Abstract
FadA, a novel adhesin of periodontal pathogen Fusobacterium nucleatum is composed of two forms, pre-FadA and mature FadA (mFadA), constituting the functional FadA complex (FadAc). By electron microscopy, we observed that mFadA formed uniformly long and thin filaments, while FadAc formed heterogeneous filaments of varying lengths and widths, as well as “knots”. Mutants in signal peptide or in the non-alpha helical loop retaining heterogeneous structures had binding activity while those forming aggregates or long filaments lost activity. These observations suggest short filaments and knots may be the active forms of FadA. This is the first demonstration that a signal peptide is required for the assembly of a bacterial adhesin.
1-Introduction
Fusobacterium nucleatum is one of the most abundant gram-negative bacilli colonizing the subgingival plaque (11) and one of the most prevalent species detected in intra-amniotic cavities, associated with pregnancy complications including preterm birth and stillbirth (3, 7). F. nucleatum can enter the blood circulation as a result of periodontal infection (dental bacteremia) and spread hematogenously to the pregnant uterus (3). Hematogenous infection of pregnant mice led to colonization of the feto-placental unit and eventually fetal demise (6, 10). F. nucleatum adheres to and invades host epithelial and endothelial cells (5, 8). It also promotes the invasion of epithelial cells by non-invasive bacteria (1). Host cell attachment and invasion play a critical role in F. nucleatum in intrauterine infection (6, 9). A novel adhesin, FadA, was identified to be involved in F. nucleatum attachment and invasion to host cells (4, 5). A fadA-deletion mutant, F. nucleatum 12230-US1, was defective in host-cell attachment and invasion as well as colonization murine placenta (5). Complementation with FadA restores host-cell adhesion and invasion, and colonization of the placenta (9). FadA is highly conserved among oral fusobacteria species (5). It exists in two forms, the non-secreted pre-FadA, consisting of 129 amino acids, and the secreted mature FadA (mFadA), consisting of 111 amino acids (13) (Supplementary Figure). Pre-FadA possess a signal peptide of 18 amino acids (MKKFLLLAVLAVSASAFA) (5). Pre-FadA is insoluble under neutral pH, unless it is mixed with mFadA (13). An inhibitory cell attachment assay shows that the pre-FadA-mFadA complex (FadAc) inhibited the attachment of F. nucleatum to host cells, while mFadA alone did not (13). This observation suggests a potentially novel role of the signal peptide. However, it was unclear how the signal peptide was involved in FadA structure and function.
The crystal structure of mFadA reveals an infinite filament with the monomers linked together in a head-to-tail pattern via a novel leucine chain motif (12). The only non-alpha helical loop is exposed at the tip of the filament. The aim of this study was to examine the role of the non-alpha helical loop region and the signal peptide in the structure and function of FadA. Our results demonstrate and that a proper oligomerization of the FadA protein is essential for its function. It also appears that the signal peptide determines the length and width of the filament and that short filaments and knots may be the active form.
2-Material and Methods
2.1-Bacterial strains and cell lines
E. coli BL21(DE3)-pYWH417-6 carrying the entire fadA gene in the pET21(b) expression vector (Novagen) with a His-tag fusion at the carboxyl terminal was used to produce FadAc (13). E. coli BL21(DE3)-pYH1490, carrying fadA without the signal peptide in pET21(b), was used to produce mFadA alone. E. coli strains were cultured aerobically in Luria Bertani broth or agar (BD Diagnostics). F. nucleatum 12230 was cultured as described previously (2). For attachment assay, F. nucleatum was sub-cultured on the day of the test and allowed to grow anaerobically for 3.5 hr at 37°C. Human Umbilical Vein Endothelial cells (HUVEC) and Chinese Hamster Ovarian (CHO) cells were cultured in F12-K supplemented with 10% fetal bovine serum (Invitrogen) and 100μg/ml Streptomycin-100U/ml Penicillin (Invitrogen). In addition, a total of 30μg/ml Endothelial Cell Growth Supplements (BD Biosciences) and 0.1mg/ml heparin (Sigma) were added for HUVEC and 1mM sodium pyruvate (Mediatech Inc.) for CHO cells.
2.2-Construction of FadA variants
The FadA variants were constructed using the QuickChange®II Site-Directed Mutagenesis Kit (Stratagene) using the primers listed in supplementary Table. Plasmids were purified, verified by PCR, and sequenced at the Genomics Core Facility (CWRU, OH). Correct plasmids were transformed into E. coli BL21(DE3) for protein expression.
2.3-Purification and western-blot analysis
Wild-type and variant recombinant FadAc were expressed and purified from E. coli BL21(DE3)-pYWH417-6 and its derivatives using denaturing conditions as previously described (13). The mFadA alone was expressed and purified from E. coli BL21(DE3)-pYH1490 using either denaturing or native conditions as previously described (13). The protein concentration was determined using the BCA kit (Pierce Biotechnology).
Protein samples were loaded onto 12% SDS-polyacrylamide gels and transferred to Immobilon-Psq PVDF membranes (Millipore). FadA-His tag fusion proteins were detected using FadA monoclonal antibody 5G11-3G8 (13) (1:2000 dilution) and horseradish peroxidase-conjugated goat anti-mouse IgG (1:2000 dilution; Pierce Biotechnology), followed by chemiluminescence using SuperSignal West Dura Extended Duration Substrate (Pierce Biotechnology).
2.4-Transmission electron microscopy
A total of 7.5 μg of FadA protein was mounted on Formvar carbon support Nickel grid (Electron Microscopy Science), incubated at room temperature for 5 min and stained with 2% uranyl acetate at room temperature for 1 min. Unmounted proteins and excess dye were removed, and the specimen grid was dried at room temperature for 16 hours. Observation was carried out as previously described, using Jeol JEM-1200EX or Zeiss Libra 200FE electron transmission microscope with 80kV electron acceleration voltage (13).
2.5-Inhibitory cell attachment assays
CHO and HUVEC cells were seeded in 24-well plates (BD Falcon) at 4×105 and 8×104 cells/well, respectively. FadA proteins (50μg/ml) were pre-incubated on the confluent monolayer for 45 min, prior to the addition of F. nucleatum at a multiplicity of infection (MOI) of 5. After one-hour incubation, the monolayers were washed 6 times with PBS pH7.4 (Invitrogen) for CHO cells or 3 times with D-PBS pH7.1 supplemented with Ca2+ and Mg2+ (Invitrogen) for HUVEC and then lysed with water. Attached bacteria were enumerated by plating serial dilutions on blood agar plates. The level of attachment was expressed as the percentage of bacteria recovered following cell lysis relative to the total number of bacteria initially added. Each experiment was performed in duplicates or more and repeated at least three times.
2.6-Statistical analysis
Attachment assays were analyzed with an analysis of variance followed by a Dunnett's multiple comparison test. These statistical tests were performed with InStat software (GraphPad).
3-Results and Discussion
FadA is a unique protein involved in F. nucleatum attachment and invasion of host cells. In this study, we have investigated the role of the non-alpha helical region and the signal peptide in its structure and function.
3.1-Construction and purifications of the FadA mutants
The crystal structure of mFadA has revealed a filamentous structure with the monomers linked in a head-to-tail pattern (12). We surmise that in vivo the only exposed non-alpha helical region, i.e. the loop, would be at the tip of the filament, which may be involved in the binding to the host-cell receptor. The crystal structure also showed that the leucine residues are involved in inter- and intra-molecular interactions (12). We speculated that intermolecular interactions involving leucine residues in the signal peptide may also be involved in the proper formation of the FadAc filaments. To test these hypotheses, eight amino-acid residues, E63, N65, T66, R67, F68, Y69, K70 and S71, in the loop region and four in the signal peptide, L-9, L-12, L-13 and L-14, were replaced by alanine by site-directed mutagenesis to avoid disruption of the alpha helical structure, if there is any. Wild-type and mutant proteins were purified by cobalt affinity column chromatography without separating pre-FadA and mFadA, thus producing pre-FadA-mFadA complex (FadAc). Wild-type mFadA was purified from E. coli BL21(DE3)-pYWH1490 expressing mFadA alone. The stability of the mutant proteins was examined by Western-blot. Of all 12 mutants constructed, two in the signal peptide (L-9A and L-12A) and four in the loop region (F68A, Y69A, K70A and S71A) produced stable proteins adequate for analysis. Alanine substitution of the other residues, L-13 and L-14 in the signal peptide and E63, N65, T66 and R67 in the loop region, resulted in no protein recovery even under denaturing conditions although they were detectable in total bacterial extract (data not shown). These mutations may affect the stability of FadA. Alternatively, mutations at L-13 and L-14 may affect FadA secretion. These mutants were not analyzed further.
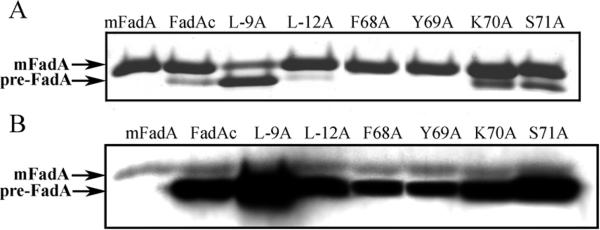
The stably expressed mutant proteins were purified and analyzed by SDS-PAGE followed by Coomassie blue staining and Western-blot. Little or no pre-FadA was detected from L-12A, F68A, or Y69A by Coomassie blue staining (Figure 1A). However, both pre-FadA and mFadA were detected from all mutants by Western-blot (Figure 1B). This was likely due to that the monoclonal anti-FadA antibodies detected pre-FadA with much higher sensitivity than mFadA as previously observed (13). Compared to the wild-type, L-9A expressed more pre-FadA and less mFadA, whereas L-12A, F68A, and Y69A each expressed less pre-FadA and comparable mFadA (Figure 1B). In K70A and S71A, both pre-FadA and mFadA were expressed at similar levels as the wild type.
Figure 1.
Analysis of mutated FadA. The pre-FadA and mFadA were co-purified and 3 μg of proteins per lane were analyzed by Coomassie Blue staining (A) or 2 μg by Western-blot with FadA monoclonal antibody Ab5G11-3G8 (B). The migration of FadA on SDS-PAGE is abnormal as the larger pre-FadA migrates faster than the smaller mFadA (13).
3.2-Activity and structure of the mutants
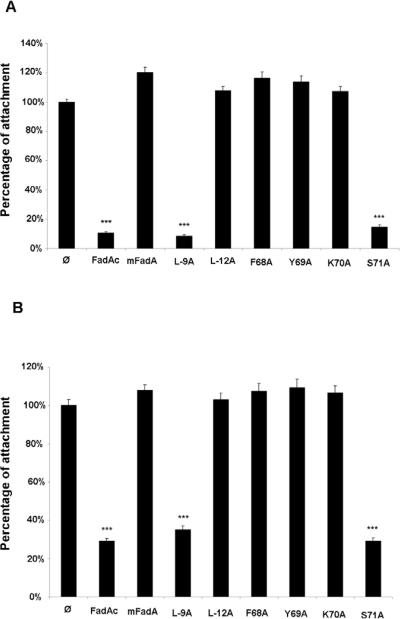
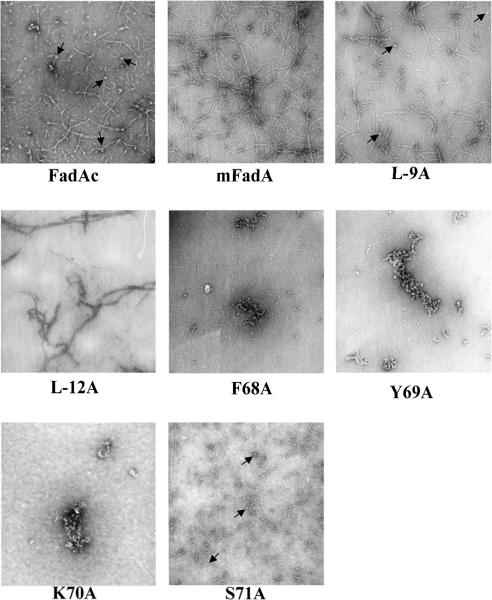
The mutant proteins’ activity was analyzed in an inhibitory cell attachment assay using CHO (Figure 2A) and HUVEC cells (Figure 2B) and their structure analyzed by transmission electron microscopy (Figure 3). For the first time, we visualized the striking difference of the filaments formed by FadAc and mFadA. The FadAc filaments were highly heterogeneous, with varying lengths and widths as well as filaments-associated “knots”, whereas the mFadA filaments were homogenously long and thin, consistent with what was observed in the mFadA crystal structure. The heterogeneous FadAc filaments exhibited binding activities with an inhibition of the attachment of F. nucleatum by approximately 90% to CHO cells and by 70% to HUVEC, while pre-incubation with the homogenous mFadA filaments did not. These results indicate that the signal peptide plays a unique role in FadA structure and function by affecting the length and width (bundling) of the filament.
Figure 2.
Cell-attachment inhibition assay using CHO (A) and HUVEC cells (B). A total of 50μg/ml of FadA proteins were incubated for 45 min prior to the addition of Fusobacterium nucleatum to the confluent monolayers at a MOI of 5. After 1 hour incubation, cells were washed, lysed and a viable count was performed on attached bacteria. Total cell-associated F. nucleatum was arbitrary set to 100% and that in the presence of the proteins was compared to it. Cell attachment inhibition assays results represent an average of three experiments performed at least in duplicate.
Figure 3.
Transmission electron microscopy pictures of FadA mutants proteins (7.5μg) mounted on grid and stained with 2% uranyl acetate using Jeol JEM-1200EX microscope. Magnification × 50000. Short filaments and knots (arrows) present in FadAc, S71A and L-9A were absent in other samples.
Similar to the wild-type proteins, L-9A and S71A formed heterogeneous filaments and retained binding activities (Figure 2A) with inhibition of attachment of F. nucleatum to CHO cells of 92% and 86%, and to HUVEC of 65% and 71%, respectively. Mutants F68A, Y69A and K70A which exhibited protein aggregates and L-12A which formed fewer and homogeneous filaments, with no short filaments or knots (Figure 3) did not inhibit F. nucleatum attachment to CHO and HUVEC cells (Figure 2A), indicating that these mutants have lost binding activity. Although mutants F68A, Y69A and K70A were stably expressed, they were defective in oligomerization and attachment, suggesting that the loop region is required for the structure that is necessary for function. Only those forming heterogeneous filaments similar to the wild type had binding activities highlighting that a correct formation of FadAc is correlated with the function of the protein.
3.3-Titration of pre-FadA required for function of FadA
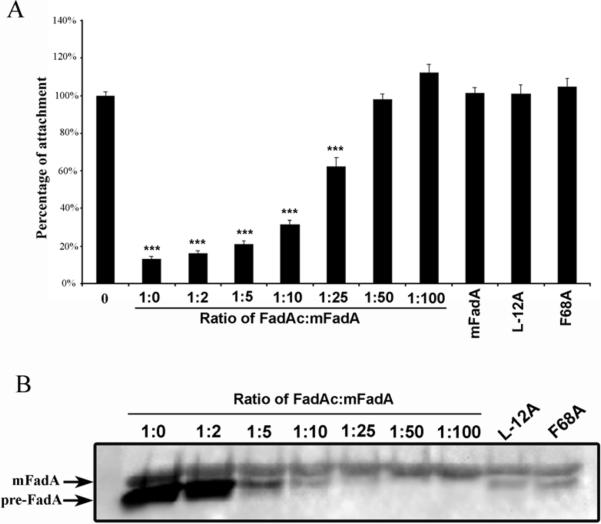
To determine the amount of pre-FadA required for activity, a titration experiment was performed. Since the proportion of preFadA/mFadA present naturally is not known, wild-type FadAc was mixed with mFadA at varying ratios of 1:2, 1:5, 1:10, 1:25, 1:50, and 1:100 to decrease pre-FadA proportions. We chose to titrate pre-FadA by mixing mFadA and FadAc rather the mixing purified mFadA and pre-FadA due to the difficulty to purify pre-FadA and its insolubility under neutral pH. The resulting mFadA- pre-FadA mixtures were examined by western-blot and cell attachment inhibition assays. Mutants L-12A and F68A, in which less pre-FadA was detected, were included for comparison. As shown in Figure 4A, attachment inhibition activities decreased with decreasing amounts of pre-FadA in the mixture. At mixing ratios of 1:2, 1:5 and 1:10 (FadAc:mFadA), attachment inhibition activity was comparable to that of the wild-type FadAc. F. nucleatum attachment was only inhibited by 40% at 1:25 and not affected at 1:50. The levels of pre-FadA in L-12A and F68A as detected by Western-blot were comparable to that in the mixture of 1:10 (Figure 4B). Thus, the lack of binding of L-12A and F68A was not due to insufficient pre-FadA expressed in these mutants.
Figure 4.
Analysis of pre-FadA involvement in cell-attachment inhibition assay on CHO cells (A) and by Western-blot (B). A total of 50 μg/ml of FadA proteins were pre-incubated on cells for 45 min, before F. nucleatum was added at a MOI of 5. Cell attachment inhibition assays results represent an average of three experiments performed in duplicate (A). A total of 5 μg of FadAc, FadAc mutants, or mixtures of FadAc:mFadA at different ratios were loaded per lane. Proteins were analyzed by Western-blot using FadA monoclonal antibody Ab5G11-3G8 (B).
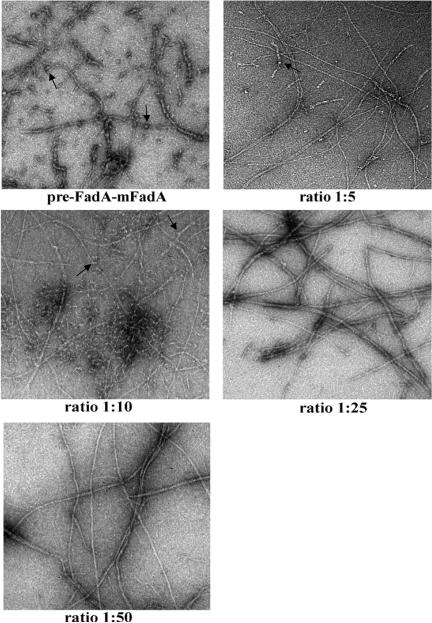
The filaments formed by FadAc:mFadA mixtures were also examined by transmission electron microscopy (Figure 5). With decreasing amounts of pre-FadA, the filaments tend to become longer and more homogeneous, with fewer short filaments and knots, and eventually becoming similar to mFadA alone. The FadAc:mFadA mixtures with binding activities (e.g. 1:5 and 1:10) formed heterogeneous structures, with both long and short filaments, as well as knots. At 1:25, very few short filaments were observed. The mixtures with no binding activity (e.g. 1:50) exhibited long filaments, similar to mFadA alone. Thus, the presence of short filaments and filaments-associated knots correlated with activity, suggesting that they may be the active forms of FadA. These observations also indicate that these active forms require pre-FadA.
Figure 5.
Transmission electron microscopy pictures of different mixing ratio of FadAc:mFadA (7.5μg) using Zeiss Libra 200FE microscope. Magnification × 25000.Short filaments and knots (arrows) present in FadAc and 1:5 and 1:10 mixtures, but not in other samples.
Interestingly, among the different strains of F. nucleatum there is some variations of the pre-FadA:mFadA ratio with more pre-FadA detected in strain 12230 than in strains ATCC-25586 or ATCC-10953 although they all exhibit attachment activities (5, 8). So far, no long filamentous appendages have been reported for F. nucleatum, which allows us to speculate that the FadA adhesin may not be exposed on the bacterial cell surface as long filaments. As few as 3 FadA monomers (Supplementary figure) are required to penetrate across the outer membrane. This is consistent with our hypothesis that short filaments and knots are the active forms. The advantage of short and thick filaments may be its strength, which ensures that the bacteria can bind to host cells despite the shearing forces from various body fluids they encounter in the host environment.
In summary, our results highlight the importance of the loop region and the signal peptide in the structure and function of FadA but more importantly that the signal peptide determines the size and shape of the filament. To the best of our knowledge, this is the first demonstration of a signal peptide being involved in the assembly of a bacterial adhesin. A correct oligomerization is required for the function of FadA but there is some flexibility in the ratio of pre-FadA:mFadA to retain structure and function. The mode by which the signal peptide modulates the FadA structure is currently under investigation.
Supplementary Material
Highlights.
-mFadA and FadAc have distinct structures observed under EM.
-mFadA forms uniformly long and thin filaments which have no binding activity
-FadAc forms heterogeneous structures, due to the presence of pre-FadA
-The heterogeneity of the FadA structure correlates with function
-The shorter filaments may be the active form of FadA
-This is the first demonstration that signal peptide may be involved in adhesin assembly
4-Acknowledgement
This work was supported in part by funds from the National Institute of Dental and Craniofacial Research RO1 DE14924, R21 DE17165, and KO2 DE 16102 to Y.W.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Structured summary of protein interactions:
mFadA and pre-FadA bind by transmission electron microscopy (View interaction)
References
- 1.Edwards AM, Grossman TJ, Rudney JD. Fusobacterium nucleatum transports noninvasive Streptococcus cristatus into human epithelial cells. Infect Immun. 2006;74:654–62. doi: 10.1128/IAI.74.1.654-662.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han YW. Laboratory maintenance of fusobacteria. Curr Protoc Microbiol. 2006 doi: 10.1002/9780471729259.mc13a01s00. Chapter 13:Unit 13A 1. [DOI] [PubMed] [Google Scholar]
- 3.Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, Redline RW. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol. 2010;115:442–5. doi: 10.1097/AOG.0b013e3181cb9955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han YW, Ikegami A, Chung P, Zhang L, Deng CX. Sonoporation is an efficient tool for intracellular fluorescent dextran delivery and one-step double-crossover mutant construction in Fusobacterium nucleatum. Appl Environ Microbiol. 2007;73:3677–83. doi: 10.1128/AEM.00428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Han YW, Ikegami A, Rajanna C, Kawsar HI, Zhou Y, Li M, Sojar HT, Genco RJ, Kuramitsu HK, Deng CX. Identification and characterization of a novel adhesin unique to oral fusobacteria. J Bacteriol. 2005;187:5330–40. doi: 10.1128/JB.187.15.5330-5340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun. 2004;72:2272–9. doi: 10.1128/IAI.72.4.2272-2279.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47:38–47. doi: 10.1128/JCM.01206-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han YW, Shi W, Huang GT, Kinder Haake S, Park NH, Kuramitsu H, Genco RJ. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun. 2000;68:3140–6. doi: 10.1128/iai.68.6.3140-3146.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikegami A, Chung P, Han YW. Complementation of the fadA mutation in Fusobacterium nucleatum demonstrates that the surface-exposed adhesin promotes cellular invasion and placental colonization. Infect Immun. 2009;77:3075–9. doi: 10.1128/IAI.00209-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu H, Redline RW, Han YW. Fusobacterium nucleatum induces fetal death in mice via stimulation of TLR4-mediated placental inflammatory response. J Immunol. 2007;179:2501–8. doi: 10.4049/jimmunol.179.4.2501. [DOI] [PubMed] [Google Scholar]
- 11.Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000. 1994;5:66–77. doi: 10.1111/j.1600-0757.1994.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 12.Nithianantham S, Xu M, Yamada M, Ikegami A, Shoham M, Han YW. Crystal structure of FadA adhesin from Fusobacterium nucleatum reveals a novel oligomerization motif, the leucine chain. J Biol Chem. 2009;284:3865–72. doi: 10.1074/jbc.M805503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem. 2007;282:25000–9. doi: 10.1074/jbc.M611567200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.