Abstract
Atopic dermatitis (AD) is a pruritic, chronic inflammatory skin disease that affects 10–20% of children and 1–3% of adults worldwide. Recent studies have indicated that the ability of Th2 cytokines such as interleukin-4 (IL-4) to regulate skin barrier function may be a predisposing factor for AD development. The present studies examined the ability of increased Th2 activity to affect cutaneous barrier function in vivo and epidermal thickening. Mice that express a constitutively active Signal Transducer and Activator of Transcription 6 (STAT6VT) have increased Th2 cells and a predisposition to allergic inflammation were used in these studies; they demonstrate that topical treatment with the irritant sodium lauryl sulfate (SLS) caused increased transepidermal water loss and epidermal thickening in STAT6VT mice over similarly treated wild-type mice. The proliferation marker Ki-67 was increased in the epidermis of STAT6VT compared to wild-type mice. However, these differences do not appear to be linked to the addition of an irritant as control-treated STAT6VT skin also exhibited elevated Ki-67 levels, suggesting that the increased epidermal thickness in SLS-treated STAT6VT mice is primarily driven by epidermal cell hypertrophy rather than an increase in cellular proliferation. Our results suggest that an environment with increased Th2 cytokines results in abnormal responses to topical irritants.
Keywords: atopic dermatitis, interleukin-4, STAT6 V547A/T548A mutation, transepidermal water loss, T-helper Type 2 cells
Introduction
Atopic dermatitis (AD) is a pruritic, chronic inflammatory skin disease with a lifetime prevalence of 10–20% in children and 1–3% in adults, worldwide. It is the most common cause of occupational skin disease of adults. In the past three decades, the prevalence of AD has increased by two to three-fold in industrialized countries, with higher incidences in urban regions compared to rural regions {Leung, 2003 #21}. The pruritic features of AD can cause mental exhaustion, mood changes, lack of concentration, and an increase in parental and child morbidity. Skin lesions tend to occur on the cheeks, scalp and extensor areas of the arms and legs in infants, and in the flexural regions of the extremities in older children and adults {Sehra, 2008 #7}. Although recent studies have resulted in an enhanced understanding of the pathogenesis of AD, there is still a need for an improved elucidative animal model.
The epidermal permeability barrier is mainly provided by the stratum corneum (SC); a complex made up of intracellular lipids and corneocytes that form a highly ordered structure {Nielsen, 2000 #24}. The SC has been likened to a “brick wall”, with the corneocytes acting as the “brick” and the lipid intracellular matrix as the “cement”. Skin transepidermal water loss (TEWL) is the rate at which water travels from the viable skin through the SC to the external environment. TEWL measurement is a widely accepted technique to evaluate the integrity of the SC and is used to study skin barrier function {Loden, 1995 #25}. An increase in TEWL is an indicator of skin barrier dysfunction. Patients with AD have abnormal skin barrier function, as measured by increased TEWL values both in lesional and normal-appearing skin {Werner, 1985 #26}.
Levels of Th2 cytokines such as IL-4 are elevated in AD lesions, and IL-4 has been shown to decrease expression of epidermal differentiation complex genes {Sehra, 2010 #27}. The cytokine IL-4 activates the signal transducer and activator of transcription 6 (STAT6) protein through the Janus kinase (Jak)/STAT pathway {Bruns, 2003 #29}. A STAT6 mutant, known as STAT6VT, contains two residue substitutions at positions V547A and T548A in the SH2 domain resulting in the constitutive phosphorylation of the critical tyrosine residue, Y641, activated independent of IL-4 stimulation {Daniel, 2000 #30}. The ability of the constitutively active STAT6 to mimic the IL-4 activated functions in vivo was evaluated by generating a transgenic mouse that expressed STAT6VT only in T cells. The STAT6VT transgenic mice have increased production of IgG1 and IgE in the serum. STAT6VT expression in T lymphocytes results in increased Th2 cell differentiation {Bruns, 2003 #29}. Approximately 40% of STAT6VT mice spontaneously develop an allergic dermatitis resembling AD. Of note, epidermal differentiation complex genes including filaggrin expression is normal in STAT6VT mice until approximately four months, the time at which skin lesions first appear {Sehra, 2010 #27}. Thus, the STAT6VT mouse is an informative model for AD in that Th2 immune abnormalities result in AD lesions. Although there is evidence for barrier abnormalities in STAT6VT mice, further characterization of the barrier and immune aberrations from this potential murine model of AD is necessary.
Sodium lauryl sulfate (SLS), an anionic detergent, is a common ingredient found in various skin and hair care products. It has been shown to trigger a prolonged barrier disruption of the skin that lasts up to a week following a single 24-hour application and thus is used in studies involving skin barrier damage {Patil, 1995 #31}. Since SLS has been shown to act in a time-and-dose dependent manner to disrupt barrier function, it is a valuable tool for studies examining irritant dermatoses {Nielsen, 2000 #24}.
The goal of the present studies was to assess the effects of topical SLS on skin in normal and STAT6VT mice. These studies tested the hypothesis that STAT6VT mice, at a young age (~2 months) before they develop skin lesions {Sehra, 2010 #27}, treated with SLS will exhibit increased barrier disruption in comparison to WT mice. Our findings indicate significant differences between the STAT6VT transgenic mice and WT littermate controls treated with SLS. These studies suggest STAT6VT mice exhibit abnormal barrier function, and provide a novel model system to explore the role of systemic Th2 cytokine overexpression on barrier function.
Materials and Methods
Animals
The STAT6VT transgenic mice on a C57/BL/6 background were generated and maintained as previously described {Sehra, 2010 #27}. All mice were maintained under specific pathogen-free conditions, and experiments were approved by the Indiana University Animal Care and Use Committee.
Induction of chronic irritation and biophysical measurements
Female WT C57BL/6 and STAT6VT mice at 8–10 weeks of age were briefly anesthetized with ketamine/xylazine (80/10 μg/kg body weight) intraperitoneally and the dorsal hair was shaved. Twenty-four hours later, baseline TEWL (g/h/m2) measurements were taken with a Multi Probe Adapter fitted with a TM300 Tewameter (Courage + Khazaka, Cologne, Germany). The mice were treated with 0.1 ml SLS, freshly prepared, (purity >98%) (BioRad Laboratories Inc., Hercules, CA) in distilled water (at 5% concentration) for six consecutive days, followed by a 14-day period with no application. This regime was repeated two more times, and a final six day treatment of 10% SLS was applied. TEWL measurements were taken at the time points indicated under climate-controlled (22±2°C at 40–50% humidity) conditions.
Tissue Processing
At the conclusion of the repeated SLS applications, the mice were euthanized and the dorsal skin of age-matched mice (STAT6VT and WT) was harvested and sectioned into 0.5 cm2 segments and fixed in 10% neutral buffered formalin for 1.5 hours for paraffin-embedding.
Quantification of epidermal thickness by IHC and positive Ki-67 immunolabeling
Images of hematoxylin and eosin (H&E) and Ki-67 stained slides were taken in an observer-blinded manner with a Nikon Eclipse E400 microscope (Nikon Corp., Melville, NY) at 200X magnification. Epidermal thickness measurements (μm) were taken from the SC base to the basement membrane of the inter-rete ridges along the entire length of each section, in 3 random fields. Positive Ki-67 immunolabeled cells were counted using Metamorph Meta Imaging Series 7.5 (Molecular Devices, Downington, PA) by thresholding on Ki-67 positive cells after normalizing to total epidermal area (shown as ratio).
Statistics
Statistical analyses were conducted using SAS version 9.2 (Cary, NC). Shapiro-Wilk’s and Levene’s tests were used to test normality and equal variance of the data. Baseline and daily TEWL data were sometimes non-normal or had unequal variance, so Mann-Whitney tests were used to compare STAT6VT vs WT. Epidermal thickness and Ki67 satisfied normality and equal variance assumptions so differences were compared using one-tailed Student’s t test (where applicable) or one-way analysis of variance (ANOVA) with significant overall tests followed by pair-wise t-tests.
Results
Chronic irritant treatment of STAT6VT mice augmented barrier disruption
Initially, we assessed whether the STAT6VT transgenic mice exhibited baseline differences in barrier function by measuring TEWL using a tewameter. These baseline TEWL levels were not statistically significant in C57BL/6 controls versus STAT6VT transgenic mice (Fig. 1B). Next, we assessed the effect of 3 sets of six-day applications with 5% SLS separated by a 14-day interval. As shown in Fig. 1A, the fold increase in TEWL was augmented in SLS-treated STAT6VT mice compared to WT mice with significance (*p<0.05, **p<0.01, ***p<0.001) observed on multiple days. Furthermore, the TEWL was increased in STAT6VT mice in each application period, whereas the TEWL in WT mice appeared less pronounced in subsequent applications (a process termed “hardening”). We also assessed whether a higher concentration of SLS would elicit a greater increase in TEWL, so 10% SLS was applied epicutaneously for 6 days. TEWL does not appear to be further increased with the higher SLS dose, and the WT mice did not develop dermatitis (data not shown). The present studies indicate that the STAT6VT transgenic murine model exhibits increased barrier disruption following chronic treatment with SLS.
Fig. 1. TEWL is augmented in STAT6VT transgenic mice with repeated SLS irritation.
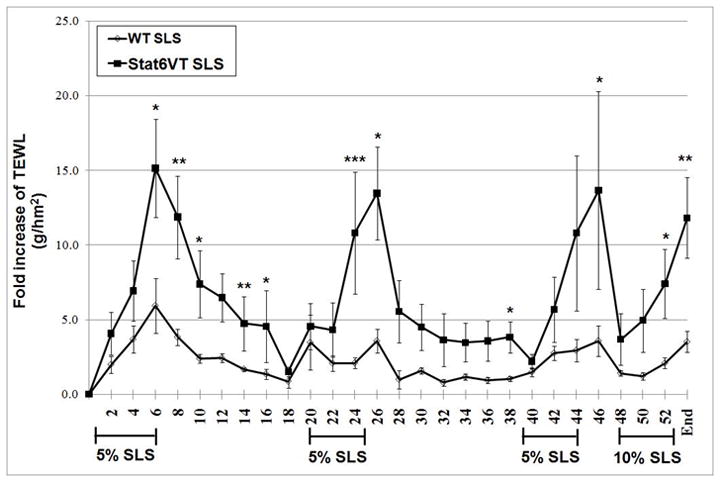
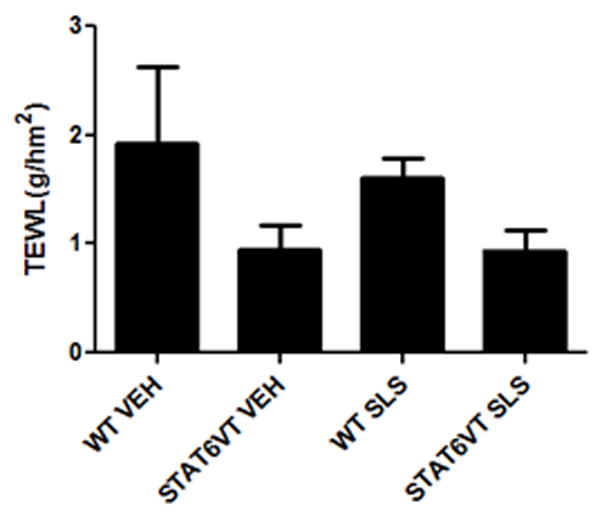
STAT6VT transgenic and WT C57BL/6 littermate controls were shaved and treated for 3 six-day periods with 5% SLS followed by a single six-day period of 10% SLS. TEWL (g/h/m2) measurements were taken with a Multi Probe Adapter fitted with a TewameterR TM 300 (Courage-Khazaka, Germany) prior to the application of the topical irritant. The dates of topical irritation were: Days 1-6, 20-25, 39-44 and 48-53, as indicated. Results are the mean ± SEM from samples of 5-8 mice. Significantly different from WT, * p<0.05, ** p<0.01, *** p<0.001, analyzed by Mann-Whitney test. A. STAT6VT mice show a marked increase in TEWL (g/h/m2 ) during each of the application periods as compared to WT mice. Barrier function is disrupted following chronic irritant treatment. TEWL (g/h/m2 ) is increased in STAT6VT and WT littermates following SLS treatment and in STAT6VT controls. B. Baseline TEWL (g/h/m2 ) levels were not statistically different in the vehicle and SLS-treated STAT6VT and WT mice.
Repeated SLS treatment results in increased epidermal thickness in STAT6VT mice
Topical acetone treatment or tape stripping has been demonstrated to induce epidermal hyperplasia in murine skin. The degree of the epidermal thickness correlated with the duration of the treatment period and was attributed to increased cell proliferation {Denda, 1996 #32}. Our next studies were designed to further characterize the increased barrier abnormalities found in SLS-treated STAT6VT mice. First, we evaluated the ability of SLS to induce a change in epidermal thickness. Quantitative epidermal measurements indicate an increase in the epidermal thickness of STAT6VT transgenic mice treated with SLS (Fig. 2A-B) with no differences noted between the STAT6VT transgenic mice treated with vehicle or the WT mice treated with SLS or vehicle; however, these results were not statistically significant (p=0.08).
Fig. 2. STAT6VT transgenic mice exhibit increased epidermal thickening following repeated SLS treatment.
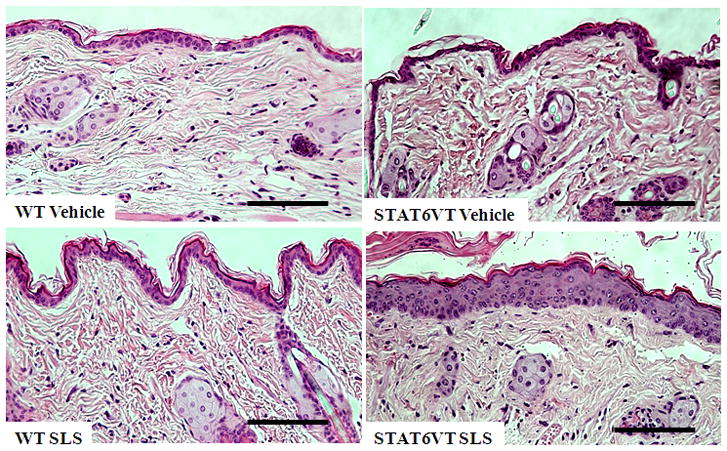
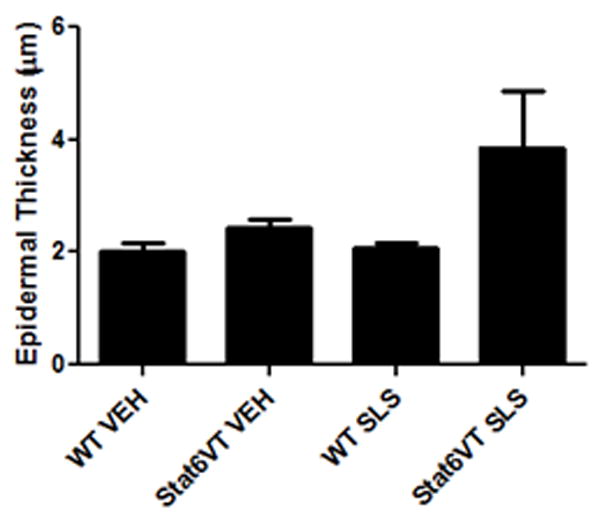
Epidermis from the dorsal side of STAT6VT transgenic and WT C57BL/6 littermate controls was chronically treated with vehicle or 5% (then 10%) SLS. A portion of the dorsal epidermis was excised, fixed in 10% buffered formalin, paraffin-embedded, and H&E stained slides were produced. Results are the mean ± SEM from samples of 6–8 mice. Significantly different from WT, * p<0.05, analyzed by 1-tailed Student t test. A, Epidermal thickness was measured in H&E stained slides by light microscopy using a micrometer. STAT6VT transgenic mice treated with SLS have a thickened epidermis and desquamation of the stratum corneum compared to littermate controls. The scale bar indicates a distance of 100 micrometers. B, STAT6VT transgenic mice treated with SLS have augmented epidermal thickness. Slides were blinded prior to analysis.
Next, we assessed whether the increased epidermal thickness in SLS-treated STAT6VT mice correlated with increased cellular proliferation (Fig. 3A). Ki-67 is a nuclear protein that provides a reliable method to evaluate the growth fraction of cells during late S, M and G2 phases {Gerdes, 1984 #33}. As shown in Fig. 3B, quantification of Ki-67 expression levels demonstrated increased levels of this nuclear protein in the epidermis of STAT6VT compared to WT mice. However, these differences do not appear to be linked to the addition of an irritant as control-treated STAT6VT skin exhibited elevated Ki-67 levels (p=0.07). These findings suggest that the increased epidermal thickness in SLS-treated STAT6VT mice is primarily driven by epidermal cell hypertrophy rather than an increase in cellular proliferation.
Fig. 3. STAT6VT transgenic mice feature active proliferation following repeated SLS treatment.
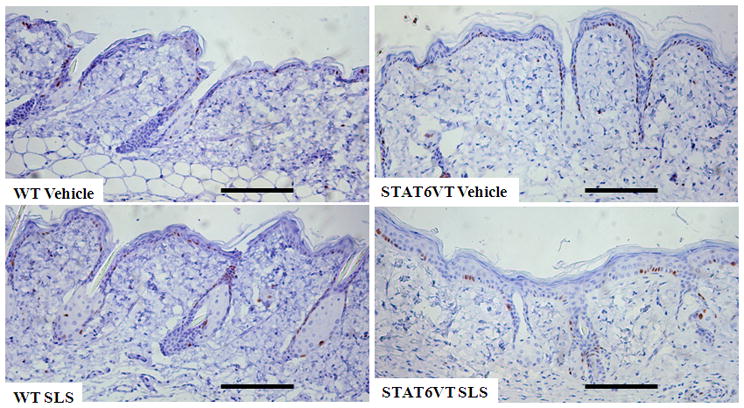
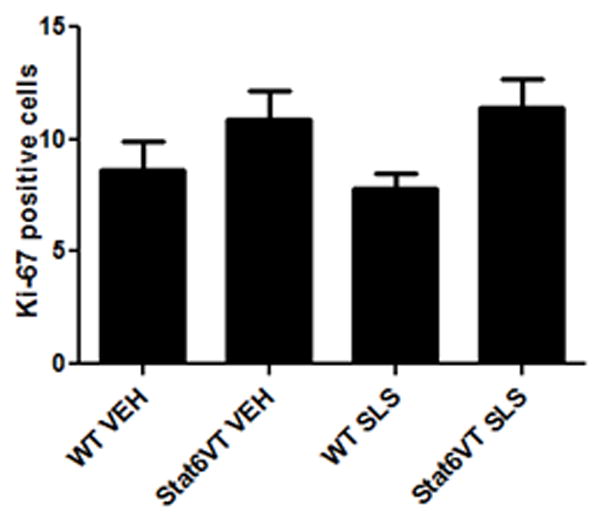
Epidermis from the dorsal side of STAT6VT transgenic and WT C57BL/6 littermate controls were treated as described in Fig. 2. Excised paraffin-embedded mouse skin was subjected to immunohistochemical analysis of Ki-67 as detailed in methods section. For each treatment group, Ki-67 positive cells were counted with MetaMorph Premium Offline software in 5 randomly chosen high power fields. Results are the mean ± SEM from samples of 6-8 mice, analyzed by ANOVA with Bonferroni post test. A, STAT6VT transgenic mice treated with SLS have positive Ki-67 staining in the basal layer. The scale bar indicates a distance of 100 micrometers. B, STAT6VT transgenic mice treated with SLS exhibit increased proliferation overall, (not statistically significant (p=0.07). Slides were blinded prior to analysis.
Discussion
The goal of this study was to compare the effects of SLS, a known irritant, on normal mice versus mice with immunological abnormalities predisposing them to allergic dermatitis. Thus, we used previously described methodologies with SLS to generate an irritant-induced dermatitis {De Jongh, 2006 #65}. Permeability barrier homeostasis was assessed using biophysical parameters to measure barrier function {Loden, 1995 #25}. TEWL measurements were elevated in the SLS-treated STAT6VT mice compared to controls during the treatment phase with significance observed on multiple days and at the conclusion of the study. The STAT6VT transgenic mice exhibited impairment in barrier recovery following SLS challenge as evidenced by a marked increase in the difference for TEWL at the study endpoint compared to baseline that was significantly higher than the WT mice (data not shown). Interestingly, the SLS-treated WT mice unlike the STAT6VT mice appeared to have reduced responses, or “hardening” after multiple treatments. Of particular note, the TEWL of the STAT6VT transgenic mice was slower to return to baseline levels during the interval period between treatments, unlike WT mice. The present studies extend recently published work that showed that although retinoic acid treatment resulted in equal levels of barrier disruption, TEWL recovery after treatment was delayed in STAT6VT transgenic mice {Sehra, 2010 #27}.
In addition to increased TEWL levels in mice treated with SLS, we noted increased hair growth in both SLS-treated WT and STAT6VT mice. Similarly, another study showed visible acceleration of hair growth on SLS-treated skin at three weeks from the start of treatment {Li, 1999 #66}, suggesting that SLS permeability is impacted by accelerated hair growth during a long-term study. It is unlikely that the increased hair growth in response to SLS was responsible for the increased barrier disruption in STAT6VT mice as both genotypes appeared to exhibit similar amounts of hair growth (data not shown).
Histology and epidermal thickness measurements support the notion that STAT6VT transgenic mice treated with a topical irritant resulted in the development of dermatitis. Immunohistochemical studies examining the expression of Ki-67 suggest that a component of the epidermal thickness could be due to increased keratinocyte proliferation. It is also possible that epidermal thickening is due to decreased apoptosis of keratinocytes, or increased differentiation of basal keratinocytes into the more mature layers of the stratum corneum. Another group has similarly shown that irritant skin reactions caused from repeated applications of SLS result in increased epidermal thickness {Anderson, 1986 #67}. Previous and current studies by our group have shown that STAT6VT murine ear tissue, even in mice without obvious lesions, exhibits a marked thickening of the dermis and epidermiswith cellular infiltration of eosinophils and lymphocytes {Sehra, 2010 #27}. Our findings further corroborate that the STAT6VT transgenic murine model is clinically relevant as this model system provides a platform for studying the complex interactions between pharmacological and immunological abnormalities observed in patients with atopic dermatitis.
References
- 1.Anderson C, Sundberg K, Groth O. Animal model for assessment of skin irritancy. Contact Dermatitis. 1986;15:143–151. doi: 10.1111/j.1600-0536.1986.tb01315.x. [DOI] [PubMed] [Google Scholar]
- 2.Bruns HA, Schindler U, Kaplan MH. Expression of a constitutively active Stat6 in vivo alters lymphocyte homeostasis with distinct effects in T and B cells. J Immunol. 2003;170:3478–3487. doi: 10.4049/jimmunol.170.7.3478. [DOI] [PubMed] [Google Scholar]
- 3.Daniel C, Salvekar A, Schindler U. A gain-of-function mutation in STAT6. J Biol Chem. 2000;275:14255–14259. doi: 10.1074/jbc.c000129200. C000129200. [DOI] [PubMed] [Google Scholar]
- 4.De Jongh CM, Verberk MM, Withagen CE, Jacobs JJ, Rustemeyer T, Kezic S. Stratum corneum cytokines and skin irritation response to sodium lauryl sulfate. Contact Dermatitis. 2006;54:325–333. doi: 10.1111/j.0105-1873.2006.00848.x. [DOI] [PubMed] [Google Scholar]
- 5.Denda M, Wood LC, Emami S, Calhoun C, Brown BE, Elias PM, Feingold KR. The epidermal hyperplasia associated with repeated barrier disruption by acetone treatment or tape stripping cannot be attributed to increased water loss. Arch Dermatol Res. 1996;288:230–238. doi: 10.1007/BF02530090. [DOI] [PubMed] [Google Scholar]
- 6.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133:1710–1715. [PubMed] [Google Scholar]
- 7.Leung DY, Bieber T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. S0140-6736(03)12193–9. [DOI] [PubMed] [Google Scholar]
- 8.Li LF, Fiedler VC, Kumar R. Induction of hair growth by skin irritants and its relation to skin protein kinase C isoforms. Br J Dermatol. 1999;140:616–623. doi: 10.1046/j.1365- 2133.1999.02759.x. [DOI] [PubMed] [Google Scholar]
- 9.Loden M. Biophysical properties of dry atopic and normal skin with special reference to effects of skin care products. Acta Derm Venereol Suppl (Stockh) 1995;192:1–48. doi: 10.2340/00015555192148. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen JB. Effects of four detergents on the in-vitro barrier function of human skin. Int J Occup Environ Health. 2000;6:143–147. doi: 10.1179/oeh.2000.6.2.143. [DOI] [PubMed] [Google Scholar]
- 11.Patil S, Singh P, Sarasour K, Maibach H. Quantification of sodium lauryl sulfate penetration into the skin and underlying tissue after topical application--pharmacological and toxicological implications. J Pharm Sci. 1995;84:1240–1244. doi: 10.1002/jps.2600841018. [DOI] [PubMed] [Google Scholar]
- 12.Sehra S, Tuana FMB, Holbreich M, Mousdicas N, Tepper RS, Chang CH, Travers JB, Kaplan MH. Scratching the surface: Towards understanding the pathogenesis of atopic dermatitis. Crit Rev Immunol. 2008;28:15–43. doi: 10.1615/critrevimmunol.v28.i1.20. [DOI] [PubMed] [Google Scholar]
- 13.Sehra S, Yao Y, Howell MD, Nguyen ET, Kansas GS, Leung DY, Travers JB, Kaplan MH. IL-4 regulates skin homeostasis and the predisposition toward allergic skin inflammation. J Immunol. 2010;184:3186–3190. doi: 10.4049/jimmunol.0901860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Werner Y, Lindberg M. Transepidermal water loss in dry and clinically normal skin in patients with atopic dermatitis. Acta Derm Venereol. 1985;65:102–105. [PubMed] [Google Scholar]


