Abstract
SDHD mutations are associated with human cancers but the mechanisms that may contribute to transformation are unknown. The hypothesis that mutations in SDHD increase levels of superoxide leading to genomic instability was tested using site-directed mutagenesis to generate a truncated SDHD cDNA that was expressed in Chinese hamster fibroblasts. Stable expression of mutant SDHD resulted in 2-fold increases in steady-state levels of superoxide that were accompanied by a significantly increased mutation rate as well as a 70-fold increase in mutation frequency at the hprt locus. Over expression of MnSOD or treatment with polyethylene glycol conjugated (PEG)-catalase suppressed mutation frequency in SDHD mutant cells by 50% (p < 0.05). Simultaneous treatment with PEG-catalase and PEG-SOD suppressed mutation frequency in SDHD mutant cells by 90% (p < 0.0005). Finally, 95% depletion of glutathione using L-buthionine-[S,R]-sulfoximine (BSO) in SDHD mutant cells caused a 4-fold increase in mutation frequency (p < 0.05). These results demonstrate that mutations in SDHD cause increased steady-state levels of superoxide which significantly contributed to increases in mutation rates and frequency mediated by superoxide and hydrogen peroxide. These results support the hypothesis that mutations in SDHD may contribute to carcinogenesis by increasing genomic instability mediated by increased steady-state levels of reactive oxygen species.
Keywords: succinate dehydrogenase mutations, mutation frequency, mutation rate, genomic instability, reactive oxygen species
Introduction
Succinate dehydrogenase (SDH; a.k.a Complex II in mitochondrial electron transport chains) is suggested to act as a tumor suppressor gene [1–3] because several neoplasias, such as paragangliomas and pheochromocytomas, are more prevalent in individuals with germ-line mutations in SDH subunits B, C, and D [1, 4–6]. This is particularly the case with missense and nonsense mutations in SDHD because the majority of human tumors containing SDH mutations involve this subunit [5]. Somatic mutations in SDHB have also been found in papillary thyroid cancers and renal cell carcinomas [7], and a decrease in SDHD expression was found in gastric and colon cancer [8]. Altered expression or mutations in SDH are hypothesized to play a causative role in neoplastic transformation; however, the mechanism is not understood.
Recent studies suggest that mutations in the gene coding for SDHB and SDHC cause Complex II to significantly increase the univalent reduction of O2 to form superoxide (O2•−) and H2O2 [9–13]. Ishii and colleagues showed that a point mutation within the CoEnzyme Q (CoQ) binding site of mev-1, homologous to SDHC, leads to rapid aging and hypersensitivity to oxygen toxicity in C. elegans [10]. This same mutation expressed in murine cells was hypothesized to lead to increased O2•− production and tumorogenesis [11]. In addition, Slane et al. showed that Chinese hamster fibroblasts expressing a truncated SDHC protein exhibited increased steady-state levels of reactive oxygen species (ROS) (i.e., O2•− and H2O2), parameters indicative of oxidative stress, and chromosomal instability [13] that were suppressed by over expression of wild type SDHC. Finally, mutations in SDHB have recently been shown to lead to accelerated aging in Drosophila and this is suggested to involve increases in steady-state levels of H2O2 [14]. These results allow for the speculation that mutations in SDHB and SDHC subunits may contribute to genomic instability by causing metabolic oxidative stress. However, direct evidence showing that SDHD mutations lead to increases in ROS that are causally involved with the genesis of a mutator phenotype that could contribute to neoplastic transformation is lacking.
In the current study site directed mutagenesis was utilized to generate a cDNA coding for a truncated form of SDHD, the mutant cDNA was over expressed in fibroblasts, and the effect this had on O2•− production as well as mutation frequencies was determined. Expression of the mutant SDHD cDNA was found to cause a 2-fold increase in steady-state levels of O2•− and significant increases in mutation frequency and mutation rate. Over expression of manganese superoxide dismutase (MnSOD) and pretreatment with PEG-catalase and PEGSOD was utilized to demonstrate the relative contribution of O2•− and H2O2 to this phenotype. Selenium supplementation and glutathione (GSH) depletion were also utilized to assess the contribution of GSH metabolism to the increased mutation frequency observed in SDHD mutant cells. The results provide strong evidence supporting the hypothesis that genomic instability demonstrated by mammalian cells expressing an SDHD mutation was caused by increased steady-state levels of O2•− and H2O2. These results also provide proof-of-principle evidence favoring a potential mechanism by which SDHD mutations may contribute to neoplastic transformation via induction of a mutator phenotype.
Experimental Procedures
Cells and culture conditions
Immortalized Chinese hamster lung fibroblasts (B1 cells, a gift from Dr. Immo Scheffler University of California San Diego, [15]) were cultured as previously described [13]. B1 cells were assayed between passages 40 to 70.
Site-directed mutagenesis of hSDHD
A premature stop codon was created at the 66th codon in human hSDHD using site-directed mutagenesis. The hSDHD cDNA was inserted into a pcDNA 3.1 plasmid (Invitrogen, Eugene, Oregon) and a point mutation was induced using the QuikChange® site-directed mutagenesis kit (Stratagene, La Jolla, California) using the forward primer (5'-TGCATCTCTCCACTGAACTAGCG AGAGGGTTG-3') and reverse primer (5'-CAACCCTCTCGCTAGTTCAGTGGAGAGATGCA-3'). The vectors were cloned into competent E. coli and isolated using the Spin Miniprep kit (QIAGEN, Valencia, California). The mutation was verified by sequencing at the University of lowa's DNA Core.
Mutated hSDHD and a vector control were stably transfected into B1 cells according to the Superfect Transfection Reagent protocol (QIAGEN). The stably transfected cells were selected in 800 μ/ml of G418 (GIBCO). Colonies of stably transfected B1 clones were isolated using the paper disk method [16].
Expression levels of W66X hSDHD mutant
To verify the expression of the W66X hSDHD gene in the B1 clones, RNA was isolated from the cells with QIAGEN's RNAeasy Isolation kit (QIAGEN), using the RNase-free DNase (QIAGEN). The RNA was quantified on a biophotometer (Eppendorf, Westbury, NY) and 1 μg of RNA was reverse transcribed into random cDNAs using the AdvantageTM RT-for-PCR kit (Clontech, Palo Alto, CA). A real time PCR single nucleotide polymorphism (SNP) assay was run to determine the expression levels of W66X hSDHD. As an endogenous control, β-actin expression was determined using a single-plex amplification reaction using a SYBR green master mix (Applied Biosystems, Foster City, CA).
Protein expression levels of W66X hSDHD mutant
To verify protein expression, samples were enriched for mitochondria using a method adapted from previous reports [17, 18]. Briefly, dry cell pellets were homogenized using a Dounce homogenizer in 0.25 M Sucrose, 5 mM HEPES, 0.1 mM EDTA pH 7.25 and centrifuged at 1000 × g for 10 min. Supernatant from this sample was then centrifuged at 10,000 × g for 10 min. Crude membrane fractions (CMFs) were then obtained from the mitochondrially enriched samples by two repeated freeze thaw cycles followed by low intensity sonication and 10,000 × g centrifugation. The supernatant (matrix) portion of this sample was also kept for study. Mutant protein expression was then verified via Western Blot analysis using Bio-Rad reagents and equipment (Hercules, CA) unless otherwise specified. 20 μg of each sample was boiled for 60 minutes in the presence of Laemlli buffer with 20 mM dithiothreitol (DTT) and then run on a 4–20% gradient Ready Gel. Blotting was done using a polyclonal, full length recognizing SDHD antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and an amino terminus recognizing voltage dependent anion channel (VDAC) antibody (Cell Signaling, Danvers, MA).
Steady-state O2•− levels
Dihydroethidium (DHE) (Invitrogen) oxidation was used to measure intracellular O2•− levels as previously described [13]. To determine if changes in DHE fluorescence were due to O2•−, cells were in incubated with 100 U/ml PEG-SOD (Sigma, St. Louis, Missouri) for 2 h prior to labeling with DHE. The DHE fluorescence with and without PEG-SOD was measured and compared (see Supplemental Figure 1). The SOD inhibitable mean fluorescent intensity (MFI) of DHE was calculated by subtracting the MFI in the presence of PEG-SOD from the MFI without PEG-SOD.
Measurements of genomic instability
To test for point mutations and deletions in nuclear DNA at the hypoxanthine-guanine phosphoribosyl transferase (hprt) locus, cells were grown in 40 μM 6-thioguanine (6-TG; Sigma) and seeded for clonogenic survival as previously described [19]. Mutation frequency was determined by the following expression: # colonies/ #cells plated in 6-TG ÷ # colonies/# cells plated in the absence of 6-TG.
To determine mutation rate, cells were selected in 1x HAT (hypoxanthine; aminopterin; thymidine) media (Cellgro, Manassas, VA) for three weeks to eradicate any cells that harbored preexisting mutations in hprt. HAT selected cells were then passed out of selection media and plated for clonogenic survival in complete DMEM media containing 40 μM 6-TG at 0, 3, 6, and 9 d after selection as described by Glaab and Tindall [20]. Mutation frequency as a function of time following HAT selection was plotted and the slope of the linear regression through the data points was used to calculate mutation rate.
To determine if ROS played a role in the mutation frequency, cells were pretreated with either 100 MOI of adenovirus containing no target gene (AdEmpty) or MnSOD (AdMnSOD) (Viraquest; North Liberty, IA, U.S.A.), 18 μM PEG (Sigma), 100 U/ml PEG-Cat (Sigma), 100 U/ml PEG-SOD, 50 U/ml PEG-Cat and 50 U/ml SOD, 250 μM L-buthionine-[S,R]-sulfoximine (BSO) (Sigma), or 30 nM selenium (Se) (Sigma) for 72 h and assayed for 6-TG resistance as described above. Duplicated treatment dishes were collected to analyze for MnSOD, catalase, CuZnSOD, and glutathione peroxidase (GPx) activity and intracellular glutathione levels as described below.
Antioxidant Analysis
SOD activity was measured in cell homogenates using the activity assay developed by Spitz and Oberley [21]. MnSOD activity was measured using 5 mM sodium cyanide to inhibit CuZnSOD (Fisher Scientific, Fair Lawn, New Jersey). Catalase activity was determined in cell homogenates 24 h after PEG-catalase treatment by measuring the disappearance of H2O2 at 240 nm in a spectrophotometric assay described by Beers and Sizer, and analyzed according to Aebi [22, 23]. Total GSH levels were determined in a recycling assay that spectrophotometrically measures the reduction of dTNB (Sigma) to TNB in the presence of glutathione reductase (GR) (Sigma) as described by Griffith and Anderson [24, 25]. Glutathione peroxidase 1 (GPx 1) was measured as described previously by monitoring NADPH oxidation in the presence of reduced GSH (Sigma), GR, and H2O2 [26]. Biochemical data was normalized to protein content as described previously [21, 27].
Statistical analyses
One-way analysis of variance was utilized for experiments with three or more groups and significance between individual groups was determined using Tukey's HSD post-hoc analysis (p < 0.05). T-tests were used to determine significance in experiments with only 2 experimental groups (p < 0.05). All statistical analyses were accomplished using SPSS 10.0 software.
Results
Expression of mutant hSDHD
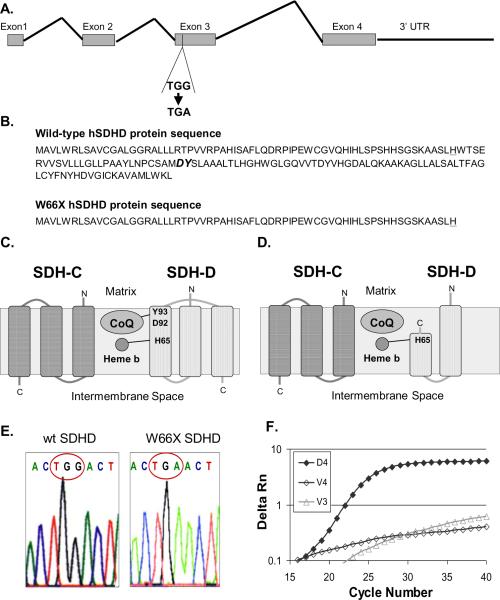
Site-directed mutagenesis was used to create a nonsense, point mutation that resulted in tryptophan 66 being mutated to a premature stop codon (W66X) in human SDHD (hSDHD) truncating 94 amino acids from the C terminus (Fig 1A and B). This mutation would be expected to disrupt the CoQ binding site of the SDH complex potentially resulting in increased one electron reductions of O2 to form O2•− (Fig 1C and D). The mutant hSDHD plasmid was sequence verified to have a nonsense mutation in the 66th codon that changes a tryptophan to a stop codon (TGG→TGA) (Figure 1E). Clonally isolated vector control cell lines (V3 and V4) as well as a mutant hSDHD clone (D4) were analyzed for mRNA expression of the mutant gene using real-time PCR (Fig 1F). The vector controls did not express W66X SDHD, while the D4 clone clearly demonstrated SDHD mutant gene expression (Fig 1F).
Figure 1.
Construction and expression of the mutant SDHD cDNA by site directed mutagenesis. Site-directed mutagenesis was used to create a nonsense, point mutation that resulted in a tryptophan mutated to a premature stop codon (W66X, tryptophan → stop at the 66th codon, truncating 94 amino acids from the C terminus) in human SDHD. A. SDHD contains 4 exons and the third exon was chosen as the site for a premature stop codon. B. Amino acid sequence of wild-type and W66X hSDHD protein. Amino acids at the CoQ binding site are in bold italics. Heme b binding site (H65) is underlined. C. Schematic of wild type SDHC and SDHD proteins in the inner mitochondrial membrane. Amino acids binding sites for heme b and CoQ are shown in SDH-D. D. Schematic of wild type SDHC and mutant SDHD proteins in the inner mitochondrial membrane. The mutation disrupts the CoQ binding site. E. The mutant hSDHD plasmid was sequence verified to have a nonsense mutation in the 66th codon that changes a tryptophan to a stop codon (TGG→TGA). Chromatogram of the wild-type SDHD sequence with the 66th codon being tryptophan (TGG) and the mutated SDHD sequence with a stop site at codon 66 (TGA). F. Real-time PCR verification of W66X expression in D4 cells. Vector controls (V3 and V4) do not express W66X hSDHD.
Steady-state O2•− levels in cell lines expressing the W66X SDHD protein
Western blotting of crude mitochondrial membrane fractions (Fig. 2A; CMF) and crude mitochondrial soluble fractions (Fig. 2A; Solu), for immuno-reactive SDHD protein revealed a ˜7 kD band (the approximate molecular weight of the truncated protein) present in CMF isolated from the D4 cell line expressing the mutant mRNA (Fig. 1F) but not in the V4 vector control that did not express the mutant mRNA (Fig. 1F). It also appeared that there was much more wild type protein being expressed relative to mutant protein (Fig. 2A). Equal loading of mitochondrial membrane protein content was verified via immuno-blotting for VDAC (Fig. 2A). The previously described DCIP method [18] of measuring mitochondrial membrane bound SDH activity was used to verify that SDH activity was similar in the cell lines (Figure 2B) which was not surprising given that equal amounts of wild type protein were being expressed in the V4 and D4 clones. Oxidation of DHE to its fluorescent product was measured to estimate steady-state level of O2•−. Figure 2C shows, that the D4 clone expressing the mutant had a 2.3-fold increase in DHE oxidation as compared to the vector controls and wild-type (p < 0.0005). There was no significant difference in DHE oxidation between the vector controls (V3 and V4) and the wild-type B1 cells (Fig 2C). To determine if the oxidation of the DHE was specifically due to O2•−, cells were incubated with PEG-SOD prior to labeling. This analysis confirmed a 2.3-fold increase in SOD inhibitable DHE oxidation (Fig 2D) (p < 0.01), clearly showing that the cell line expressing the W66X SDHD mutation demonstrated increased steady-state levels of intracellular O2•−.
Figure 2.
Expression of W66X SDHD protein does not diminish SDH activity however steady-state levels of O2•− are increased in cells expressing the SDHD mutation. A. Western blot for SDHD using a polyclonal antibody that recognizes both the wild type SDHD protein in V4 and D4 crude mitochondrial membrane fractions (CMFs) as well as the ~ 7 kD mutant protein in only the D4 CMFs. Atibodies to VDAC were used as a mitochondrial membrane protein loading control. B. Isolated mitochondria were used to measure membrane bound complex II activity by the DCIP method as previously described [17, 18]. Error bars represent ± 1SD from 3 separate experiments. The differences were not statistically significant (p>0.05), indicating complex II activity was intact in the D4 cells. C. Background fluorescence in DMSO controls was subtracted from fluorescence of DHE labeled cells in the experimental groups. Data points represent the mean of each clone normalized to the vector control + 1 SEM, † P < 0.0005 as compared to V4. D. DHE oxidation measured as in panel A with and without 100 U/ml PEG-SOD. Superoxide specific DHE oxidation (MFI) was calculated by subtracting the MFI with PEG-SOD from the MFI without PEG-SOD. The data prior to doing this subtraction to calculate the superoxide specific MFI is shown in supplemental Figure 1. Data represent the mean of each clone normalized to vector control ± 1 SEM, * P < 0.01 as compared to vector control.
Genetic instability and mutator phenotype induced by the expression of mutant SDHD
The mutation frequency in nuclear DNA was assayed by clonogenic survival in 6-TG which detects mutations at the hprt locus. D4 cells expressing the SDHD mutation demonstrated a 70-fold increase in survival in 6-TG as compared to vector controls, indicating a 70-fold increase in mutation frequency in these cell populations (p < 0.0005 vs. controls) (Fig 3A). To determine if the mutation frequency in the D4 cells was increased; B1, V3, V4, and D4 clones were selected in HAT media to eradicate any cells in the population harboring hprt mutations. Cells were then plated for clonogenic survival in 6-TG at various times out of HAT selection. Mutation frequency was calculated as a function of time following removal from HAT selection (Fig. 3B). There was a linear increase in the mutation frequency in D4 cells up to nine days out of HAT selection with a slope of 119 × 10−8 mutations/day, which represents the mutation rate in populations of cells expressing the SDHD mutation (Fig 3B, Table 1). In contrast to D4, B1 (8 × 10−8 mutations/day), V3 (16 × 10−8 mutations/day), and V4 (4 × 10−8 mutations/day) cells had significantly lower mutation rates. (Fig 3B, Table 1). These results show that hprt mutations developed in the D4 cell populations as the result of a dynamic process that occurred at a significantly higher rate than in the parental or vector controls (Table 1) (p < 0.0005).
Figure 3.
Cells expressing the SDHD mutation have increased mutation frequency and mutation rate as determined by 6-thioguanine (6-TG) resistance. Clones were grown in 40 μM 6-TG for 14 d and assayed for clonogenic survival. A. 6-TG resistance before selection in HAT media. Data represent the mean mutation frequency for each clonal population ± 1 SEM, † P < 0.0005 as compared to V4. B. Mutation frequency determined as a function of time after selection for 3 weeks in HAT media. Cells were grown in HAT free media for 0 to 9 d before being analyzed for clonogenic survival in 6-TG. Data points represent the mean mutation frequency ± 1 SEM at each time point.
Table 1.
Mutation rates in cell populations following HAT selection
Mutation rate (mutation frequency/day after HAT selection was calculated from the slope of the linear regression in Figure 3B
p < 0.05, as compared to vector control
To determine if the increased mutation frequency in D4 cells was causally related to the increase in steady-state levels of ROS, cells were pretreated with adenovirus MnSOD, PEG-catalase, PEG-SOD, or PEG-catalase + PEG-SOD in combination, 3 d prior to analysis with 6-TG. AdMnSOD increased MnSOD activity 8-fold and decreased the mutation frequency of D4 cells by 50%, compared to D4 AdEmpty (p < 0.05) (Fig 4A). PEG-catalase treatment increased catalase activity 4.5-fold and decreased the mutation frequency of D4 cells by 50% compared to PEG alone control. (p < 0.005) (Fig 4B). PEG-SOD caused a slight decrease in the mutation frequency of D4 cells that did not reach statistical significance; however, simultaneous treatment with PEG-catalase and PEG-SOD significantly decreased mutation frequency to a greater extent (91%) than either pegylated-enzyme alone (p < 0.0005) (Fig 4B). PEG alone treatment had no effect on mutation frequency, and treatment with either AdMnSOD, or PEG-catalase had no significant effect on the mutation frequency of vector control cells. These data show that the increase in hprt mutations seen in the D4 cells is at least in part caused by increases in ROS in cells expressing the SDHD mutation.
Figure 4.
Increasing antioxidant enzyme activity decreased the mutation frequency and depletion of glutathione increased the mutation frequency in cells expressing the SDHD mutation. Antioxidants were added 3 d prior to treatment with 6-TG. A. Treatment of V4 and D4 cells with 100 MOI AdMnSOD was compared to cells treated with 100 MOI of empty adenovirus (AdEmpty). MnSOD activities of infected cells was reported as U/mg protein. Data represents the mean for each treatment normalized to its appropriate control ± 1 SEM, ND = non-detectable, * P < 0.05 as compared to AdEmpty. B. Treatment of V4 and D4 cells with 100 U/ml PEG-catalase, 100 U/ml PEG-SOD, or 50 U/ml PEG-Cat and 50 U/ml PEG-SOD was compared to cells treated with 18 μM PEG. Catalase activities were reported as mk units/mg protein. Total SOD activities were reported as U/mg protein. Data represents the mean for each treatment normalized to its appropriate control ± 1 SEM, ND = non-detectable, † P < 0.005, ‡ P < 0.0005 as compared to PEG alone,* P < 0.05 as compared to PEG-Cat C. Treatment of V4 and D4 cells with 250 μM BSO was compared to untreated control. Total cellular glutathione levels were reported as nmol/mg protein. Data represents the mean for each treatment normalized to its appropriate control ± 1 SEM, * P < 0.05 as compared to no BSO control. D. Treatment of V4 and D4 cells with 30 nM selenium was compared to untreated control. Selenium dependent glutathione peroxidase activities were reported as mU/mg protein. Data represents the mean for each treatment normalized to its appropriate control ± 1 SEM, ‡ P < 0.0005 as compared to no selenium control.
To determine if hydroperoxide metabolism by the glutathione/glutathione peroxidase system played a significant role in the accumulation of hprt mutations, cells were pretreated with BSO, a relatively specific inhibitor of GSH synthesis, to deplete GSH [28, 29]. Treatment with BSO decreased intracellular total GSH levels by 95% and significantly increased the mutation frequency of D4 cells 4-fold (p < 0.05), but did not affect mutation frequency in V4 controls (Fig 4C). As a further test of the role of the glutathione/glutathione peroxidase system in the formation of hprt mutations, cells were grown in the presence and absence of 30 nM Se which is required for the active site of glutathione peroxidase (GPx) and was found to increase GPx activity 2 to 3-fold. Se-supplementation resulted in a 60% decrease in mutation frequency in the D4 cells (p < 0.0005) but did not affect mutation frequency in the V4 cells (Fig 4D). Overall, the results in Figure 4 clearly demonstrate that manipulations of ROS scavengers can significantly alter the mutation frequency of cells harboring the SDHD mutation and strongly support the hypothesis that the mutator phenotype seen in these cells is caused by increased steady-state levels of O2•− and H2O2.
Discussion
Mutations in genes coding for mitochondrial electron transport chain (ETC) proteins are hypothesized to lead to dysfunctional mitochondrial metabolism resulting in increased steady-state levels of O2•− and H2O2, that contribute to genetic instability and carcinogenesis [30]. Mutations in SDHD are some of the most prevalent germline mutations in genes coding for ETC proteins that are associated with human tumor formation [5], but the mechanism(s) by which these mutations might contribute to tumorigenesis are not clearly defined. In the current study a premature stop codon resulting in a truncation mutation, expected to disrupt the CoQ binding site, was introduced into the human gene coding for SDHD. When this mutant SDHD cDNA was stably expressed in hamster fibroblasts, increased steady-state levels of O2•− and a mutator phenotype characterized by increased mutation frequency and increased mutation rate was induced despite the continued presence of the wild type protein. Furthermore, antioxidant enzymes specific for scavenging O2•− and H2O2 (SOD and catalase, respectively) were able to significantly suppress the increased mutation frequency in the cells expressing the SDHD mutation and both enzymes together were more effective than either enzyme alone. In addition, increasing non-specific hydroperoxide metabolizing enzyme activity (i.e., GPx) via Se supplementation also suppressed mutation frequency and depleting GSH (an essential cofactor for GPx activity) dramatically increased mutation frequency in the SDHD mutants. These results unambiguously show that mutations in the gene coding for SDHD in mammalian cells are capable of resulting in increased steady-state levels of O2•− which causes a persistent heritable mutator phenotype that is mediated by both O2•− and H2O2.
The most logical explanation for the fact that PEG-SOD and PEG-catalase together were more effective than either enzyme alone in SDHD mutants involves the reaction of O2•− and H2O2 commonly known as the Haber-Weiss reaction [31, 32]. In this scenario, excess O2•− produced by the mutant SDHD containing ETC Complex II, could reduce redox active metal ions [i.e., Fe(III) or Cu(II) + O2•− → Fe(II) or Cu (I) + O2] which could then readily react with H2O2 (also formed by the dismutation of O2•−) to produce hydroxyl radicals (•OH) capable of causing DNA damage [31, 33]. In addition O2•− is not only capable of reducing metal ions; it has also been shown to result in the mobilization of Fe from metal storage proteins such as ferritin and Fe-S complexes, leading to an increase in labile iron that is capable of diffusing to critical cellular targets such as DNA and catalyzing damaging reactions [31, 34]. Therefore, increasing SOD activity in the SDHD mutants would be expected to remove O2•− inhibiting the reduction and release of metal ions while increasing catalase activity would be expected to remove H2O2. The combination of SOD and catalase would be expected to act in at least an additive fashion by inhibiting the Haber-Weiss reaction.
The potential mechanisms by which mutations in SDHD (or other ETC proteins) could result in increased one electron reductions of O2 to form O2•− are a subject of intense interest in cancer biology and aging. Recent studies with SDHC and SDHD missense mutations in the CoQ binding site have shown that the yeast expressing the mutant proteins become sensitive to cell killing mediated by hyperoxia and respiratory directed substrates such as galactose as well as showing evidence of increased O2•− production [12]. Consistent with these findings in yeast, our previous work in mammalian cells with SDHC truncation mutants and the current work with SDHD truncation mutants (both thought to disrupt the CoQ binding site) have led us to speculate that mutations in ETC proteins may cause an increase in the probability of one electron reduction of O2 to form O2•−. In cases where SDHD or SDHC mutations result in the decreased or increased expression of specific ETC protein subunits, increased O2•− could be the result of stochiometric mismatches between complexes resulting in increased residence time of electrons at sites that are normally accessible to O2. In cases where truncation mutations or missense mutations that disrupt the tightly coupled flow of electrons from one subunit to another (as in the CoQ binding site) are expressed, increases in the accessibility of electrons to O2 would be expected to lead to excess O2•− production. In either case the rate at which one electron reductions of O2 occur would be expected to increase. In the current work we speculate that by truncating the SDHD protein at codon 66 (Fig 1C and D), only one and a half of the three transmembrane domains will be translated and inserted into the inner membrane of the mitochondria. We speculate that this truncated SDHD subunit could either cause the destabilization of Complex II such that it isn't anchored properly in the membrane coupling Complex II to CoQ or open up the CoQ binding site thereby increasing the accessibility of O2 to undergo one electron reductions. These changes in Complex II structure would be anticipated to make the Fe-S clusters in SDHB or the heme binding site at the interface of SDHC and SDHD more accessible to O2 increasing the probability of O2•− formation.
Several studies have associated mutations in SDH with oxidative stress, cancer, and aging [10, 11, 13, 14]. To our knowledge, the current study is the first to show that increased steady-state levels of O2•− and reactive oxygen species (ROS) derived from O2•− (i.e., H2O2 and/or organic hydroperoxides) in cells expressing SDHD mutations are directly responsible for causing a mutator phenotype in mammalian cells. This provides a potential mechanism for how mutations in SDHD may contribute to neoplastic transformation by leading to ROS-mediated cellular damage and genomic instability [35–38]. Consistent with the mutator phenotype hypothesis [39] and the fact that in general tumor cells have much higher mutation frequencies than their normal cell counterparts [40], the propagation of persistent genomic instability via mutations in genes coding for ETC proteins may be a common feature leading to genomic instability and carcinogenesis. Also this paradigm potentially supports a general mechanism by which mutations in genes involved with oxidative metabolic processes may contribute to the well known mechanistic link between neoplastic transformation and aging.
Supplementary Material
Acknowledgements
The authors would like to thank Gareth Smith for editorial assistance. This work was supported in part by NIH R01CA133114, NIH R01CA100045, NIH P30CA086862, DOE-DE-SC0000830, and NIH T32CA078586.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Douwes Dekker PB, Hogendoorn PCW, Kuipers-Dijkshoorn N, Prins FA, van Duinen SG, Taschner PEM, van der Mey AGL, Cornelisse CJ. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. Journal of Pathology. 2003;201:480–486. doi: 10.1002/path.1461. [DOI] [PubMed] [Google Scholar]
- [2].Pollard PJ, Briere JJ, Alam NA, Barwell J, Barclay E, Wortham NC, Hunt T, Mitchell M, Olpin S, Moat SJ, Hargreaves IP, Heales SJ, Chung YL, Griffiths JR, Dalgleish A, McGrath JA, Gleeson MJ, Hodgson SV, Poulsom R, Rustin P, Tomlinson IPM. Accumulation of Krebs cycle intermeiates and over-expression of HIF1a in tumours which result from germline FH and SDH mutations. Human Molecular Genetics. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- [3].Selak MA, Armour SA, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottieb E. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-a prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- [4].Gimenez-Roqueplo AP, Favier J, Rustin P, Mourad JJ, Plouin PF, Corvol P, Rotig A, Jeunemaitre X. The R22X mutation of the SDHD gene in hereitary paraganglioma abolishes the enzymatic activiy of Complex II in the mitochondrial respiratory chain and activities the hypoxia pathway. American Journal of Human Genetics. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baysal BE. On the association of succinate dehydrogenase mutations with herediary paraganlioma. Trends in Endocrinology and Metabolism. 2003;14:453–459. doi: 10.1016/j.tem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- [6].Rotig A, Rustin P. Inborn errors of complex II - Unusual human mitochondrial diseases. Biochimica et Biophysica Acta. 2002;1553:117–122. doi: 10.1016/s0005-2728(01)00228-6. [DOI] [PubMed] [Google Scholar]
- [7].Neumann HP, Pawlu C, Peczkowaska M, Bausch B, McWinney SR, Muresan M, Cuchta M, Franke G, Klisch J, Bley TA, et al. Distinct clinical features of paraglioma syndromes associated with SDHB and SDHD gene mutations. JAMA. 2004;292:943–951. doi: 10.1001/jama.292.8.943. [DOI] [PubMed] [Google Scholar]
- [8].Habano W, Sugai T, Nakamura S, Uesugi N, Higuchi T, Terashima M, Horiuchi S. Reduced expression and loss of heterozygosity of the SDHD gene in colorectal and gastric cancer. Oncology Report. 2003;10:1375–1380. [PubMed] [Google Scholar]
- [9].Yankovskaya V, Horsefield R, Tornroth S, Luna-Chevez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- [10].Ishii N, Fujii M, Hartman PS, Tsuda M, Yasuda K, Senoo-Matsuda N, Yanase S, Ayusawa D, Suzuki K. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature. 1998;394:694–697. doi: 10.1038/29331. [DOI] [PubMed] [Google Scholar]
- [11].Ishii T, Yasuda K, Akatsuka A, Hino O, Hartman PS, Ishii N. A mutation in the SDHC gene of Complex II increasees oxidative stress, resulting in apoptosis and tumorigenesis. Cancer Research. 2005;65:203–209. [PubMed] [Google Scholar]
- [12].Szeto SSW, Reinke SN, Sykes BD, Lemire BD. Ubiquinone-binding site mutations in the Saccharomyces cerevisiae succinate dehydrogenase generate superoxide and lead to the accumulation of succinate. Journal of Biological Chemistry. 2007;282:27518–27526. doi: 10.1074/jbc.M700601200. [DOI] [PubMed] [Google Scholar]
- [13].Slane BG, Aykin-Burns N, Smith B, Kalen AK, Goswami PC, Domann FE, Spitz DR. Mutation of succinate dehydrogenase subunit C results in increased O2•−, oxidative stress, and genomic instability. Cancer Research. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- [14].Walker DW, Hajek P, Muffat J, Knoepfle D, Cornelison S, Attardi G, Benzer S. Hypersensitivity to oxygen and shortened lifespan in a Drosophila mitochondrial complex II mutant. Proc. Nat. Acad. Sci. U.S.A. 2006;103:16382–16287. doi: 10.1073/pnas.0607918103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Oostveen FG, Au HC, Meijer PJ, Scheffler IE. A Chinese hamster mutant cell line with a defect in the integral membrane protein CII-3 of Complex II of the mitochondrial electron transport chain. Journal of Biological Chemistry. 1995;270:26104–26108. doi: 10.1074/jbc.270.44.26104. [DOI] [PubMed] [Google Scholar]
- [16].Domann FE, Martinez J. Alternative to cloning cylinders for isolation of adherent cell clones. Biotechniques. 1995;18:594–595. [PubMed] [Google Scholar]
- [17].Dayal D, Martin SM, Owens KM, Aykin-Burns N, Zhu Y, Boominathan A, Pain D, Limoli CL, Goswami PC, Domann FE, Spitz DR. Mitochondrial complex II dysfunction can contribute significantly to genomic instability after exposure to ionizing radiation. Radiat Res. 2009;172:737–745. doi: 10.1667/RR1617.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Singer TP. Determination of the activity of succinate, NADH, choline, and alphaglycerophosphate dehydrogenases. Methods Biochem Anal. 1974;22:123–175. doi: 10.1002/9780470110423.ch3. [DOI] [PubMed] [Google Scholar]
- [19].Hei T, Liu SX, Walden C. Mutagenicity of arsenic in mammalian cells: Role of reactive oxygen species. Proc. Natl. Acad. Sci. USA. 1998;95(14):8103–8107. doi: 10.1073/pnas.95.14.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Glaab WE, Tindall KR. Mutation rate at the hprt locus in human cancer cell lines with specific mismatch repair-gene defects. Carcinogenesis. 1997;18:1–8. doi: 10.1093/carcin/18.1.1. [DOI] [PubMed] [Google Scholar]
- [21].Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Analytical Biochemistry. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- [22].Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of H2O2 by catalase. Journal of Biological Chemistry. 1952;195:133–140. [PubMed] [Google Scholar]
- [23].Aebi H. Catalase in vitro. Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- [24].Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Analytical Biochemistry. 1980;106:207–212. doi: 10.1016/0003-2697(80)90139-6. [DOI] [PubMed] [Google Scholar]
- [25].Anderson ME. Determination of Glutathione in Biological Tissues. In: Greenwald RA, editor. Handbook for Oxygen Radical Research. CRC press; Boca Raton, Florida: 1985. p. 11. [Google Scholar]
- [26].Lawrence R, Burk R. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- [27].Lowry OH, J TN, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- [28].Bailey HH. L-S,R-buthionine sulfoximine: historical development and clinical issues. Chem Biol Interact. 1998;111–112:239–254. doi: 10.1016/s0009-2797(97)00164-6. [DOI] [PubMed] [Google Scholar]
- [29].Arrick BA, Griffith OW, Cerami A. Inhibition of glutathione synthesis as a chemotherapeutic strategy for trypanosomiasis. Journal of Experimental Medicine. 1981;153:720–725. doi: 10.1084/jem.153.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: A unifiying concept in stress response biology. Cancer and Metastasis Reviews. 2004;23:311–322. doi: 10.1023/B:CANC.0000031769.14728.bc. [DOI] [PubMed] [Google Scholar]
- [31].Halliwell B. Role of free radicals in the neurodegenerative diseases. Drugs & Aging. 2001;18:685–716. doi: 10.2165/00002512-200118090-00004. [DOI] [PubMed] [Google Scholar]
- [32].Haber F, Weiss J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Ednib. Sect. A. (Math. Phys. Sci.) 1934;201 [Google Scholar]
- [33].Fridovich I. The biology of oxygen radicals. Science. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- [34].Keyer K, Imlay JA. Superoxide accelerates DNA damage by elevating free-iron levels. Proc. Natl. Acad. Sci. USA. 1996;93 doi: 10.1073/pnas.93.24.13635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Martinez-Cayuela M. Oxygen free radicals and human disease. Biochimie. 1995;77:147–161. doi: 10.1016/0300-9084(96)88119-3. [DOI] [PubMed] [Google Scholar]
- [36].Emerit I. Reactive oxygen species, chromosome mutation, and cancer: Possible role of clastogensic factors in carcinogenesis. Free Radical Biology & Medicine. 1994;16:99–109. doi: 10.1016/0891-5849(94)90246-1. [DOI] [PubMed] [Google Scholar]
- [37].Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the mutliple mutations in cancer. Mutation Research. 2001;477:7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- [38].Christians FC, Newcomb TG, Loeb LA. Potential sources of multiple mutations in human cancers. Preventive Medicine. 1995;24:329–332. doi: 10.1006/pmed.1995.1054. [DOI] [PubMed] [Google Scholar]
- [39].Loeb LA, Springgate CF, Battula N. Errors in DNA replication as a basis of malignant change. Cancer Research. 1974;34:2311–2321. [PubMed] [Google Scholar]
- [40].Bielas JH, Loeb KR, Rubin BP, True LD, Loeb LA. Human cancers express a mutator phenotype. Proc. Natl. Acad. Sci. USA. 2006;103:18238–18242. doi: 10.1073/pnas.0607057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.