Abstract
The purpose of this study was to determine the relationship between mitochondrial DNA (mtDNA) deletions, mtDNA content and aging in rhesus monkeys. Using 2 sets of specific primers, we amplified an 8 kb mtDNA fragment covering a common 5.7 kb deletion and the entire 16.5 kb mitochondrial genome in the brain and buffy-coats of young and aged monkeys. We studied a total of 66 DNA samples: 39 were prepared from a buffy-coat and 27 were prepared from occipital cortex tissues. The mtDNA data were assessed using a permutation test to identify differences in mtDNA, in the different monkey groups. Using real-time RT-PCR strategy, we also assessed both mtDNA and nuclear DNA levels for young, aged and male and female monkeys. We found a 5.7 kb mtDNA deletion in 81.8% (54 of 66) of the total tested samples. In the young group of buffy-coat DNA, we found 5.7 kb deletions in 7 of 17 (41%), and in the aged group, we found 5.7 kb deletions in 12 of 22 (54%), suggesting that the prevalence of mtDNA deletions is related to age. We found decreased mRNA levels of mtDNA in aged monkeys relative to young monkeys. The increases in mtDNA deletions and mtDNA levels in aged rhesus monkeys suggest that damaged DNA accumulates as rhesus monkeys age and these altered mtDNA changes may have physiological relevance to compensate decreased mitochondrial function.
Introduction
Healthy aging is a natural phenomenon that is not affected by age-related diseases. Several hypotheses have been proposed in order to explain the aging process, including telemerase shortening, DNA methylation, and mitochondrial free radical production [1–7]. Among these hypotheses, mitochondrial oxidative damage has been extensively studied, using lower organisms, including yeast, worms, flies, rodents, and higher organisms such as nonhuman primates and humans, to determine the relationship between mitochondrial oxidative damage, and aging and age-related diseases [1,6,8–12]. Although the mitochondrial theory of aging has been supported by convincing evidence to explain causes of aging and death [13,14], this theory has been challenged repeatedly because research investigating this hypothesis has resulted in inconsistent evidence in different species. However, this is the only theory that has thus far survived all challenges and has provided convincing evidence that mitochondria are responsible for these natural causes of death.
Mitochondria are cytoplasmic organelles that arise from a symbiotic association between glycolytic prokaryotic cells and oxidative bacteria [12,15]. Mitochondria are present in every eukaryotic cell, are essential for cell survival and cell death, and are involved in several important functions: 1) production of cellular ATP, 2) intracellular calcium regulation, and 3) regulation of apoptotic cell death. In addition, mitochondria are the primary source of endogenous reactive oxygen species (ROS) and are responsible for altering the reduction-oxidation potential of cells.
Every mitochondrion contains 2–5 copies of its genome. The mitochondrial genome is 16.5 kb and encodes polypeptides that make up the electron transport chain (ETC), which serves as the primary source of ROS. Alterations in mtDNA can lead to changes in ETC efficiency. Using PCR and DNA sequencing analysis, Gokey et al. [16] studied mtDNA deletions in laser, micro-dissected muscle fibers from rhesus monkeys that exhibited abnormalities in their ETC. They found that muscle fibers with ETC abnormalities harbored large deletions in the mitochondrial genome, suggesting that mtDNA changes, particularly large deletions in the mtDNA, lead to abnormalities in the ETC [16]. These abnormalities may exacerbate the normal tendency of the ETC to serve as the primary source of ROS, which may in turn implicate deletions in mtDNA.
It is widely held that mtDNA deletions are caused by errors that occur during mtDNA replication, since the majority of deletions and point mutations in mtDNA occur during replication of mtDNA [17]. Recently, however, it has been suggested that large-scale mtDNA deletions occur during the repair of damaged mtDNA [18]. Most mtDNA deletions begin as a “response” to a break in double-stranded mtDNA. These breaks have been found to occur much more frequently in mitochondria than in nuclear DNA due to the proximity of mtDNA to the origin of ROS and mtDNA’s lack of protective histones and fewer repair enzymes [19,20].
mtDNA mutations have been observed to accumulate with age in a variety of species [7], and an increase in age-dependent mtDNA defects was found to induce free radical production. Age-dependent free radicals can further damage mtDNA, leading to large deletions in the mitochondrial genome. A specific mtDNA deletion that becomes more prevalent with age is the “common deletion”, a 4,977 base pair deletion in the human mitochondrial genome [18]. Several lines of evidence suggest that mtDNA deletions are responsible for diseases in humans [21,22]. Jessie et al. [23] found high numbers of mtDNA deletions in tissues from prostate cancer compared to the number of mtDNA deletions in non-cancerous tissues, indicating that mtDNA may be related to prostate cancer [21,23]. Lim et al. [21]. Jessie et al [23] also found high numbers of mtDNA deletions in patients with end-stage renal disease.
The rhesus monkey is an excellent candidate to study mitochondrial deletions and aging for several reasons. Physiologically, the age-related decline in metabolic rate is strikingly similar between rhesus monkeys and humans [24], and the rhesus monkey’s innate defenses against oxidative damage are also remarkably homologous to those of humans [25]. Genetically, the mitochondrial genome shares 80.4% homology with humans [16]. Further, the ‘common deletion’ is widely characterized in humans, but preliminary research only suggests that in the rhesus monkey, it increases with age in the rhesus monkey [16].
The precise link between mtDNA deletions in aging in nonhuman primates, particularly rhesus monkeys, is not completely understood. Further, whether mtDNA content accumulates in aged rhesus monkeys relative to young rhesus monkeys is also unclear. To understand the mitochondrial theory of aging in nonhuman primates, additional research is needed. Based on current knowledge of aging and mitochondrial deletions in nonhuman primates, we hypothesize that mtDNA deletions increase with age in rhesus monkeys and that mtDNA content increases with age, as a physiological adaptation or compensation for the loss of mitochondrial function in aged monkeys. To investigate these hypotheses, we studied 1) mtDNA deletions and 2) mtDNA content in young (4–10 years) and aged (21–33 years) rhesus monkeys. We also studied 3) gender difference in terms of mtDNA deletions and mtDNA content and 4) mtDNA expressions relative to nuclear DNA in young and aged, and male and female rhesus monkeys.
To determine mtDNA deletions and mtDNA content in rhesus monkeys, we used long-range PCR and amplified an 8 kb PCR covering ‘a common 5.7 kb mtDNA deletion’ in DNA prepared from the occipital cortex and buffy-coat of the rhesus monkey. The position of the ‘5,704 base-pair common deletion’ is between 14,849 and 8,966 [26]. We confirmed the 8 kb PCR results, using full-length mtDNA PCR analysis. We visualized the mtDNA PCR product and quantified the mtDNA PCR using densitometry, and using a permutation test, we determine the difference in the mtDNA PCR band intensity across rhesus monkeys in terms of gender and age.
Materials and Methods
Rhesus macaqhes, source of DNA, and number of samples studied
All monkeys were in good health and had not been used previously in studies of genomic and neurotoxic related studies. Because monkeys reach sexual maturity at approximately 3–5 years of age and have a maximum life span of approximately 35 years [27], we used occipital cortex tissues that were collected during necropsies of young (4–10 years) and aged (21–33 years) rhesus monkeys. Table 1 identifies the numbers of rhesus monkeys studied, by gender. We also studied DNA samples prepared from buffy-coats of young and aged rhesus monkeys. These animals were from our rhesus colony for primate genetic studies at the Oregon National Primate Research Center at Oregon Health & Science University. Our primary interest was to study the mtDNA deletions and mtDNA content in both rhesus brain and peripheral nervous system tissues.
Table 1.
Number of samples studied for mtDNA PCR analysis in the present study
| Location | Age Group | Sex | Number |
|---|---|---|---|
| Blood (39) | Young (22) | Female | 7 |
| Male | 15 | ||
| Aged (17) | Male | 10 | |
| Female | 7 | ||
| Brain (27) | Young (13) | Female | 3 |
| Male | 10 | ||
| Aged (14) | Male | 9 | |
| Female | 5 |
As shown in Table 1, a total of 66 DNA samples were studied for mtDNA deletions and content, 39 were prepared from peripheral buffy-coat DNA, and 27 were prepared from occipital cortex tissues of the brains of young (4 to 10 years) and aged (21 to 33 years) male and female rhesus monkeys.
Oligonucleotide primers for mtDNA
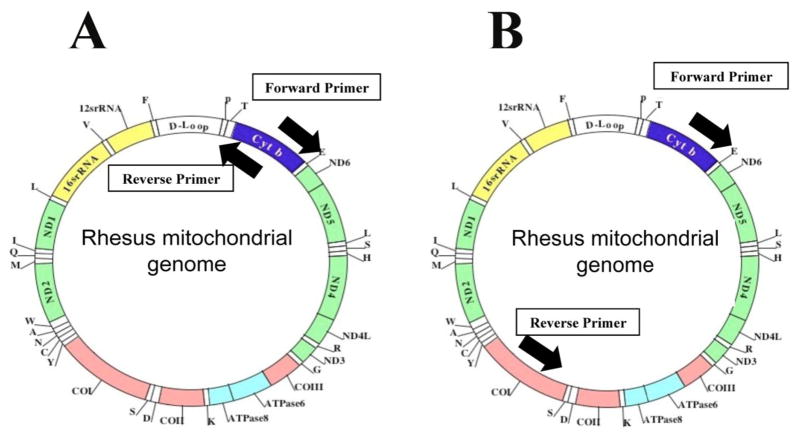
To assess mtDNA deletions and content, we used 2 sets of oligonucleotide primers and amplified the rhesus monkey mitochondrial genome: set 1, 8 kb DNA covering a common ~5 Kb deletion; and set 2, the entire 16.5 kb (Figs. 1A and 1B). For 8 kb amplification: To amplify the common mitochondrial deletion, we used oligonucleotide primers covering half of the mitochondrial genome in rhesus monkeys (Fig. 1A and Table 2). 16 kb amplification: To amplify the entire mitochondrial genome, we designed the oligonucleotide primers targeted to the cytochrome b gene of the rhesus mitochondrial genome, using Invitrogen (OligoPerfect™ Designer). The location of these primers allowed for the replication of the entire mitochondrial genome (~16.5 kb) (Fig. 1B, Table 2).
Figure 1.
mtDNA in rhesus monkeys. Figure 1A shows the location of oligonucleotide primers used to amplify full-length mtDNA. Figure 1B shows the location of oligonucleotide primers used to amplify 8 kb mtDNA. Detailed localization of mitochondrial primers are given in Table 2.
Table 2.
Sequences and locations of primers used to amplify either the entire or about half of the rhesus mitochondrial genome.
| Primer Label | Sequence (5′-3′) | Location in Genome |
|---|---|---|
| Full-length mtDNA PCR | ||
| Forward Primer | CAA CAG TAA TCA CAA ACC TGC TAT CA | 15,168–15,193 |
| Reverse Primer | GTG ATT CAG CCG TAC TTT ACA TCT CG | 14,976–14,951 |
| 8 Kb mtDNA PCR | ||
| Forward Primer | GGA ATA CCC CGA CGC TAC TCT G | 7,152–7173 |
| Reverse Primer | AAG TAT AGG GAT GGC TGC TAG AAT G | 15,661–15,647 |
PCR Conditions
8 kb amplification: Twenty μL samples were prepared for PCR with 300 ng of DNA, 0.230 μM of each (forward and reverse – Fig. 1A) primer, 1U Platinum Taq polymerase, 1X PCR Buffer, 0.2 mM of each dNTP, and 2 mM MgCl2. PCR conditions consisted of an initial denaturation phase of 94°C for 5 min, followed by 35 cycles of 94°C for 30 sec, 60°C for 45 sec, and 68°C for 12 min. The PCR process concluded with a final elongation period of 72°C for 10 min. 16 kb amplification: Twenty μL samples were prepared for PCR with 300 ng of DNA, 0.230 μM of each (forward and reverse) primer, 1U Platinum Taq high fidelity polymerase, 1X PCR buffer, 0.2 mM of each dNTP, and 2 mM MgSO4. PCR conditions consisted of an initial denaturation phase of 94°C for 30 sec, followed by 35 cycles of 94°C for 20 sec, 55°C for 30 sec, and 68°C for 16 min. The PCR process concluded with a final elongation period of 72°C for 10 min.
Real-time RT-PCR to determine mtDNA and nDNA expressions
To determine the mtDNA expression relative to nuclear DNA, using quantitative Real time RT-PCR, we measured mRNA levels of mitochondrial-encoded genes NADH subunit 1 and NADH subunit 4 and nuclear gene, beta actin as described earlier [28–30]. Using Primer Express software (Applied Biosystems), we designed the oligonucleotide primers for the housekeeping genes, β-actin, mitochondrial genes NADH subunit 1 and NADH subunit 4. Oligonucleotide primer sequences for mitochondrial genes, NADH subunit 1 and NADH subunit 4 and beta-actin genes are as follows: NADH subunit 1- forward primer 5′ CCCTAAAACCCGCCACATCT 3′ reverse primer 5′ CGATGGTGAGAGCTAAGGTC 3′; NADH subunit 4 –forward primer 5′ CAAACACACACTTCCCCCTAGTC 3′ and reverse primer – 5′ TTCACAAGCAGCGAATACTAGCA 3; Beta-actin forward primer 5′ GCGCGGCTACAGCTTCA 3′ and reverse primer 5′ CTTAATGTCACGCACGATTTCC 3′. NADH subunit 4 subunit is a part a 5.7 kb common deletion, and this allowed us to verify mtDNA expression with and without 5.7 kb common deletion in rhesus mtDNA. Briefly, total RNA was isolated from neurons representing three independent cultures, in 6-well plates, using TRIzol. Reverse transcription was performed with 2 μg of total RNA from each sample, using the Superscript III First Strand Synthesis System for RT-PCR (Invitrogen). RNA was combined with oligo-dT20, one μl of oligo (dt), and one μl dNTPs (10 mM each) in a total volume of 12 μl; and then heated to 65°C for 5 min. The mixture was chilled on ice, and then 4 μl of 5× first strand buffer, 2 μl of 0.1M DTT, and one μl RNAse out were added. Samples were incubated at 42°C for 2 min, and then one μl of Superscript III (40 U/ml) was added. After a 50-min incubation at 42°C, the reaction was inactivated by heating at 70°C for 15 min.
Real-time quantitative PCR was performed, using an ABI PRISM 7900HT Sequence Detection System (Applied Biosystems, Carlsbad, CA) in a 25-μl volume. The reaction mixture for each primer was comprised of 1x PCR buffer, 2 mM MgCl2, 250 μM dNTPs, 0.3x SYBR Green, 3.12% DMSO, 0.015 U/μl GoldTaq, 50 ng cDNA, 200 nM. β-actin was used as nuclear gene and CT values of beta-actin (nuclear gene) were compared with NADH subunit 1 (mitochondrial-encoded gene), and beta-actin, with NADH subunit 4. We use cycle-threshold (CT) values as a measure for mRNA expression between nuclear DNA (100%) to mitochondrial-encoded DNA (NADH subunit 1 and 4).
Product visualization of mtDNA deletion
PCR products were visualized using horizontal gel electrophoresis. 10 μL of the amplified DNA samples were run for 1.5 hr at 120 volts on TAE agarose gels stained with ethidium bromide. Eight kb products were run on 1.5% gels, and 16 kb products were run on 0.8% gels.
Quantification of mtDNA content and 8kb PCR
In addition to scoring the presence/absence of the common deletion, the net intensity of the PCR band at 8 kb was quantified using Kodak imaging software (Kodak Digital Science, Kennesaw, GA). We confirmed the presence of the 5.7 kb common deletion using 16 Kb amplification for all samples. The common deletion and additional deletions were scored as present or absent. We analyzed the mtDNA PCR quantification data from brain and buffy-coats of young and aged rhesus monkeys. We analyzed the data between the male and female rhesus monkeys of both age groups.
Statistical analysis
A permutation test was used to determine the difference in the mtDNA PCR band intensity across the rhesus monkeys in terms of gender and age. A permutation test provides a non-parametric test to compare groups. When comparing groups of monkeys, we generated the null distribution of test statistics by enumerating the test statistics under every possible permutation of groupings, with groups the same size as original sample. Then we compared an observed test to the null distribution to determine the probability of getting achieving a test as extreme as observed. Permutation was based on probability statements derived solely from observed data sets of mtDNA PCR densitometry values. These tests were robust and nonparametric since no assumptions about theoretical sampling distributions were needed.
Results
The purpose of this study was to determine the relationship between mtDNA deletions/mtDNA content and the effects of aging in rhesus monkeys. Using 2 sets of oligonucleotide primers, we amplified the rhesus monkey mitochondrial genome: set 1, 8 kb DNA covering a common 5.7 Kb deletion (in between 14,849 and 8,966 base-pair of mtDNA); and set 2, the entire 16.5 kb. As shown in Table 1, we studied a total of 66 DNA samples: 39 were prepared from a peripheral buffy-coat and 27 were prepared from occipital cortex tissues from the brains of young and aged rhesus monkeys. mtDNA content and mtDNA deletions were evaluated by visualization of PCR products and densitometry analysis of PCR products. The mtDNA data were statistically assessed using a permutation test in order to identify the differences in mtDNA in young and aged, male and female rhesus monkeys.
PCR analysis of 8 kb mtDNA in rhesus monkeys
We found 5.7 Kb mtDNA deletions in 81.8% (54 of 66) total tested samples. However, all 27 samples prepared from the occipital cortex of young and aged monkeys showed 5.7 kb deletions, and 17 of 39 (43.6%) DNA samples prepared from the buffy-coat in young and aged monkeys showed 5.7 kb deletion. In the young group of buffy-coat DNA, we found 5 Kb deletions in 7 of 17 (41%), and in the aged group, we found 5.7 kb deletions in 12 of 22 (54%), suggesting that mtDNA deletions increase with age.
Brain mtDNA
We analyzed 8 kb mtDNA PCR data for brain DNA and buffy-coat DNA for gender and age using permutation analysis. Our densitometry analysis of mtDNA PCR amplified from brain DNA revealed a significant increase in mtDNA in aged (P<0.0001) compared to young monkeys (Table 3).
Table 3.
Summary of densitometry values of 8 Kb mtDNA PCR in rhesus monkeys
| Location | Comparison | Group | Estimated Densitometry Value | Difference | Standard Error | Permutation Test p-value |
|---|---|---|---|---|---|---|
| Blood | Gender Effect | Female | 373.03 | 20.60 | 45.99 | 0.5248 |
| Male | 352.44 | |||||
| Age Effect | Aged | 387.75 | 50.02 | 45.99 | 0.2655 | |
| Young | 337.72 | |||||
| Gender Affect for Aged Group | Female | 428.14 | 80.78 | 67.40 | 0.3276 | |
| Male | 347.36 | |||||
| Gender Affect for Young Group | Female | 317.93 | −39.59 | 62.60 | 0.4473 | |
| Male | 357.52 | |||||
| Age Effect for Female | Aged | 428.14 | 110.21 | 67.40 | 0.0920 | |
| Young | 317.93 | |||||
| Age Effect for Male | Aged | 347.36 | −10.16 | 62.60 | 0.8829 | |
| Young | 357.52 | |||||
| Brain | Gender Effect | Female | 604.56 | −0.94 | 109.62 | 0.2612 |
| Male | 605.50 | |||||
| Age Effect | Aged | 787.92 | 365.79 | 109.62 | <0.0001* | |
| Young | 422.13 | |||||
| Gender Affect for Aged Group | Female | 711.44 | −152.96 | 141.73 | 0.3148 | |
| Male | 864.40 | |||||
| Gender Affect for Young Group | Female | 497.67 | 151.07 | 167.27 | 0.4350 | |
| Male | 346.60 | |||||
| Age Effect for Female | Aged | 711.44 | 213.78 | 169.40 | 0.2341 | |
| Young | 497.67 | |||||
| Age Effect for Male | Aged | 864.40 | 517.80 | 139.17 | 0.0019* | |
| Young | 346.60 |
Indicates statistical significance
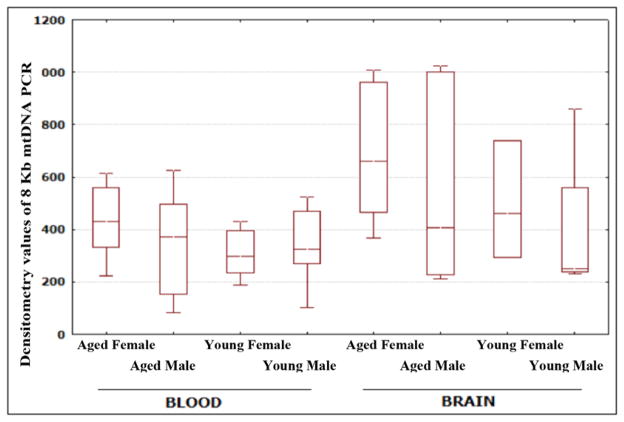
Using PCR analysis, we did not find a significant difference between males and females, in terms of mtDNA. However, we found a significant increase in densitometry values of mtDNA PCR in aged male monkeys (P=0.0019) compared to aged female monkeys (Table 3 and Fig. 3).
Figure 3.
Densitometry values of mtDNA PCR. The figure presents a graphical representation of densitometry values of mtDNA from young and aged, and male and female groups of rhesus monkeys.
Buffy-coat mtDNA
Using PCR analysis, we found increased densitometry values of mtDNA in aged monkeys compared to young monkeys, but the values were not statistically significant. In terms of gender difference, we found increased mtDNA in aged monkeys compared to young monkeys, but these values were also not statistically significant (Table 3).
16 kb mtDNA PCR
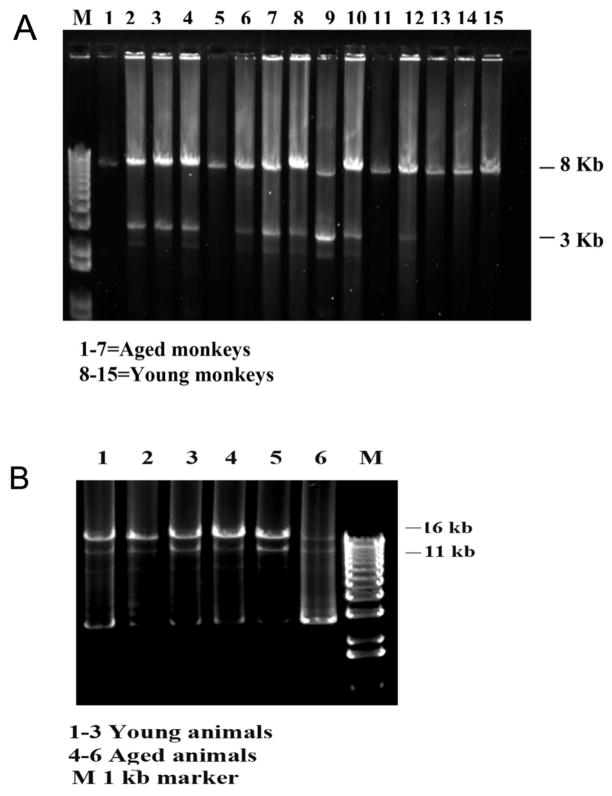
To validate the 8 kb mtDNA data, we also amplified full-length mtDNA for all 66 DNA samples (Fig. 2B). We confirmed the mtDNA deletions in the same samples, using the 8 kb mtDNA amplification.
Figure 2.
PCR amplification of 8 kb and full-length mtDNA. Figure 2A shows a representative 8 kb mtDNA. Samples 1–7 represent DNA from aged rhesus monkeys and samples 8–15, from young rhesus monkeys. Figure 2B shows representative, full-length mtDNA PCR. Samples 1–3 show DNA from young rhesus monkeys, and 4–6, from aged rhesus monkeys.
Nuclear DNA to mitochondrial DNA
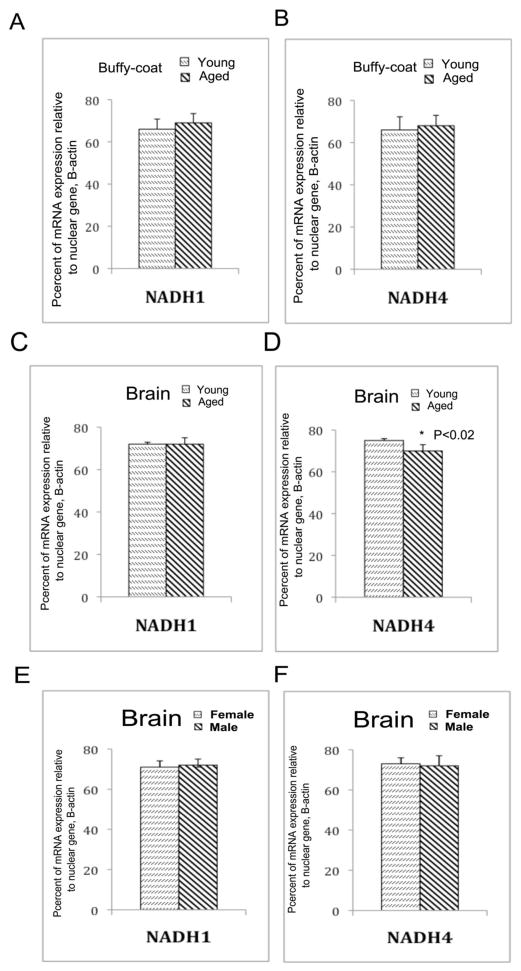
To determine the expression difference between nuclear DNA to mitochondrial-encoded DNA in the brain and buffy-coats of young and aged, and male and rhesus monkeys, we used real-time RT-PCR strategy and amplified nuclear DNA (beta-actin) and mitochondrial-encoded DNA (NADH subunit 1 and 4). We used cycle-threshold (CT) values as a measure for expression between nuclear DNA (100%) to mitochondrial-encoded DNA (NADH subunit 1 and 4). As shown in Figure 5A and B, we found expression levels of NADH subunit 1 in the buffy-coats of young and aged monkeys are 72% of each to beta-actin, 100%. The mRNA expression levels of NADH subunit 4 is significantly decreased in the brains of aged monkeys (70%) relative to young monkeys (75%, P<0.02) (Figure 5B and C), indicating the presence of increased mtDNA deletions in the brains of aged monkeys. As shown in Figure 5E and F, mRNA expression levels of NADH subunit 1 and 4 are same for both male and female monkeys, indicating that gender did not play a role in mRNA expression of mitochondrial-encoded genes investigated in this study.
Figure 5.
mRNA expression of mitochondrial-encoded genes, NADH subunit 1 and 4 relative to nuclear gene beta-actin. (A) Represents percent mRNA expression of NADH subunit 1 in buffy-coat of aged monkeys relative to beta-actin in young and aged rhesus monkeys. (B) Represents percent mRNA expression of NADH subunit 4 in buffy-coat relative to beta-actin in buffy-coat in young and aged rhesus monkeys. (C) Represents percent mRNA expression of NADH subunit 1 in the occipital cortex relative to beta-actin in young and aged rhesus monkeys. (D) Represents percent mRNA expression of NADH subunit 4 in the occipital cortex relative to beta-actin in young and aged rhesus monkeys. mRNA levels of NADH subunit 4 were significantly decreased in aged monkeys relative to young monkeys. (E) Represents percent mRNA expression of NADH subunit 1 in the occipital cortex relative to beta-actin in male and female rhesus monkeys. (F) Represents percent mRNA expression of NADH subunit 4 in the occipital cortex relative to beta-actin in male and female rhesus monkeys.
Discussion
The purpose of our study was to understand mtDNA deletions and mtDNA content in brain and peripheral tissues, as a part of our investigation of mtDNA in nonhuman primates. The sources of DNA in our study were from the occipital cortex and blood derived the buffy-coats of the rhesus monkey. To study the mtDNA common 5.7 kb deletion and mtDNA content, we used a long-range PCR technique and amplified 8 kb mtDNA derived from 66 DNA samples of buffy-coat and occipital cortices from young and aged, male and female rhesus monkeys. Our 8 kb mtDNA PCR harbored a 5.7 common deletion (Fig. 1). The position of the 5,704 kb common deletion was between the 14,849 and 8,966 base-pair mtDNA [26]. mtDNA content and mtDNA deletions were evaluated, using PCR visualization products, and densitometry analysis was also conducted, using the PCR visualization products. The mtDNA data were statistically assessed using a permutation test, to identify differences in mtDNA from the young/aged and male/female rhesus monkeys groups.
mtDNA deletions in rhesus monkeys
We found a 5.7 kb mtDNA deletion in 81% DNA samples; we cross-checked our findings using full-length and 8 Kb mtDNA PCR. These findings suggest that a 5.7 kb common mtDNA deletion is common in both the peripheral and central nervous systems of rhesus monkeys. Our initial findings agree with other researchers’ reports of mtDNA deletions in oocytes [26] and skeletal muscle tissues [22,31,32] from rhesus monkeys. Our findings of 5.7 kb deletions in blood and brain samples from rhesus monkeys – together with these earlier studies [26,31–33] – suggest that the 5.7 kb deletion is present in different tissues in the rhesus monkey.
However, the connection between mtDNA deletion, and mitochondrial function – particularly free radical production, mitochondrial enzyme activities, and ATP production in both central and peripheral nervous systems of young and aged rhesus monkeys – is still not completely understood. In nonhuman primates, the connection between mtDNA deletions and aging [18,34] and diseases [21–23] that were reported in humans has not yet been studied. Further research is needed to elucidate the connection between mtDNA deletion(s) and mitochondrial dysfunction; and the connection between mtDNA deletion and diseases, in rhesus monkeys.
mtDNA deletions in humans and their relationship to human diseases
In humans, 2 major common deletions have been described:
(1) The most commonly described deletion covering a 4,977 base-pair fragment comprises 5 tRNA genes and polypeptide genes disrupting the function of mitochondrial ETC complexes 1, IV, and V. The probable origin of this large deletion is due to an intra-genomic recombination event that occurs between 2, 13-base-pair repeats (at 8,470–8,482, and 13,447–13,459 positions). This common deletion is reported to be associated with an increased production of free radicals and a decreased mitochondrial membrane potential [35]. Further, this common deletion is linked to several diseases, such as prostate cancer, end-stage renal disease, multiple sclerosis and Alzheimer’s disease (AD) [21–23,36]. In humans, Porteous et al. [18] found a 4,977 bp common deletion linked to aging. This large mtDNA was also found in several organs in humans, including skeletal muscles, the heart, and different parts of the human brain [34]. This deletion has been studied in high-energy demand organs, including skeletal muscles, the heart, and the brain. An increase in the prevalence of this deletion in each of these tissues was observed with age [18]. In addition, Hirai et al [36] found increased levels of 4.977 bp common mtDNA deletion in cortical and hippocampal neurons from AD patients relative to age-matched control subjects, indicating that neurons with increased mtDNA deletions are damaged in AD.
(2) The second major common deletion is a 3,895 bp found in human mtDNA, first reported in diseased muscle by Moraes and colleagues [37]. This deletion is linked to photoaging [38], spans between 547 and 4,443 of mtDNA covering 12s rRNA, 16s rRNA and complex I gene, ND1, and the promoters of transcription of both outer and inner strands. This deletion is associated with a point mutation 414T-G, which is found in skin fibroblasts in individuals older than 65 years of age [39].
Overall, both of these deletions may play a large role in the aging process and age-related diseases in humans. However, in nonhuman primates, the relationship among mtDNA deletions, aging, and age-related diseases is not completely understood. Currently, we have initiated a large-scale study to elucidate these relationships.
Increased mtDNA content in aged rhesus monkeys
For the first time, we found a significant increase in mtDNA in aged rhesus monkeys (P<0.0001) compared to young rhesus monkeys in our densitometry analysis of mtDNA content, suggesting that aged rhesus monkeys harbor degraded mtDNA. This increased mtDNA content, in both the peripheral and central nervous systems, may be due to a local compensatory response of decreased mitochondrial function and low ATP production. Interestingly, this feature may be tissue-specific, in rat it has shown that a substantial age-related decline in mtDNA copy number proportional to tissue oxidative capacities is demonstrated in skeletal muscle and liver. mtDNA levels are in contrast preserved or slightly increased in the aging heart muscle, presumably due to its incessant aerobic activity [40]. It may be similar mechanism to heart, brain is another high oxygen demanded organelle, we consider these adopted changes in the key organelles as physiological compensatory responses rather than pathological markers.
There is growing literature indicating that, in rodents and humans, mitochondrial function decreases with an increase in age, which may be primarily due to the accumulation of defective mtDNA (including point mutations and large deletions), and which may be accompanied by an increase in free radical production [13,14,19,41]. Our real-time RT-PCR data show that NADH subunit 4 mRNA expression is reduced in the cortices of old monkeys relative to young monkeys, indicating that the mitochondrial function is going down with aging, at least it occurs in some key mtDNA-encoded genes and related functions. To compensate for the loss of mitochondrial function and the associated reduction in ATP production in aged tissues, mitochondria may replicate rapidly and may try to produce more ATP. However, during the process of balancing the supply (more mitochondria) and demand (ATP) in high-energy consuming tissues, such as brain tissues, excessive defective mitochondria and mtDNA may accumulate. This possibility may be the explanation for the increase in mtDNA content that we found in this study, in tissues from aged rhesus monkeys compared to young rhesus monkeys.
This phenomenon – that is, the accumulation of defective mitochondria and a corresponding decrease in mitochondrial function with the increase of age – has been observed: 1) in postmortem brains from patients with AD [36] and in transgenic mice with over-expressed amyloid precursor protein [42], and 3) in a presenilin 1 transgenic mouse model [43]. Briefly, Hirai et al. [41] found an increase in damaged mitochondria and defective mtDNA, including increased mtDNA deletions in hippocampal and cortical neurons, from postmortem brains from AD patients, compared to the mtDNA in postmortem hippocampal and cortical neurons from healthy control subjects, indicating that mitochondrial abnormalities are evident in AD brains [36]. In a time-course gene expression study of APP transgenic mice, Reddy et al. [42] found increased mRNA expression of mitochondrially encoded genes in APP mice that were 2, 5, and 18 months old, compared to age-matched, non-transgenic wild-type mice, indicating that mitochondrial toxicity may increase with age and may also be due to over-expressed APP and an increase in the production of amyloid beta. This increase in mitochondrial gene expression may be cautiously interpreted as a compensatory response to a decrease in mitochondrial function [28,42].
In a recent proteomics study, Fu et al. [43] found that mitochondrial proteins of oxidative phosphorylation, mitochondrial permeability transition pores, and energy metabolism increased with age in PS1 transgenic mice, compared to age-matched, non-transgenic wild type mice, indicating that increased mitochondrial proteins may be a compensatory response to PS1 mutations in aged PS1 mice. Findings from those studies clearly suggest that increased mtDNA, RNA, and proteins are compensatory responses – that is, physiological adaptations – to the loss of mitochondrial function, also in support of our current findings.
We found a greater increase in mtDNA in the rhesus brain than in the rhesus blood, suggesting that, in general, brain tissue may be oxidatively more damaged by mtDNA deletions than peripheral tissue, such as blood, in aging process. These mtDNA deletions may be due to mitochondria dividing and producing a greater number of defective mitochondria and defective mtDNA in aging. Further research is needed to evaluate these initial interpretations, using a large number of samples and also a different species of nonhuman primate, such as the Cynomolgus monkey.
Gender difference in mtDNA content
Our combined brain DNA and buffy-coat DNA analysis of mtDNA PCR suggests that mtDNA accumulates significantly both in aged male rhesus monkeys (P=0.0172) and in young and aged female monkeys (P=0.0385), suggesting an age-dependent accumulation of degraded mtDNA in both genders (Table 5). Such age-dependency may be due to a compensatory response to the decrease in mitochondrial function in aged monkeys, particularly in males more than in females. However, additional research using large numbers of samples and different species of nonhuman primates is needed to determine, with statistical significance, the connection between mtDNA deletions and gender differences.
Table 5.
Analysis of Gender and Age Effects with a combined 8 kb mtDNA PCR data (blood + brain)
| Comparison | Group | Estimated Densitometry Values | Difference | Standard Error | Permutation test p-value |
|---|---|---|---|---|---|
| Overall Gender Effect | Female | 467.09 | 9.12 | 61.22 | 0.2315 |
| Male | 457.97 | ||||
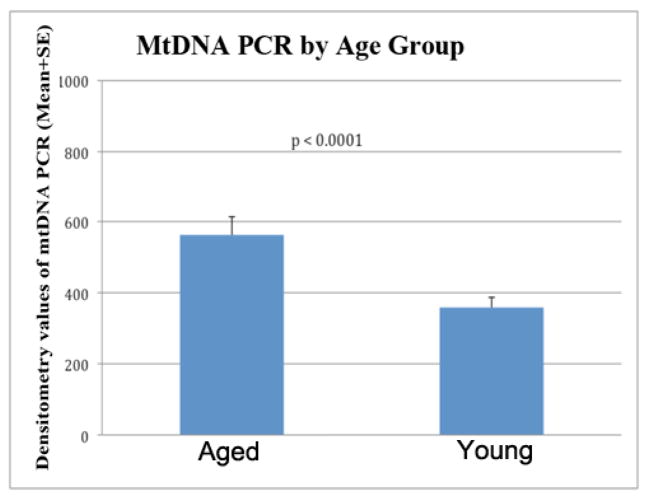
| Overall Age Effect | Aged | 562.56 | 200.06 | 61.22 | <0.0001* |
| Young | 362.50 | ||||
| Gender Affect for Aged Group | Female | 562.34 | −0.45 | 85.94 | 0.9972 |
| Male | 562.79 | ||||
| Gender Affect for Young Group | Female | 371.85 | 18.70 | 87.21 | 0.7835 |
| Male | 353.15 | ||||
| Age Effect for Female | Aged | 562.34 | 190.49 | 91.06 | 0.0385* |
| Young | 371.85 | ||||
| Age Effect for Male | Aged | 562.79 | 209.64 | 81.85 | 0.0172* |
| Young | 353.15 |
Indicates statistical significance
Regarding the relationship of mtDNA deletions and mtDNA defects to gender, there are no published reports on nonhuman primates. However, in rodents, Vina and colleagues [6] reported a decrease in mitochondrial free radical production and in oxidative damage, in female rats relative to age-matched male rats. Those results indicate that female rats are more resistant to developing mtDNA defects (including deletions and point mutations) and are more resistant to mitochondrial dysfunction. However, the numbers of mitochondria in ovariectomized female rats were found to be similar to the numbers in age-matched males, further supporting the possibility that females are more resistant than males, in developing mitochondrial dysfunction [6]. Our initial findings in the present study revealed that mtDNA deletions are similar in male and female, young and aged rhesus monkeys. Further research is needed to understand these issues, using a larger number of monkeys.
In summary, we amplified full-length and 8 kb mtDNA in brain and buffy-coat derived DNA from young and aged rhesus monkeys. A 5.7 kb mtDNA deletion was observed in a majority of the DNA samples that we tested. Our PCR analysis of mtDNA revealed that mtDNA content was significantly increased in aged rhesus monkeys, compared to the young rhesus monkeys. This increase may be a compensatory physiological response to decreased mitochondrial function in the rhesus monkey as it ages. These age-dependent mtDNA changes may be useful biomarkers for aging in monkeys and by extension, possibly in humans. Additional research using greater numbers of nonhuman human primates is needed to determine: 1) the relationship between mtDNA deletion and mitochondrial dysfunction, 2) mtDNA deletion and disease in monkeys, 3) to determine different types of deletions, if any (in addition to the 5.7 kb deletion) in mtDNA, and 3) gender differences associated with mtDNA content.
Figure 4.
Densitometry of mtDNA PCR by age. Significantly increased mtDNA (combined analysis of buffy-coat DNA and brain DNA samples) was observed in aged rhesus monkeys, compared to young rhesus monkeys.
Table 4.
Summary of densitometry values of 8 Kb mtDNA PCR combined (young + aged)
| Age Group | Sex | No. of Samples | Mean Densitometry Values | Standard Dev |
|---|---|---|---|---|
| Aged | Female | 19 | 562.34 | 251.66 |
| Male | 12 | 562.79 | 339.94 | |
| Young | Female | 10 | 371.85 | 155.32 |
| Male | 25 | 353.15 | 175.57 |
Highlights.
Investigated the mtDNA deletions and mRNA expression in young, aged, male and female rhesus monkeys.
Found increased mtDNA deletions in aged monkeys relative to young monkeys.
mtDNA content is increased in the brains of aged monkeys, probably to compensate defective mitochondrial function.
Discussed the compensatory aspects related to aged-dependent mitochondrial dysfunction.
Acknowledgments
This research presented was supported by NIH grants AG028072, RR00163 and Alzheimer Association IIRG grant, IIRG-09-92429
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beckman KB, Ames BN. Mitochondrial aging: open questions. Ann NY Acad Sci. 1998;854:118–127. doi: 10.1111/j.1749-6632.1998.tb09897.x. [DOI] [PubMed] [Google Scholar]
- 2.de Carvalho CV, Payao SL, Smith MA. DNA methylation, ageing and ribosomal genes activity. Biogerontology. 2000;1:357–361. doi: 10.1023/a:1026542618182. [DOI] [PubMed] [Google Scholar]
- 3.Mattson MP. Emerging neuroprotective strategies for Alzheimer’s disease: dietary restriction, telomerase activation, and stem cell therapy. Exp Gerontol. 2000;35:489–502. doi: 10.1016/s0531-5565(00)00115-7. [DOI] [PubMed] [Google Scholar]
- 4.Vijg J. Somatic mutations and aging: a re-evaluation. Mutat Res. 2000;447:117–135. doi: 10.1016/s0027-5107(99)00202-x. [DOI] [PubMed] [Google Scholar]
- 5.Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, Castellani RJ, Atwood CS, Aliev G, Sayre LM, Takeda A, Smith MA. Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med. 2002;33:1475–1479. doi: 10.1016/s0891-5849(02)01113-9. [DOI] [PubMed] [Google Scholar]
- 6.Sastre J, Borras C, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondrial damage in aging and apoptosis. Ann NY Acad Sci. 2002;959:448–451. doi: 10.1111/j.1749-6632.2002.tb02114.x. [DOI] [PubMed] [Google Scholar]
- 7.Tonska K, Solyga A, Bartnik E. Mitochondria and aging: innocent bystanders or guilty parties? J Appl Genet. 2009;50:55–62. doi: 10.1007/BF03195653. [DOI] [PubMed] [Google Scholar]
- 8.Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. doi: 10.1126/science.283.5407.1482. [DOI] [PubMed] [Google Scholar]
- 9.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 10.Sedensky MM, Morgan PG. Mitochondrial respiration and reactive oxygen species in mitochondrial aging mutants. Exp Gerontol. 2006;41:237–245. doi: 10.1016/j.exger.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 11.DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 12.Reddy PH. Mitochondrial medicine for aging and neurodegenerative diseases. Neuromolecular Med. 2008;10:291–315. doi: 10.1007/s12017-008-8044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly-Y M, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 14.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 15.Swerdlow RH. The neurodegenerative mitochondriopathies. J Alzheimers Dis. 2009:737–751. doi: 10.3233/JAD-2009-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gokey NG, Cao Z, Pak JW, Lee D, McKiernan SH, McKenzie D, Weindruch R, Aiken JM. Molecular analyses of mtDNA deletion mutations in microdissected skeletal muscle fibers from aged rhesus monkeys. Aging Cell. 2004;3:319–326. doi: 10.1111/j.1474-9728.2004.00122.x. [DOI] [PubMed] [Google Scholar]
- 17.Krishnan KJ, Reeve AK, Samuels DC, Chinnery PF, Blackwood JK, Taylor RW, Wanrooij S, Spelbrink JN, Lightowlers RN, Turnbull DM. What causes mitochondrial DNA deletions in human cells? Nat Genet. 2008;40:275–279. doi: 10.1038/ng.f.94. [DOI] [PubMed] [Google Scholar]
- 18.Porteous WK, James AM, Sheard PW, Porteous CM, Packer MA, Hyslop SJ, Melton JV, Pang CY, Wei YH, Murphy MP. Bioenergetic consequences of accumulating the common 4977-bp mitochondrial DNA deletion. Eur J Biochem. 1998;257:192–201. doi: 10.1046/j.1432-1327.1998.2570192.x. [DOI] [PubMed] [Google Scholar]
- 19.Reddy PH, Beal MF. Amyloid beta, mitochondrial dysfunction and synaptic damage: implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol Med. 2008;14:45–53. doi: 10.1016/j.molmed.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LeDoux SP, Druzhyna NM, Hollensworth SB, Harrison JF, Wilson GL. Mitochondrial DNA repair: a critical player in the response of cells of the CNS to genotoxic insults. Neuroscience. 2007;145:1249–1259. doi: 10.1016/j.neuroscience.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim PS, Cheng YM, Wei YH. Large-scale mitochondrial DNA deletions in skeletal muscle of patients with end-stage renal disease. Free Radic Biol Med. 2000;29:454–463. doi: 10.1016/s0891-5849(00)00334-8. [DOI] [PubMed] [Google Scholar]
- 22.Mao P, Reddy PH. Is multiple sclerosis a mitochondrial disease? Biochim Biophys Acta. 2009 doi: 10.1016/j.bbadis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jessie BC, Sun CQ, Irons HR, Marshall FF, Wallace DC, Petros JA. Accumulation of mitochondrial DNA deletions in the malignant prostate of patients of different ages. Exp Gerontol. 2001;37:169–174. doi: 10.1016/s0531-5565(01)00153-x. [DOI] [PubMed] [Google Scholar]
- 24.Roth GS, Mattison JA, Ottinger MA, Chachich ME, Lane MA, Ingram DK. Aging in rhesus monkeys: relevance to human health interventions. Science. 2004;305:1423–1426. doi: 10.1126/science.1102541. [DOI] [PubMed] [Google Scholar]
- 25.Kapahi P, Boulton ME, Kirkwood TB. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic Biol Med. 1999;26:495–500. doi: 10.1016/s0891-5849(98)00323-2. [DOI] [PubMed] [Google Scholar]
- 26.Gibson TC, Kubisch HM, Brenner CA. Mitochondrial DNA deletions in rhesus macaque oocytes and embryos. Mol Hum Reprod. 2005;11:785–789. doi: 10.1093/molehr/gah227. [DOI] [PubMed] [Google Scholar]
- 27.Fernandes A, Bradley DV, Tigges M, Tigges J, Herndon JG. Ocular measurements throughout the adult life span of rhesus monkeys. Invest Ophthalmol Vis Sci. 2003;44:2373–2380. doi: 10.1167/iovs.02-0944. [DOI] [PubMed] [Google Scholar]
- 28.Manczak M, Park BS, Jung Y, Reddy PH. Differential expression of oxidative phosphorylation genes in patients with Alzheimer’s disease: implications for early mitochondrial dysfunction and oxidative damage. Neuromolecular Med. 2004;5:147–162. doi: 10.1385/NMM:5:2:147. [DOI] [PubMed] [Google Scholar]
- 29.Manczak M, Mao P, Nakamura K, Bebbington C, Park B, Reddy PH. Neutralization of granulocyte macrophage colony-stimulating factor decreases amyloid beta 1–42 and suppresses microglial activity in a transgenic mouse model of Alzheimer’s disease. Hum Mol Genet. 2009;18:3876–3893. doi: 10.1093/hmg/ddp331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutala RV, Reddy PH. The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease post-mortem brains. J Neurosci Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Lopez ME, Van Zeeland NL, Dahl DB, Weindruch R, Aiken JM. Cellular phenotypes of age-associated skeletal muscle mitochondrial abnormalities in rhesus monkeys. Mutat Res. 2000;452:123–138. doi: 10.1016/s0027-5107(00)00059-2. [DOI] [PubMed] [Google Scholar]
- 32.Schwarze SR, Lee CM, Chung SS, Roecker EB, Weindruch R, Aiken JM. High levels of mitochondrial DNA deletions in skeletal muscle of old rhesus monkeys. Mech Ageing Dev. 1995;83:91–101. doi: 10.1016/0047-6374(95)01611-3. [DOI] [PubMed] [Google Scholar]
- 33.Mehmet D, Ahmed F, Cummins JM, Martin R, Whelan J. Quantification of the common deletion in human testicular mitochondrial DNA by competitive PCR assay using a chimaeric competitor. Mol Hum Reprod. 2001;7:301–306. doi: 10.1093/molehr/7.3.301. [DOI] [PubMed] [Google Scholar]
- 34.Meissner C, Bruse P, Mohamed SA, Schulz A, Warnk H, Storm T, Oehmichen M. The 4977 bp deletion of mitochondrial DNA in human skeletal muscle, heart and different areas of the brain: a useful biomarker or more? Exp Gerontol. 2008;43:645–652. doi: 10.1016/j.exger.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Peng TI, Yu PR, Chen JY, Wang HL, Wu HY, Wei YH, Jou MJ. Visualizing common deletion of mitochondrial DNA-augmented mitochondrial reactive oxygen species generation and apoptosis upon oxidative stress. Biochim Biophys Acta. 2006;1762:241–255. doi: 10.1016/j.bbadis.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 36.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer’s disease. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moraes CT, Ricci E, Petruzzella V, Shanske S, DiMauro S, Schon EA, Bonilla E. Molecular analysis of the muscle pathology associated with mitochondrial DNA deletions. Nat Genet. 1992;1:359–367. doi: 10.1038/ng0892-359. [DOI] [PubMed] [Google Scholar]
- 38.Krishnan KJ, Harbottle A, Birch-Machin MA. The use of a 3895 bp mitochondrial DNA deletion as a marker for sunlight exposure in human skin. J Invest Dermatol. 2004;123:1020–1024. doi: 10.1111/j.0022-202X.2004.23457.x. [DOI] [PubMed] [Google Scholar]
- 39.Michikawa Y, Mazzucchelli F, Bresolin N, Scarlato G, Attardi G. Aging-dependent large accumulation of point mutations in the human mtDNA control region for replication. Science. 1999;286:774–779. doi: 10.1126/science.286.5440.774. [DOI] [PubMed] [Google Scholar]
- 40.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275:3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 41.Reddy PH. Mitochondrial dysfunction in aging and Alzheimer’s disease: strategies to protect neurons. Antioxid Redox Signal. 2007;9:1647–1658. doi: 10.1089/ars.2007.1754. [DOI] [PubMed] [Google Scholar]
- 42.Reddy PH, McWeeney S, Park BS, Manczak M, Gutala RV, Partovi D, Jung Y, Yau V, Searles R, Mori M, Quinn J. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer’s disease. Hum Mol Genet. 2004;13:1225–1240. doi: 10.1093/hmg/ddh140. [DOI] [PubMed] [Google Scholar]
- 43.Fu YJ, Xiong S, Lovell MA, Lynn BC. Quantitative proteomic analysis of mitochondria in aging PS-1 transgenic mice. Cell Mol Neurobiol. 2009;29:649–664. doi: 10.1007/s10571-009-9359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]