Abstract
Liposomes consisted of phosphatidylinositol (PI) and phosphatidylcholine (PC) have been utilized as delivery vehicle for drugs and proteins. In the present work, we studied the effect of soy PI on physical properties of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) liposomes such as phase state of lipid bilayer, lipid packing and phase properties using multiple orthogonal biophysical techniques. The 6-dodecanoyl-2-dimethylamino naphthalene (Laurdan) fluorescence studies showed that presence of PI induces the formation of fluid phases in DMPC. Differential scanning calorimetry (DSC), temperature dependent fluorescence anisotropy measurements, and generalized polarization values for Laurdan showed that the presence of as low as 10 mol% of PI induces substantial broadening and shift to lower temperature of phase transition of DMPC. The fluorescence emission intensity of DPH labeled, PI containing DMPC lipid bilayer decreased possibly due to deeper penetration of water molecules in lipid bilayer. In order to further delineate the effect of PI on the physico chemical properties of DMPC is due to either significant hydrophobic mismatch between the acyl chains of the DMPC and that of soy PI or due to the inositol head group, we systematically replaced soy PI with PC species of similar acyl chain composition (DPPC and 18:2 (Cis) PC) or with diacylglycerol (DAG) respectively. The anisotropy of PC membrane containing soy PI showed largest fluidity change compared to other compositions. The data suggests that addition of PI alters structure and dynamics of DMPC bilayer in that it promotes deeper water penetration in the bilayer, induces fluid phase characteristics and causes lipid packing defects that involve its inositol head group.
Keywords: phosphatidylinositol, Laurdan fluorescence, drug delivery, phase transition, lamellarity, phosphatidylcholine
1. Introduction
Liposome based drug carriers have been recognized to enhance potency, reduce toxicity, and modulate pharmacokinetics of the encapsulated therapeutic agent [1–2]. The role of phosphatidylinositol (PI) in liposomes for drug delivery applications has been extensively investigated [3–5]. The pharmacological disposition of PI-containing liposomes showed reduced uptake by the reticuloendothelial system (RES) which resulted in prolonged circulation time in rats and mice compared with other liposome formulations. This is possibly due to shielding of the negative charge on the particles by inositol ring that prevents binding of blood proteins such as opsonizing proteins onto the liposome surface, a major mechanism for liposome clearance [6–7]. Recently, we have shown that the inclusion of PI in 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) lipid vesicles improved loading efficiency, prolonged in vivo circulation and reduced immunogenicity of an associated therapeutic protein, human recombinant Factor VIII (FVIII) [5, 8]. The sandwich ELISA study indicated that substantial protein surface is shielded by PI-containing particles [5] in contrast to the less protein binding observed in phosphatidylserine (PS)-containing liposomes [9].
As the stability and biological fate of lipid particles is closely related to the liposome characteristics [10], a thorough characterization of PI-containing liposomes is important to understand the in vivo behavior and to rationally design PI-based therapeutic preparations. Prior studies have focused on the orientation of the inositol ring using neutron diffraction [11–13], X-ray diffraction [11] and nuclear magnetic resonance (NMR) [14]. The inositol head group extends into the aqueous environment and is perpendicular to the membrane. Other groups have investigated the membrane fusion induced by cations (calcium, magnesium, and lanthanum) in lipidic particles containing PI [15–17]. The effect of PI on physical properties of binary model membranes has also been studied [18–19]. It has been reported previously that the inclusion of PI in dipalmitoylphosphatidylcholine (DPPC) liposomes resulted in the broadening and unclear phase transition in ESR and NMR analysis [18–20]. The phase transition of soy bean PI, a naturally occurring PI, and its effect on Disteroyl Phosphstidyl Choline (DSPC) has been studied [21]. Soy PI did not show any transition above 0°C but its addition to DSPC lowers the transition temperature of DSPC. Additional details on structural and dynamic properties of soy PI bilayer such as water penetration and dynamics near head group, lipid packing defects, phase properties of the bilayer, would be useful for designing therapeutic delivery vehicle as soy PI with base lipid PC is extensively used for drug delivery applications. Further, these studies may shed some light on biological role of PI in trans membrane signaling and protein anchoring. PI and its phosphorylated derivatives play important roles as signal mediators in cellular signal transduction processes [22–23]. In addition, glycosylphosphatidylinositol is recognized as major means of anchoring several surface proteins including cell adhesion molecules and cellular receptors to cell membranes [24–25].
In the present study, we investigated the dynamic properties of PI-containing model membranes using biophysical techniques. The results suggested that the inclusion of PI in DMPC model membrane induces fluid phase formation, alters lipid packing, and promotes deeper water penetration in the bilayer, and this involves inositol head group.
2. Material and methods
2.1. Materials
DMPC, soybean PI, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1,2-dilinoleoyl-sn-glycero-3-phosphocholine (DLPC; 18:2 (Cis) PC), and 1-stearoyl-2-linoleoyl-sn-glycerol (DAG) were purchased from Avanti Polar Lipids (Alabaster, AL, USA) as a chloroform solution, stored at −80°C, and used without further purification. The fluorescent probes, DPH and Laurdan, were obtained from Molecular Probes Inc. (Eugene OR, USA). All buffer salts and solvents used in this study were obtained from Fisher Scientific (Hanover Park, IL, USA).
PI is isolated from commercially available soybeans and purified using high-pressure column chromatography. The resulting PI product is a mixture of saturated and unsaturated PI with majority of the species as 18:2 (46.8%) and 16:0 (33.2).
2.2. Preparation of liposomes
Thin lipid films consisted of DMPC:PI (100:0, 90:10, 70:30, or 50:50 molar ratio), DMPC:DAG (70:30 molar ratio), DMPC:DPPC:18:2 (Cis) PC (70:15:15 molar ratio), and DMPC:PS (70:30 molar ratio) were prepared by evaporating required amounts of lipids in chloroform under N2 stream using a rotary evaporator (Buchi-R200, Fisher Scientific). The lipid film which was formed in a Kimax glass tube, was rehydrated with Tris buffer (25 mM Tris, 150 mM NaCl, pH 7.0) and vortexed at 37°C for 15~20 min, forming multilamellar vesicles (MLV). DPH or Laurdan was incorporated into MLV by mixing the probe and the lipids in chloroform solution giving probe:lipid ratio of 1:500 and 1:1,000, respectively, prior to thin lipid film formation. All sample preparations were used immediately for the experiments.
2.3. Differential scanning calorimetry
The phase transition temperatures of DMPC liposomes in the presence and in the absence of 10% PI were measured by a Perkin Elmer DSC-2 calorimeter (Perkin Elmer, Waltham, MA, USA). Each differential heat capacity scan was measured at a lipid concentration of 80 mM with a heating rate of 5 K/min. 13 μL of MLV suspension was added and sealed in aluminum pans, and degassed under N2 stream. The sample was then equilibrated at ~ 0°C for at least 10 min before each thermogram which was scanned from 290~315 K. The instrument was calibrated with an indium standard. To ensure the reproducibility of the transition temperature, several cycles of heating and cooling were applied on the same samples. In addition, the experiment was repeated on different days with freshly prepared samples. A representative DSC thermogram from three independent measurements and samples is presented.
2.4. Fluorescence
Fluorescence measurement was performed using a PTI-Quantamaster fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ, USA) which is equipped with photon counting electronics and a xenon arc lamp. Each sample analysis was carried out over the temperature range of 4 to 37°C at a heating rate 1°C/min using circulating water from an external water bath unit (Peltier unit) that is connected to the cuvette chamber. An I-shaped cuvette was used to minimize any inner filter effect. The sample was placed into the cuvette chamber and equilibrated at the desired temperature for at least 10 min prior to each measurement. Scattering from the particles was minimized by using a long pass filter on emission path.
2.4.1. DPH fluorescence and polarization measurements
Each MLV sample containing 0.13 mM total lipid concentration was excited at 351 nm, and the emission spectra were measured from 380 to 560 nm with a slit width of 2 nm [26]. For fluorescence polarization measurement, samples were excited at 351 nm and the emission monochromator set at 430 nm. Fluorescence polarization (P) was calculated using the following equation:
| (Eq. 1) |
where I(0,0) represents emission intensity with the emission polarizer parallel to the excitation polarizer, and I(0,90) represents emission intensity at which the emission polarizer is perpendicular to the excitation polarizer. G is the instrument-specific grating correction factor which is expressed as the ratio of the efficiencies of the detection system for vertically and horizontally polarized light:
| (Eq. 2) |
A representative figure of fluorescence polarization as a function of temperature from three independent measurements (and samples) is presented. The transition temperature is estimated by fitting the data to a sigmoid function using WinNonlin (Pharsight Corporation, Cary, NC).
2.4.2. Steady-state Laurdan fluorescence measurements
MLV samples containing 0.5 mM of total lipid concentration was used for each measurement. The excitation spectra were acquired from 320–420 nm with emission wavelength of 440 nm or 490 nm. Two sets of emission spectra were acquired, one with excitation wavelength of 340 nm and the other at 410 nm. Excitation and emission slit widths were set at 2 nm. The excitation generalized polarization (GPex) was calculated according to the following equation:
| (Eq. 3) |
where I440 and I490 represent the intensities obtained from 320–420 nm at fixed wavelengths of 440 and 490 nm, respectively. The emission generalized polarization (GPem) was calculated as:
| (Eq. 4) |
where I410 and I340 represent the emission intensities detected from 425–550 nm at fixed wavelengths of 410 and 340, respectively [27–29]. A Representative figure of temperature-dependent excitation spectra of Laurdan and Laurdan GPex from three independent measurements (and samples) is presented.
3. Results
The physicochemical properties of lipid vesicle are critical for its stability and in vivo behavior. The effect of PI on structural and dynamics properties of DMPC is studied by multiple orthogonal biophysical techniques.
3.1. The effect of PI on DMPC lamellar phase properties using Laurdan probe
3.1.1. Temperature dependent excitation spectra
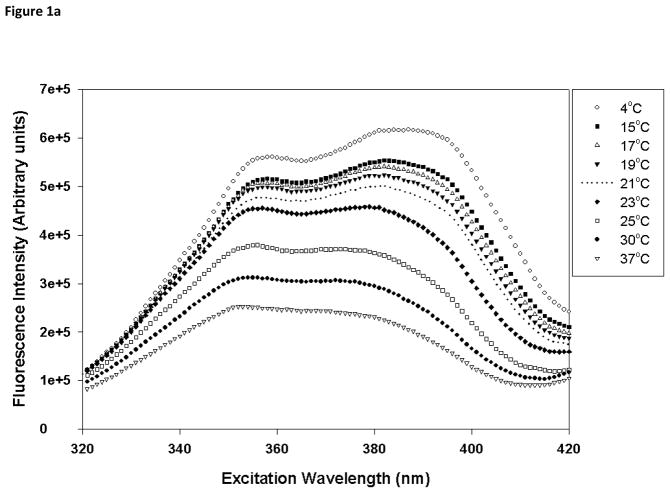
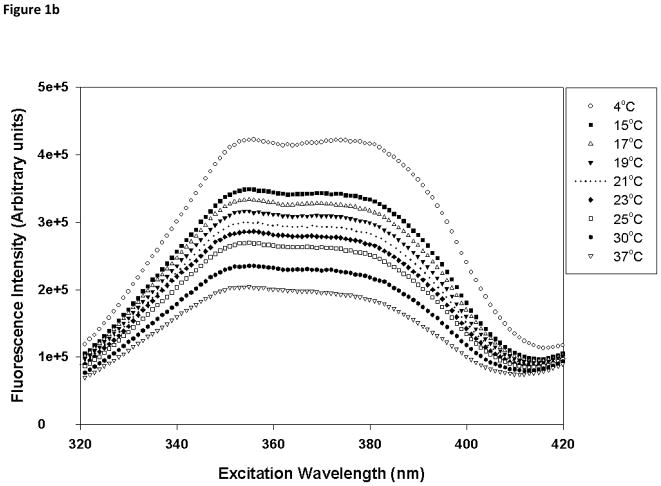
Laurdan is a fluorescent probe that has been used to monitor the polarity of the environment at the membrane interfacial region and also to understand lamellar domains [30]. Temperature-dependent excitation spectra of Laurdan in DMPC and DMPC/PI were acquired from 4 to 37°C, spanning the phase transition of DMPC membrane (Fig. 1). The probe displays two peaks in the excitation spectra, one at ~360 nm and the other at ~390 nm, and the relative intensity of these peaks is very sensitive to the phase state of the membrane. In the gel phase, the intensity at 390 nm is greater than that at 360 nm whereas the intensity at 360 nm would be more intense in the fluid liquid crystalline (LC) phase. In the temperature range of 4 to 21°C, DMPC bilayer exists in the gel phase (Fig 1a), and as the temperature is further increased, the ratio of the intensities at 390 to 360 nm becomes less than 1, suggesting the formation of the fluid LC phase in DMPC bilayer. However, in the presence of PI, no clear temperature dependent structural changes were observed (Fig. 1b). For liposomes composed of 50 mol% PI, even at 4°C, the lowest temperature that displayed gel phase in DMPC membrane, the bilayer showed substantial fluid LC characteristics (ratio of intensities at 390:360 nm <1). This indicated that PI partition in DMPC bilayer and induces the formation of the fluid phase. As the mol% of PI increased from 0–50%, a gradual change in Laurdan spectral properties were observed but no reversal of these characteristics suggest that no demixing of PI and PC rich domains in the PC:PI range studied.
Fig. 1.
Temperature-dependent excitation spectra of Laurdan in (a) DMPC (100 mol%), and (b) DMPC/PI (50/50 mol%) vesicles. The excitation spectra were monitored at 440 nm from 4 to 37°C spanning the phase transition temperature of DMPC. Each vesicle sample contains 0.5 mM total lipid concentration and probe:lipid ratio was maintained at 1:1,000.
3.1.2. Generalized polarization (GP)
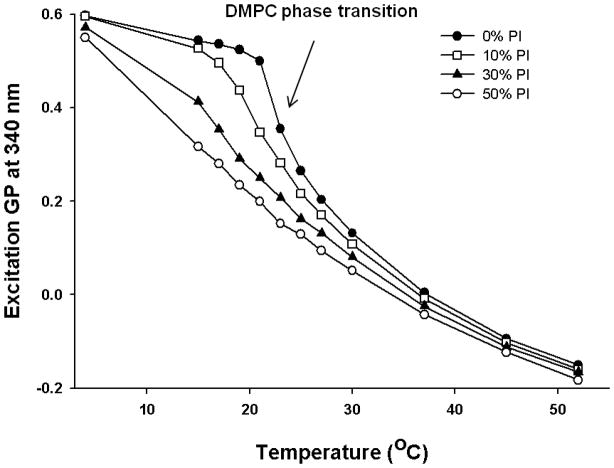
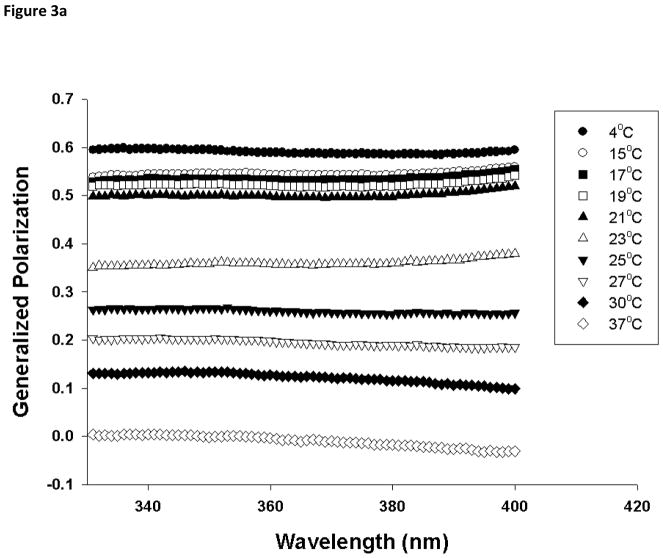
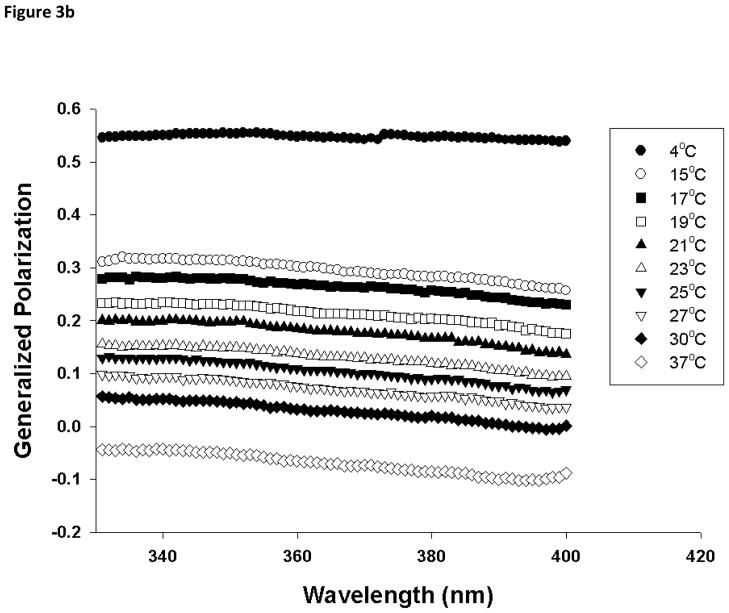
In order to provide additional information on the membrane dynamics, the generalized polarization (GP) of Laurdan was analyzed. GP values for the gel phase and fluid LC phase are distinct from each other, and can be used to determine the relative fractions of gel and fluid LC phase when coexisted [27]. A temperature dependent GP spectra for DMPC in the presence and in the absence of various molar ratio of PI was constructed using excitation spectra as described in experimental section. In gel phase (4°C), 100 mol% DMPC liposomes have a GP value of about 0.6 and this drops below 0 as the phase transition occurs (Fig 2). For 100 mol% DMPC vesicles, there is a sharp transition in the GP values around 23°C, but the phase transition is shifted to a lower temperature (~21°C) and broadened as 10 mol% of PI was incorporated into the DMPC liposomes. Further increase in mol% of PI abolished this transition suggesting that presence of PI fluidizes DMPC liposomes and induces formation of fluid LC phase. This is consistent with the GP spectra constructed as a function of wavelength (Fig 3). The GPex values showed wavelength independence in the gel phase with GP value ~0.6 for DMPC but changes to characteristics that is wavelength dependent above the phase transition temperature (23°C) [27, 29, 31], exhibiting decreasing values with increasing excitation wavelength. This wavelength dependence is a characteristic property of Laurdan partitioned in fluid LC phases and is attributed to the dipolar relaxation phenomena [27, 32]. On the other hand, GPex values displayed a stronger dependence on wavelength at most temperatures for DMPC MLV containing 50 mol% PI, suggesting fluid LC phase formation in PI containing vesicles. While the fluid LC phase for DMPC MLVs was observed only beyond its transition temperature (23°C), the addition of PI in DMPC membrane displayed fluid LC state formation starting from 15°C. The coexistence of gel and fluid phases between 15 to 23 °C was observed in pure DMPC membranes but this clear co-existence was not observed in PI containing DMPC model membranes.
Fig. 2.
Laurdan excitation generalized polarization (GP) values at 340 nm as a function of temperature for DMPC and DMPC/PI MLVs. GP values for DMPC vesicles containing 0, 10, 30 and 50 mol% PI were plotted as a function of temperature for each vesicle composition. Each vesicle sample contains 0.5 mM total lipid concentration and probe:lipid ratio was maintained at 1:1,000.
Fig. 3.
Laurdan excitation generalized polarization (GP) spectra as a function of wavelength in (a) DMPC (100 mol%), and (b) DMPC/PI (50/50 mol%) vesicles. Each formulation was acquired from 4 to 37°C spanning the phase transition temperature of DMPC, using emission wavelength of 440 nm and 490 nm. Excitation GP values were calculated using Eq. 3 in Section 2.4.2. Each vesicle sample contains 0.5 mM total lipid concentration and probe:lipid ratio was maintained at 1:1,000.
3.2. Effect of PI on the phase behavior of DMPC membrane by DSC
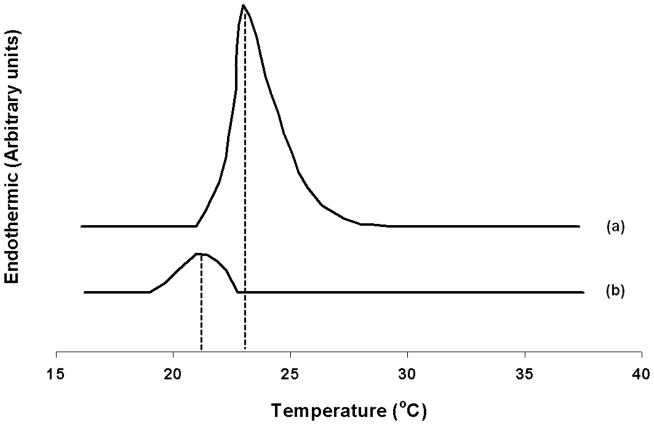
The effect of PI on the phase behavior of DMPC was evaluated using DSC. Fig. 4 shows the thermal transition of DMPC vesicles in the presence and in the absence of 10 mol% fraction of PI. In the absence of PI, a sharp peak due to transition of lamellar phase from gel to fluid LC phase was observed around 23°C and is consistent with previously reported results [33]. The incorporation of 10 mol% PI resulted in the broadening and shift of the main transition of DMPC to lower temperature suggesting acyl chain packing changes [34–36]. A broadening and shift of the main transition corresponds to a type “A” change in the bilayer [37]. Such profile would be expected if type “A” molecules, which are relatively large and moderately amphipathic, were to be localized in the region of the C1–C8 carbon atoms of the acyl chain. This is consistent with the structure of PI; a bulky inositol head group that has great propensity for polar interactions and the hydrophobic tails. Further increase in mol% of PI (above 30 mol%), no detectable transition was observed in this temperature range studied and could be due to further broadening and shift in the Tm. Moreover, analysis of the thermograms also showed that addition of PI did not result in reversal of changes in peak position or observation of transition that correspond to DMPC. These studies clearly suggest that incorporation of PI did not result in demixing of PC and PI rich domains. The addition of PI results in partitioning in DMPC bilayer that induces changes in phase transition and acyl chain packing of DMPC.
Fig. 4.
Differential scanning calorimetry (DSC) thermogram of (a) DMPC (100 mol%), and (b) DMPC/PI (90/10 mol%). Each differential heat capacity scan was measured at a lipid concentration of 80 mM with a heating rate of 5 K/min as described in Section 2.4.
3.3. PI effect on the structure and dynamics of DMPC as studied by DPH fluorescence
3.3.1. DPH fluorescence emission spectra
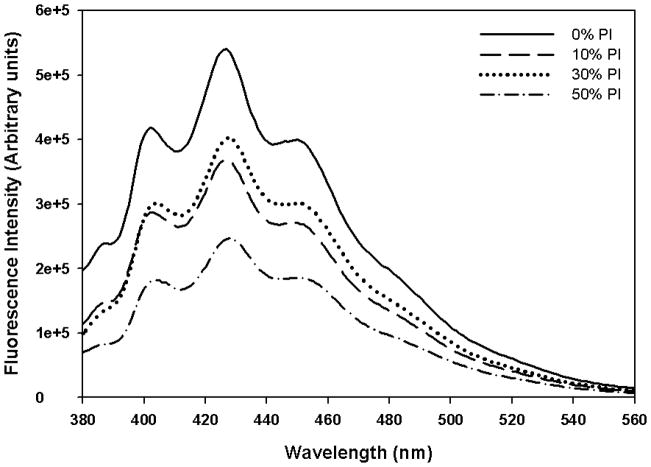
DPH is a very useful fluorescent probe to study membrane dynamics. Further, fluorescent intensity of the DPH labeled bilayers have been extensively used to investigate water penetration and polarity of the bilayer [26]. The fluorescence intensity of DPH labeled DMPC bilayer in the absence and in the presence of various mol% of PI was studied to investigate the water penetration in the bilayer (Fig. 5). These studies were also carried out below (4°C) and above (37°C) phase transition temperature of DMPC. The fluorescence emission spectrum of DPH in DMPC bilayer in the absence of PI showed three emission peaks centered at 430 nm that is consistent with previously reported results [26]. The intensity of the DPH fluorescence is sensitive to the polarity of its environment. As clear from the figure, the addition of PI resulted in reduction in fluorescence intensity at 430 nm and this reduction appeared to be dependent on mol% of PI. This is due to deep bilayer penetration of water leading to quenching of DPH fluorescence. The fluorescence intensity of DPH in DMPC and DMPC/PI system is also found to be temperature dependent. As temperature increased from 4°C to 37°C, fluorescence intensity of DPH associated with DMPC was reduced due to more water penetration, associated with phase transition. However, even at 4°C, upon inclusion of PI as low as 10 mol%, the fluorescence intensity of the probe was reduced and is possibly due to the penetration of water deeper in the bilayer of PI containing vesicles.
Fig. 5.
Emission spectra of DPH in DMPC and DMPC/PI vesicles measured at (a) 4°C, and (b) 37°C. DMPC MLVs containing 0, 10, 30 and 50 mol% PI were prepared. Each MLV sample contains 0.13 mM total lipid concentration and was excited at 351 nm, and the emission spectra were measured from 380 to 560 nm with a slit width of 2 nm.
3.3.2. DPH fluorescence polarization
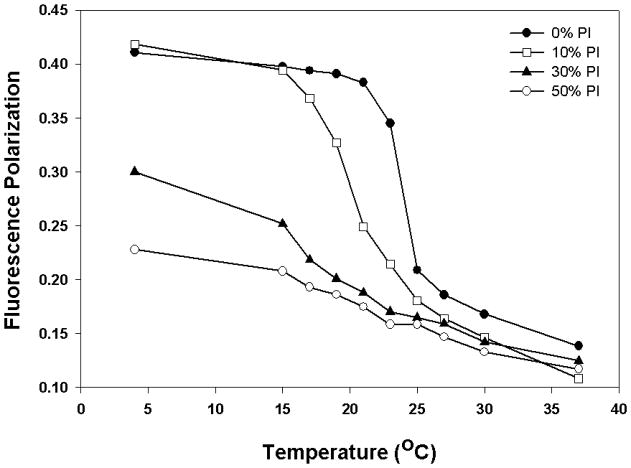
Due to its hydrophobic nature, DPH distributes in the hydrophobic interior of the bilayer and aligns parallel to the lipid acyl chain tail portion [38–39]. This partitioning of the probe provides dynamic properties in the acyl chain region of the bilayer such as fluidity. In order to investigate the effect of PI on membrane dynamics in DMPC bilayer, the fluorescence anisotropy of membranes labeled with DPH was monitored. Fluorescence polarization of DPH is sensitive to lamellar phase changes and has been used to monitor membrane order [36, 40–42]. As the bilayer phase transition occurs from gel to fluid LC, the fluorescence polarization drops due to the increase in the rotational freedom of the probe. Fig. 6 shows the effect of PI on phase transition of DMPC as measured by temperature dependent fluorescence polarization study. At 4°C, DPH fluorescence polarization was observed around 0.41 for DMPC vesicles in the gel phase. There is no difference in fluorescence polarization below 15°C for 0 and 10% PI vesicles because both liposomal membranes exist in the gel phase with little mobility in the bilayer. However, in the presence of PI above 10 mol%, there was a significant reduction in fluorescence polarization, even at 4°C, due to formation of fluid LC phase. For instance, the DPH fluorescence polarization was diminished by about 44% when 50 mol% PI was included in DMPC bilayer suggesting that PI induces fluid phase formation when added to DMPC bilayer in gel phase. Furthermore, as is clear from the figure, the polarization of DPH labeled DMPC was decreased as a function of increasing temperature, with a sharp transition observed around 23°C. This is the transition temperature for DMPC at which it undergoes phase transition from the gel to the fluid LC phase [33]. This indicated that the presence of DPH at low concentration (~0.2 mol%) in DMPC vesicles does not interfere with the lamellar phase properties of the bilayer and can accurately reflect the phase transition. In the presence of 10 mol% PI, the transition temperature is slightly broadened and shifted to a lower temperature of ~21°C. Above 10 mol% PI, the phase transition of DMPC vesicles becomes broader and clear transition temperature is no longer detectable, suggesting that PI alters the organization and dynamics of DMPC model membrane from a highly ordered state to a more disordered phase. The transition profile was fitted to a sigmoid function to determine Tm and γ hill coefficient (Table 1). The γ function provides information on broadening of the transition. The data show that as the mol% of PI is increased, both Tm and γ decreased. For example, for DMPC, the γ is found to be 8.11 but in the presence of 30 mol% PI it decreases to 3. It is clear that the addition of PI induces broadening and lowering of melting profile. This is consistent with the results obtained from DSC and Laurdan studies (Fig. 1–4) and the previously published reports. Ohki et al showed that yeast PI (33 mol%) included in DPPC liposomes lowered and broadened the phase transition temperature of DPPC [18]. Larijani et al reported similar observations in which membrane fluidity is enhanced dramatically and non-lamellar structures are promoted with increasing molar ratios of PI in PI/DPPC binary model membranes.
Fig. 6.
Fluorescence polarization as a function of temperature for DMPC and DMPC/PI vesicles. Fluorescence polarization values for DPH in DMPC vesicles containing 0, 10, 30 and 50 mol% PI were plotted as a function of temperature for each vesicle composition. Each MLV sample contains 0.13 mM total lipid concentration and the fluorescence polarization was measured with excitation wavelength at 351 nm and emission wavelength at 430 nm.
Table 1.
Estimation of Tm and hill coefficient (γ) determined by fitting fluorescence polarization as a function of temperature to a sigmoid function using WinNonlin 5.2 (Pharsight Corporation, Cary, NC).
| Lipid Composition | Parameter | Estimate | Standard Error | CV% |
|---|---|---|---|---|
| DMPC (100%) | Tm1 | 23.1 | 0.41 | 1.83 |
| γ1 | 8.11 | 0.91 | 11.08 | |
| PI (10%) | Tm2 | 21.0 | 0.39 | 1.89 |
| γ2 | 7.11 | 0.89 | 12.32 | |
| PI (30%) | Tm3 | 18.1 | 1.09 | 5.39 |
| γ3 | 3.00 | 0.46 | 18.30 | |
| PI (50%) | Tm4 | 17.2 | 1.05 | 4.55 |
| γ4 | 2.04 | 0.91 | 20.49 |
3.4. Effect of mismatched acyl chains and head group membrane dynamics of DMPC
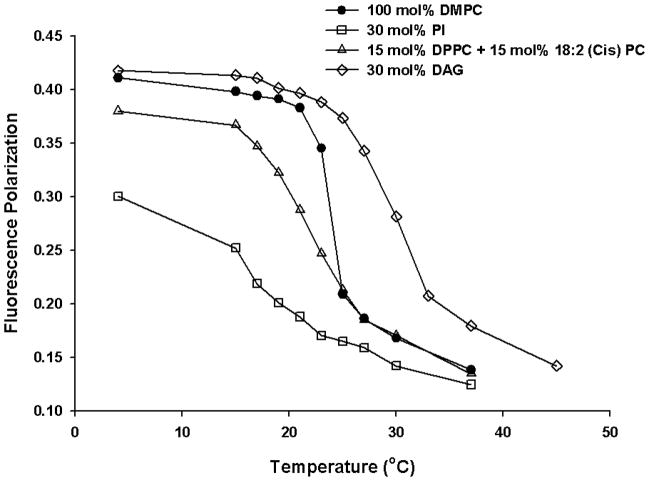
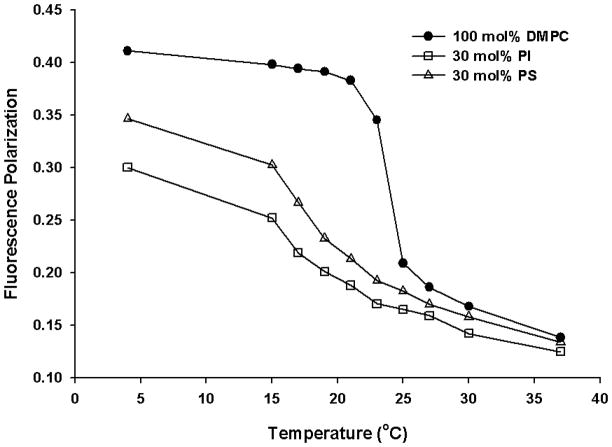
Soy PI employed in the present study is a natural phospholipid that contains heterogeneous fatty acyl chains with a high level of unsaturation. It is possible that the observed effect of soy PI on the physicochemical property changes in DMPC model membrane is from the significant hydrophobic mismatch between the acyl chains of the DMPC (14:0) and that of soy PI (16:0 and 18:2), and not necessarily from the inositol head group. To investigate whether the observed effect was due to acyl chain mismatch, control experiments were carried out. First, to delineate the effect of head group, we replaced soy PI with DPPC and 18:2 (Cis) PC (DLPC) that carry acyl chains 16:0 and 18:2, respectively. Then, the effect of head group was further investigated by replacing PI with diacylglycerol (DAG). From the temperature dependent DPH fluorescence polarization study (Fig. 7), the transition temperature for DMPC is slightly broadened and shifted to a lower temperature of ~21°C in the presence of DPPC (15 mol%) and 18:2 (Cis) PC (15 mol%), but in the presence of 30 mol% of PI, much larger changes such as abolishment of the phase transition was observed. The data suggest that acyl chain mismatch alone cannot account for the substantial changes observed with PI. When PI was replaced with charge matched phosphatidylserine (PS) carrying mismatched and unsaturated acyl chains, the changes observed were smaller than that observed for PI (Fig. 8).
Fig. 7.
Fluorescence polarization as a function of temperature for DMPC (100 mol%; filled circles), PI (30 mol%; empty squares), DMPC/DPPC/18:2 (Cis) PC (70/15/15 mol%; empty triangles) and DMPC/DAG (70/30 mol%; empty diamonds) vesicles. Fluorescence polarization values for DPH labelled vesicles were plotted as a function of temperature for each vesicle composition. Each MLV sample contains 0.13 mM total lipid concentration and the fluorescence polarization was measured with excitation wavelength at 351 nm and emission wavelength at 430 nm.
Fig. 8.
Fluorescence polarization as a function of temperature for DMPC (100 mol%; filled circles), PI (30 mol%; empty squares) and PS (30 mol%; empty triangles) vesicles. Fluorescence polarization values for DPH labelled vesicles were plotted as a function of temperature for each vesicle composition. Each MLV sample contains 0.13 mM total lipid concentration and the fluorescence polarization was measured with excitation wavelength at 351 nm and emission wavelength at 430 nm.
In order to further analyze the effect of head group properties such as molecular volume, the effect of inositol head group on membrane dynamics in pure DMPC model membrane was investigated by replacing PI with the same amount of DAG, a hydrolyzed product of PI. DAG has a small polar head group which allows close contact packing in the phospholipid membranes. In contrast to the PI effect, the addition of DAG did not abolish the phase transition of the pure DMPC vesicles as observed with PI, but slightly broadened the phase transition that was associated with increased transition temperature (Fig. 6). Overall, the results suggest that PI mediated effects involve head group of PI and its molecular architecture/volume and organization in the interfacial region could alter the lipid packing.
4. Discussions
PI-containing liposomes have been reported as potential drug delivery vehicles that provide long circulating properties [3–5, 43] as well as the capability to reduce immunogenicity [5]. As the in vivo behavior of liposomes depends on the fundamental physical characteristics of the bilayer, it is important to understand the structure and dynamic properties of PI-containing lipid bilayer organization. Based on several orthogonal biophysical techniques, it is found that the inclusion of natural PI broaden and eventually eliminate the gel/LC phase transition of the DMPC membrane, indicating that PI promotes formation of fluid LC phases, causes packing defects and allows deeper water penetration in DMPC bilayer. These biophysical changes may contribute to our understanding of lipid-lipid and lipid-protein interaction, pharmacology and stability of PI containing delivery vehicles.
The acyl chain mismatch caused by the incorporation of soy PI (18:2 and 16:0) to DMPC (14:0) membranes was investigated by replacing PI with PC of similar acyl chain characteristics. This was investigated by replacing PI with DPPC (16:0) and DLPC (18:2) in the temperature dependent DPH fluorescence polarization study. Due to the high level of unsaturated acyl chains, the close packing of acyl chains was hindered, leading to the broadening and shift of the phase transition for DMPC to a lower temperature. However, the extent of changes induced by DPPC/18:2 (Cis) PC replacement was not as dramatic as with PI suggesting that mismatch in acyl chain alone cannot explain the observed changes. Moreover, similarly charged lipid (PS and PG) also could not explain for the observed changes. Our preliminary results showed that the extent of changes in phase transition in DMPC/PI is much higher than that observed in DMPC/porcine brain PS binary mixtures (Fig. 8). Phase behaviors of binary mixtures of PG and PC have been extensively investigated [44]. PG is highly miscible with PC [45] and mixtures of DMPC and DMPG produced phase transition that is similar to that observed with either of the two components alone. Mixing of PC and PG with different chain lengths only slightly increased the phase transition width. Based on these studies, the mismatch in the acyl chain region alone and charge effect could not explain for the membrane dynamic changes induced by PI, but may also involve head group-mediated effects.
The bulky head group of PI and its effect on lipid packing is consistent with studies carried out with DAG. The replacement of PI with DAG at the same concentration resulted in the shift of the phase transition that is exactly opposite to what we observed in DMPC/PI binary system. This is possible due to the small polar head group from DAG which allows close contact lipid packing of the bilayer, resulting in the reduction of the membrane fluidity [18]. On the other hand, the orientation of the head group of PI in the model membrane has been shown to project perpendicularly from the membrane surface into the aqueous environment and this allows maximum hydration of the inositol ring [11–13]. Due to potential of substantial hydration and hydrogen bond formation involving six hydroxyl groups of inositol group, the head group is bulky. The surface area of this highly hydrated head group is expected to be larger than the cross-sectional area of the acyl chains, and this might lead to the crowding of the head group and looser packing of the acyl chains as compared to lipids with matching head group and acyl chain cross-sectional area. Thus, at the molecular level, we speculate that the head group orientation with respect to bilayer, its hydration and relative ratio of area of the head group to acyl chain cause changes in the interfacial region, permitting deeper penetration of water and lipid packing defects. The presence of PI head group may cause changes in the interfacial area of the membrane and influence water penetration, and this is consistent with our DPH and Laurdan studies. The fluorescence intensity of DPH in DMPC/PI vesicles is reduced suggesting deeper penetration of the water molecules into the bilayer structure. Further, Laurdan probe that is located in the interfacial region of the membrane showed that presence of PI induces the formation of fluid phases in DMPC.
Such head group-mediated effect can explain several other biological and biochemical observations. Recently, we showed that PI improved loading efficiency of FVIII possibly due to altered protein topology compared to liposomes composed of similarly charged anionic lipids [5, 9]. The interaction between FVIII and PI involves several domains in addition to the lipid binding C2 domain of FVIII as a substantial surface area of the protein was shielded by PI binding possibly by deeper penetration of protein into the lipid particles. This is in contrast to the binding between FVIII and PS that is limited to the amino acid residues 2303–2332 in the lipid binding C2 domain of FVIII [9, 46–47]. The association efficiency between FVIII and PS-containing liposomes was found to be about 50% [9]. However, the replacement of PS with similarly charged PI resulted in about 72% association efficiency that involved several other domains of FVIII in addition to lipid binding domain [5]. This is puzzling given that the binding affinity of FVIII with PS is 10 times higher than that observed for PI [48]. We believe that the physico chemical changes in PC/PI system caused by PI mediated packing defects might contribute to the improved loading efficiency of the protein. Such lipid packing defects caused by PI could create more loading space for FVIII and the water penetration deeper in the bilayer could satisfy some solvent interactions required to stabilize protein structure located deeper in the bilayer. This could also explain our sandwich ELISA [5] and acylamide quenching studies (data not shown) that showed substantial shielding of the protein surface area in PI-containing bilayer compared to the shielding observed with PS-containing model membranes.
PI- and PS-containing liposomes have been shown to fuse and aggregate in the presence of Ca2+ ions, leading to instability in vitro [15–17, 49–50] and possibly in vivo. However, the threshold concentration of cation and relative mol% of PI required for fusion is higher than that is required for PS-containing liposomes [15, 49]. The bulky inositol group could impart fusion resistance in PI containing vesicles. The fusion-inhibitory characteristics of PI even in the presence of metal ions may be due to the steric hindrance of the bulky inositol ring that prevents close approach of PI-containing bilayers [15–17]. However, aggregation of PI-containing vesicles with higher mol% and in the presence of higher threshold Ca2+ or Mg2+ have been documented [15].
The PI mediated effect on dynamics of PC bilayer could alter lipid-protein interaction by altering both hydrophilic and hydrophobic interactions. The bulky head group may interfere with hydrophilic interactions by charge shielding. The bulky head group could shield negative charge imparted by PI and could interfere with interaction between charged groups and opsonin proteins. This interaction has been implied for reduced phagocytic uptake of PI vesicles by RES leading to reduced clearance and improved plasma survival of PI containing liposomes [4].
It is appropriate to mention here that microscopic molecular phase separated clusters are possible and that the interpretation of protein-lipid interaction in terms of the generalized fluidization concept is an over-simplification. Our conclusions inferred from the Laurdan and DPH fluorescence studies and DPH fluorescence polarization studies are based on the assumption that the fluorescent probes accurately reflect the order of homogenous macroscopic lamellar phase. Our observation might be an oversimplification and we should not rule out the possibility of existing microscopic molecular phase separated clusters in which the phase behavior differs significantly from the macroscopic phase.
5. Conclusions
In the present work, we investigated structural and dynamics of binary mixtures of DMPC and soy PI using phase diagram generated from DSC and fluorescent molecules (DPH and Laurdan) as probes of membrane dynamics. Due to its head group, PI causes packing defects in the interfacial region, allows deeper penetration of water molecules and promotes fluid domain formation. These physico chemical changes could contribute to its prolonged circulation and improve protein interaction for optimal drug delivery.
Higlights.
PI causes packing defects in the interfacial region of DMPC bilayer.
PI promotes deeper water penetration in DMPC bilayer.
PI induces fluid phase formation in DMPC bilayer.
PI alters structure and dynamics of DMPC bilayer that involve its inositol head group.
Acknowledgments
This work was supported by the National Institutes of Health grant (R01 HL-70227) to SVB. The authors thank Pharmaceutical Sciences Instrumentation Facility, University at Buffalo, State University of New York, for the use of fluorescence and DSC instruments. The fluorometer was obtained by shared Instrumentation Grants S10-RR013665 and S10-RR15877 from the National Center for Research Resources, National Institute of Health.
Abbreviations
- DAG
diacylglycerol
- DMPC
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DLPC
1,2-dilinoleoyl-sn-glycero-3-phosphocholine
- DPH
1,6-diphenyl-1,3,5-hexatriene
- DPPC
1,2-dipalmitoyl-sn-glycero-3-phosphocholine
- DSC
differential scanning calorimetry
- Laurdan
6-dodecanoyl-2-dimethylamino naphthalene
- FVIII
factor VIII
- LC
liquid crystalline
- MLV
multilamellar vesicles
- NMR
nuclear magnetic resonance
- PC
phosphatidylcholine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- RES
reticuloendothelial system
- SUV
unilamellar vesicles
- TEM
transmission electron microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim S. Liposomes as carriers of cancer chemotherapy. Current status and future prospects. Drugs. 1993;46:618–638. doi: 10.2165/00003495-199346040-00004. [DOI] [PubMed] [Google Scholar]
- 2.Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010;99:2557–2575. doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kao YJ, Loo TL. Pharmacological disposition of negatively charged phospholipid vesicles in rats. J Pharm Sci. 1980;69:1338–1340. doi: 10.1002/jps.2600691126. [DOI] [PubMed] [Google Scholar]
- 4.Gabizon A, Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988;85:6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng A, Straubinger RM, Balu-Iyer SV. Phosphatidylinositol containing lipidic particles reduces immunogenicity and catabolism of factor VIII in hemophilia a mice. AAPS J. 2010;12:473–481. doi: 10.1208/s12248-010-9207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassef NM, Alving CR. Complement-dependent phagocytosis of liposomes. Chem Phys Lipids. 1993;64:239–248. doi: 10.1016/0009-3084(93)90068-e. [DOI] [PubMed] [Google Scholar]
- 7.Chonn A, Semple SC, Cullis PR. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J Biol Chem. 1992;267:18759–18765. [PubMed] [Google Scholar]
- 8.Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thromb Haemost. 2010;104:1073–1075. doi: 10.1160/TH10-06-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Purohit VS, Ramani K, Kashi RS, Durrani MJ, Kreiger TJ, Balasubramanian SV. Topology of factor VIII bound to phosphatidylserine-containing model membranes. Biochim Biophys Acta. 2003;1617:31–38. doi: 10.1016/j.bbamem.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Lian T, Ho RJ. Trends and developments in liposome drug delivery systems. J Pharm Sci. 2001;90:667–680. doi: 10.1002/jps.1023. [DOI] [PubMed] [Google Scholar]
- 11.Bradshaw JP, Bushby RJ, Giles CC, Saunders MR. Orientation of the headgroup of phosphatidylinositol in a model biomembrane as determined by neutron diffraction. Biochemistry. 1999;38:8393–8401. doi: 10.1021/bi990338+. [DOI] [PubMed] [Google Scholar]
- 12.Bradshaw JP, Bushby RJ, Giles CC, Saunders MR, Reid DG. Neutron diffraction reveals the orientation of the headgroup of inositol lipids in model membranes. Nat Struct Biol. 1996;3:125–127. doi: 10.1038/nsb0296-125. [DOI] [PubMed] [Google Scholar]
- 13.Bradshaw JP, Bushby RJ, Giles CC, Saunders MR, Saxena A. The headgroup orientation of dimyristoylphosphatidylinositol-4-phosphate in mixed lipid bilayers: a neutron diffraction study. Biochim Biophys Acta. 1997;1329:124–138. doi: 10.1016/s0005-2736(97)00093-x. [DOI] [PubMed] [Google Scholar]
- 14.Hansbro PM, Byard SJ, Bushby RJ, Turnbull PJ, Boden N, Saunders MR, Novelli R, Reid DG. The conformational behaviour of phosphatidylinositol in model membranes: 2H-NMR studies. Biochim Biophys Acta. 1992;1112:187–196. doi: 10.1016/0005-2736(92)90391-x. [DOI] [PubMed] [Google Scholar]
- 15.Sundler R, Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. I. Phosphatidate/phosphatidylinositol specificity. Biochim Biophys Acta. 1981;649:743–750. doi: 10.1016/0005-2736(81)90179-6. [DOI] [PubMed] [Google Scholar]
- 16.Sundler R, Duzgunes N, Papahadjopoulos D. Control of membrane fusion by phospholipid head groups. II. The role of phosphatidylethanolamine in mixtures with phosphatidate and phosphatidylinositol. Biochim Biophys Acta. 1981;649:751–758. doi: 10.1016/0005-2736(81)90180-2. [DOI] [PubMed] [Google Scholar]
- 17.Muller M, Zschornig O, Ohki S, Arnold K. Fusion, leakage and surface hydrophobicity of vesicles containing phosphoinositides: influence of steric and electrostatic effects. J Membr Biol. 2003;192:33–43. doi: 10.1007/s00232-002-1062-0. [DOI] [PubMed] [Google Scholar]
- 18.Ohki K, Sekiya T, Yamauchi T, Nozawa Y. Effect of phosphatidylinositol replacement by diacylglycerol on various physical properties of artificial membranes with respect to the role of phosphatidylinositol response. Biochim Biophys Acta. 1982;693:341–350. doi: 10.1016/0005-2736(82)90441-2. [DOI] [PubMed] [Google Scholar]
- 19.Larijani B, Dufourc EJ. Polyunsaturated phosphatidylinositol and diacylglycerol substantially modify the fluidity and polymorphism of biomembranes: a solid-state deuterium NMR study. Lipids. 2006;41:925–932. doi: 10.1007/s11745-006-5045-2. [DOI] [PubMed] [Google Scholar]
- 20.Ohki K, Sekiya T, Yamauchi T, Nozawa Y. Physical properties of phosphatidylcholine-phosphatidylinositol liposomes in relation to a calcium effect. Biochim Biophys Acta. 1981;644:165–174. doi: 10.1016/0005-2736(81)90372-2. [DOI] [PubMed] [Google Scholar]
- 21.Neill SDO, Leopold AC. An Assesment of phase transition in Soy bean membranes. Plant Physiol. 1982;70:1405–1409. doi: 10.1104/pp.70.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 23.Di Paolo G, De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 24.Low MG, Saltiel AR. Structural and functional roles of glycosyl-phosphatidylinositol in membranes. Science. 1988;239:268–275. doi: 10.1126/science.3276003. [DOI] [PubMed] [Google Scholar]
- 25.Udenfriend S, Kodukula K. How glycosylphosphatidylinositol-anchored membrane proteins are made. Annu Rev Biochem. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- 26.Nambi P, Rowe ES, McIntosh TJ. Studies of the ethanol-induced interdigitated gel phase in phosphatidylcholines using the fluorophore 1,6-diphenyl-1,3,5-hexatriene. Biochemistry. 1988;27:9175–9182. doi: 10.1021/bi00426a015. [DOI] [PubMed] [Google Scholar]
- 27.Parasassi T, De Stasio G, Ravagnan G, Rusch RM, Gratton E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys J. 1991;60:179–189. doi: 10.1016/S0006-3495(91)82041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramani K, Balasubramanian SV. Fluorescence properties of Laurdan in cochleate phases. Biochim Biophys Acta. 2003;1618:67–78. doi: 10.1016/j.bbamem.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Parasassi T, Di Stefano M, Loiero M, Ravagnan G, Gratton E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys J. 1994;66:120–132. doi: 10.1016/S0006-3495(94)80763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parasassi T, De Stasio G, d’Ubaldo A, Gratton E. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys J. 1990;57:1179–1186. doi: 10.1016/S0006-3495(90)82637-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gratton TPaE. Membrane Lipid Domains and Dynamics as Detected by Laurdan Fluorescence. Journal of Fluorescence. 1995;5:59–69. doi: 10.1007/BF00718783. [DOI] [PubMed] [Google Scholar]
- 32.Balasubramanian SV, Campbell RB, Straubinger RM. Propofol, a general anesthetic, promotes the formation of fluid phase domains in model membranes. Chem Phys Lipids. 2002;114:35–44. doi: 10.1016/s0009-3084(01)00199-2. [DOI] [PubMed] [Google Scholar]
- 33.Small DM. Handbook of Lipid Research. Plenum Press; London: 1988. [Google Scholar]
- 34.Korkmaz F, Severcan F. Effect of progesterone on DPPC membrane: evidence for lateral phase separation and inverse action in lipid dynamics. Arch Biochem Biophys. 2005;440:141–147. doi: 10.1016/j.abb.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Biruss B, Dietl R, Valenta C. The influence of selected steroid hormones on the physicochemical behaviour of DPPC liposomes. Chem Phys Lipids. 2007;148:84–90. doi: 10.1016/j.chemphyslip.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 36.Campbell RB, Balasubramanian SV, Straubinger RM. Phospholipid-cationic lipid interactions: influences on membrane and vesicle properties. Biochim Biophys Acta. 2001;1512:27–39. doi: 10.1016/s0005-2736(01)00290-5. [DOI] [PubMed] [Google Scholar]
- 37.Jain MKaW. Norm Min, Effect of Small Molecules on the Dipalmitoyl Lecithin Liposomal Bilayer: III. Phase Transition in Lipid Bilayer. Journal of Membrane Biology. 1977;34:157–201. [Google Scholar]
- 38.Davenport L, Dale RE, Bisby RH, Cundall RB. Transverse location of the fluorescent probe 1,6-diphenyl-1,3,5-hexatriene in model lipid bilayer membrane systems by resonance excitation energy transfer. Biochemistry. 1985;24:4097–4108. doi: 10.1021/bi00336a044. [DOI] [PubMed] [Google Scholar]
- 39.Lentz BR. Membrane “fluidity” as detected by diphenylhexatriene probes. Chemistry and Physics of Lipids. 1989;50:171–190. [Google Scholar]
- 40.Lentz BR, Barenholz Y, Thompson TE. Fluorescence depolarization studies of phase transitions and fluidity in phospholipid bilayers. 1. Single component phosphatidylcholine liposomes. Biochemistry. 1976;15:4521–4528. doi: 10.1021/bi00665a029. [DOI] [PubMed] [Google Scholar]
- 41.Hitzemann RJ. Effect of ganglioside-GM1 on the order of phosphatidylcholine-cholesterol multilamellar liposomes. A fluorescence polarization study. Chem Phys Lipids. 1987;43:25–38. doi: 10.1016/0009-3084(87)90014-4. [DOI] [PubMed] [Google Scholar]
- 42.Van Blitterswijk WJ, Van Hoeven RP, Van der Meer BW. Lipid structural order parameters (reciprocal of fluidity) in biomembranes derived from steady-state fluorescence polarization measurements. Biochim Biophys Acta. 1981;644:323–332. doi: 10.1016/0005-2736(81)90390-4. [DOI] [PubMed] [Google Scholar]
- 43.Pisal DS, Balu-Iyer SV. Phospholipid binding improves plasma survival of factor VIII. Thrombosis and Haemostasis. 2010;104 doi: 10.1160/TH10-06-0422. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Findlay EJ, Barton PG. Phase behavior of synthetic phosphatidylglycerols and binary mixtures with phosphatidylcholines in the presence and absence of calcium ions. Biochemistry. 1978;17:2400–2405. doi: 10.1021/bi00605a023. [DOI] [PubMed] [Google Scholar]
- 45.Lee YC, Taraschi TF, Janes N. Support for the shape concept of lipid structure based on a headgroup volume approach. Biophys J. 1993;65:1429–1432. doi: 10.1016/S0006-3495(93)81206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stoilova-McPhie S, Villoutreix BO, Mertens K, Kemball-Cook G, Holzenburg A. 3-Dimensional structure of membrane-bound coagulation factor VIII: modeling of the factor VIII heterodimer within a 3-dimensional density map derived by electron crystallography. Blood. 2002;99:1215–1223. doi: 10.1182/blood.v99.4.1215. [DOI] [PubMed] [Google Scholar]
- 47.Pratt KP, Shen BW, Takeshima K, Davie EW, Fujikawa K, Stoddard BL. Structure of the C2 domain of human factor VIII at 1.5 A resolution. Nature. 1999;402:439–442. doi: 10.1038/46601. [DOI] [PubMed] [Google Scholar]
- 48.Kemball-Cook G, Barrowcliffe TW. Interaction of factor VIII with phospholipids: role of composition and negative charge. Thromb Res. 1992;67:57–71. doi: 10.1016/0049-3848(92)90258-c. [DOI] [PubMed] [Google Scholar]
- 49.Papahadjopoulos D, Vail WJ, Newton C, Nir S, Jacobson K, Poste G, Lazo R. Studies on membrane fusion. III. The role of calcium-induced phase changes. Biochim Biophys Acta. 1977;465:579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- 50.Papahadjopoulos D, Bangham AD. Biophysical properties of phospholipids. II. Permeability of phosphatidylserine liquid crystals to univalent ions. Biochim Biophys Acta. 1966;126:185–188. doi: 10.1016/0926-6585(66)90053-7. [DOI] [PubMed] [Google Scholar]