Abstract
Rationale
Chronic ethanol (EtOH) treatment decreases the motor-impairing effects of cannabinoids and down-regulates the CB1 receptor. However, these studies have been limited to measures of ataxia and analysis of CB1 expression from whole-brain or hippocampal preparations.
Objective
To more fully assess the interactions between ethanol and cannabinoids, a tetrad of four well-characterized cannabinoid-induced behaviors (hypolocomotion, antinociception, hypothermia, and catalepsy) was measured in mice following EtOH treatment. Additionally, immunoblotting assessed CB1 protein in tissue from nine brain regions associated with these behaviors and the addiction neurocircuitry.
Materials and Methods
Male C57Bl/6J mice were administered EtOH (0, 2, or 4 g/kg; i.p) twice daily for ten days. Tetrad behaviors induced by the CB1 agonist WIN 55,212-2 (3 mg/kg, i.p.) were measured in subjects 1 or 10 days following the last EtOH injection. In a separate group of animals, tissue was collected at the same time points for immunoblot analysis.
Results
EtOH treated mice were less sensitive to the hypothermic, hypolocomotive, and antinociceptive effects of WIN and this effect reversed to control levels over a 10-day abstinence period. EtOH treatment did not affect WIN-induced catalepsy. CB1 protein expression was significantly altered in several brain areas including hypothalamus, periaqueductal grey, ventral tegmental area, and cerebellum.
Conclusions
These results show that chronic EtOH treatment significantly affects the behavioral sensitivity to cannabinoid drugs and alters CB1 expression in several brain regions. Furthermore, these effects are selective as some behaviors and brain regions display an altered response while others do not.
Keywords: Ethanol, CB1, Mouse Tetrad, Tolerance, WIN 55, 212-2, Cannabinoids, nociception, catalepsy
Introduction
Alcohol dependence and abuse are devastating disorders that affect an estimated 1 in 10 Americans and cost the United States economy in excess of $185 billion annually (NIAAA 2009). Alcohol use disorders are often comorbid with abuse of other substances including cannabis or marijuana, and both animal and human studies suggest that alcohol and cannabinoids interact in ways that contribute to addiction pathology. For example, it has long been known that cannabinoids and ethanol (EtOH) produce a cross-tolerance to one another (da Silva et al. 2001; Lemos et al. 2007; MacAvoy and Marks 1975; Newman et al. 1974; Newman et al. 1972; Siemens and Doyle 1979; Sprague and Craigmill 1976). The majority of this work used rodents and examined the ataxic effects of each drug using either a rotorod (da Silva et al. 2001; Siemens and Doyle 1979; Sprague and Craigmill 1976) or tilting plane apparatus (Lemos et al. 2007). Two studies have also reported cross-tolerance to a learned escape behavior in a one-way avoidance apparatus (Newman et al. 1974; Newman et al. 1972). Despite these interesting findings, it is not known whether cross-tolerance develops between EtOH and cannabinoids for well-characterized CB1-dependent behaviors.
In rodents, acute administration of a CB1 agonist produces a syndrome of four behaviors known as the tetrad. The tetrad consists of decreased locomotor activity, hypothermia, antinociception, and catalepsy (Martin et al. 1991). These effects are produced via activation of the CB1 receptor, a G-protein coupled receptor that is highly expressed throughout the brain (Zimmer et al. 1999). Because the tetrad is convenient and highly reproducible, it is often employed as a behavioral assay to screen novel compounds for cannabinomimetic activity (Compton et al. 1992) and to assess cross-tolerance between cannabinoid substances (Fan et al. 1994). To our knowledge, the mouse tetrad has not been used previously to test for cross-tolerance between cannabinoids and EtOH.
Therefore, in this study, we used the mouse tetrad to assess whether repeated exposures to EtOH leads to cross-tolerance to the effects of an acute CB1 agonist. The results from these studies suggest that chronic EtOH produces a significant cross-tolerance to three of the behaviors in the tetrad. Chronic EtOH treatment produces parallel reductions in both CB1 receptor expression (Basavarajappa et al. 1998; Mitrirattanakul et al. 2007) and function (Basavarajappa and Hungund 1999b). Accordingly, in the present study, western blots were used to determine if changes in CB1 expression represents a possible mechanism for the altered behavioral response to a CB1 agonist following repeated EtOH treatment.
Methods
Subjects
Male C57BL/6J mice (22–30g) were purchased from Jackson Laboratories (Bar Harbor, ME) and group housed (4 per cage). CNR1−/− mice on a C57BL/6 background were kindly provided by Dr. David Lovinger, NIAAA, Rockville, MD and were housed under similar conditions. Mice were maintained on a 12:12 light:dark cycle (lights on at 0600 hr), and given ad libitum access to food and water throughout the experiment. For behavioral experiments mice were removed from the colony at the end of the light cycle and experiments continued through beginning of the dark cycle. All experimental protocols were approved by the Institutional Animal Care and Use Committee at MUSC and were in accordance with the Guide for Care and Use of Laboratory Animals (NIH Publication No. 80-23, revised 1996).
Drugs
EtOH was administered i.p. in a volume of 0.03 mL/g in order to keep the concentration of EtOH below 18%. The CB1 agonist WIN 55,212-2 mesylate (WIN; Tocris Bioscience, Ellisville, MO), was first dissolved in Tocrisolve 100 and then diluted with 0.9% saline to give a final concentration of 1.5% Tocrisolve. WIN solutions were injected i.p. in 0.02 mL/g injection volumes.
10-Day EtOH Treatment
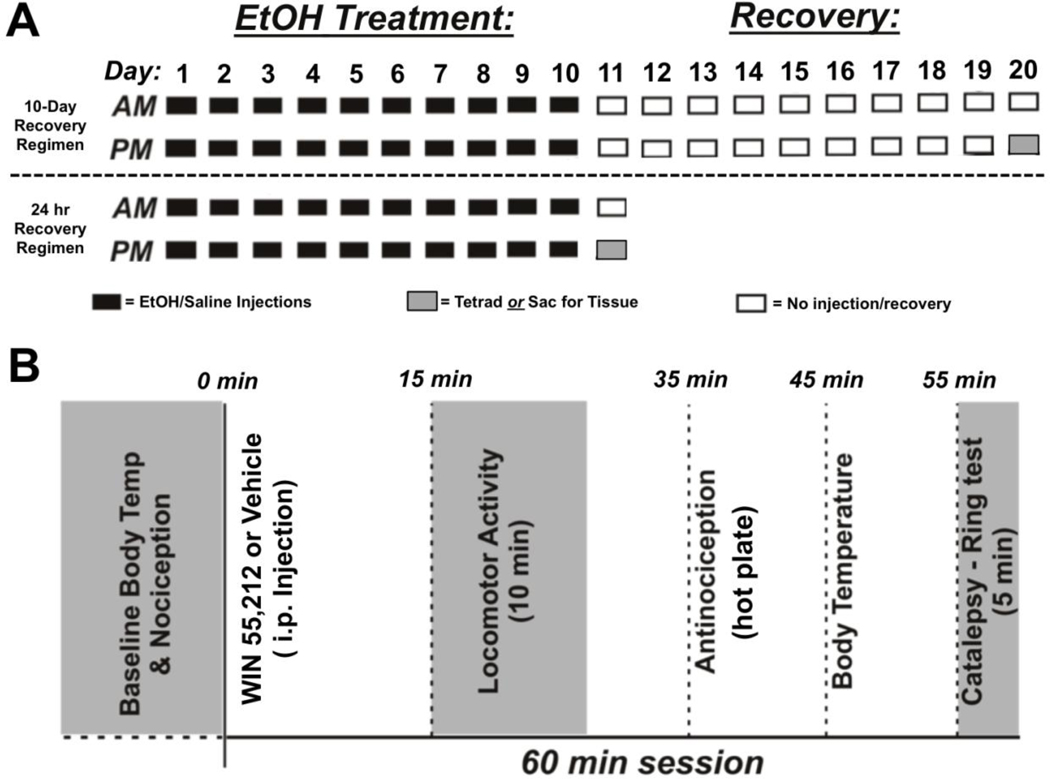
Mice were handled daily for 1 week prior to experimental manipulation and then given injections of EtOH (2 or 4 g/kg; i.p.) or saline twice daily for 10 consecutive days. Injections were spaced eight hours apart such that the second injection coincided with the onset of the dark cycle. One or 10 days following the 10-day treatment, mice were used for behavioral testing or tissue collection (Figure 1A).
Fig. 1.
Summary of EtOH treatment and testing regimen. A: Mice were administered saline or EtOH (2 or 4 g/kg) i.p. twice per day for 10 consecutive days. Following the last injection, subjects were returned to their home cage for 24 hrs (bottom panel) or 10 days prior to behavioral testing or tissue collection for CB1 protein quantification (top panel). B: Timeline of behavioral measures taken as part of the mouse tetrad assay.
Subjects used for western blot analysis underwent retro-orbital bleeding during the first ethanol injection on day 1 and 10 of treatment for analysis of blood ethanol concentration (BEC). BEC analysis was performed using a modified version of the colorimetric alcohol oxidase assay described by Prencipe et al (1987). The 4.0 g/kg dose of EtOH yielded BECs of 414.1±17.8 mg/dL 20 min following the injection on day 1 of treatment, and 490.2±12.3 mg/dL at the same time point on day 10. The change in BEC between the 20 and 60 min time points was calculated for Day 1 and Day 10 of treatment to estimate the rate of change in BEC before and after treatment. There was no significant decrease in this measure following 10-Day EtOH treatment (paired t-test, p=0.1209) suggesting metabolic tolerance to EtOH does not develop over the 10-day treatment regimen.
Mouse Tetrad
Mice were tested for locomotor activity, nociception, body temperature and immobility (ie. catalepsy) as previously described (Figure 1B; Martin et al. 1991). Each subject was used in the tetrad only once as tolerance can develop to CB1 agonists even after a single administration (unpublished observation). Prior to drug administration, baseline measurements of body temperature and nociception were determined, and mice were habituated to the locomotor activity boxes (Digiscan Animal Activity Monitors, Omnitech Electronics, Inc., Columbus, OH) for 30 min. After injection with WIN (0, 1, 3, or 7 mg/kg), mice were immediately placed back in the activity boxes for 25 min during which locomotor data were collected (VersaMax Software v4, Accuscan Instruments, Inc., Columbus, OH). Horizontal locomotor data during the last 10 min were used for analysis and are presented as the number of optical beam breaks.
Nociception was measured 35 min after WIN injection using a Hot Plate Analgesia Meter (Columbus Instruments, Columbus, OH) set to 56°C. Latency to respond to the heated surface was measured and included jumping or hind-paw licking or fanning. Forepaw responses were not counted because these can be confounded by grooming behaviors (Bannon and Malmberg 2007). Subjects were removed from the hot plate immediately after making one of the three qualifying responses or at the end of 30 sec if they failed to respond. Antinociception data are presented as the percent maximal possible effect (%MPE) where:
Body temperature was measured 45 min post-injection using a rectal probe (Physitemp Instruments, Clifton, NJ), and is expressed as the change in body temperature from the pre-drug baseline.
Catalepsy was assessed 55 min after the WIN injection using the ring test described by Pertwee (1972). Briefly, mice were placed on the ring with their forepaws gripping one side and their hindpaws on the other. Catalepsy was measured as the time spent immobile in a 5 min period with exception to movements associated with respiration and regaining their starting position. In rare cases, subjects displayed hyper-reflexive behaviors when placed on the ring. In these instances, data was excluded from analysis if an animal fell or jumped five or more times the 5-minute observation period. Data for WIN-induced catalepsy are expressed as the percent of total time spent immobile to the total time on the ring.
CB1 Protein
Following a 10-day treatment with 4.0 g/kg EtOH or saline, mice were placed back in their home cages for one or 10 days prior to sacrifice. Animals were rapidly euthanized by decapitation, and brains were immediately immersed for 1–2 min in ice-cold dissection buffer containing (in mM): 200 sucrose, 1.9 KCl, 6 MgCl2, 0.5 CaCl2, 10 glucose, 0.4 ascorbic acid, 25 HEPES, pH 7.3 with KOH. Brains were sectioned into 2 mm thick coronal slices using an adult mouse brain matrix (ASI Instruments, Warren, MI). From each mouse, nine brain regions were collected for western blot analysis (Supplemental Figure 1). For the frontal cortices, the olfactory bulbs were first removed and the medial portion of frontal cortex (mFC) containing prelimbic, cingulate, medial orbital, and secondary motor cortex was separated from the lateral aspect (lFC) containing primary motor, lateral orbital, and frontal association cortices. A 2 mm punch of dorsal striatum (DStr) and a 1 mm punch of the nucleus accumbens (NAc) were made from the next slab. Dorsal hippocampus (DHC) and the hypothalamus (HyTh) were dissected from the third slab, and 2 mm punches from the fourth slab were used to separate tissue containing periaqueductal grey (PAG) and ventral tegmental area (VTA). Finally, the cerebellum (Cereb) was dissected from the brain stem. Tissue samples were also prepared from CNR1−/− mice to test the specificity of the anti-CB1 antibody.
Following dissection, a crude membrane fraction was prepared and tissue samples were used for western blotting as previously described (Mulholland and Chandler 2010). After separation on NuPage Novex gels (4–12% Bis-Tris; Invitrogen Corp., Carlsbad, CA) protein was transferred to Immobilon-P PVDF membranes (Millipore Corporation, Billerica, MA). Membranes were blocked in 1% non-fat dry milk plus 5% BSA and incubated at 4°C overnight in a 1:500 dilution of the L15 anti-CB1 primary antibody (kindly provided by Dr. Ken Mackie, Indiana University, Bloomington, IN). A horseradish peroxidase (HRP) conjugated goat anti-rabbit secondary antibody was then added and bands were detected using enhanced chemiluminescence (ECL). The band corresponding to the molecular weight of CB1 (≈52 kDa) was quantified using computer-assisted densitometry with ImageJ v1.41 (National Institutes of Health, USA). Following development with ECL, each membrane was stained with Coomasie Brilliant Blue R-250 (Fisher Scientific, Pittsburgh, PA), air-dried overnight and scanned at 600 dpi to measure total protein (Aldridge et al. 2008). These values were used to normalize the optical density value of the CB1 band from the corresponding lane. Data are expressed as the fraction of control for each condition.
Statistics
All analyses were performed using Prism GraphPad Software v4.0c (La Jolla, CA). Data from studies assessing tetrad behaviors and CB1 protein expression was analyzed using analysis of variance (ANOVA). Post-hoc analyses (Bonferroni post-tests) were conducted when appropriate. Significance criteria were established as α = 0.05 (two-tailed) for all analyses.
Results
Dose-Dependence of CB1R-Induced Behaviors
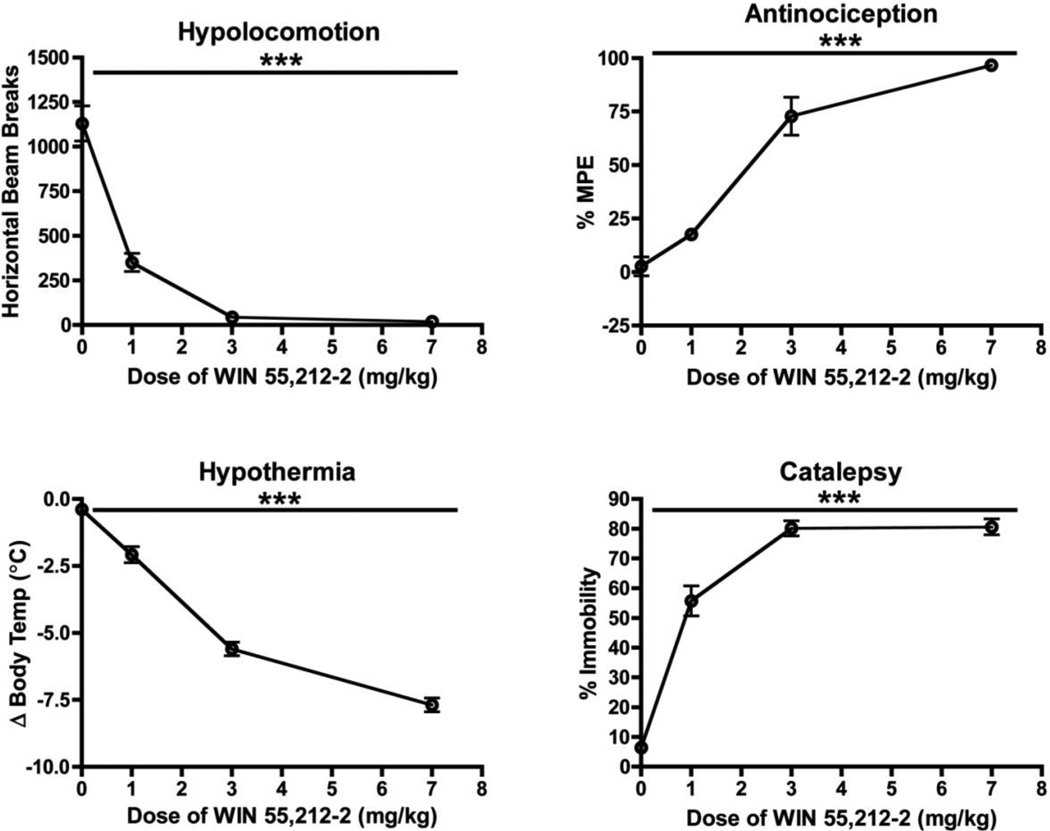
As previously reported (Compton et al. 1992), acute injection of mice with the CB1 agonist WIN produced hypolocomotion, antinociception, hypothermia, and catalepsy (Figure 2; One-Way ANOVA, p<0.0001). The hypothermic effects of WIN were significantly different from the baseline at all doses tested (Bonferroni’s Test, p<0.001), and the anti-nociceptive effects differed between all doses (p<0.01 – 0.001) except 0 and 1 mg/kg (p>0.05). For measures of catalepsy and locomotor activity, Bonferroni’s Multiple Comparison Test revealed significant differences between all doses tested (p<0.05 – 0.001) except between the 3 mg/kg and 7 mg/kg dose (p > 0.05), indicating a ceiling effect at the higher doses. Since the 3 mg/kg dose was at or very near the maximal observed effect for all tetrad behaviors (Figure 2) and because we hypothesized a decrease in sensitivity to CB1 agonists following EtOH treatment, we used 3 mg/kg WIN for all subsequent studies.
Fig. 2.
Effects of WIN on the mouse tetrad in ethanol-naïve mice. Ordinates represent the mean (± SEM, N=15–24) value for each measurement and are expressed as number of beam breaks per session (locomotor activity), percent maximum possible effect (antinociception), change in body temperature (hypothermia), and percent immobility on the ring stand (catalepsy). Abscissae represents the dose (mg/kg) of WIN administered to a group of subjects. Symbol: (***), values significantly different from the vehicle control (p<0.0001, One-way ANOVA)
10-Day EtOH Treatment Decreases Sensitivity to WIN
A separate group of naïve male C57 mice were administered EtOH (0, 2, or 4 g/kg i.p.) twice daily for ten consecutive days. Twenty-four hr following the last EtOH injection, the mouse tetrad was used to assess the effects of WIN (Figure 3). Baseline measures of body temperature for the control, 2 g/kg, and 4 g/kg EtOH groups were 37.63±0.17, 37.48±0.13, & 37.99± 0.14 (Mean±SEM in ºC), respectively, and these values were not significantly different (One-Way ANOVA, p=0.0552). Similarly, baseline measures of hot plate latency did not differ between groups (One-way ANOVA, p=0.3922). These values were 6.392±0.6728, 6.500±0.5177, & 7.431±0.5698 (Mean±SEM in sec) for the control, 2 g/kg, and 4 g/kg EtOH groups, respectively. Overall, the 10-day EtOH treatment significantly reduced the hypolocomotor effects of WIN (One-Way ANOVA, p<0.001). This effect was statistically significant for the 4.0 g/kg dose and mice treated with the 2.0 g/kg EtOH showed a clear trend towards reduced sensitivity. The antinociceptive effect of WIN was also reduced by EtOH treatment (One-Way ANOVA, p<0.0001), and there were significant differences in WIN-sensitivity between all treatment groups (Bonferroni post-test, p<0.01 – 0.001). WIN-induced hypothermia was also blunted in mice that had been previously treated with EtOH (One-Way ANOVA, p<0.001), and this effect was similar for both doses of ethanol (Bonferroni post-test, p>0.05). In contrast to these findings, repeated treatment of mice with either dose of ethanol failed to alter WIN-induced catalepsy (One-Way ANOVA, p=0.7607).
Fig. 3.
Effects of repeated EtOH treatments on WIN-induced effects in the mouse tetrad. Bars represent the mean (± SEM; N=11–13) value for each measure. The scales on the ordinates are expressed as number of beam breaks per session (locomotor activity), percent maximum possible effect (antinociception), change in body temperature (hypothermia), and percent immobility on the ring stand (catalepsy). Values on the abscissae represent the dose of EtOH administered for pre-treatment. Symbols: value significantly different from saline control (*p<0.05, **p<0.01, ***p<0.001, One-way ANOVA with Bonferroni post-tests)
EtOHs Effect on WIN-Induced Behaviors Is Reversible
Two separate groups of mice were administered either saline or 4.0 g/kg EtOH twice daily for ten days followed by a 10-day no-treatment period. On day 20, baseline measures of body temperature and hot plate latency were assessed prior to measurement of WIN-induced hypolocomotion, antinociception, hypothermia, and catalepsy. To determine if there was an interaction between days of recovery and treatment, tetrad data from day 11 and day 20 for the saline and 4.0 g/kg EtOH treatments were analyzed by two-way ANOVAs (Figure 4). Data from the saline and 4.0 g/kg groups in the previous experiment (Figure 3) were used in this comparison and are show in Figure 4 as Day 11. As previously mentioned, one day after the repeated EtOH treatment, WIN’s effects on locomotor activity, nociception, and body temperature were significantly blunted (Bonferroni post-tests, p<0.001). There was a significant interaction of day and treatment for measurements of locomotor activity (p<0.05) and body temperature (p<0.01). There was a significant main effect of treatment (p<0.05) but not for day for locomotor activity, and there were significant main effects of treatment (p<0.0001) and day (p<0.01) for body temperature. There was no overall interaction between day and treatment on WIN-induced antinociception (p=0.1141), but there was a significant main effect of treatment (p<0.0001) that was probably driven by the difference in WIN-sensitivity between EtOH and saline mice tested on Day 11 of the study. Furthermore, like other tetrad behaviors tested on day 20 of the experiment, there was no difference between EtOH and saline treated mice with regard to their sensitivity to the antinociceptive effects of WIN (Bonferroni post-test, p>0.05). These data suggest that the change in WIN sensitivity following EtOH treatment reverses within a 10-day recovery period. However, there was no overall interaction between treatment and day on WIN-induced catalepsy (p=0.0582) although EtOH-treated mice showed a trend to being more cataleptic than controls on Day 20 (Bonferroni post-test, p=0.0591).
Fig. 4.
Effects of repeated 4.0 g/kg EtOH treatments on WIN-Induced behaviors are reversible. Bars represent the mean (± SEM; N=8–13) value for each measure. The scales on the ordinates are expressed as number of beam breaks per session (locomotor activity), percent maximum possible effect (antinociception), change in body temperature (hypothermia), and percent immobility on the ring stand (catalepsy). Values on the abscissae represent the day behavioral testing was performed. Symbol: value significantly different from corresponding saline control (***p<0.001, Two-way ANOVA with Bonferroni post-test)
CB1 Protein Expression is Altered in HyTh, VTA, & PAG Following 10-day EtOH Treatment
Previous studies have reported decreases in CB1 protein (Mitrirattanakul et al. 2007), receptor binding (Basavarajappa et al. 1998; Vinod et al. 2006), and G-protein cycling (Basavarajappa and Hungund 1999b) in rodents chronically exposed to EtOH. Western blot analysis was used investigate whether the 10-day EtOH treatment used in the present study also induced changes in CB1 receptor expression. Anti-CB1 antibody specificity was confirmed in western blots using crude membrane fractions from both wild-type and CB1 KO mice. In wild-type mice, all brain regions tested showed a band that corresponded to the predicted molecular weight of full-length CB1 (≈52 kDa). This band was completely absent in samples from CB1 KO mice (Figure 5A) confirming antibody specificity. Experiments assessing changes to CB1 expression used tissue collected from mice after determination of BECs following repeated EtOH injections. These animals were not used for behavioral measures of the tetrad as WIN administration by itself can down-regulate levels of CB1 protein (Martini et al. 2010; Martini et al. 2007; Tappe-Theodor et al. 2007). As shown in the summary panels (Figure 5B), CB1R levels were altered in a region-dependent manner in animals treated chronically with ethanol. Ethanol decreased CB1 levels in HyTh while it increased CB1 expression in PAG and VTA. There was a significant main effect of EtOH treatment in the VTA (Two-way ANOVA, p<0.05) attributable to a significant increase in CB1 protein expression in the EtOH-treated group on day 11 (Bonferroni post-test, p<0.05) that returned to control levels by day 20. In the HyTh, there was a significant overall interaction (Two-way ANOVA, p<0.05) and main effects of EtOH treatment (p<0.01) and day (p<0.05). This was due to a significant decrease in CB1 protein on day 11 (Bonferroni post-test, p<0.01) that returned to baseline levels on day 20. In the PAG, there was a significant main effect of EtOH treatment to increase CB1 protein expression on days 11 and 20 (Two-way ANOVA, p<0.05). Finally, there was a trend for interaction between treatment and day on CB1 protein expression in the Cereb (Two-way ANOVA, p=0.0522) with a reduction in CB1 protein on day 11. While EtOH treatment appeared to reduce CB1 levels in other brain regions in a time-dependent manner (frontal cortex, DStr, NAc), these changes were not statistically significant.
Fig. 5.
Effects of repeated EtOH treatments on expression of CB1 receptor protein. A) Representative gel showing immunoreactive band corresponding to CB1 receptor (MW≈52 kDa) in wild-type (WT) that is lacking in knockout (KO) mice. B) Quantification of CB1 receptor expression in saline and EtOH treated animals. Bars show mean (± SEM, N=4–5) fraction of control for optical density of CB1R immunoreactive band for each region shown. Values on the abscissae represent the day tissue was havested. Representative blots for each region are shown below the corresponding graph. Symbols: significant interaction (§) and main effect of treatment (†), p<0.05, two-way ANOVA; value significantly different from corresponding saline control (*p<0.05, **p<0.01, Two-way ANOVA with Bonferroni post-test)
Discussion
The major finding of this study is that a 10-day treatment of mice with EtOH reduces the hypolocomotive, antinociceptive, and hypothermic effects of an acute dose of the cannabinoid agonist WIN. The magnitude of this response was dependent on the dose of EtOH, and importantly, was selective as no effects of EtOH were seen for WIN-induced catalepsy. In addition, the effects of EtOH treatment on WIN-induced hypolocomotion, antinociception, and hypothermia were reversible and returned to baseline levels 10-days after the last ethanol treatment. Finally, the changes in WIN-induced behaviors following the 10-day EtOH treatment protocol were associated with altered CB1 receptor expression in HyTh, PAG, and VTA.
Repeated Exposures to EtOH Alters the Behavioral Response to WIN
A behavioral interaction between EtOH and CB1 agonists has been previously reported but these studies are limited to measures of ataxia or shock avoidance (da Silva et al. 2001; Lemos et al. 2007; Newman et al. 1974; Newman et al. 1972; Siemens and Doyle 1979; Sprague and Craigmill 1976). In addition, considerable work has demonstrated a clear effect of alcohol on CB1 receptor expression/binding and EC signaling in both in vitro and in vivo studies (Basavarajappa et al. 1998; Basavarajappa and Hungund 1999a; Basavarajappa and Hungund 1999b; Basavarajappa et al. 2000; Basavarajappa et al. 2003; Caillé et al. 2007; Ferrer et al. 2007; Mitrirattanakul et al. 2007; Vinod et al. 2006). These data suggest that alcohol treatment, by altering CB1 expression, should induce changes in the sensitivity of mice to other CB1 mediated behaviors. In the present study, 10 days of twice-daily EtOH injections significantly attenuated the effect of the CB1 agonist WIN on three of the four measured behaviors. To our knowledge, these data are the first to show that there is behavioral specificity with respect to the effects of chronic EtOH on cannabinoid-induced behaviors.
While WIN is a highly potent agonist at CB1 receptors, it can also activate CB2 receptors, and more selective CB2 agonists are known to have analgesic properties in certain models of inflammatory and neuropathic pain (Curto-Reyes et al. 2010; Jhaveri et al. 2007; Rahn et al. 2008). However, the role CB2 plays in mediating the antinociceptive effects of WIN to acute pain is somewhat contentious. One study using CB1 and CB2 KO mice found the acute antinociceptive effects of WIN were due exclusively to its effects at CB2 (Ibrahim et al. 2006). However, the doses of WIN used to test CB1 KO mice in that study were an order of magnitude lower than those used to test CB2 KOs making it difficult to truly compare genotypic differences. In contrast, mice with a targeted deletion of CB1 in the periphery that spared central CB1 receptors demonstrated a suppression of WIN-induced antinociception at the 3.0 mg/kg dose (Agarwal et al. 2007). These findings strongly suggest that the antinociceptive effects of WIN observed in the present work resulted from activation of the CB1 receptor.
Several observations suggest that the reduction in WIN-induced behaviors following EtOH treatment are likely to result from changes in pharmacodynamic rather than pharmacokinetic processes. If EtOH treatment enhanced metabolism of WIN, then all behaviors should be similarly affected; a finding that was not observed in the present study as measures of WIN-induced catalepsy were unaffected. In addition, the decreased responsiveness of EtOH-treated mice to WIN was dependent on the dose of EtOH administered over the 10-day treatment, but the magnitude of this effect varied between measures of locomotor activity, nociception, and body temperature. For measures of nociception, there was a significant difference in the sensitivity to WIN between all treatment groups. However, cross-tolerance to the hypolocomotive effects of WIN were only observed in mice treated with 4.0 g/kg EtOH, while the tolerance to WIN-induced hypothermia was not different between subjects given 2.0 or 4.0 g/kg EtOH. This suggests that cross-tolerance to the hypothermic effects of WIN reaches its upper threshold at lower doses of EtOH than do the other behaviors. In other words, WIN-induced hypothermia may be more sensitive to pre-treatment with EtOH than other measures. These data argue that changes in CB1 receptor expression or function underlie the development of tolerance to the WIN-induced behaviors following EtOH treatment. They also suggest that that the mechanisms and brain regions underlying these changes are differentially sensitive to EtOH treatment.
The Reversibility of EtOH-induced Changes in WIN Sensitivity
Results from previous studies indicate that changes in CB1 expression following chronic EtOH treatment are reversible. For example, CB1 protein expression in rat hippocampus is reduced after ethanol exposure and this change recovers during withdrawal (Mitrirattanakul et al. 2007). Similarly, ethanol-induced decreases in CB1 binding in mouse cortex, hippocampus, cerebellum, and striatum revert to control levels over a 24 hr withdrawal period (Vinod et al. 2006). In contrast, the results from the present study indicate that the effects of the CB1 agonist on tetrad behaviors remain attenuated one day following the last EtOH injection. This indicates that the 10-day treatment regimen produces longer lasting effects on CB1 expression than those observed after 3 days of continuous vapor inhalation (Vinod et al. 2006). Following ten days of recovery from the ethanol treatment, we found a significant interaction between the recovery time and treatment on WIN-induced hypolocomotion and hypothermia. Importantly, there was no interaction between these factors for WIN-induced antinociception, and the EtOH-treated mice seemed to return to baseline levels more slowly for this measure when compared to locomotor activity and body temperature. These results suggest that over the 10-day recovery period, CB1 receptor expression or function may return to control values in a region-dependent manner.
Repeated EtOH Treatment Alters CB1 Expression in Brain Nuclei that Underlie Tetrad Behaviors
In the present study, EtOH treated mice showed alterations in CB1 receptor expression that varied across brain regions. For example, the trend for decreased CB1 expression in the cerebellum of EtOH-treated mice suggests that the cross-tolerance that develops to the ataxic effects of cannabinoids following chronic ethanol exposure (da Silva et al. 2001; Lemos et al. 2007; Siemens and Doyle 1979; Sprague and Craigmill 1976) may be due to decreased expression of cerebellar CB1 receptors. Similarly, EtOH treatment decreased expression of CB1 protein in the hypothalamus and levels recovered to control values over the 10-day recovery period. These changes mirror the attenuation and recovery of WIN-induced hypothermia observed in EtOH-treated mice. As the anterior preoptic region of the hypothalamus is strongly implicated in mediating the hypothermic effects of CB1 agonists (Fitton and Pertwee 1982; Rawls et al. 2002), these findings support the idea that the decrease in WIN-induced hypothermia following EtOH treatment follows the down-regulation of CB1 receptors in this area.
Cannabinoid-mediated catalepsy is thought to reflect activation of CB1 receptors in the dorsal striatum (Gough and Olley 1977; 1978), although the PAG has also been implicated (Lichtman et al. 1996). In the present study, EtOH treatment increased CB1 protein expression in the PAG and these mice showed a trend towards enhanced sensitivity to the cataleptic effects of WIN. Paradoxically, WIN-induced antinociception, typically thought to involve the PAG, was reduced in EtOH-treated subjects, and this effect only partially returned to baseline levels following the recovery period. Although these findings seem to be at odds, the brain nuclei associated with the antinociceptive effects of cannabinoids are numerous and widespread. In addition to the PAG (Lichtman et al. 1996; Martin et al. 1995), the rostral ventromedial medulla (Martin et al. 1998), amygdala, lateral posterior and submedial nuclei of the thalamus, superior colliculus, and noradrenergic A5 region are also thought to be involved in mediating the antinoiceptive effects of CB1 agonists (Martin et al. 1999). It is possible that reduced expression of CB1 receptors in one or more of these supraspinal structures, in the spinal cord itself (Lichtman and Martin 1991), or in the periphery (Agarwal et al. 2007) could underlie the attenuation in WIN-induced antinociception following EtOH treatment.
While cannabinoids display antinociptive properties in and of themselves, the cannabinoid system also has substantial interactions with the opioid system. This occurs both via the formation of heterodimers between CB1 and the μ-opioid receptor (Hojo et al. 2008) as well as through down-stream modulation of Gαz subunits. This latter mechanism contributes to the analgesic cross-tolerance that forms between opioid and cannabinoid drugs (Garzón et al. 2009). In addition, cross-tolerance has been reported for the antinociceptive effects of ethanol and morphine (Malec et al. 1987). Although WIN is not known to directly interact with the μ-opioid receptor, it is possible that EtOH-induced alterations in μ-opioid expression or in shared signaling pathways between CB1 and the μ receptor may contribute to the cross-tolerance to WIN-induced antinociception observed in this study.
The neural substrates associated with cannabinoid-induced hypolocomotion are perhaps the least well studied of the tetrad behaviors. However, recent work indicates that cannabinoids may reduce locomotor activity via a striatal mechanism that involves A2A receptors (Carriba et al. 2007; Lerner et al. 2010). In the present study, there were no significant changes in CB1 expression in tissue punches containing either the DStr or NAc in ethanol treated mice. Although these results are limited by modest anatomical resolution of the western blot studies, it is possible that decreased CB1 expression in a sub-population of neurons (e.g. indirect pathway cells) could be masked by increased expression in another cell population.
Lastly, there was a significant increase in CB1 protein expression in the VTA 24 hr following the chronic EtOH treatment, and levels returned to control values over the recovery period. The effect of chronic EtOH on VTA CB1 expression is particularly interesting given the high prevalence of co-abuse of EtOH and marijuana in humans mentioned earlier. These findings suggest that chronic ethanol exposure may enhance the rewarding properties of cannabinoids by increasing the sensitivity of VTA neurons to CB1 agonists. Conversely, cannabinoids are known to mediate certain aspects of ethanol reward and consumption. For example, CB1 antagonists including rimonabant (SR141716A) block EtOH-induced increases in the firing rate of VTA dopamine (DA) neurons (Perra et al. 2005), and CB1 KO mice show reduced place conditioning for EtOH (Houchi et al. 2005). CB1 KO mice also consume less alcohol in a two-bottle choice paradigm, and unlike control animals, they do not show an increased release of DA in the NAc following acute EtOH administration (Hungund et al. 2003). Interestingly, mice that undergo repeated cycles of chronic ethanol vapor exposure and withdrawal that results in dependence show increased voluntary EtOH consumption (Becker and Lopez 2004; Griffin et al. 2009; Lopez and Becker 2005). Combined with the data from the present study, these results suggest the possibility that increases in CB1-mediated expression and signaling in VTA neurons may underlie this change in drinking.
In conclusion, the results of this study expand upon previous work demonstrating an interaction between the ataxic effects of EtOH and cannabinoids. We demonstrate for the first time that repeated EtOH treatment results in decreased sensitivity to the hypolocomotive, antinociceptive, and hypothermic effects of the CB1 agonist WIN. Furthermore, our data indicate that these effects are accompanied by alterations in CB1 protein expression in neural substrates that are thought to underlie these behaviors. Future studies to determine the mechanism behind the observed changes in CB1 receptor expression following CET will contribute to the basic understanding of physiological processes underlying alcohol abuse and may provide insight into novel therapeutic targets.
Acknowledgments
This work was supported by National Institutes of Health Grants P50AA10761 (Charleston Alcohol Research Center; JJW), F31AA018908 (MJP), and R00AA017922 (PJM). The L15 antibody was a kind gift of Dr. Ken Mackie (NIH Grant DA011322).
Footnotes
Disclosure/Conflicts of Interest: The authors declare no conflicts of interest.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nature Neuroscience. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. The use of total protein stains as loading controls: an alternative to high-abundance single-protein controls in semi-quantitative immunoblotting. J Neurosci Methods. 2008;172:250–254. doi: 10.1016/j.jneumeth.2008.05.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon AW, Malmberg AB. Models of nociception: hot-plate, tail-flick, and formalin tests in rodents Current protocols in neuroscience / editorial board, Jacqueline N Crawley [et al] Chapter 8. 2007 doi: 10.1002/0471142301.ns0809s41. Unit 8.9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999a;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTP gamma S binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999b;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2- arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur J Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriba P, Ortiz O, Patkar K, Justinova Z, Stroik J, Themann A, Müller C, Woods AS, Hope BT, Ciruela F, Casadó V, Canela EI, Lluis C, Goldberg SR, Moratalla R, Franco R, Ferré S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32:2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther. 1992;263:1118–1126. [PubMed] [Google Scholar]
- Curto-Reyes V, Llames S, Hidalgo A, Menéndez L, Baamonde A. Spinal and peripheral analgesic effects of the CB2 cannabinoid receptor agonist AM1241 in two models of bone cancer-induced pain. British Journal of Pharmacology. 2010;160:561–573. doi: 10.1111/j.1476-5381.2009.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva GE, Morato GS, Takahashi RN. Rapid tolerance to Delta(9)-tetrahydrocannabinol and cross-tolerance between ethanol and Delta(9)-tetrahydrocannabinol in mice. Eur J Pharmacol. 2001;431:201–207. doi: 10.1016/s0014-2999(01)01449-2. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta 9- tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Ferrer B, Bermúdez-Silva FJ, Bilbao A, Alvarez-Jaimes L, Sanchez-Vera I, Giuffrida A, Serrano A, Baixeras E, Khaturia S, Navarro M, Parsons LH, Piomelli D, Rodríguez de Fonseca F. Regulation of brain anandamide by acute administration of ethanol. Biochem J. 2007;404:97–104. doi: 10.1042/BJ20061898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitton AG, Pertwee RG. Changes in body temperature and oxygen consumption rate of conscious mice produced by intrahypothalamic and intracerebroventricular injections of delta 9- tetrahydrocannabinol. British Journal of Pharmacology. 1982;75:409–414. doi: 10.1111/j.1476-5381.1982.tb08802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garzón J, de la Torre-Madrid E, Rodríguez-Muñoz M, Vicente-Sánchez A, Sánchez-Blázquez P. Gz mediates the long-lasting desensitization of brain CB1 receptors and is essential for cross-tolerance with morphine. Molecular pain. 2009;5:11. doi: 10.1186/1744-8069-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough AL, Olley JE. delta9-Tetrahydrocannabinol and the extrapyramidal system. Psychopharmacology (Berl) 1977;54:87–99. doi: 10.1007/BF00426547. [DOI] [PubMed] [Google Scholar]
- Gough AL, Olley JE. Catalepsy induced by intrastriatal injections of delta9-THC and 11-OH-delta9- THC in the rat. Neuropharmacology. 1978;17:137–144. doi: 10.1016/0028-3908(78)90126-0. [DOI] [PubMed] [Google Scholar]
- Griffin WC, Lopez MF, Yanke AB, Middaugh LD, Becker HC. Repeated cycles of chronic intermittent ethanol exposure in mice increases voluntary ethanol drinking and ethanol concentrations in the nucleus accumbens. Psychopharmacology (Berl) 2009;201:569–580. doi: 10.1007/s00213-008-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor electrophysiological and FRET assay analysis. Journal of pharmacological sciences. 2008;108:308–319. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- Houchi H, Babovic D, Pierrefiche O, Ledent C, Daoust M, Naassila M. CB1 receptor knockout mice display reduced ethanol-induced conditioned place preference and increased striatal dopamine D2 receptors. Neuropsychopharmacology. 2005;30:339–349. doi: 10.1038/sj.npp.1300568. [DOI] [PubMed] [Google Scholar]
- Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Rude ML, Stagg NJ, Mata HP, Lai J, Vanderah TW, Porreca F, Buckley NE, Makriyannis A, Malan TP. CB2 cannabinoid receptor mediation of antinociception. Pain. 2006;122:36–42. doi: 10.1016/j.pain.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Jhaveri MD, Sagar DR, Elmes SJR, Kendal DA, Chapman V. Cannabinoid CB2 receptor-mediated anti-nociception in models of acute and chronic pain. Molecular neurobiology. 2007;36:26–35. doi: 10.1007/s12035-007-8007-7. [DOI] [PubMed] [Google Scholar]
- Lemos JI, Takahashi RN, Morato GS. Effects of SR141716 and WIN 55,212-2 on tolerance to ethanol in rats using the acute and rapid procedures. Psychopharmacology (Berl) 2007;194:139–149. doi: 10.1007/s00213-007-0804-1. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Horne EA, Stella N, Kreitzer AC. Endocannabinoid signaling mediates psychomotor activation by adenosine A2A antagonists. J Neurosci. 2010;30:2160–2164. doi: 10.1523/JNEUROSCI.5844-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Cook SA, Martin BR. Investigation of brain sites mediating cannabinoid-induced antinociception in rats: evidence supporting periaqueductal gray involvement. J Pharmacol Exp Ther. 1996;276:585–593. [PubMed] [Google Scholar]
- Lichtman AH, Martin BR. Spinal and supraspinal components of cannabinoid-induced antinociception. J Pharmacol Exp Ther. 1991;258:517–523. [PubMed] [Google Scholar]
- Lopez MF, Becker HC. Effect of pattern and number of chronic ethanol exposures on subsequent voluntary ethanol intake in C57BL/6J mice. Psychopharmacology (Berl) 2005;181:688–696. doi: 10.1007/s00213-005-0026-3. [DOI] [PubMed] [Google Scholar]
- MacAvoy MG, Marks DF. Divided attention performance of cannabis users and non-users following cannabis and alcohol. Psychopharmacology (Berl) 1975;44:147–152. doi: 10.1007/BF00445566. [DOI] [PubMed] [Google Scholar]
- Malec D, Kotlińska J, Langwiński R. Cross-tolerance between morphine and ethanol and their antinociceptive effects. Journal of studies on alcohol. 1987;48:507–510. doi: 10.15288/jsa.1987.48.507. [DOI] [PubMed] [Google Scholar]
- Martin BR, Compton DR, Thomas BF, Prescott WR, Little PJ, Razdan RK, Johnson MR, Melvin LS, Mechoulam R, Ward SJ. Behavioral, biochemical, and molecular modeling evaluations of cannabinoid analogs. Pharmacol Biochem Behav. 1991;40:471–478. doi: 10.1016/0091-3057(91)90349-7. [DOI] [PubMed] [Google Scholar]
- Martin W, Patrick S, Coffin P, Tsou K, Walker J. … examination of the central sites of action of cannabinoid-induced antinociception …. Life Sci. 1995 doi: 10.1016/0024-3205(95)00195-c. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Coffin PO, Attias E, Balinsky M, Tsou K, Walker JM. Anatomical basis for cannabinoid-induced antinociception as revealed by intracerebral microinjections. Brain Res. 1999;822:237–242. doi: 10.1016/s0006-8993(98)01368-7. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Tsou K, Walker JM. Cannabinoid receptor-mediated inhibition of the rat tail-flick reflex after microinjection into the rostral ventromedial medulla. Neurosci Lett. 1998;242:33–36. doi: 10.1016/s0304-3940(98)00044-5. [DOI] [PubMed] [Google Scholar]
- Martini L, Thompson D, Kharazia V, Whistler JL. Differential regulation of behavioral tolerance to WIN55,212-2 by GASP1. Neuropsychopharmacology. 2010;35:1363–1373. doi: 10.1038/npp.2010.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini L, Waldhoer M, Pusch M, Kharazia V, Fong J, Lee JH, Freissmuth C, Whistler JL. Ligand-induced down-regulation of the cannabinoid 1 receptor is mediated by the G-protein-coupled receptor-associated sorting protein GASP1. FASEB J. 2007;21:802–811. doi: 10.1096/fj.06-7132com. [DOI] [PubMed] [Google Scholar]
- Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, Spigelman I. Bidirectional alterations of hippocampal cannabinoid 1 receptors and their endogenous ligands in a rat model of alcohol withdrawal and dependence. Alcohol Clin Exp Res. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ. Inhibition of glutamate transporters couples to Kv4.2 dephosphorylation through activation of extrasynaptic NMDA receptors. Neuroscience. 2010;165:130–137. doi: 10.1016/j.neuroscience.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman LM, Lutz MP, Domino EF. Delta9-tetrahydrocannabinol and some CNS depressants: evidence for cross-tolerance in the rat. Archives internationales de pharmacodynamie et de thérapie. 1974;207:254–259. [PubMed] [Google Scholar]
- Newman LM, Lutz MP, Gould MH, Domino EF. 9 -Tetrahydrocannabinol and ethyl alcohol: evidence for cross-tolerance in the rat. Science. 1972;175:1022–1023. doi: 10.1126/science.175.4025.1022. [DOI] [PubMed] [Google Scholar]
- NIAAA. Five year strategic plan. 2009 FY 09-14.110. [Google Scholar]
- Perra S, Pillolla G, Melis M, Muntoni AL, Gessa GL, Pistis M. Involvement of the endogenous cannabinoid system in the effects of alcohol in the mesolimbic reward circuit: electrophysiological evidence in vivo. Psychopharmacology (Berl) 2005;183:368–377. doi: 10.1007/s00213-005-0195-0. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The ring test: a quantitative method for assessing the 'cataleptic' effect of cannabis in mice. Br J Pharmacol. 1972;46:753–763. doi: 10.1111/j.1476-5381.1972.tb06900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe L, Iaccheri E, Manzati C. Enzymic ethanol assay: a new colorimetric method based on measurement of hydrogen peroxide. Clinical Chemistry. 1987;33:486–489. [PubMed] [Google Scholar]
- Rahn EJ, Zvonok AM, Thakur GA, Khanolkar AD, Makriyannis A, Hohmann AG. Selective activation of cannabinoid CB2 receptors suppresses neuropathic nociception induced by treatment with the chemotherapeutic agent paclitaxel in rats. The Journal of pharmacology and experimental therapeutics. 2008;327:584–591. doi: 10.1124/jpet.108.141994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawls SM, Cabassa J, Geller EB, Adler MW. CB1 receptors in the preoptic anterior hypothalamus regulate WIN 55212-2[(4,5-dihydro-2-methyl-4(4-morpholinylmethyl)-1-(1-naphthalenyl carbonyl)-6H-pyrrolo[3,2,1ij]quinolin-6-one]-induced hypothermia. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- Siemens AJ, Doyle OL. Cross-tolerance between delta9-tetrahydrocannabinol and ethanol: the role of drug disposition. Pharmacol Biochem Behav. 1979;10:49–55. doi: 10.1016/0091-3057(79)90168-0. [DOI] [PubMed] [Google Scholar]
- Sprague GL, Craigmill AL. Ethanol and delta-9-tetrahydrocannabinol: mechanism for cross-tolerance in mice. Pharmacol Biochem Behav. 1976;5:409–415. doi: 10.1016/0091-3057(76)90104-0. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, Swiercz J, Mackie K, Monyer H, Parolaro D, Whistler J, Kuner T, Kuner R. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–4177. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Xie S, Cooper TB, Hungund BL. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochem Int. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]