Abstract
Firing rates of dopamine (DA) neurons in substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) control DA release in target structures such as striatum and prefrontal cortex. DA neuron firing in the soma and release probability at axon terminals are tightly regulated by cholinergic transmission and nicotinic acetylcholine receptors (nAChRs). To understand the role of α6* nAChRs in DA transmission, we studied several strains of mice expressing differing levels of mutant, hypersensitive (L9′S) α6 subunits. α6 L9′S mice harboring six or more copies of the hypersensitive α6 gene exhibited spontaneous home cage hyperactivity and novelty-induced locomotor activity, whereas mice with an equal number of WT and L9′S α6 genes had locomotor activity resembling that of control mice. α6-dependent, nicotine-stimulated locomotor activation was also more robust in high-copy α6 L9′S mice versus low-copy mice. In wheel running experiments, results were also bi-modal; high-copy α6 L9′S animals exhibited blunted total wheel rotations during each day of a nine day experiment, but low-copy α6 L9′S mice ran normally on the wheel. Reduced wheel running in hyperactive strains of α6 L9′S mice was attributable to a reduction in both overall running time and velocity. ACh and nicotine-stimulated DA release from striatal synaptosomes in α6 L9′S mice was well-correlated with behavioral phenotypes, supporting the hypothesis that augmented DA release mediates the altered behavior of α6 L9′S mice. This study highlights the precise control that the nicotinic cholinergic system exerts on DA transmission, and provides further insights into the mechanisms and consequences of enhanced DA release.
Keywords: dopamine, cholinergic, nicotinic, acetylcholine, transgenic, mouse
1 - Introduction
α6-Containing nAChRs (α6*) represent a group of ACh-gated cys-loop family ion channels characterized by high sensitivity to ACh or nicotine, and a discrete expression pattern in the central nervous system. α6 subunits are selectively expressed in midbrain DA neurons of the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA), norepinephrine neurons of the locus coeruleus, and glutamatergic retinal ganglion cells (Lena et al., 1999, Whiteaker et al., 2000, Azam et al., 2002, Champtiaux et al., 2002). In contrast to the widespread expression of α4β2* nAChRs, the well-defined and specific expression of α6* nAChRs in these neuronal types suggests they may be candidate drug targets for manipulation of these neurotransmitter systems (Quik and McIntosh, 2006). Functional α6* nAChRs typically include β2 and β3 subunits (Cui et al., 2003, Salminen et al., 2004), and in midbrain DA neurons they often assemble with α4 subunits to produce high-sensitivity α6α4β2β3 pentamers (Champtiaux et al., 2003, Gotti et al., 2005, Drenan et al., 2010). α6* nAChRs, like most CNS nAChRs, are localized in axons and/or presynaptic terminals where they participate in cholinergic modulation of neurotransmitter release (Kulak et al., 1997). We previously reported that α6 functional responses were specifically excluded from midbrain GABAergic neurons and striatal GABA release (Drenan et al., 2008). Recent work implicates α6* nAChRs in modulation of GABA release onto midbrain DA neurons (Yang et al., 2011). However, the source of these α6-containing GABAergic afferents remains undetermined, and α6*-dependent mIPSCs were non-existent in recordings made in native brain slices (Yang et al., 2011).
Rodent DA striatal synaptosomes have been used to study native, presynaptic α6* nAChR function, stoichiometry, and pharmacology. Recent studies implicate α6* nAChRs in DA release and reward phenotypes, although results are complex and have not yielded a consistent conclusion on the role of α6 subunits. For example, α6 subunits are not necessary for intracranial nicotine self-administration, but are partially responsible for activity-dependent DA release in NAc (Exley et al., 2011). NAc DA release in response to acute, systemic nicotine is intact in α6 KO mice (Champtiaux et al., 2003), but these mice do not participate in acute I.V. nicotine self-administration (Pons et al., 2008). α6 KO mice with α6 subunits selectively restored to the VTA – but not SNc – have normal acute I.V. nicotine self-administration (Pons et al., 2008). Furthermore, intra-VTA perfusion of α6 antagonists blocks nicotine-self administration, nicotine-stimulated locomotion, and DA release in rat nucleus accumbens (NAc) (Gotti et al., 2010), and intra-NAc injection of α-conotoxin MII (αCtxMII) suppresses nicotine self-administration in a progressive ratio schedule of reinforcement, while similar injections into NAc do not reduce nicotine-stimulated locomotor activation (Brunzell et al., 2009).
We have recently produced transgenic mice expressing α6 nAChR subunits with a leucine 9′ to serine (L9′S) mutation in the second transmembrane domain (Drenan et al., 2008). The resulting hypersensitive α6* nAChRs, which are expressed with WT cellular selectivity, allow for amplification and isolation of α6*-dependent behavioral and physiological responses. α6 L9′S mice exhibit a number of altered locomotor phenotypes, including home cage hyperactivity, novelty-induced locomotor activity, and nicotine-stimulated locomotor activation (Drenan et al., 2008). In brain slice recordings from these mice, brief applications of nicotine to midbrain DA - but not GABA – neurons elicit large inward currents and transient firing responses that are blocked by αCtxMII (Drenan et al., 2008). These data suggest that selective activation of hypersensitive α6* nAChRs produces increased locomotor activity. In addition, electrochemical measurements of evoked DA release show that α6 L9′S mice also display a modified DA release profile in striatal slices where α6* nAChRs are localized presynaptically on DA axons. In these experiments, single spikes in DA axons produce less DA release whereas spike trains (mimicking phasic firing in DA neurons) produce augmented and/or prolonged DA release (Drenan et al., 2010). Genetic deletion of α4 subunits from α6 L9′S mice, which eliminates high-sensitivity α6α4* nAChR pentamers, abolishes this DA release result as well as α6 L9′S-associated locomotor hyperactivity (Drenan et al., 2010). Collectively, these results suggest that α6 L9′S mice will be useful for investigating the causes and consequences of disrupted dopaminergic transmission patterns that may be associated with human disorders such as ADHD, bipolar disorder, and schizophrenia. Here, we studied the locomotor activity and agonist-evoked DA release of six transgenic lines of mice with varying α6 L9′S BAC transgene copy numbers. Our results support a novel mechanism for producing enhanced DA release and locomotor hyperactivity by severely augmenting the nicotinic cholinergic system.
2 - Experimental Procedures
2.1 - Mice
All experiments were conducted in accordance with the guidelines for care and use of animals established by the Office of Laboratory Animal Welfare at the National Institutes of Health, and our protocols were approved by the Institutional Animal Care and Use Committee at the California Institute of Technology or the University of Colorado at Boulder. Mice were kept on a standard 12/12 or 13/11 h light/dark cycle at 22°C and given food and water ad libitum. On postnatal day 21, mice were weaned and housed with same-sex littermates. At 21 to 28 days, tail biopsies were taken for genotype analysis by PCR as previously described (Drenan et al., 2008). α6 L9′S mice from line 2 and line 5 have been described previously (Drenan et al., 2008, Drenan et al., 2010, Grady et al., 2010). Lines 9, 11, 15, and 29 were generated simultaneously with lines 2 and 5 and have been maintained identically to lines 2 and 5 but have not been previously reported. All transgenic lines were backcrossed to C57BL/6J mice for at least 10 generations. Because all transgenic lines were congenic C57BL/6, non-transgenic littermates from all lines were used interchangeably as control subjects for behavior and DA release experiments. For behavior, mice were three to six months old at the beginning of an experiment, and groups of mice were matched as closely as possible for age and sex.
2.2 - α6 L9′S BAC transgene copy number analysis
Mouse genomic DNA was isolated from tail samples using the DNeasy Blood and Tissue Kit (Qiagen). Samples from several α6 L9′S and control mice were analyzed for each line. For each real time PCR reaction, a master mix, including 5 μM each primer (RTa6Set3_F 5′CTG TGA ATC TGA AGA GCA GC 3′ and RTa6Set3_R 3′GAG GCA CTC ACC ACA TTG GC 5′) and LightCycler 480 SYBR Green I Master mix (Roche Applied Science), was aliquoted into LightCycler 480 96-well plates. Sample DNA was then aliquoted into each well. The LightCycler 480 program was pre-incubation for 10 mins at 95° C followed by 45 cycles of amplification (10 sec at 95° C, 20 sec at 58° C,10 sec at 72° C). A melting curve analysis was completed with each reaction using the following protocol: 5 sec at 95° C, 1 min at 65° C, a continuous ramp to 97° C (2.5° C per sec), and a cooling cycle of 10 sec at 40° C. α6 L9′S BAC copy number was calculated using the comparative CT method following real time PCR (Livak and Schmittgen, 2001, Ballester et al., 2004, Lee et al., 2006, Schmittgen and Livak, 2008).
2.3 - Mouse locomotor activity
Horizontal locomotor activity was measured with an infrared photobeam activity cage system (San Diego Instruments; San Diego, CA). Ambulation events were recorded when two contiguous photobeams were broken in succession. Acute locomotor activity in response to novelty or nicotine was studied by recording ambulation events during four 15 sec intervals per min for a designated number of min. For all behavioral measures in this study, activity cages were located in the same isolated and sound-proof room. For nicotine-induced locomotion experiments, groups of eight mice were placed in activity cages (18 × 28 cm) and their baseline level of activity was recorded for eight min. Mice were then removed, injected (100 μL per 25 g body mass), and returned to the cage within 15 sec. For injection experiments, saline injections were administered once daily for 3 to 5 days to habituate the animals to the injection procedure, which isolated the specific effect of nicotine. Additionally, we have previously shown that saline does not produce a significant locomotor response in α6 L9′S mice (Drenan et al., 2008, Drenan et al., 2010, Grady et al., 2010). For novelty-induced locomotor activity experiments, mice were removed from their home cage, placed in an activity cage, and locomotor activity was recorded for 30 min. For 48 h home cage monitoring, mice were isolated in their own cage and habituated to the test room and cage for 24 h. Following this, locomotor activity was recorded in 15 min intervals for 48 h. Locomotor activity methods are also described in our previous papers (Drenan et al., 2008, Drenan et al., 2010, Grady et al., 2010).
2.4 - Wheel-running activity
Spontaneous wheel-running activity was measured using an automated monitoring system (Mini Mitter system by Philips Respironics; Bend, OR). Mice were housed individually in activity cages equipped with a stainless-steel running wheel (11.5 cm dia) and allowed free access to the running wheel, food, and water. Running-wheel activity (total revolutions) within a five min period was recorded continuously for 9 days via magnetic switches attached to the wheels. Mice were placed in running-wheel cages 3 to 4 h before the onset of lights off on the first recording day to allow them to become accustomed to the cage. We started measuring activity at the beginning of the first dark phase.
Wheel running occurs in episodes ranging from minutes to hours, separated by periods of inactivity. We used ClockLab software (Coulbourn Instruments; Whitehall, PA) to measure the time that mice spent running during the dark phase. Our analysis was restricted to this phase because almost all wheel-running (~96%) occurred during lights off. We computed the time spent running during the dark phase for each mouse and recording day, and used these values to test for significant differences in running time between lines. Mean wheel-running velocity (rpm) during lights off was calculated by dividing the number of rotations during this period by the time spent running.
2.5 - Dopamine release from striatal synaptosomes
After a mouse was sacrificed by cervical dislocation, its brain was removed and placed immediately on an ice-cold platform and brain regions were dissected. Tissues from each mouse were homogenized in 0.5 ml of ice-cold 0.32 M sucrose buffered with 5 mM HEPES, pH 7.5. A crude synaptosomal pellet was prepared by centrifugation at 12,000 g for 20 min. The pellets were resuspended in “uptake buffer”: 128 mM NaCl, 2.4 mM KCl, 3.2 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM HEPES, 10 mM glucose, 1 mM ascorbic acid, and 10 μM pargyline at pH 7.5. Synaptosomes were incubated at 37° C in uptake buffer for 10 min before addition of 100 nM [3H]-dopamine (1 μCi per 0.2 ml of synaptosomes), and the suspension was incubated for an additional 5 min.
All experiments were conducted at ambient room temperature using methods described previously (Nashmi et al., 2007, Salminen et al., 2007) with modifications for collection into 96-well plates. In brief, aliquots of synaptosomes (80 μl) were distributed onto filters and perfused with buffer (uptake buffer containing 0.1 % bovine serum albumin and 1 μM atropine with 1 μM nomifensine) at 0.7 ml/min for 10 min, or buffer for 5 min followed by buffer with 50 nM αCtxMII for 5 min. Aliquots of synaptosomes were then exposed to ACh, nicotine, or high K+ (20 mM) in buffer for 20 sec to stimulate release of [3H]-dopamine followed by buffer. Fractions (~ 0.1 ml) were collected for 4 min into 96-well plates starting 1 min before stimulation, using a Gilson FC204 fraction collector with a multicolumn adapter (Gilson, Inc.; Middleton, WI). Radioactivity was determined by scintillation counting using a 1450 MicroBeta Trilux scintillation counter (Perkin Elmer Life Sciences) after addition of 0.15 ml Optiphase ‘SuperMix’ scintillation cocktail. Instrument efficiency was 40%.
Data were analyzed using SigmaPlot 5.0 for DOS or the open source program, R. Perfusion data were plotted as counts per min versus fraction number. Fractions collected before and after the peak were used to calculate baseline as a single exponential decay. The calculated baseline was subtracted from the experimental data. Fractions that exceeded baseline by 10% or more were summed to give total released cpm and then normalized to baseline to give units of release [(cpm-baseline)/baseline] (Salminen et al., 2007).
2.6 - Statistical Analysis
Following a square-root transform of the data, statistical significance for 48 h home cage locomotor activity (Figure 1), novelty-induced locomotor activity (Figure 2), and nicotine-stimulated locomotor activity (Figure 3) experiments were computed using one-way analysis of variance (ANOVA) followed by a Dunnett’s post-hoc test. For daily wheel running experiments (Figure 4), ANOVA was performed as follows: mean values for each line on each day were computed and one-way repeated measures analysis was performed using the mouse line as the treatment variable and the mean values for each line on each day as the measured values. A Dunnett’s post-hoc test set for an alpha level of 0.01 was used for individual comparisons on wheel running data. This procedure was used to test for overall differences between number of rotations per day, nocturnal running time, and nocturnal velocity of the WT and α6 L9′S mouse lines during the 9 d experiment. For synaptosomal DA release experiments, responses from each α6 L9′S line were compared with results obtained from control synaptosomes tested on the same day using one-way ANOVA followed by a Duncan’s post-hoc test for individual comparisons.
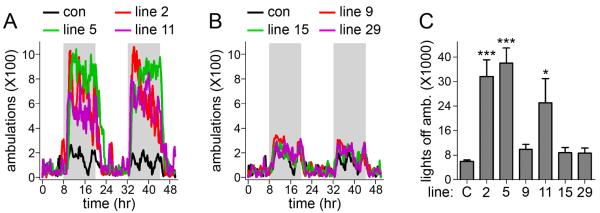
Figure 1.
Home cage locomotor activity in six lines of α6 L9′S mice. A, B. Six α6 L9′S transgenic lines were tested for home-cage locomotor activity over 48 h. Average 48 h activity traces for groups of mice from each line are plotted compared to traces from control, non-transgenic mice. Shaded background indicates lights off and white background indicates lights on. Results fell into two categories and were plotted on separate graphs for clarity: locomotor activity elevated compared to control (A), and locomotor activity comparable to control (B). C. Plots of total ambulations during lights off for control and six α6 L9′S lines. Total ambulations during both lights-off periods during the 48 h experiment were averaged for each individual, and the average for each line is plotted. The number of mice for home-cage hyperactivity was as follows: control, n = 14; line 2, n = 24; line 5, n = 24; line 9, n = 8; line 11, n = 9; line 15, n = 7; line 29, n = 7. Data are mean ± SEM of untransformed data whereas p values are reported for Dunnett’s tests on square-root transformed data. * p < 0.05, *** p < 0.001
Figure 2.
Novelty-induced locomotor activity in six lines of α6 L9′S mice. A, B. Six lines of α6 L9′S mice were tested for novelty-induced locomotor activity over a 30 min period. Average 30 min activity traces for groups of mice from each line are plotted compared to traces from control, non-transgenic mice. Results fell into two categories and were plotted on separate graphs for clarity: locomotor activity elevated compared to control (A), and locomotor activity comparable to control (B). C. Plot of ambulations for the 30 min period for each line compared to control. Mean ambulation values during mins 25 to 30 of the 30 min period for control and six α6 L9′S lines are plotted. The number of mice for novelty-induced locomotor activity was as follows: control, n = 17; line 2, n = 18; line 5, n = 18; line 9, n = 14; line 11, n = 20; line 15, n = 14; line 29, n = 13. Data are mean ± SEM of untransformed data whereas p values are reported for Dunnett’s tests on square-root transformed data. * p < 0.05, *** p < 0.001
Figure 3.
Nicotine-stimulated locomotor activity in six lines of α6 L9′S mice. A, B. Six lines of α6 L9′S mice were tested for nicotine-stimulated locomotor activity. Mice were habituated for 8 mins followed by nicotine administration (0.15 mg/kg; i.p.) at a dose designed to specifically activate hypersensitive α6* receptors. After injection, mice were monitored for an additional 30 mins. Average activity traces for groups of mice from each line are plotted with traces from control, non-transgenic mice. Results fell into two categories and were plotted on separate graphs for clarity: locomotor activity elevated compared to control (A), and locomotor activity slightly elevated or not elevated comparable to control (B). C. Plot of ambulations for nicotine-stimulated locomotor activity for each line compared to control. Mean ambulation values for control and six α6 L9′S lines are plotted for the peak response period (mins 9-20). The number of mice for nicotine-induced locomotor activity was as follows: control, n = 9; line 2, n = 7; line 5, n = 7; line 9, n = 8; line 11, n = 8; line 15, n = 8; line 29, n = 5. Data are mean ± SEM of untransformed data whereas p values are reported for Dunnett’s tests on square-root transformed data. * p < 0.05, *** p < 0.001
Figure 4.
Wheel running locomotor activity in six lines of α6 L9′S mice. A, B. Six lines of α6 L9′S mice were tested for acquisition of wheel running behavior over 9 d. Mice were habituated to cages fit with running wheels for 4 h, followed by continuous recording of running behavior for 9 d beginning at the onset of the dark phase each day. Total revolutions per day over 9 d for each α6 L9′S line are plotted compared to the same control group for comparison. The data for each group were fit to a single exponential function. Results fell into two categories and were plotted on separate graphs for clarity: wheel running activity depressed compared to control (A), and wheel running activity comparable to control (B). C, D. Daily wheel running time for six lines of α6 L9′S mice. Average time spent on the wheel per day is plotted for each α6 L9′S mouse line compared to control. Similar to (A) and (B), results were plotted on separate graphs for clarity: lines 2, 5, and 11 compared to control (C), and lines 9, 15, and 29 compared to control (D). The data for each group were fit to a linear regression function. E, F. Daily running speed for six lines of α6 L9′S mice. Average running velocity per day is plotted for each α6 L9′S mouse line compared to control. The data for each group were fit to a single exponential function. Results fell into two categories and were plotted on separate graphs for clarity: running velocity depressed compared to control (E), and running velocity comparable to control (F). The number of mice for wheel running was as follows: control, n = 18; line 2, n = 18; line 5, n = 10; line 9, n = 10; line 11, n = 20; line 15, n = 10; line 29, n = 10. Data are mean ± SEM.
2.7 - Materials
All radioactive compounds were obtained from Perkin Elmer (Boston, MA). αCtxMII was synthesized as previously described (Cartier et al., 1996). Ultra centrifugation grade sucrose was obtained from Fisher Chemicals (Fairlawn, NJ). Sigma-Aldrich (St. Louis, MO) was the source for the following compounds: ascorbic acid, atropine sulfate, bovine serum albumin (BSA), (-)-nicotine tartrate, nomifensine, cytisine, and pargyline. Optiphase ‘SuperMix’ scintillation fluid was from Perkin Elmer Life Sciences.
3 - Results
3.1 - α6 L9′S BAC Copy Number Analysis
To gain a better understanding of the causes of elevated DA release and locomotor hyperactivity in two lines of α6 L9′S transgenic mice, we studied an additional four α6 L9′S lines in this paper. Although α6 L9′S lines 2 and 5 have been studied in some assays (Drenan et al., 2008, Drenan et al., 2010, Grady et al., 2010), we now describe new results obtained from these lines as well as lines “9”, “11”, “15” and “29”, which together provide a more informative range of transgene copy number. We used relative, quantitative real-time PCR to measure the α6 L9′S transgene copy number in these lines. The transgenic lines studied here were created on a WT genetic background, which includes two WT alleles of the Chrna6 gene. By utilizing a pan-α6 primer set (which recognizes WT and L9′S Chrna6 genes equally), we determined the fold-increase in Chrna6 gene number per diploid genome, relative to non-transgenic control mice which have two Chrna6 genes per diploid genome. From these data we then calculated the number of α6 L9′S BAC transgenes in each mouse line. Line 2 and 11 have the highest copy number (18.0 ± 0.9 and 16.3 ± 0.5 copies, respectively), line 5 has an intermediate copy number (5.5 ± 0.1 copies), and lines 9, 15, and 29 have similar low copy number values (2.3 ± 0.1, 2.0 ± 0.2, and 2.0 ± 0.3 copies, respectively) (data not shown).
3.2 - Ambulatory Locomotion and Hyperactivity in α6 L9′S Mice
We previously reported that α6 L9′S lines 2 and 5 mice exhibit home-cage hyperactivity when monitored over 48 h (Drenan et al., 2008, Drenan et al., 2010). This phenotype is largely confined to the dark phase, and is likely mediated by elevated DA release from midbrain DA neurons. The number of transgenes may influence the number of L9′S α6 subunits produced and therefore the final α6* nAChR sensitivity (Labarca et al., 1995, Labarca et al., 2001). To determine whether a relationship exists between putative transgene expression and hyperactivity, we repeated this study with all six available α6 L9′S mouse lines. The results of this analysis largely segregated into two groups: lines 2 and 5 again showed home cage hyperactivity (Figure 1A), and line 11 also displayed a strongly hyperactive phenotype relative to control mice (Figure 1A). In contrast, lines 9, 15, and 29 showed little to no home cage hyperactivity (Figure 1B). We quantified mean ambulations during the dark phase for each mouse line, and found a statistically significant increase in ambulation for lines 2, 5, and 11 compared to control (line 2: p < 0.001; line 5: p < 0.001; line 11: p < 0.05; one-way ANOVA of square-root transformed data and Dunnett’s post-hoc analysis) (Figure 1C), and no statistically significant difference for lines 9, 15, and 29 (one-way ANOVA of square-root transformed data and Dunnett’s post-hoc analysis) (Figure 1C).
In a previous study, we also reported a novelty-induced locomotor activity phenotype in line 2 and line 5 α6 L9′S mice (Drenan et al., 2008). In these experiments, α6 L9′S mice removed from their home cage and placed into an identical cage do not habituate to their new environment as control mice do. This phenotype has been previously reported for other mice with mutations causing increased DA release (Giros et al., 1996, Zhuang et al., 2001), and could be mediated in α6 L9′S mice by enhanced cholinergic facilitation of DA release. We studied the ability of each of the six lines of α6 L9′S mice to habituate to a novel environment. The results of this experiment also segregated into a hyperactive and non-hyperactive group of transgenic lines. Consistent with our previous results (Drenan et al., 2008), lines 2 and 5 showed increased locomotor activity behavior during the 30 min test period (Figure 2A). Line 11 also did not habituate to the novel cage (Figure 2A). In contrast, lines 9, 15, and 29 habituated to the novel cage in a manner similar to control mice (Figure 2B). When we quantified ambulations during mins 25 to 30 of the locomotor test period, we found a statistically significant increase for lines 2, 5, and 11 (line 2: p < 0.05; line 5: p < 0.001; line 11: p < 0.05; one-way ANOVA of square-root transformed data and Dunnett’s post-hoc analysis) (Figure 2C). There was no significant increase in ambulations for lines 9, 15, and 29 compared to control (one-way ANOVA of square-root transformed data and Dunnett’s post-hoc analysis) (Figure 2C), consistent with their ability to habituate to the novel cage (Figure 2B). When we quantified ambulations during mins 0 to 5, however, there was no significant difference in locomotor activity between strains (data not shown).
Nicotine suppresses locomotion in WT mice at moderate doses (0.5 to 1.5 mg/kg) (Tapper et al., 2007, Drenan et al., 2008) and induces seizures and/or death at high doses (~10 mg/kg) (Fonck et al., 2005). In contrast, high-copy α6 L9′S mice exhibit locomotor activation in response to low doses of nicotine (0.08 to 0.15 mg/kg) that selectively stimulate α6α4β2β3 nAChRs on DA neurons (Drenan et al., 2008, Drenan et al., 2010, Grady et al., 2010). Here, we tested all six α6 L9′S lines for locomotor responses following injection of 0.15 mg/kg nicotine – a maximal dose for lines 2 and 5 based on previous studies (Drenan et al., 2008, Drenan et al., 2010). As with home cage locomotion and novelty-induced locomotion, results of this experiment segregated into two groups, with lines 2, 5, and 11 responding with strong locomotor activation (Figure 3A), and lines 9, 15, and 29 responding with little to no locomotor activation (Figure 3B). When we quantified ambulations during the peak response period (mins 9 to 20; the first 11 mins following injection), we found significant differences from control for line 2 (p < 0.001), line 5 (p < 0.001), line 9 (p < 0.05), and line 11 (p < 0.001) using one-way ANOVA of square-root transformed data and Dunnett’s post-hoc analysis (Figure 3C). Nicotine-stimulated locomotor responses in lines 15 and 29 were not significantly different from control (Figure 3C).
3.3 - Wheel Running in α6 L9′S Mice
The results described in Figures 1-3 suggest that, like our study of α6 L9′S mice lacking α4 subunits (Drenan et al., 2010), ambulatory locomotor activity in α6 L9′S mice is sensitive to genetic manipulations that modify receptor sensitivity. In this case, fewer copies of the α6 L9′S transgene (such as in lines 9, 15, and 29) may be insufficient to result in the expression of a α6* nAChR pool with elevated sensitivity. The neural substrates that underlie complex locomotor activities such as wheel running may differ from those underlying simple ambulation. Therefore, we tested the ability of control and α6 L9′S mice to initiate and sustain wheel running behavior, with the hypothesis that hyperactive α6 L9′S mice (lines 2, 5, and 11) – in contrast to non-hyperactive α6 L9′S mice (lines 9, 15, and 29) – may have altered running wheel locomotor activity.
We housed mice individually in cages fitted with running wheels, and recorded wheel rotations over a 9 d period during which the mice had continuous access to the wheels. Control mice steadily increased their daily wheel running over the 9 d period, approaching a plateau by the end of the experiment. We measured rotations per day for all six transgenic α6 L9′S mouse lines and plotted the daily total of each line compared to the control group. Strikingly, lines 2, 5, and 11 ran substantially less across the entire 9 d period compared to control mice (Figure 4A). In contrast, the wheel-running profiles of lines 9, 15, and 29 were not qualitatively different from control mice (Figure 4B). All α6 L9′S lines exhibited a similar gradual increase during the 9 d test period, but with variable starting and ending values.
We quantified wheel rotations during each day of the wheel running experiment and performed a statistical analysis to detect overall differences between the different α6 L9′S mouse lines across all 9 d. For rotations, there was a significant difference in mean values among all α6 L9′S lines (p < 0.001; one-way repeated-measures ANOVA), with line 2 (p < 0.01), line 5 (p < 0.01), and line 11 (p < 0.01) differing significantly from control (Dunnett’s post-hoc analysis). In contrast, rotations for lines 9, 15, and 29 were not significantly different from the control group over the 9 d experiment. Thus, similar to ambulatory locomotion, the wheel running results fell largely into two categories, with low-copy α6 L9′S mice and control mice having similar wheel rotations, while high-copy α6 L9′S mice (lines 2, 5, and 11) exhibited reduced wheel running.
Almost all rodent wheel running occurs during lights off. To determine whether α6 L9′S mice (lines 2, 5, and 11) run less because of a disruption in light/dark entrainment, we calculated the percentage of wheel rotations occurring in the dark phase. None of the α6 L9′S lines differed significantly from control following Dunnett’s post-hoc testing (data not shown). From these data, we concluded that α6 L9′S mice do not have disrupted light/dark entrainment compared to control mice, and we therefore restricted the subsequent analysis to dark phase wheel running.
When given free access to the wheel, mice run in bouts lasting from minutes to hours, interspersed with periods of no wheel-running activity. Thus, the number of wheel rotations for lines 2, 5, and 11 could be less than control either because (1) the mutant mice spend less time running on the wheel or (2) they run more slowly once they are on the wheel. To determine the relative contributions of these two factors to the effects seen for lines 2, 5, and 11 (Figure 4A), we measured the time spent running during lights off and the nocturnal running speed. Lines 2, 5, and 11 spent less time on the wheel (Figure 4C) and ran at slower speeds than the control (Figure 4E) over the 9 d period of the experiment. In contrast, lines 9, 15, and 29 ran for similar times (Figure 4D) and at similar speeds compared to control (Figure 4F). Quantification of 9 d mean running time revealed a significant difference (p < 0.001; one-way repeated-measures ANOVA followed by Dunnett’s post-hoc analysis) between lines 2 (p < 0.01), 5 (p < 0.01), and 11 (p < 0.01) versus control. Similarly, quantification of 9 d mean running speed also revealed a significant difference (p < 0.001; one-way repeated-measures ANOVA followed by Dunnett’s post-hoc analysis) between lines 2 (p < 0.01), 5 (p < 0.01), and 11 (p < 0.01) versus control. Thus, both factors contributed to the reduced wheel running seen in lines 2, 5, and 11.
Interestingly, wheel-running velocity increased over the 9 d experimental period in a fashion similar to that of overall activity (Figure 4E-F). In contrast, the time spent running remained relatively constant (Figure 4C-D). Although both factors contributed to the reduced wheel-running activity of lines 2, 5, and 11 at the beginning of the experiment, the reduction in velocity made a larger contribution by the end. The data therefore suggest that, from the beginning, high-copy number α6 L9′S mutant mice (lines 2, 5, 11) are less interested in exploring other forms of exercise besides simple ambulation and, when they do use the wheel, they find running on it less rewarding than control animals.
3.4 - ACh and Nicotine-Stimulated Dopamine Release from α6 L9′S Striatal Synaptosomes
α6 L9′S mice exhibit a number of spontaneous behavioral phenotypes, indicating that endogenous neurotransmitter systems (i.e. dopaminergic or cholinergic) may be altered. Native α6* and α4*(non-α6) nAChR function is readily studied using synaptosome preparations from striatum and olfactory tubercle (Grady et al., 2002). The striatum is organized such that a greater percentage of the total input is received from A9 SNc DA neurons as one moves dorsolateral, and a greater percentage of total input is received form A10 VTA DA neurons as one moves ventromedial (Voorn et al., 2004, Ikemoto, 2007). We fractionated the striatum into dorsal striatum (ST) and ventral striatum/olfactory tubercle (OT), thus attempting to roughly distinguish between SNc-derived DA terminals and VTA-derived DA terminals. We measured ACh-stimulated DA release from control and α6 L9′S synaptosomes prepared from ST and OT. DA release responses were distinguished as α6*-dependent and α4(non-α6)*-dependent components using 50nM αCtxMII (Drenan et al., 2008, Drenan et al., 2010), and responses for each line were compared to a contemporaneous control experiment and plotted as a percentage of control (set to 100%). Although αCtxMII has antagonist activity at α3* and α6* nAChRs, there is no significant expression of α3* nAChRs in mouse DA neurons or DA presynaptic terminals, thus making αCtxMII specific for α6* nAChRs in this assay. In experiments with ST synaptosomes, several α6 L9′S lines exhibited augmented DA release via α6* nAChRs when stimulated with 0.1 μM ACh (p < 0.05, one-way ANOVA with Duncan’s post-hoc analysis) (Figure 5A, left panel). For OT, all α6 L9′S lines except line 15 exhibited augmented α6*-dependent ACh-stimulated release relative to control (p < 0.05, one-way ANOVA with Duncan’s post-hoc analysis) (Figure 5A, right panel).
Figure 5.
ACh-stimulated and nicotine-stimulated DA release from synaptosomes in six lines of α6 L9′S mice. A. For control and six α6 L9′S mouse lines, αCtxMII-sensitive (α6*-dependent) DA release from caudate/putamen (CPu) and olfactory tubercle (OT) synaptosomes was measured in response to stimulation with 0.1 μM ACh. B. For control and six α6 L9′S mouse lines, αCtxMII-sensitive (α6*-dependent) DA release from caudate/putamen (CPu) and olfactory tubercle (OT) synaptosomes was measured in response to stimulation with 0.1 μM nicotine. For (A) and (B), data are expressed as a percentage of the response from a control group (set to 100%) of mice assayed on the same day. The number of mice for ACh-stimulated and nicotine-stimulated DA release was as follows: control, n = 8; line 2, n = 8; line 5, n = 8; line 9, n = 6; line 11, n = 8; line 15, n = 8; line 29, n = 6. Data are mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001
Nicotine-stimulated locomotor activation (Figure 3) may be related to augmented DA release (Drenan et al., 2008). We previously studied nicotine-stimulated DA release from striatal synaptosomes in α6 L9′S line 2 and line 5 mice (Drenan et al., 2008, Drenan et al., 2010), and here we performed this analysis on all six lines of α6 L9′S mice under investigation. In ST synaptosome preparations, α6 L9′S line 2, 5 and 11 exhibited augmented α6*-dependent, nicotine-stimulated (0.1 μM) DA release compared to control (p < 0.05, one-way ANOVA with Duncan’s post-hoc analysis) (Figure 5B, left panel). For OT tissue stimulated with nicotine (0.1 μM), results were similar (p < 0.05, one-way ANOVA with Duncan’s post-hoc analysis) for α6*-dependent DA release (Figure 5B, right panel). The difference in DA release between OT and ST at 0.1 μM nicotine is consistent with our previous studies (Drenan et al., 2008, Drenan et al., 2010), and likely reflects a more sensitive α6* nAChR pool in VTA-derived DA terminals (OT) versus SNc-derived DA terminals (ST). α6* nAChR numbers are also increased in OT versus ST (Drenan et al., 2010). These neurochemical data strongly support the hypothesis that excess DA release may be responsible for increased ambulatory locomotion in hyperactive α6 L9′S mice, while also suggesting that participation in wheel running behavior may be inhibited by increased DA release.
4 - Discussion
4.1 - Gene dosage effects on hypersensitive α6* nAChRs
In this study, we investigated several behavioral and biochemical consequences of introducing different numbers of hypersensitive α6 nAChR subunits into mice. Introduction of two copies of the L9′S α6 allele, in combination with two WT α6 alleles (all transgenic mice described here are produced on a WT genetic background), produces a modest gain-of-function in synaptosomal DA release experiments (Figure 5), little or no locomotor hyperactivity (Figures 1-2), a modestly enhanced locomotor response of nicotine (Figure 3), and normal wheel running behavior (Figure 4). In contrast, six or more copies of the L9′S allele is sufficient to produce statistically significant increases in ambulatory locomotion (Figures 1-3), a significant reduction in wheel running behavior (Figure 4), and an increase in α6-dependent ACh-stimulated and nicotine-stimulated DA release from striatal synaptosomes (Figure 5).
We previously demonstrated that α6 L9′S lines 2 and 5, while exhibiting behavioral hyperactivity and hypersensitive receptors, show no major change in receptor numbers as determined by radioligand binding (Drenan et al., 2008, Drenan et al., 2010). Therefore, because β2* receptor numbers are apparently fixed, we interpret the data in this study to indicate that adding α6 L9′S alleles to two WT α6 alleles produces a concomitant increase in the overall sensitivity of the receptor population. This likely occurs via production of α6 subunit polypeptides with the L9′S mutation, which competes with WT α6 subunits for incorporation into β2* receptor pentamers. It is well known that the number of functional, pentameric α6* nAChRs assembled is ultimately limited by the number of available β2, (Picciotto et al., 1998), β3 (Cui et al., 2003, Salminen et al., 2007) and α4 subunits (Champtiaux et al., 2003, Drenan et al., 2010). Increasing the sensitivity of the receptor pool modestly by adding two α6 L9′S alleles (i.e. lines 9, 15, and 29) begins to produce increases in DA release yet little behavioral effect, whereas adding six or more α6 L9′S alleles drastically increases the sensitivity of the receptor pool, with profound physiological and behavioral consequences (Figure 6A, 6B). We expect that increasing the agonist sensitivity of the receptor pool by adding α6 L9′S alleles causes a left-shift in the ACh concentration-response curve, lowering the threshold for endogenous ACh (or similarly for exogenous nicotine) to produce DA increased DA release and ultimately lead to behavioral changes such as locomotor hyperactivity (Figure 6C).
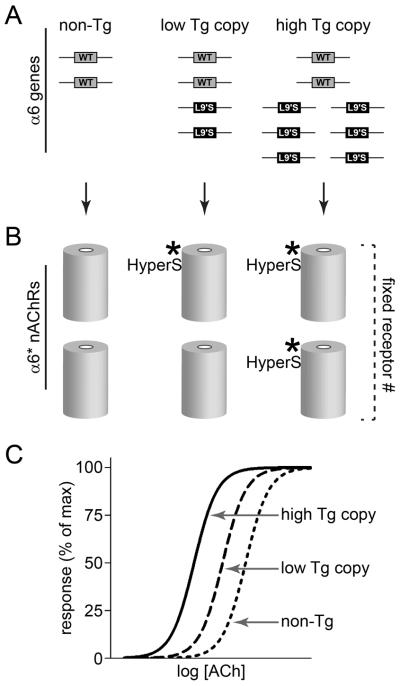
Figure 6.
A putative model describing the mechanism of α6 L9′S transgene-induced hypersensitivity. A. Comparison of α6 L9′S allele copy numbers among the strains studied in this paper. B. Effects on sensitivity of the α6* nAChRs in DA neurons. Elevated α6 L9′S allele copy number produces a greater fraction of hypersensitive nAChRs (abbreviated HyperS) in the α6β2* receptor pool. However, previous data show that even the greatest copy numbers do not substantially alter the size of the pool, presumably because this is limited by other factors (perhaps β2 or β3 subunit pools). Results in this study indicate that addition of two α6 L9′S alleles (e.g. α6 L9′S lines 9, 15, and 29) to the two endogenous WT α6 alleles produces modest hypersensitivity at α6* nAChRs.. In contrast, six or more α6 L9′S alleles (e.g. α6 L9′S lines 2, 5, and 11) is sufficient to out-compete production of WT α6 subunits from the two endogenous α6 alleles, resulting in a pool of α6β2* receptors with marked sensitivity. C. Idealized ACh concentration-response curve for behavioral or physiological responses, comparing response magnitude in mice with zero (“non-Tg”), two (“low Tg copy”; lines 9, 15, and 29), and six or more (“high Tg copy”; lines 2, 5, and 11) α6 L9′S alleles.
4.2 - Locomotor Phenotypes in α6 L9′S Mice
Previously, we showed that DA neurons in midbrain slices of α6 L9′S mice (line 2 and 5) are characterized by an elevated resting membrane conductance that results from tonic activation of hypersensitive α6* nAChRs (Drenan et al., 2008). Also, we demonstrated that α6 L9′S line 2 mice have altered frequency-dependent DA release in striatal slices, with burst stimulation of DA axons causing increased DA release relative to control (Drenan et al., 2010). These results suggest that ACh released at baseline levels – or circulating choline (Labarca et al., 2001) – is capable of more strongly activating hypersensitive α6* nAChRs on the soma and/or the axons/presynaptic terminals of DA neurons, ultimately leading to augmented DA release and altered locomotor behavior. This hypothesis is further supported by results in this study: locomotor hyperactivity phenotypes in various α6 L9′S transgenic lines are strongly correlated with α6-dependent DA release, and α6-dependent DA release depends on the number of α6 L9′S alleles (Figure 5). This relationship is consistent with data showing that levels of ACh are higher during the dark phase and/or during spontaneous locomotor activity (Kametani and Kawamura, 1990, Day et al., 1991, Mizuno et al., 1991). Further experiments are required to elucidate the precise mechanism, as DA release in striatum can be altered by modifying the rate of burst-firing in the soma of DA neurons, and/or it may be changed by selectively altering the function of DA presynaptic terminals. If this hypothesis regarding ACh-driven activation of α6* nAChRs is true, then other agonists for α6 should cause similar results. Indeed, we do find a very similar pattern of results for nicotine: both nicotine-stimulated locomotor activation (Figure 3) and nicotine-stimulated DA release (Figure 5) results are very similar to those attributable to endogenous ACh. The present data, based on transgene copy number, thus complement our previous data based on pharmacology, which showed a relationship between a drug’s ability to elicit α6*-dependent DA release from α6 L9′S striatal synaptosomes, and the drug’s ability to induce locomotor activity (Drenan et al., 2008).
Although the effect of excess DA on ambulatory locomotion in rodents is well-documented, the connection between altered DA release and rodent wheel running is not as well established. Here we find that hyperactive α6 L9′S mice with elevated striatal DA release, in contrast to control mice or low-copy, non-hyperactive α6 L9′S mice, engage in significantly less wheel running. Wheel running is a form of rewarding, voluntary physical exercise. Our analysis shows that reduced wheel rotations in the high-copy mutants (Figure 4A) arise from both 1) less time spent running on the wheel (Figure 4C) and 2) a slower velocity of running once on the wheel (Figure 4E). Interestingly, mice selectively bred for high voluntary wheel running run faster on the wheel (Rhodes et al., 2005) and this increase correlates with changes in their pharmacological response to DA drugs but not in their physiological capacity for exercise, suggesting that genetic alterations in the DA pathway dramatically affect wheel-running velocity. Hyperactive α6 L9′S mice did learn to run on the wheel at the same rate as control and non-hyperactive α6 L9′S mice (Figure 4A and B), and there was no evidence to indicate that gross motor or fine motor coordination was impaired in any of the mice under investigation (data not shown).
The reduction in running velocity and time spent on the wheel (Figure 4C and E), may indicate that reward associated with wheel running is blunted in high-copy α6 L9′S mice (lines 2, 5, and 11). The reduction in time spent on the wheel at the outset of the experiment further suggests that high-copy α6 L9′S mice are less receptive to reward opportunities in their environment than control mice. DA signal to noise ratio – and not necessarily absolute DA release – is critical for DA’s ability to communicate reward prediction errors (Tsai et al., 2009). We suggest, therefore, that baseline DA release in α6 L9′S mice (lines 2, 5, and 11) may be sufficiently elevated to interfere with the ability of a stimulus with reward or motivational salience to further increase DA levels. It is unclear whether augmented baseline DA release in α6 L9′S mice might be mediated by increased release during DA neuron pacemaker firing, or by more frequent episodes of burst firing. The latter may be more likely than the former, as single spikes elicit reduced DA release whereas spike trains produce elevated DA release relative to control in α6 L9′S slices (Drenan et al., 2010). Access to a running wheel gives mice a choice: exercise by ambulating on the cage floor or by running on the wheel. WT mice spend significantly more time running on the wheel, and run more rapidly once they are on the wheel, than lines 2, 5, and 11. It is not yet clear why WT mice show a greater preference for wheel-running than α6 L9′S mutants. One explanation is that WT mice find wheel running more rewarding than lines 2, 5, and 11. In this case, the choice to run on the wheel or ambulate in the cage is an active choice involving DA reward mechanisms. An alternative explanation is that enhanced ambulatory activity becomes established prior to introduction of the wheel and continues to dominate the animal’s behavior. In this case, the selection of which action to take, once the alternative option is presented (wheel running), may be largely predetermined. Further experiments will be required to address these possibilities.
DA neurons and their presynaptic terminals must coordinately couple to excitatory/facilitative cholinergic inputs through nAChRs, as well as to inhibitory GABAergic inputs. DA neurons express α6* nAChRs, whereas midbrain GABAergic neurons express mainly α4*(non-α6) nAChRs. By selectively augmenting cholinergic α6* nAChR function in α6 L9′S mice, DA neuron activity has become “hypercoupled” to local cholinergic tone and consequently uncoupled from the influence of GABAergic efferents whose activity depends in part on ACh and nAChRs. By exploring mice with perturbed cholinergic regulation of neurotransmitter release, we show the precise balance between excitation and inhibition that normally exists to maintain proper levels of DA. Further, these experiments contribute to our knowledge of the DA system, including some of the cellular and molecular mechanisms regulating this neurotransmitter system that may be dysregulated in DA-related diseases or disorders in humans.
Acknowledgements
We thank members of the Lester laboratory for helpful discussion. This work was supported by National Institutes of Health (NIH) grants (DA17279, DA12242, DA015663, DA03194, MH53631, GM48677, and DA030396). R.M.D. was supported by a fellowship from the California Tobacco Related Disease Research Program (15FT-0030), an NIH National Research Service Award (DA021492), and an NIH Pathway to Independence Award (DA030396).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Azam L, Winzer-Serhan UH, Chen Y, Leslie FM. Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol. 2002;444:260–274. doi: 10.1002/cne.10138. [DOI] [PubMed] [Google Scholar]
- Ballester M, Castello A, Ibanez E, Sanchez A, Folch JM. Real-time quantitative PCR-based system for determining transgene copy number in transgenic animals. Biotechniques. 2004;37:610–613. doi: 10.2144/04374ST06. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM. α-Conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology. 2009;35:665–673. doi: 10.1038/npp.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new alpha-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J Biol Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Champtiaux N, Gotti C, Cordero-Erausquin M, David DJ, Przybylski C, Lena C, Clementi F, Moretti M, Rossi FM, Le Novere N, McIntosh JM, Gardier AM, Changeux JP. Subunit composition of functional nicotinic receptors in dopaminergic neurons investigated with knock-out mice. J Neurosci. 2003;23:7820–7829. doi: 10.1523/JNEUROSCI.23-21-07820.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champtiaux N, Han ZY, Bessis A, Rossi FM, Zoli M, Marubio L, McIntosh JM, Changeux JP. Distribution and pharmacology of α6-containing nicotinic acetylcholine receptors analyzed with mutant mice. J Neurosci. 2002;22:1208–1217. doi: 10.1523/JNEUROSCI.22-04-01208.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Booker TK, Allen RS, Grady SR, Whiteaker P, Marks MJ, Salminen O, Tritto T, Butt CM, Allen WR, Stitzel JA, McIntosh JM, Boulter J, Collins AC, Heinemann SF. The β3 nicotinic receptor subunit: a component of α-Conotoxin MII-binding nicotinic acetylcholine receptors that modulate dopamine release and related behaviors. J Neurosci. 2003;23:11045–11053. doi: 10.1523/JNEUROSCI.23-35-11045.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Steele AD, McKinney S, Patzlaff NE, McIntosh JM, Marks MJ, Miwa JM, Lester HA. Cholinergic modulation of locomotion and striatal dopamine release is mediated by α6α4* nicotinic acetylcholine receptors. J Neurosci. 2010;30:9877–9889. doi: 10.1523/JNEUROSCI.2056-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenan RM, Grady SR, Whiteaker P, McClure-Begley T, McKinney S, Miwa JM, Bupp S, Heintz N, McIntosh JM, Bencherif M, Marks MJ, Lester HA. In vivo activation of midbrain dopamine neurons via sensitized, high-affinity α6* nicotinic acetylcholine receptors. Neuron. 2008;60:123–136. doi: 10.1016/j.neuron.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux JP, Maskos U, Cragg SJ, Faure P. Distinct contributions of nicotinic acetylcholine receptor subunit α4 and subunit α6 to the reinforcing effects of nicotine. Proc Natl Acad Sci U S A. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonck C, Cohen BN, Nashmi R, Whiteaker P, Wagenaar DA, Rodrigues-Pinguet N, Deshpande P, McKinney S, Kwoh S, Munoz J, Labarca C, Collins AC, Marks MJ, Lester HA. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive α4* nicotinic receptors. J Neurosci. 2005;25:11396–11411. doi: 10.1523/JNEUROSCI.3597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area α6β2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Clementi F, Riganti L, McIntosh JM, Collins AC, Marks MJ, Whiteaker P. Expression of nigrostriatal α6-containing nicotinic acetylcholine receptors is selectively reduced, but not eliminated, by β3 subunit gene deletion. Mol Pharmacol. 2005;67:2007–2015. doi: 10.1124/mol.105.011940. [DOI] [PubMed] [Google Scholar]
- Grady SR, Drenan RM, Breining SR, Yohannes D, Wageman CR, Fedorov NB, McKinney S, Whiteaker P, Bencherif M, Lester HA, Marks MJ. Structural differences determine the relative selectivity of nicotinic compounds for native α4β2*-, α6β2*-, α3β4*- and α7-nicotine acetylcholine receptors. Neuropharmacology. 2010;58:1054–1066. doi: 10.1016/j.neuropharm.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady SR, Murphy KL, Cao J, Marks MJ, McIntosh JM, Collins AC. Characterization of nicotinic agonist-induced [3H]dopamine release from synaptosomes prepared from four mouse brain regions. J Pharmacol Exp Ther. 2002;301:651–660. doi: 10.1124/jpet.301.2.651. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametani H, Kawamura H. Alterations in acetylcholine release in the rat hippocampus during sleep-wakefulness detected by intracerebral dialysis. Life Sci. 1990;47:421–426. doi: 10.1016/0024-3205(90)90300-g. [DOI] [PubMed] [Google Scholar]
- Kulak JM, Nguyen TA, Olivera BM, McIntosh JM. α-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci. 1997;17:5263–5270. doi: 10.1523/JNEUROSCI.17-14-05263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca C, Nowak MW, Zhang H, Tang L, Deshpande P, Lester HA. Channel gating governed symmetrically by conserved leucine residues in the M2 domain of nicotinic receptors. Nature. 1995;376:514–516. doi: 10.1038/376514a0. [DOI] [PubMed] [Google Scholar]
- Labarca C, Schwarz J, Deshpande P, Schwarz S, Nowak MW, Fonck C, Nashmi R, Kofuji P, Dang H, Shi W, Fidan M, Khakh BS, Chen Z, Bowers BJ, Boulter J, Wehner JM, Lester HA. Point mutant mice with hypersensitive α4 nicotinic receptors show dopaminergic deficits and increased anxiety. Proc Natl Acad Sci U S A. 2001;98:2786–2791. doi: 10.1073/pnas.041582598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Kim J, Shin SG, Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol. 2006;123:273–280. doi: 10.1016/j.jbiotec.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Lena C, de Kerchove D’Exaerde A, Cordero-Erausquin M, Le Novere N, del Mar Arroyo-Jimenez M, Changeux JP. Diversity and distribution of nicotinic acetylcholine receptors in the locus ceruleus neurons. Proc Natl Acad Sci U S A. 1999;96:12126–12131. doi: 10.1073/pnas.96.21.12126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC(T) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Endo Y, Arita J, Kimura F. Acetylcholine release in the rat hippocampus as measured by the microdialysis method correlates with motor activity and exhibits a diurnal variation. Neuroscience. 1991;44:607–612. doi: 10.1016/0306-4522(91)90081-x. [DOI] [PubMed] [Google Scholar]
- Nashmi R, Xiao C, Deshpande P, McKinney S, Grady SR, Whiteaker P, Huang Q, McClure-Begley T, Lindstrom JM, Labarca C, Collins AC, Marks MJ, Lester HA. Chronic nicotine cell specifically upregulates functional α4* nicotinic receptors: basis for both tolerance in midbrain and enhanced long-term potentiation in perforant path. J Neurosci. 2007;27:8202–8218. doi: 10.1523/JNEUROSCI.2199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pons S, Fattore L, Cossu G, Tolu S, Porcu E, McIntosh JM, Changeux JP, Maskos U, Fratta W. Crucial role of α4 and α6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci. 2008;28:12318–12327. doi: 10.1523/JNEUROSCI.3918-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, McIntosh JM. Striatal α6* nicotinic acetylcholine receptors: potential targets for Parkinson’s disease therapy. J Pharmacol Exp Ther. 2006;316:481–489. doi: 10.1124/jpet.105.094375. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Gammie SC, Garland T. Neurobiology of Mice Selected for High Voluntary Wheel-running Activity. Integrative and Comparative Biology. 2005;45:438–455. doi: 10.1093/icb/45.3.438. [DOI] [PubMed] [Google Scholar]
- Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of α-Conotoxin MII-Sensitive Subtypes of Nicotinic Acetylcholine Receptors Isolated by Breeding of Null Mutant Mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, Grady SR. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Marks MJ, Lester HA. Nicotine responses in hypersensitive and knockout α4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics. 2007;31:422–428. doi: 10.1152/physiolgenomics.00063.2007. [DOI] [PubMed] [Google Scholar]
- Tsai HC, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Whiteaker P, McIntosh JM, Luo S, Collins AC, Marks MJ. [125I]-α-Conotoxin MII identifies a novel nicotinic acetylcholine receptor population in mouse brain. Mol Pharmacol. 2000;57:913–925. [PubMed] [Google Scholar]
- Yang K, Buhlman L, Khan GM, Nichols RA, Jin G, McIntosh JM, Whiteaker P, Lukas RJ, Wu J. Functional Nicotinic Acetylcholine Receptors Containing α6 Subunits Are on GABAergic Neuronal Boutons Adherent to Ventral Tegmental Area Dopamine Neurons. J Neurosci. 2011;31:2537–2548. doi: 10.1523/JNEUROSCI.3003-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]