client proteins, had lower surface hydrophobicity than the Wt protein.
Most small heat shock proteins exist in oligomeric forms. Whether oligomerization is a pre-requisite for their chaperone function is debated. Saha and Das [47] demonstrated that the chaperone activity of human αA- and αB-crystallin depends more on the alpha-crystallin domain and hydrophobic clefts on the protein surface than on their oligomeric size. However, Gu et al. [48] reported that dissociation of the oligomeric structure is essential for the chaperone-like activity of Hsp16.3. In this study, we found that acetylation with Ac2O improved the chaperone function of αA-crystallin despite the fact that the overall structural integrity/assembly of the protein was destabilized. Exactly how acetylation improves the chaperone function is not known. The data from the MLS analyses that acetylated protein has lower molar mass when compared to the unmodified protein hints to the possibility that acetylation resulted in partial dissociation of the oligomeric protein and led improvement in the chaperone function. A similar improvement in the chaperone function as a result of a reduction in oligomeric size has been previously observed for αB-crystallin [39].
In summary, the present study showed that the acetylation of αA-crystallin occurs in the human lens. Whether the acetylation of αA-crystallin increases the binding and disposal of damaged proteins through ubiquitination is to be determined. Acetylation could also be a strategy to limit other modifications of lysine residues in proteins.
Highlights.
Lysine acetylation occurs in α-crystallin, a major protein of the human lens.
The chaperone function of αA-crystallin is enhanced by lysine acetylation.
The surface hydrophobicity αA-crystallin is increased by lysine acetylation.
The acetylation mimic of αA-crystallin (K70Q) shows increased chaperone function.
Acetylation could regulate the chaperone function of small heat shock proteins.
Supplementary Material
Fig. 1S. Effect of acetylation on the chaperone function of αA-crystallin.αA-crystallin was acetylated using 0.01 and 0.05 molar excess (relative to lysine) of Ac2O as described in Methods and the ELISA results (A) show that the protein is acetylated (1.0 µg protein was used for the assay).The chaperone function of αA-crystallin was assessed using (B) citrate synthase (αA:CS ratio=1:10, w/w) and (C) βL-crystallin (αA:βL ratio=1:57 w/w) as client proteins.
Fig. 2S. SDS-PAGE of purified recombinant human wild type (Wt) and K70Q αA-crystallin. M=molecular weight markers
Fig. 3S. Multiangle light scattering analysis of wild type αA, K70Q and acetylated αA-crystallin. The molar mass distribution across UV peaks was analyzed from the MLS data using the ASTRA software and the molar mass at the peak apex is reported. The data suggest that there is a 20% decrease in molar mass in acetylatedαA-crystallin.
Acknowledgements
This study was supported from NIH grants R01EY-016219 and R01EY-09912 (RHN), P30EY-11373 (the Visual Sciences Research Center of CWRU); Research to Prevent Blindness, NY, the Ohio Lions Eye Research Foundation and DST SERC FAST TRACK, India grant SR/FT/CS-039/2009 (AB).
Abbreviations
- PBST
phosphate-buffered saline with 0.05% Tween-20
- NFDM
non-fat dry milk
- SIRT
silent information regulator
- WS
water-soluble lens protein
- SWI
sonicated water-insoluble lens protein
- Ac2O
acetic anhydride
- TNS
6-(p-toluidinyl)naphthalene-2-sulfonic acid
- HRP
horseradish peroxidase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hildmann C, Riester D, Schwienhorst A. Histone deacetylases--an important class of cellular regulators with a variety of functions. Appl Microbiol Biotechnol. 2007;75:487–497. doi: 10.1007/s00253-007-0911-2. [DOI] [PubMed] [Google Scholar]
- 2.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annu Rev Biochem. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 3.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 2007;17:195–211. doi: 10.1038/sj.cr.7310149. [DOI] [PubMed] [Google Scholar]
- 4.Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning ZB, Zeng R, Xiong Y, Guan KL, Zhao S, Zhao GP. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327:1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, Yao J, Zhou L, Zeng Y, Li H, Li Y, Shi J, An W, Hancock SM, He F, Qin L, Chin J, Yang P, Chen X, Lei Q, Xiong Y, Guan KL. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris KL, Lee JY, Yao TP. Acetylation goes global: the emergence of acetylation biology. Sci Signal. 2009;2:pe76. doi: 10.1126/scisignal.297pe76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science. 2009;325:834–840. doi: 10.1126/science.1175371. [DOI] [PubMed] [Google Scholar]
- 8.Allis CD, Berger SL, Cote J, Dent S, Jenuwien T, Kouzarides T, Pillus L, Reinberg D, Shi Y, Shiekhattar R, Shilatifard A, Workman J, Zhang Y. New nomenclature for chromatin-modifying enzymes. Cell. 2007;131:633–636. doi: 10.1016/j.cell.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 9.Sadoul K, Wang J, Diagouraga B, Khochbin S. The tale of protein lysine acetylation in the cytoplasm. J Biomed Biotechnol. 2011 doi: 10.1155/2011/970382. article id. 970382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng T, Lu Y. Changes in SIRT1 Expression and Its Downstream Pathways in Age-Related Cataract in Humans. Curr Eye Res. 2011;36:449–455. doi: 10.3109/02713683.2011.559301. [DOI] [PubMed] [Google Scholar]
- 11.Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- 12.Andley UP, Hamilton PD, Ravi N, Weihl CC. A knock-in mouse model for the R120G mutation of alphaB-crystallin recapitulates human hereditary myopathy and cataracts. PLoS One. 6:e17671. doi: 10.1371/journal.pone.0017671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in alphaB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274:33235–33243. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- 14.Kumar LV, Ramakrishna T, Rao CM. Structural and functional consequences of the mutation of a conserved arginine residue in alphaA and alphaB crystallins. J Biol Chem. 1999;274:24137–24141. doi: 10.1074/jbc.274.34.24137. [DOI] [PubMed] [Google Scholar]
- 15.Shroff NP, Cherian-Shaw M, Bera S, Abraham EC. Mutation of R116C results in highly oligomerized alpha A-crystallin with modified structure and defective chaperone-like function. Biochemistry. 2000;39:1420–1426. doi: 10.1021/bi991656b. [DOI] [PubMed] [Google Scholar]
- 16.Pasupuleti N, Matsuyama S, Voss O, Doseff AI, Song K, Danielpour D, Nagaraj RH. The anti-apoptotic function of human alphaA-crystallin is directly related to its chaperone activity. Cell Death Dis. 2010;1:e31. doi: 10.1038/cddis.2010.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andley UP. Effects of alpha-crystallin on lens cell function and cataract pathology. Curr Mol Med. 2009;9:887–892. doi: 10.2174/156652409789105598. [DOI] [PubMed] [Google Scholar]
- 18.Mao YW, Liu JP, Xiang H, Li DW. Human alphaA- and alphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 19.Liu JP, Schlosser R, Ma WY, Dong Z, Feng H, Lui L, Huang XQ, Liu Y, Li DW. Human alphaA- and alphaB-crystallins prevent UVA-induced apoptosis through regulation of PKCalpha, RAF/MEK/ERK and AKT signaling pathways. Exp Eye Res. 2004;79:393–403. [PubMed] [Google Scholar]
- 20.Alge CS, Priglinger SG, Neubauer AS, Kampik A, Zillig M, Bloemendal H, Welge-Lussen U. Retinal pigment epithelium is protected against apoptosis by alphaB-crystallin. Invest Ophthalmol Vis Sci. 2002;43:3575–3582. [PubMed] [Google Scholar]
- 21.Moyano JV, Evans JR, Chen F, Lu M, Werner ME, Yehiely F, Diaz LK, Turbin D, Karaca G, Wiley E, Nielsen TO, Perou CM, Cryns VL. AlphaB-crystallin is a novel oncoprotein that predicts poor clinical outcome in breast cancer. J Clin Invest. 2006;116:261–270. doi: 10.1172/JCI25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kase S, He S, Sonoda S, Kitamura M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton DR. alphaB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asomugha CO, Gupta R, Srivastava OP. Identification of crystallin modifications in the human lens cortex and nucleus using laser capture microdissection and CyDye labeling. Mol Vis. 2010;16:476–494. [PMC free article] [PubMed] [Google Scholar]
- 24.Driessen HP, Ramaekers FC, Vree Egberts WT, Dodemont HJ, de Jong WW, Tesser GI, Bloemendal H. The function of N alpha-acetylation of the eye-lens crystallins. Eur J Biochem. 1983;136:403–406. doi: 10.1111/j.1432-1033.1983.tb07756.x. [DOI] [PubMed] [Google Scholar]
- 25.MacCoss MJ, McDonald WH, Saraf A, Sadygov R, Clark JM, Tasto JJ, Gould KL, Wolters D, Washburn M, Weiss A, Clark JI, Yates JR., 3rd Shotgun identification of protein modifications from protein complexes and lens tissue. Proc Natl Acad Sci U S A. 2002;99:7900–7905. doi: 10.1073/pnas.122231399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin PP, Barry RC, Smith DL, Smith JB. In vivo acetylation identified at lysine 70 of human lens alphaA-crystallin. Protein Sci. 1998;7:1451–1457. doi: 10.1002/pro.5560070622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lapko VN, Smith DL, Smith JB. In vivo carbamylation and acetylation of water-soluble human lens alphaB-crystallin lysine 92. Protein Sci. 2001;10:1130–1136. doi: 10.1110/ps.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Groenen PJ, Merck KB, de Jong WW, Bloemendal H. Structure and modifications of the junior chaperone alpha-crystallin. From lens transparency to molecular pathology. Eur J Biochem. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- 29.Hasan A, Smith JB, Qin W, Smith DL. The reaction of bovine lens alpha A-crystallin with aspirin. Exp Eye Res. 1993;57:29–35. doi: 10.1006/exer.1993.1095. [DOI] [PubMed] [Google Scholar]
- 30.Pal JK, Bera SK, Ghosh SK. Acetylation of alpha-crystallin with N-acetylimidazole and its influence upon the native aggregate and subunit reassembly. Current Eye Research. 1999;19:358–367. doi: 10.1076/ceyr.19.4.358.5299. [DOI] [PubMed] [Google Scholar]
- 31.Swamy MS, Abraham EC. Inhibition of lens crystallin glycation and high molecular weight aggregate formation by aspirin in vitro and in vivo. Investigative Ophthalmology and Visual Science. 1989;30:1120–1126. [PubMed] [Google Scholar]
- 32.Rao GN, Cotlier E. Aspirin prevents the nonenzymatic glycosylation and carbamylation of the human eye lens crystallins in vitro. Biochem Biophys Res Commun. 1988;151:991–996. doi: 10.1016/s0006-291x(88)80463-7. [DOI] [PubMed] [Google Scholar]
- 33.Sharma KK, Kumar RS, Kumar GS, Quinn PT. Synthesis and characterization of a peptide identified as a functional element in alphaA-crystallin. J Biol Chem. 2000;275:3767–3771. doi: 10.1074/jbc.275.6.3767. [DOI] [PubMed] [Google Scholar]
- 34.Chellan P, Nagaraj RH. Early glycation products produce pentosidine cross-links on native proteins. novel mechanism of pentosidine formation and propagation of glycation. J Biol Chem. 2001;276:3895–3903. doi: 10.1074/jbc.M008626200. [DOI] [PubMed] [Google Scholar]
- 35.Udenfriend S, Stein S, Bohlen P, Dairman W, Leimgruber W, Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972;178:871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- 36.Gangadhariah MH, Wang B, Linetsky M, Henning C, Spanneberg R, Glomb MA, Nagaraj RH. Hydroimidazolone modification of human alphaA-crystallin: Effect on the chaperone function and protein refolding ability. Biochim Biophys Acta. 2010;1802:432–441. doi: 10.1016/j.bbadis.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biswas A, Lewis S, Wang B, Miyagi M, Santoshkumar P, Gangadhariah MH, Nagaraj RH. Chemical modulation of the chaperone function of human alphaA-crystallin. J Biochem. 2008;144:21–32. doi: 10.1093/jb/mvn037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswas A, Goshe J, Miller A, Santhoshkumar P, Luckey C, Bhat MB, Nagaraj RH. Paradoxical effects of substitution and deletion mutation of Arg56 on the structure and chaperone function of human alphaB-crystallin. Biochemistry. 2007;46:1117–1127. doi: 10.1021/bi061323w. [DOI] [PubMed] [Google Scholar]
- 39.Santhoshkumar P, Murugesan R, Sharma KK. Deletion of (54)FLRAPSWF(61) residues decreases the oligomeric size and enhances the chaperone function of alphaB-crystallin. Biochemistry. 2009;48:5066–5073. doi: 10.1021/bi900085v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scroggins BT, Robzyk K, Wang D, Marcu MG, Tsutsumi S, Beebe K, Cotter RJ, Felts S, Toft D, Karnitz L, Rosen N, Neckers L. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Hayes JJ. Acetylation mimics within individual core histone tail domains indicate distinct roles in regulating the stability of higher-order chromatin structure. Mol Cell Biol. 2008;28:227–236. doi: 10.1128/MCB.01245-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stengel F, Baldwin AJ, Painter AJ, Jaya N, Basha E, Kay LE, Vierling E, Robinson CV, Benesch JL. Quaternary dynamics and plasticity underlie small heat shock protein chaperone function. Proc Natl Acad Sci U S A. 2009;107:2007–2012. doi: 10.1073/pnas.0910126107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaya N, Garcia V, Vierling E. Substrate binding site flexibility of the small heat shock protein molecular chaperones. Proc Natl Acad Sci U S A. 2009;106:15604–15609. doi: 10.1073/pnas.0902177106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Wang SY, Zhang XH, Zhao M, Hou CM, Xu YJ, Du ZY, Yu XD. FK228 inhibits Hsp90 chaperone function in K562 cells via hyperacetylation of Hsp70. Biochem Biophys Res Commun. 2007;356:998–1003. doi: 10.1016/j.bbrc.2007.03.076. [DOI] [PubMed] [Google Scholar]
- 45.Bhattacharyya J, Srinivas V, Sharma KK. Evaluation of hydrophobicity versus chaperonelike activity of bovine alphaA- and alphaB-crystallin. J Protein Chem. 2002;21:65–71. doi: 10.1023/a:1014187300930. [DOI] [PubMed] [Google Scholar]
- 46.Reddy GB, Kumar PA, Kumar MS. Chaperone-like activity and hydrophobicity of alpha-crystallin. IUBMB Life. 2006;58:632–641. doi: 10.1080/15216540601010096. [DOI] [PubMed] [Google Scholar]
- 47.Saha S, Das KP. Relationship between chaperone activity and oligomeric size of recombinant human alphaA- and alphaB-crystallin: a tryptic digestion study. Proteins. 2004;57:610–617. doi: 10.1002/prot.20230. [DOI] [PubMed] [Google Scholar]
- 48.Gu L, Abulimiti A, Li W, Chang Z. Monodisperse Hsp16.3 nonamer exhibits dynamic dissociation and reassociation, with the nonamer dissociation prerequisite for chaperone-like activity. J Mol Biol. 2002;319:517–526. doi: 10.1016/S0022-2836(02)00311-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. 1S. Effect of acetylation on the chaperone function of αA-crystallin.αA-crystallin was acetylated using 0.01 and 0.05 molar excess (relative to lysine) of Ac2O as described in Methods and the ELISA results (A) show that the protein is acetylated (1.0 µg protein was used for the assay).The chaperone function of αA-crystallin was assessed using (B) citrate synthase (αA:CS ratio=1:10, w/w) and (C) βL-crystallin (αA:βL ratio=1:57 w/w) as client proteins.
Fig. 2S. SDS-PAGE of purified recombinant human wild type (Wt) and K70Q αA-crystallin. M=molecular weight markers
Fig. 3S. Multiangle light scattering analysis of wild type αA, K70Q and acetylated αA-crystallin. The molar mass distribution across UV peaks was analyzed from the MLS data using the ASTRA software and the molar mass at the peak apex is reported. The data suggest that there is a 20% decrease in molar mass in acetylatedαA-crystallin.