Abstract
Nonhuman primates appear to capitalize more effectively on visual cues than corresponding auditory versions. For example, studies of inferential reasoning have shown that monkeys and apes readily respond to seeing that food is present (“positive” cuing) or absent (“negative” cuing). Performance is markedly less effective with auditory cues, with many subjects failing to use this input. Extending recent work, we tested eight captive tufted capuchins (Cebus apella) in locating food using positive and negative cues in visual and auditory domains. The monkeys chose between two opaque cups to receive food contained in one of them. Cup contents were either shown or shaken, providing location cues from both cups, positive cues only from the baited cup, or negative cues from the empty cup. As in previous work, subjects readily used both positive and negative visual cues to secure reward. However, auditory outcomes were both similar to and different from those of earlier studies. Specifically, all subjects came to exploit positive auditory cues, but none responded to negative versions. The animals were also clearly different in visual versus auditory performance. Results indicate that a significant proportion of capuchins may be able to use positive auditory cues, with experience and learning likely playing a critical role. These findings raise the possibility that experience may be significant in visually based performance in this task as well, and highlight that coming to grips with evident differences between visual versus auditory processing may be important for understanding primate cognition more generally.
Introduction
Studies of captive primates have revealed that at least some tasks that are readily mastered based on visual stimuli can be significantly more challenging when presented in the auditory domain. That difference has, for example, appeared in recent tests of inferential reasoning in both apes and monkeys, even for species argued to exploit auditory-based cuing under natural foraging circumstances. In captive settings, when primate subjects are required to indicate the location of food reward based on the occurrence (“positive” cuing) or non-occurrence (“negative” cuing) of visual or auditory events (Call 2004), results have consistently shown much more effective performance from seeing that food is present or absent than in responding to analogous auditory cues. Results with auditory stimuli have also varied, particularly when capuchin monkeys (Cebus apella) perform this task (e.g., Sabbatini and Visalberghi 2008; Paukner et al. 2009). These inconsistencies prompted us to conduct additional experiments with capuchins using the same paradigm, which replicated earlier findings in the visual domain while showing spontaneous utilization of positive, but not negative, cues in auditory presentations.
Visual versus auditory cuing in an inferential task
Cognitive and perceptual experiments with laboratory primates have shown for some time that subjects may perform quite differently in a given task depending on whether visual versus auditory stimuli are involved (reviewed by Schmitt and Fischer 2009). This kind of difference has been demonstrated by D’Amato and Colombo (1985; Colombo and D’Amato 1986), who found that capuchin monkeys had difficulty remembering target sounds across delay intervals in a matching-to-sample task that the animals could readily perform with visual stimuli. Such performance differences are well-documented in rhesus macaques (Macaca mulatta) in particular, with these monkeys being much slower to master auditory compared to visual short-term memory tasks, and showing dramatically shorter retention durations in the former than the latter (see Ng et al. 2009 for a recent review).
A similar discrepancy has appeared in recent, “inference-by-exclusion” tests, designed to shed light on the overarching question of whether primates show basic reasoning about food location based on positive versus negative cuing. This question is important in a larger sense because reasoning is considered central to human intelligence, and has even been proposed to be the aspect of cognition that sets humans apart from all other animals (e.g., Hume 1909–1914). Although many different kinds of reasoning have been identified in humans (e.g., Holyoak and Morrison 2005), inferential reasoning may be particularly pertinent to primates. It refers to the ability to use available information to draw conclusions about circumstances that are not directly observable. Having at least rudimentary inferential reasoning capabilities would be of potential value in a variety of circumstances faced by wild primates, including extractive foraging (Paukner et al. 2009), predator avoidance (Seyfarth and Cheney 1990), and even attribution of mental states to others (Adolphs 2009). However, evidence of inferential reasoning in primates has been mixed, with the modality of testing playing a surprising role.
For example, in Call’s (2004) seminal test of inference in great apes, each trial required subjects to choose between an opaque cup that was baited with food and a second opaque cup that was empty. Positive visual cuing consisted of showing the contents of the baited cup, while positive auditory cuing involved shaking that cup to produce a rattling sound. Negative cuing consisted of either showing the empty cup or shaking it to demonstrate that no sound was produced. After baiting the container out of sight of the subject, the experimenter either showed or shook one or both containers. When seeing the contents of both cups, thereby having both positive and negative cues available, all 30 subjects in Call’s study performed accurately. However, only nine of these individuals consistently selected the correct cup on trials combining positive and negative auditory cues. A second experiment focusing on these particular subjects tested positive and negative cues separately and manipulated only one cup on a given trial. All nine performed significantly above chance levels in both positive visual and positive auditory testing, and also responded effectively to negative visual cuing. However, just three of the nine performed above chance levels in the negative auditory condition.
As at least some individuals had been successful in all conditions, Call (2004) concluded that great apes in general show inference-by-exclusion capabilities (also see Bräuer et al. 2006). That conclusion must be subject to the caveat that the ape subjects were notably less successful with auditory than with visual cuing, which has since been found for various monkey species as well. For example, tests with Tonkean macaques (M. tonkeana; Petit et al. 2005) and baboons (Cynocephalus anubis; Schmitt and Fischer 2009) have demonstrated analogous evidence of inference-by-exclusion with visual stimuli, but no effective responding to auditory cuing – whether positive or negative. The basic question of whether apes and monkeys can show inferential reasoning in the task has become intertwined with the seemingly orthogonal issue of stimulus modality.
Auditory inference in capuchins and a possible role of learning
While still showing performance asymmetries, capuchins tested in inference-by-exclusion studies have produced better auditory-domain results than have other monkeys. These outcomes have highlighted the potential importance of learning and experience in the task. In one study, Sabbatini and Visalberghi (2008) reported that initially, only one of eight individual capuchins tested was able to use auditory cues to retrieve hidden food. That number rose to four after the subjects were allowed to directly explore baited and unbaited containers – even though these containers were different from those used in testing. The researchers further found that two of the four animals were also utilizing negative auditory cuing after that experience. In a second study, Paukner et al. (2009) found that two of nine capuchins made use of positive auditory cues in the most comparable of several conditions tested. In this case, the subjects had not had an opportunity to explore or manipulate any apparatus. Unlike Sabbatini and Visalberghi, these researchers found no learning effects even with continued experience in the task.
Results with capuchins indicate that the initial probability of observing responses to auditory cuing of food location in inference-by-exclusion tasks is low, including 3 of 17 total subjects tested by Sabbatini and Visalberghi (2008) and Paukner et al. (2009). However, that proportion is not zero, raising the question of whether additional testing would tend to weaken or strengthen confidence in this modest, but positive outcome. Furthermore, based on improved performance observed after their capuchins had handled other kinds of containers that were either baited or empty, Sabbatini and Visalberghi suggested that experience could be a critical factor in the task. Paukner et al. also embraced this position, although without finding evidence of a role of experience in their own work.
To further examine both issues, the current Experiment 1 began by replicating earlier inference-by-exclusion testing with positive cuing, in this case comparing outcomes for visual and auditory cues provided both together and separately. As expected, all animals responded to visual cuing. Unexpectedly, a majority of the individuals tested also made use of auditory cues. Experiment 2 then tested positive and negative cuing separately in both visual and auditory domains, which revealed effective responding to positive auditory cues in all subjects – including those previously performing at chance rates. However, because none of the animals showed reliable responding to negative auditory cues, the third and last study was designed to probe for any evidence of learning about the absence of sound as a cue to look for food elsewhere. Here, the critical condition included two empty cups. One was shaken as before, while the other was rotated. We hypothesized that if even simple learning about the absence of sound had occurred during the previous experiment, the capuchins would have formed a negative association with the empty, shaken cup – having consistently failed to receive a reward when choosing it. If so, the animals should then show a detectable tendency to avoid that choice when offered a second cup whose movement was unfamiliar to them.
General Material and Methods
Subjects
Subjects were eight tufted capuchins, captive-born, and ranging from 3 to 16 years of age at the beginning of testing (see Table 1). The animals were housed in two groups at the Language Research Center (LRC) at Georgia State University, with each group in their own indoor-outdoor enclosure. Group 1 consisted of five individuals, of which one 18-month-old was deemed too young to be included in the study. Group 2 consisted of four monkeys, all of whom participated. These monkeys had extensive cognitive testing experience, including making choices in computer-based testing and interacting manually with experimenters in token-exchange tasks. To our knowledge, previous testing had focused on visual stimuli, with little or no experience in specifically auditory-based tasks. Subjects were fed their usual diet of fruits, vegetables, and monkey chow in the morning, afternoon, and evening throughout testing, as well as having unlimited access to water.
Table 1.
Age, sex, and group membership of the eight capuchin subjects
| Subject | Age (years) | Sex | Group |
|---|---|---|---|
| Wren | 4 | Female | 1 |
| Lily | 10 | Female | 1 |
| Griffin | 10 | Male | 1 |
| Drella | 16 | Male | 1 |
| Liam | 3 | Male | 2 |
| Nala | 4 | Female | 2 |
| Gabe | 9 | Male | 2 |
| Gambit | 10 | Female | 2 |
Apparatus
Two opaque, 6-oz plastic cups were used to present food to the subjects. The reward on each trial consisted of a single piece of Cocoa Puffs (General Mills, Inc., Minneapolis, MN), an air-filled, chocolate-flavored breakfast cereal. The cups were shielded from view behind an opaque screen between trials and presented on a platform approximately 0.5-m high placed in front of the test cage.
Procedure
Subjects were tested individually in a 1.5 × 1 m area that was part of their normal indoor caging. Other group members were locked in an adjacent, but separate area during this time. Each testing session included multiple conditions, as appropriate for the various experiments. The order of trial conditions for each session was randomized, with some restrictions noted separately for each study. The first session began at about 2 PM on testing days, with a given individual being tested for 10 to 15 min. On each trial, an experimenter sitting approximately 1 m from the cage first concealed both cups behind the screen while baiting one of them. Both cups were subsequently brought out from behind the screen and placed on the presentation platform with the tops covered by the experimenter’s hands. The experimenter then provided food-location cues for 3 sec, with the nature of that cuing varying by condition. Finally, both cups were held up about 2/3-m apart for 3 sec, with the monkey making its selection by touching one or the other using either hand. All monkeys were able to select a cup in this way from the beginning, likely due to having experience in previous tasks involving making choices as well as performing manual exchanges with an experimenter.
Cuing of reward location was considered “Positive” when a subject was shown the contents of a cup containing food or heard the food rattling as the cup was shaken. Negative cuing consisted of being shown an empty cup or hearing no sound when the cup was shaken. On “Visual-Both” and “Auditory-Both” trials, the experimenter either tilted and showed the contents of both cups or shook both cups, respectively. Only one cup was shown or shaken on “Positive” versus “Negative” trials, thereby providing only one kind of cue. In these cases, the experimenter’s hand remained in contact with, and covering the top of, the other cup. On “Control” trials, neither cup was shown or shaken during the cuing segment. Across all conditions, capuchins choosing correctly when the cups were held up received the food from the baited cup, with both cups then being moved behind the screen.
Data analysis
Performance was measured by tallying each individual’s correct choices in each condition and calculating percentage-correct across sessions. Group outcomes were then computed as means and standard errors derived from these values. Group-level analyses included comparing percentage-correct on Control trials to chance-level performance of 50% correct using one-sample t-tests. Testing was one-tailed, in order to maximize potential to detect evidence of possible inadvertent cuing effects. Outcomes were compared across conditions using the Friedman test, with planned post-hoc comparisons performed using two-tailed, Wilcoxon matched-pair, signed-rank tests with Bonferroni correction. Individual-level analyses included comparing proportion-correct outcomes in experimental conditions to chance performance levels using binomial tests. These tests were one-tailed, as below-chance performance was not relevant to the hypotheses being tested. To look for evidence of learning, we tallied the number of correct responses occurring on “First Trials,” meaning the first instance in which a given animal was tested in a given condition. However, because there were only eight subjects, all but one of the animals had to be correct to in order to produce a statistically significant outcome. We therefore considered this measure only modestly informative, and also compared percentage-correct performance over blocked sessions using Wilcoxon testing. The first block was comprised of the first half of the sessions (1 to 4 in Experiment 1, 1 to 6 in Experiment 2), while the second block included the remaining sessions (5 to 8 in Experiment 1, 7 to 12 in Experiment 2). Testing was one-tailed, as hypotheses specifically concerned increases, rather than possible decreases in performance.
Experiment 1
The first experiment tested subject responses to “Both” cuing in visual versus auditory presentations. Based on comparable comparisons in capuchins (Sabbatini and Visalberghi 2008; Paukner et al. 2009), we expected subjects to perform very well in response to visually based testing, with a subset of animals also potentially able to utilize auditory cuing.
Material and Methods
Subjects
All eight monkeys participated.
Procedure
This experiment included testing with Visual-Both, Auditory-Both, and Control trials. One of the cups was baited on each trial in all three conditions. In visual testing, the experimenter showed the contents of both cups simultaneously, while shaking them one at a time with left-right order randomized across trials in auditory testing. All subjects completed 8 sessions with 12 trials in each. Sessions included four Visual, four Auditory, and four Control trials conducted in randomized order, with left-right placement of food also randomized, but neither trial-type nor reward placement could be the same more than twice in a row.
Results and Discussion
Results are illustrated in Fig. 1, showing that mean percentage-correct on Control trials was indistinguishable from chance performance, t7 = 0.16, P = 0.439. Comparing across the three conditions using a Friedman test revealed an overall effect, χ22 = 16.0, P < 0.001. Post-hoc comparisons among the conditions were conducted using a Bonferroni adjusted alpha value of 0.017. Percentage-correct in Visual-Both testing was greater than in either Auditory-Both or Control trials (Z = −2.53, P = 0.011, in both cases), and subjects also performed statistically better in Auditory-Both than in Control testing (Z = −2.53, P = 0.012).
Fig. 1.
Mean percentage-correct (with standard errors) for the Visual-Both, Auditory-Both, and Control conditions in Experiment 1. The dashed line shows the 50% performance level expected by chance.
Individual subjects all scored at least 94% correct in Visual-Both testing, proportions that were statistically above chance performance level in each case (binomial tests, all Ps < 0.001). Scores in auditory testing were more variable, ranging from 56% to 84% correct. However, five of eight subjects performed statistically better than expected by chance (P < 0.05 in each such case). First-Trial tallies were not different from chance performance and a significant performance increase was found between the first and second session blocks in Visual-Both (Z = −1.86, P = 0.032) as well as Auditory-Both conditions (Z = −2.05, P = 0.021).
These outcomes show that all capuchins could routinely locate the food when seeing which of two cups contained this reward, and that the majority could also do so when hearing one of the cups rattling when shaken. The monkeys showed evidence of learning in the task, with improved performance across blocks in both experimental conditions. As expected, percentage-correct outcomes were statistically higher for positive visual than positive auditory cues, although monkeys showed more accurate performance with positive auditory cues than was shown in comparable experiments conducted by Sabbatini and Visalberghi (2008) and Paukner et al. (2009). Across these three studies, 8 of 26 individual capuchins tested have demonstrated use of positive auditory cues without specific experience other than during test trials. This proportion is comparable to that reported by Call (2004) for great apes.
Experiment 2
In the first experiment, subjects were able to locate food items when cued using both cups, but their performance was less efficient if the cues were auditory rather than visual in nature. We next tested the monkeys with positive versus negative cues presented separately in each domain, checking in particular for continued, experience-based performance gains in response to positive auditory cues, as well as for any evidence of coming to respond to negative versions. The former could be expected based both on improved overall performance shown by subjects in Experiment 1 and on Sabbatini and Visalberghi’s (2008) results. The latter showed the importance of experience for at least some animals, although in that case gained by exploring and manipulating different containers than those used in the experiments. In addition to finding improved performance with positive auditory cues, Sabbatini and Visalberghi reported that two animals came to show significant use of negative auditory cuing as well. In the current experiment, experience consisted of continued testing alone, which could potentially produce improvement for individuals already responding to some positive auditory cues, for those who were not responding to positive auditory cues, and for any animal when tested with negative auditory cues. However, the outcomes could also resemble results reported by Paukner et al. (2009), whose capuchins showed no effect of continued testing with either positive or negative auditory cuing.
Material and Methods
Subjects
All eight subjects participated.
Apparatus
The apparatus was the same as in Experiment 1.
Procedure
There were six experimental conditions and one control condition. Visual-Both, Auditory-Both, and Control trials were the same as in Experiment 1. In the “Visual-Positive” and “Auditory-Positive” conditions, only the cup containing food was shown or shaken. In the “Visual-Negative” and “Auditory-Negative” conditions, only the empty cup was shown or shaken. All subjects completed 12 sessions of 12 trials each, including eight experimental and four control trials. Two of the six experimental conditions were tested in each session, with trial types randomized across sessions. Each experimental condition included 16 trials, with the overall total of 144 trials for a given subject, consisting of 96 experimental and 48 control trials. Other procedures were the same as in Experiment 1.
Results and Discussion
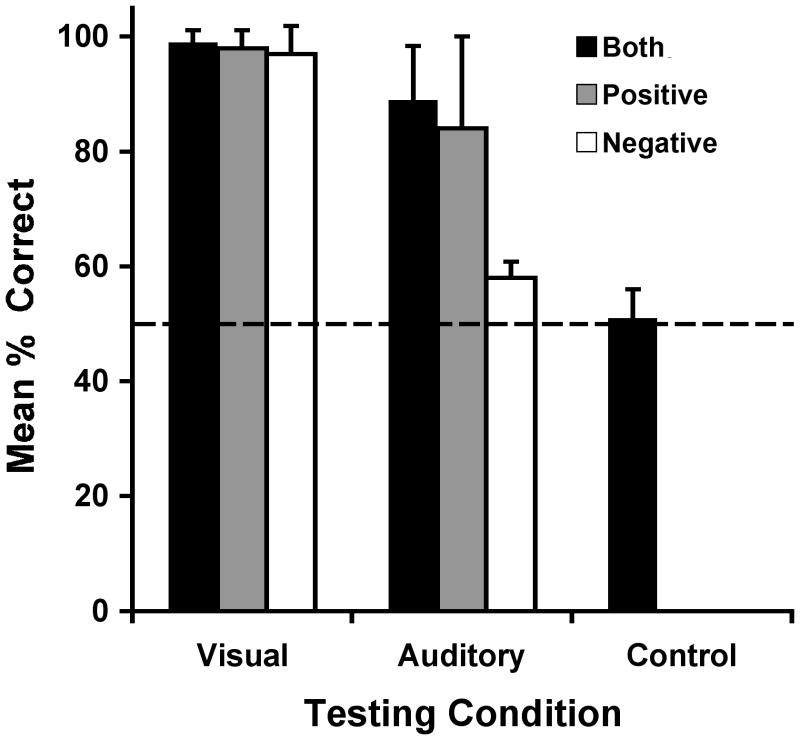
Results from Experiment 2 are shown in Fig. 2. Mean percentage-correct for Control trials was again indistinguishable from 50% (t7 = 0.71, P = 0.250), while Friedman testing revealed an overall effect across the seven conditions, χ26 = 37.9, P < 0.001. Post-hoc comparisons were limited to four specific comparisons in order to avoid imposing unduly strict Bonferroni correction and concomitant likelihood of Type II error. These comparisons included both versions of Positive and Negative cuing versus performance in the Control condition, and used a corrected alpha value of 0.0125. Results showed significant effects for Visual-Positive and Visual-Negative trials (Z = −2.52, P = 0.012, in both cases), as well as for Visual-Negative testing (Z = −2.52, P = 0.012). However, there was no difference between the Auditory-Negative and Control conditions (Z = −1.26, P = 0.104).
Fig. 2.
Mean percentage-correct (with standard errors) for Experiment 2. Conditions included Visual-Both and Auditory-Both, Visual-Positive and Visual-Negative, Auditory-Positive and Auditory-Negative, as well as a Control condition. The dashed line shows the 50% performance level expected by chance.
All subjects showed at least 94% correct in each of the three visual conditions (Visual-Positive, Visual-Negative, and Visual-Both). As in Experiment 1, these were all statistically significant proportions. In addition, First-Trial performance was statistically significant in both Positive and Negative visual conditions, with seven of eight subjects choosing correctly the first time out (binomial tests, P = 0.035). In contrast, First-Trial performance did not reveal any statistically significant effects in the auditory conditions, and there was no statistical improvement between the first and second session blocks in any of the six experimental conditions. All subjects now performed above chance levels in Auditory-Both testing, including the three who had not done so in Experiment 1 (binomial tests, all Ps < 0.001). Six of eight individuals also performed above chance in the Auditory-Positive condition (binomial tests, P < 0.05 in each such case). However, performance on Auditory-Negative trials was not different than in Control testing and no individual performed above chance levels.
Results included that the animals performed well in visually based testing, whether seeing into both cups or into only one cup, and whether or not that cup was baited or empty. Performance was also good in auditory testing when positive cues were available. All subjects now made use of auditory cues when both cups were shaken, and the majority generalized that performance to trials with only the baited cup. Although there was no direct statistical evidence of continued learning in Experiment 2, it seems safe to conclude that beneficial effects of experience gained in Experiment 1 carried through in this study as well. In contrast, there was no indication that any individual could respond effectively to only the absence of sound when an empty cup was shaken. In other words, outcomes suggested that while some capuchins may be able to immediately capitalize on positive auditory cues in inference-by-exclusion testing, specific experience with such cuing may routinely be helpful in performing the task. However, that experience had no evident impact on responses to negative auditory cuing.
Experiment 3
Performance in Auditory-Both and Auditory-Positive testing in Experiment 2 relative to Experiment 1 provided evidence that the subject responses to hearing the sound of the food being shaken in the baited cup continued to improve. This improvement occurred even though the animals never received direct access to the cups or other containers. Furthermore, some of the capuchins that were given direct, exploratory opportunities with containers in Sabbatini and Visalberghi’s (2008) study were helped not only with positive auditory cuing, but also in responding to negative auditory cues. Given that all eight monkeys were successful with positive auditory cues here, it seemed unlikely that they would have learned nothing at all about the negative ones. The simplest detectable form of such learning could be formation of negative associations with the empty, shaken cup, as that option had been selected many times by each animal in the Auditory-Both and Auditory-Negative conditions. However, the testing procedure may not have been suitable to revealing such learning. For example, any negative associations to the empty, shaken cup would not have been apparent in the context of the rewarded option of a rattling sound from the other cup on Auditory-Both trials. In addition, the alternative during Auditory-Negative testing was a cup that was neither shown nor – shaken matching a condition that was present on many other kinds of trials. This non-manipulated alternative would have had no predictive value concerning food reward, which the capuchins could also have learned (e.g., Domjan 2009).
Specifically, any learning about the empty, shaken cup might or might not have had an impact on the capuchins’ behavior, with negative associations to the one cup being contrasted with learned irrelevance for the other. Capitalizing on a condition also tested by Call (2004), Experiment 3 probed for potential learning about negative auditory cues in a different way. Here, shaking an empty cup was contrasted with a new, readily discriminable rotation movement. Therefore, testing in Experiment 3 included using two empty cups on Negative-Auditory trials, with one cup being shaken and the other rotated in the vertical plane. We reasoned that any existing, negative associations with the empty, shaken cup would be expressed as a tendency to select this new, presumably association-free, alternative. Even weak learning that might have occurred in the negative auditory cuing condition might thereby become detectable.
Material and Methods
Subjects
All eight subjects participated in Experiment 3.
Apparatus
The apparatus was the same as in Experiments 1 and 2.
Procedure
Subjects completed a single session of 24 trials in Experiment 3, which included eight trials in each of two experimental conditions, as well as eight Control trials. One of the experimental conditions was equivalent to Auditory-Both testing in earlier experiments, but here was called “Shake-Shake.” In a new “Shake-Rotate” procedure, one empty cup was shaken as before, while the other empty cup was turned upside-down and then rightside-up. Control trials were the same as in earlier experiments, as were randomization procedures.
Results and Discussion
Results are shown in Fig. 3, with mean percentage-correct for Control trials again showing no difference from 50%, t7 = 0.36, P = 0.365. Friedman testing did reveal an overall effect across the three conditions (χ22, P = 0.002), with mean accuracy in the Shake-Shake condition exceeding 90% correct. Post-hoc comparisons using a Bonferroni corrected alpha value of 0.017 showed this outcome to be significantly higher than the mean on Control trials, (Z = −2.54, P = 0.011), and very similar to that of Auditory-Both testing in Experiment 2. On Shake-Rotate trials, subjects chose the shaken cup about 47% of the time, and the rotated cup about 53% of the time. While this difference was in the predicted direction, it was not statistically significant (t7 = −0.45, P = 0.668). Furthermore, percentage-correct on Shake-Rotate trials was not statistically different from performance on Control trials, but was better than on Shake-Shake trials (Z = −2.53, P = 0.011). These results indicate that while the monkeys were able to continue to use the presence of positive auditory cues as a guide to food location, they did not show convincing evidence of even simple association learning about the empty, shaken cup. These findings thus support the conclusion that the subjects had learned nothing of import about that cup and the absence of food over the first two experiments. This evident lack of learning is discussed in the next section.
Fig. 3.
Mean percentage-correct (with standard errors) for the Shake-Shake (both cups shaken, one containing food) and Control conditions (neither cup manipulated, one containing food), as well as mean percentage-selected for the Shake-Rotate condition (one cup shaken, one cup rotated, neither containing food).
General Discussion
The current work confirms that capuchin subjects were able to solve the problem of locating hidden food based on either positive or negative visual cues, as well as with positive auditory cuing. These outcomes are unlikely to have resulted from the animals being able to see into the cups or attending to inadvertent cuing by the experimenter, as control testing in each experiment revealed only chance-level accuracy when deliberate cuing was omitted. As predicted, performance was notably better in visual than in auditory versions of the task. Nonetheless, a larger-than-expected proportion of subjects were able to utilize positive auditory cues in Experiment 1, and all animals were able to do so in both subsequent experiments. Performance improved both during initial testing and between the first and second experiment, and was particularly evident with positive auditory cues. In Experiment 2, the three individuals that had not previously exceeded chance levels came to do so, and the remaining subjects showed higher percentage-correct outcomes than in Experiment 1. In contrast, Experiments 2 and 3 provided no evidence of the animals responding to or forming associations to negative auditory cues.
Comparisons to other primate inference-by-exclusion studies
Capuchins
The current findings are in agreement with some, but not all aspects of the two previous inference-by-exclusion experiments performed with capuchins. On the one hand, these animals have performed effectively with both positive and negative visual cues in each of the three studies conducted to date, while being notably worse with auditory cuing. On the other hand, whereas a significant proportion of the capuchins tested here quickly made use of positive auditory cues, only one of Sabbatini and Visalberghi’s (2008) and two of Paukner et al.’s (2009) animals did so. The current subjects also showed improvement based solely on experiencing the reward contingencies involved. In contrast, Sabbatini and Visalberghi found that being able to access and explore baited and empty containers was key in facilitating better performance, whereas Paukner et al. found no evident effect of experience.
As there is no reason to believe that the tufted capuchins tested here are inherently different from others of their species, these discrepancies may reflect differences in testing procedures or extraneous factors affecting the various studies. If so, however, it is not immediately clear what the important discrepancies were and why they would most affect performance with auditory cuing in this kind of task. The studies appear quite similar in many critical ways, for instance in isolating subjects from their cage mates, having them approach and attend to an experimenter, and providing preferred food rewards. Individual variation seems a more likely explanation, although difficult to specifically substantiate. For example, individual primates are known to vary in critical psychological traits, including cognitive capabilities, attention capacities, and personality (e.g., Locurto 2007). Differences in motivational state and attention during the task could also have critically affected the outcomes, including across the various studies and among individual animals within each study.
As further discussed below, however, differences in previous experience may be the most important source of performance variation within and across studies with capuchins. For example, while all capuchins likely have extensive experience with positive versus negative cues to food location, experience with auditory cuing may vary significantly across individuals and across settings. In the current work, none of the capuchins were known to have previous experience in auditory tasks or to have experienced an unusual amount of relevant, auditory-based cuing during the course of daily life in the colony. However, there are many potential sources of relevant experience with sound even in captive environments. Our capuchins may have had a greater number of earlier learning opportunities with sound than their conspecifics in the other inference-by-exclusion experiments with this species. This interpretation appears consistent with Sabbatini and Visalbergi’s (2008) overall finding that experience even with different containers was important in facilitating improved performance with both positive and negative auditory cuing. In their study, that experience also occurred at a point in time when it was directly relevant to the testing being conducted, with the containers potentially being similar enough to those used in the experiment itself to create particularly salient learning opportunities.
Other monkeys
While capuchins in two inference-by-exclusion studies have now been shown to respond to auditory cuing, neither Tonkean macaques (Petit et al. 2005) nor baboons (Schmitt and Fischer 2009) have been found to do so. In the latter, even specific training designed to foster associations between auditory cues and food location failed to produce improvement. Taken together, these various studies raise the possibility that there are meaningful differences in the cognitive capabilities of these respective monkey species. This interpretation is consistent with previous arguments that capuchins are among the most cognitively capable of all monkeys – even to the point of rivaling great apes (Anderson 1996; Visalberghi and Limongelli 1996; Mitchell and Anderson 1997; Visalberghi 1997).
However, it is difficult to draw strong conclusions from negative evidence. Often primates are found to be capable of learning specific responses to positive auditory cues in other kinds of circumstances. Both wild monkeys and lemurs can show effective, differentiated responses to heterospecific alarm calls (e.g., Seyfarth and Cheney 1990; Ramakrishnan and Coss 2000; Zuberbühler 2000; Fichtel 2004) – reactions that must have been learned through experience with these sounds. It is also clear that a variety of captive primates can learn differential responses to sound, including both species-specific vocalizations and more arbitrary kinds of auditory events (reviewed by Owren et al. 2011). Other studies described here also show that baboons, Tonkean macaques, and even capuchins can have difficulty in the auditory domain in particular. As discussed earlier, nonhuman primates that readily master a given memory task in the visual domain can routinely struggle when the same test is conducted with analogous auditory stimuli.
Great apes
Results from capuchins may also have implications for interpreting the performance of great apes in the inference-by-exclusion task. Call’s (2004) original study with the design used here found that 9 of 30 individual apes responded effectively to positive auditory cues. Of those nine, three were able to utilize negative auditory cuing (see also Hashiya and Kojima 2001). Bräuer et al. (2006) also tested many of the same chimpanzees and bonobos that had participated in Call’s (2004) study, raising the question of whether animals showed experience-based improvement. Unfortunately, the data reported do not allow clear conclusions to be drawn on that point. For capuchins, Sabbatini and Visalberghi (2008), Paukner et al. (2009), and the current studies have cumulatively shown that 14 of 26 individuals tested in comparable conditions could eventually use positive auditory cues, with two animals also responding to negative versions. In light of this performance, one could argue that capuchins and great apes fall on one side of a cognitive divide, with monkeys such as Tonkean macaques, baboons, and rhesus falling on the other side. However, it may be premature to draw this kind of conclusion, for example given the role that factors such as motivation and attention may play across species and study settings. Furthermore, the possibility of important variation in relevant auditory experience cannot be ruled out, which could critically contribute to differences in individual- and species-level performance both in visual versus auditory, as well as positive versus negative cuing conditions.
Species-specialization, experience, or both?
The possible role of specialization
Previous investigators have made a number of pertinent points concerning differences between performance with visual and auditory cues in inference-by-exclusion studies (reviewed by Schmitt and Fischer 2008). In particular, it has been noted that wild capuchins, as well as many other primates, rely primarily on herbivorous foraging that targets fruits, leaves, tubers, and the like. Visual cuing is probably predominant for these foods, with audition playing little if any direct role (e.g., Brown and Zunino 1990; see also Melin et al. 2007). As a result, many species may have an evolved propensity to attend more to visual than to auditory cues in food-related contexts, reflecting differentiated, modality-specific neural circuitry (reviewed by Ng et al. 2009). Less bias would be expected in species that do rely on auditory cuing during foraging, which is consistent with the relative success reported for capuchins in two of three studies. While primarily herbivorous, capuchins are also known to use auditory cues to find some kinds of insect prey (e.g., Phillips et al. 2003; Phillips et al. 2004), as well as in tapping a nut and evaluating its likely content based on the sound produced (Visalberghi and Neel 2003). Evolved differences in neural processing of auditory cues by capuchins versus other monkeys could contribute to discrepancies observed in inference-by-exclusion testing.
The possible role of experience
On the other hand, studies of both lower- and higher-level auditory processing in primates have found important neural integration with other sensory modalities, including vision, olfaction, and touch (e.g., Kohler et al. 2002; Ghazanfar et al. 2005; Kayser et al. 2009). It is therefore not obvious that discrepancies between visual and auditory cuing necessarily implicate immutable, species-wide specializations. Both attentional biases and any observed neural differences in visual versus auditory processing could instead reflect differential experiences with these two kinds of cues. If food availability is routinely more powerfully associated with visual than auditory input, a lifetime of experience with this asymmetry could become deeply ingrained in the attentional and cognitive processing of individual animals. Furthermore, when auditory cues are available, they are probably often redundant with visual cuing. Resulting cue-competition effects (see Domjan, 2009 for a recent review) may well be applicable to both foraging and inference-by-exclusion situations.
As Schmitt and Fischer (2009) point out, visual cues may overshadow concurrent auditory ones. In this well-known effect, the presence of a more salient cue interferes with learning that would otherwise occur to a less-salient one – even if the latter has significant predictive value (e.g., Pavlov, 1927). Blocking is another relevant form of cue-competition (e.g., Kamin 1969a,b), occurring when an individual first learns about one cue and a second cue is then compounded with it. Unless the compound has a different predictive value than the original, little is learned about the new, functionally redundant cue. Both capuchins and other primates may experience overshadowing and blocking with visual versus auditory cues in both natural and captive foraging conditions. Visual input is virtually always present, providing differential cuing of not only the presence or absence of food, but also what kind of food it is. Even when food is detected by purely auditory means, vision inevitably comes into play. In other words, visual cues are virtually omnipresent, while auditory cuing is not only less prevalent, but also probably much less important in many circumstances.
A final important and relevant learning phenomenon is the so-called feature-positive effect (Allan and Jenkins 1980; Hearst 1991). Here, a large body of experiments with both birds and mammals has shown that stimuli that are present are easier to associate with food reward than are stimuli that are absent. In all probability, the feature-positive effect influences ease of learning about positive versus negative cuing in both visual and auditory domains. However, given massive experience in seeing that food is either present or not present, primates and other animals may routinely overcome feature-positive influences in food-related contexts. In contrast, there is likely much less opportunity to do so with auditory cues. While the presence of food is probably often associated with at least some kinds of sounds, the absence of sound is not a strong or frequent cue to the absence of food. Even in cases where capuchins rely on auditory cues during foraging, it is the sounds that occur – rather than the sounds that do not occur – that likely guide their behavior.
Conclusions: Implications for inference-by-exclusion testing
Overall, it is difficult to draw definitive conclusions from available primate inference-by-exclusion experiments. All nonhuman primates tested in Call’s (2004) elegant design have performed well with both positive and negative visual cues. From this performance, one might conclude that each of the species has shown inferential reasoning. However, none of the species have performed as well with auditory cues. Whereas great apes and capuchins have demonstrated partial success, Tonkean macaques, baboons, and rhesus monkeys have shown little. While it is possible that between-species discrepancies may reflect differences in inferential capabilities, modality-related effects are almost certainly also involved. As yet, we do not know how or why modality is affecting performance, which could reflect either or both phylogenetic history and individual experience. At this point, the safest conclusion appears to be that it is difficult to draw strong conclusions about cognitive abilities based on the auditory conditions of inference-by-exclusion testing.
A larger question then becomes whether the same concern also applies to testing in the visual domain. For instance, Penn and Povinelli (2007) have proposed that animals can potentially perform well in the inference-by-exclusion task based on associative learning alone. In other words, they suggest that performing the task may not require inferential reasoning at all. Consistently effective performance with visual cues could instead be attributable to the animals being highly “over-learned” in this domain, with poorer performance with auditory cuing being traceable to relative inexperience in relevant aspects of auditory cuing. We suggest that investigating the origin of the differences between performance with visual and auditory cues may therefore be key in also understanding whether inference is truly occurring in this particular task. From this perspective, finding that experience can importantly affect an animal’s performance with auditory cuing in the inference-by-exclusion task may be quite important. Specifically, one has to ask whether a long history of seeing that food is present or absent may be the critical factor in visually based inference-by-exclusion performance rather than reasoning abilities per se. Definitive conclusions may depend on learning more about primate processing of visual versus auditory input and how both phylogenetic and experiential factors affect how well the animals can capitalize on cuing in each of these forms.
Acknowledgments
We thank Sarah Hunsberger, Betty Chan, Dan Rice, and John Kelley for expert care of the animals. Four anonymous reviewers provided insightful comments on an earlier version of this work. The studies were supported by Grant HD-38051 from the National Institute of Child Health and Human Development, with additional support from the Center for Behavioral Neuroscience under the STC Program of the National Science Foundation under Agreement No. IBN-9876754. Lisa A. Heimbauer was a Research Challenges in Language and Literacy Fellow and Duane M. Rumbaugh Fellow, while Rebecca L. Antworth was a Brains & Behavior Fellow. Experiments were conducted in accordance with applicable laws and regulations of the United States and the state of Georgia, including compliance with institutional animal care and use guidelines at Georgia State University. The authors declare they have no conflict of interest.
References
- Adolphs R. The social brain: neural basis of social knowledge. Ann Rev Psych. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan RM, Jenkins HM. The judgement of contingency and the nature of the response alternatives. Canad J Psych. 1980;34:1–11A. [Google Scholar]
- Anderson JR. Chimpanzees and capuchin monkeys: comparative cognition. In: Russon A, Bard K, Parker S, editors. Reaching into thought. Cambridge University Press; Cambridge: 1996. pp. 2–47. [Google Scholar]
- Bräuer J, Kaminski J, Riedel J, Call J, Tomasello M. Making inferences about the location of hidden food: social dog, causal ape. J Comp Psych. 2006;120:38–47. doi: 10.1037/0735-7036.120.1.38. [DOI] [PubMed] [Google Scholar]
- Brown AD, Zunino GE. Dietary variability in Cebus apella in extreme habitats: evidence for adaptability. Folia Primatolog. 1990;54:187–195. doi: 10.1159/000156443. [DOI] [PubMed] [Google Scholar]
- Call J. Inferences about the location of food in the great apes (Pan paniscus, Pan troglodytes, Gorilla gorilla, and Pongo pygmaeus) J Comp Psych. 2004;118:232–241. doi: 10.1037/0735-7036.118.2.232. [DOI] [PubMed] [Google Scholar]
- Colombo M, D’Amato MR. A comparison of visual and auditory short-term memory in monkeys (Cebus apella) Q J Exp Psychol. 1986;38B:425–448. [PubMed] [Google Scholar]
- D’Amato MR, Colombo M. Auditory matching-to-sample in monkeys (Cebus apella) Anim Learn Behav. 1985;13:375–382. [Google Scholar]
- Domjan M. The principles of learning and behavior. 6. Wadsworth; Belmont, California: 2009. [Google Scholar]
- Fichtel C. Reciprocal recognition of sifaka (Propithecus verreauxi verreauxi) and redfronted lemur (Eulemur fulvus rufus) alarm calls. Anim Cog. 2004;7:45–52. doi: 10.1007/s10071-003-0180-0. [DOI] [PubMed] [Google Scholar]
- Ghazanfar AA, Maier JX, Hoffman KL, Logothetis NK. Multisensory integration of dynamic faces and voices in rhesus monkey auditory cortex. J Neurosci. 2005;25:5004–5012. doi: 10.1523/JNEUROSCI.0799-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashiya K, Kojima S. Hearing and auditory-visual intermodal recognition in the chimpanzee. In: Matsuzawa T, editor. Primate origins of human cognition and behavior. Springer-Verlag; Berlin: 2001. pp. 155–189. [Google Scholar]
- Hearst E. Psychology and nothing. Am Sci. 1991;79:432–443. [Google Scholar]
- Holyoak KJ, Morrison RG, editors. The Cambridge handbook of thinking and reasoning. Cambridge University Press; Cambridge UK: 2005. [Google Scholar]
- Hume D. An enquiry concerning human understanding. Collier; New York: 1909–1914. [Google Scholar]
- Kamin LJ. Predictability, surprise, attention, and conditioning. In: Campbell BA, Church RM, editors. Punishment and aversive behavior. Appleton-Century-Crofts; New York: 1969a. pp. 279–296. [Google Scholar]
- Kamin LJ. Selective association and conditioning. In: Mackintosh NJ, Honig WK, editors. Fundamental issues in associative learning. Dalhousie University Press; Halifax: 1969b. pp. 42–64. [Google Scholar]
- Kayser C, Petkov CI, Logothetis NK. Multisensory interactions in primate auditory cortex: fMRI and electrophysiology. Hearing Res. 2009;258:80–88. doi: 10.1016/j.heares.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Kohler E, Keysers C, Umiltà MA, Fogassi L, Gallese V, Rizzolatti G. Hearing sounds, understanding actions: action representation in mirror neurons. Science. 2002;297:847–848. doi: 10.1126/science.1070311. [DOI] [PubMed] [Google Scholar]
- Locurto C. Individual differences and animal personality. Comp Cog Behav Rev. 2007;2:67–78. [Google Scholar]
- Melin AD, Fedigan LM, Hiramatsu C, Sendall CL, Kawamura S. Effects of colour vision phenotype on insect capture by a free-ranging population of white-faced capuchins, Cebus capucinus. Anim Behav. 2007;73:205–214. [Google Scholar]
- Mitchell RW, Anderson JR. Pointing, withholding information, and deception in captive capuchin monkeys (Cebus apella) J Comp Psych. 1997;111:351–361. doi: 10.1037/0735-7036.111.4.351. [DOI] [PubMed] [Google Scholar]
- Ng C-W, Plakke B, Poremba A. Primate auditory recognition memory performance varies with sound type. Hear Res. 2009;256:64–74. doi: 10.1016/j.heares.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owren MJ, Amoss RT, Rendall D. Two organizing principles of vocal production: implications for nonhuman and human primates. Am J Primatol. 2011;73:530–544. doi: 10.1002/ajp.20913. [DOI] [PubMed] [Google Scholar]
- Paukner A, Huntsberry ME, Suomi SJ. Tufted capuchin monkeys (Cebus apella) spontaneously use visual, but not acoustic information to find hidden food items. J Comp Psychol. 2009;123:26–33. doi: 10.1037/a0013128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. In: Conditioned reflexes. Anrep GV, translator. Oxford University Press; London: 1927. [Google Scholar]
- Penn DC, Povinelli DJ. Causal cognition in human and nonhuman animals: a comparative, critical review. Ann Rev Psych. 2007;58:97–118. doi: 10.1146/annurev.psych.58.110405.085555. [DOI] [PubMed] [Google Scholar]
- Petit O, Call J, Thierry B. Inferences about food locations in Tonkean macaques. Primate Rep. 2005;72:76–77. [Google Scholar]
- Phillips KA, Grafton B, Haas ME. Tap-scanning for invertebrates by capuchins (Cebus apella) Folia Primatolog. 2003;74:162–164. doi: 10.1159/000070650. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Shauver Goodchild LM, Haas ME, Ulyan MJ, Petro S. Use of visual, acoustic, and olfactory information during embedded invertebrate foraging in brown capuchins (Cebus apella) J Comp Psychol. 2004;118:200–205. doi: 10.1037/0735-7036.118.2.200. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U, Coss RG. Recognition of heterospecific alarm vocalizations by bonnet macaques (Macaca radiata) J Comp Psychol. 2000;114:3–12. doi: 10.1037/0735-7036.114.1.3. [DOI] [PubMed] [Google Scholar]
- Sabbatini G, Visalberghi E. Inferences about the location of food in capuchin monkeys (Cebus apella) in two sensory modalities. J Comp Psychol. 2008;122:156–166. doi: 10.1037/0735-7036.122.2.156. [DOI] [PubMed] [Google Scholar]
- Schmitt V, Fischer J. Inferential reasoning and modality dependent discrimination learning in olive baboons (Papio hamadryas anubis) J Comp Psych. 2009;123:316–325. doi: 10.1037/a0016218. [DOI] [PubMed] [Google Scholar]
- Seyfarth R, Cheney D. The assessment by vervet monkeys of their own and other species’ alarm calls. Anim Behav. 1990;40:754–764. [Google Scholar]
- Visalberghi E, Limongelli L. Action and understanding: tool use revisited through the mind of capuchin monkeys. In: Russon A, Bard K, Parker S, editors. Reaching into thought. Cambridge University Press; Cambridge: 1996. pp. 57–79. [Google Scholar]
- Visalberghi E. Success and understanding in cognitive tasks: a comparison between Cebus apella and Pan troglodytes. Int J Primatol. 1997;18:811–830. [Google Scholar]
- Visalberghi E, Neel C. Tufted capuchins (Cebus apella) use weight and sound to choose between full and empty nuts. Ecolog Psychol. 2003;15:215–228. [Google Scholar]
- Zuberbühler K. Interspecies semantic communication in two forest primates. Proc R Soc Lond B. 2000;267:713–718. doi: 10.1098/rspb.2000.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]