Abstract
There have been many reports of mitochondrial DNA (mtDNA) mutations associated with human malignancies. We have observed allelic instability in UV-induced cutaneous tumors at the mt-Tr locus encoding the mitochondrial tRNA for arginine. We examined the effects of somatic alterations at this locus by modeling the change in a uniform nuclear background by generating cybrids harboring allelic variation at mt-Tr. We utilized the naturally occurring mtDNA variation at mt-Tr within the BALB/cJ (BALB) and C57BL/6J (B6) strains of Mus musculus to transfer their mitochondria into a mouse ρ0 cell line that lacked its own mtDNA. The BALB haplotype containing the mt-Tr 9821insA allele produced significant changes in cellular respiration (resulting in lowered ATP production), but increased rates of cellular proliferation in cybrid cells. Furthermore, the mtDNA genotype associated with UV-induced tumors endowed the cybrid cells with a phenotype of resistance to UV-induced apoptosis and enhanced migration and invasion capabilities. These studies support a role for mtDNA changes in cancer.
Keywords: mtDNA mutation, proliferation, reactive oxygen species, antioxidants, UV-induced apoptosis, migration and invasion
1. Introduction
Although mtDNA mutations have been observed frequently in human malignancies (1–2) their functional significance is not fully understood. One limitation of the approach of studying mtDNA mutations in humans is that there is abundant mtDNA sequence heterogeneity in the form of inherited polymorphisms. Although this naturally occurring variation is helpful for the study of maternal ancestry (3), it confounds studies to examine the effects of specific mutations given that they arise in a background that is inherently heterogeneous.
We previously observed somatic alterations in the mitochondrial tRNAArg gene in UV induced mouse skin tumors and modeled the effects of somatic variation at mt-Tr by harvesting mitochondria from brain synaptosomes of B6 and BALB mice and transferring them to a mouse fibroblast ρ0 cell line (LMEB3ρ0) that lacked its own mtDNA (4). The resulting cybrid cell lines LMEB3(mtBALB) and LMEB3(mtB6) contain the same nuclear genotype and differ in their mitochondria at three nucleotides. The locations of the mtDNA differences between B6 (the mouse reference sequence) and BALB are a T to C polymorphism at 9461 and a 9348G to A change resulting in the amino acid change V248I which is thought to be a neutral polymorphism (5–6). The final difference between the two strains is an additional A insertion in the mt-Tr locus resulting in the expansion of a homopolymeric A tract in the pseudouridine loop of the tRNAArg molecule (from 8 consecutive A residues (the B6 reference sequence) to 9 consecutive As (9821insA)). Alterations of this molecule potentially affect the synthesis of all mitochondrially-encoded proteins that contain arginine. An acquired somatic alteration at the locus would produce heteroplasmy of both B6 and BALB mitochondrial tRNAArg alleles. Inherited mtDNA changes at mt-Tr are associated with sensorineural hearing loss (7), and modulation of complex phenotypes such as learning (8).
We analyzed the effects of this nucleotide insertion in the mtDNA by studying cybrids that harbor alterations at this locus. Our results show that the mtBALB haplotype cybrid cells harboring 9821insA [LMEB3(mtBALB)] has readily observable increased rates of cellular proliferation that were associated with alterations in respiratory activity. This haplotype was also associated with diminished levels of complex I protein resulting in lower levels of baseline oxygen consumption and lower cellular ATP levels (4). The mtBALB haplotype was also associated with higher levels of reactive oxygen species (ROS) and striking changes in cellular motility such as increased migration through transwell pore openings and increased invasion through matrigel matrix. Finally, the cybrids that contain the BALB mtDNA haplotype also were endowed with a resistance to UV-induced apoptosis compared to the B6 haplotype. These altered biochemical and behavioral changes produced by the mtBALB haplotype containing the mt-Tr 9821insA allele result in phenotypes that are consistent with those of tumor cells.
2. Materials and Methods
2.1 Cell lines and media
All cell lines were grown in high glucose (4.5g/L) DMEM medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 10% FBS (Invitrogen Corporation, Carlsbad, CA). Where indicated, cells were grown in DMEM lacking glucose but containing 4.5g/L of galactose supplemented with 10% FBS. LMEB3(mtBALB) and LMEB3(mtB6) cells were generated by harvesting the mitochondria from brain synaptosomes of B6 and BALB mice and electrofusing them to a mouse fibroblast LMEB3ρ0 cell line that lacked its own mtDNA (4). We produced two cybrid cell lines which differ only in their mtDNA but have identical nuclear background from murine LA9 cells which are of C3H origin. The genetics of LA9 cells is discussed in the study of Bayona-Bafaluy et al. (6). A mouse LMEB3ρ0 cell line was produced by exposure of LM(TK−) cells to ethidium bromide as described previously by Trounce et al. (9). Clonal isolates were obtained by picking single colonies after fusion and growing them through successive passaging for over a month prior to beginning experiments. The continued propagation of the cells in culture eliminates any potential contribution of small molecules in the mitochondria of the donor cell line. All cellular compartments would be expected to consist of only molecules synthesized from the LMEB3 nuclear DNA and the donated mtDNA.
2.2 Proliferation assay
Both mtB6 and mtBALB cybrid cells were plated in the 6-well plates at concentration 1 × 105 per well. Cells were incubated in either high glucose DMEM with or without 10μg/ml Mito C (Sigma Aldrich, St. Louis, MO) or in DMEM supplemented with 4.5 g/L galactose. Cybrid cells were also grown in high glucose DMEM supplemented with antioxidants such as 200μM vit E (Sigma Aldrich, St. Louis, MO) and 10mM NAC (Sigma Aldrich, St. Louis, MO) for 24 h, 48 h and 72 h, respectively. Where indicated, cells were pre-incubated for 7 d in the presence of 10 mM NAC and then plated to test their ability to grow in DMEM containing glucose or galactose plus NAC. Viable cells were counted with a hemocytometer every other day by the trypan blue exclusion method.
2.3 ROS levels measurement
ROS production was detected by using the fluorescent probe DCFH2-DA (Molecular Probes, Carlsbad, CA). Cybrid cells were seeded in 96-well plates (5 × 104/well) and allowed to attach for 4h, cells were washed with Hanks' Balanced Salt Solution (Invitrogen Corporation, Carlsbad, CA) and preloaded with 10 μM DCFH2-DA at 37°C for 60 min. Next, the loading buffer was removed and the cells were returned to prewarmed DMEM without phenol red, fluorescence measurements (excitation and emission wavelengths of 490 and 525 nm, respectively) were carried out with a Synergy HT fluorimetric plate reader (Biotek, Winooski, VT).
2.4 UVA Radiation Induced Apoptosis
Cybrid cells at concentration 2 × 105 cells/well growing in 6-well plate (50–60% confluent) were irradiated with 15 J UVA from our panel (Solarc Systems Inc., Minesing (Barrie), Ontario, Canada) of ultraviolet bulbs (six F72T12-BL-HO UVA) that deliver broadband UVA with peak output between 340–368 nm. The UVA dose was monitored with a UVA meter (National Biological Corporation, Beachwood, OH) (10). Treated cells were harvested and stained with Annexin V and 7-AAD included in the Annexin V-PE apoptosis detection kit I (BD Pharmingen, San Diego, CA), according to manufacturer’s protocol. Flow cytometry analysis was performed on FACScan flow cytometer (Becton–Dickinson, Franklin Lakes, NJ) equipped with a 488 nm argon laser with appropriate filters and data were collected from gated cells of appropriate size. Obtained results were analyzed using Cellquest software (Becton–Dickinson, Franklin Lakes, NJ).
2.5 Transwell Migration and Invasion Assay
Eight micrometer pore size translucent transwell migration chambers (BD Biosciences, Bedford, MA) in 24-well plate were used for migration analysis. Briefly, 600ul of Invasion buffer (DMEM containing 0.5% FBS and 0.1% BSA) was added to the bottom of each well, and a total of 2.5 × 104 mtB6 or mtBALB cybrid cells resuspended in 150μl of Invasion buffer with or without 10μg/ml Mito C or in the presence of antioxidants such as vit E (200μM) or NAC (10mM) were seeded on the top of the membrane. After 18 h incubation at 37°C, 5% CO2, non-invading cells were removed by wiping the upper side of the membrane, and invading cells were fixed with methanol and stained with crystal violet (Sigma Aldrich, St. Louis, MO) for 1 min. The number of invading cells was quantified by counting 10 random fields per filter at 400x magnification. Three membrane filters were used for each condition within one experiment.
Cell invasion was performed using modified Boyden chambers consisting of transwells with pre-coated Matrigel™ membrane filters inserted in 24-well tissue culture plates (BD Biosciences, Bedford, MA). A total of 2.5 × 104 cybrid cells (75% confluence) were resuspended in 300μl of serum free media containing only 0.1% BSA and placed on the top of each chamber. After 24 h incubation, invading cybrid cells were stained with crystal violet and then quantified as described above.
2.6 Mitochondrial DNA/Nuclear DNA Copy Number Ratio
Total DNA was extracted from mtB6 and mtBALB cybrid cells using DNeasy Tissue DNA Isolation kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. mtDNA copy numbers/cell were determined using the iQ SYBR Green Supermix (Bio-Rad, Hercules, CA). The quantification assay was designed as a multiplex analysis of mtDNA and nuclear DNA (nDNA) simultaneously. Primers were designed and these sequences were used: COI.F: 5’-GCCTTTCAGGAATACCACGA-3’, COI.R: 5’-AGGTTGGTTCCTCGAATGTG-3’; CIpB.F: 5’-CACCGAGATCCCATACCTGA-3’, CIpB.R: 5’-GCTGTAGCACTGAACCACCA-3’. The mitochondrial target spanned the genes for COX I, and the nuclear target was the mouse CIpB caseinolytic peptidase B homolog (E. coli). The PCR was performed in a total volume of 25 μL containing DNA extracted from cybrids, 400 nm of each primer. Following thermal profile was used: initial denaturation step for 5 min at 95°C, followed by 40 cycles of 95 °C for 10 sec, 65 °C 30 sec and 72 °C 30 sec. The amplification and detection were performed in an iCycler real-time PCR detection system (Bio-Rad, Hercules, CA). Standard curves from 10-fold serial dilutions of plasmid-cloned mitochondrion COX I and nuclear CIpB DNA with known concentrations were generated to estimate the mtDNA and nDNA copy numbers in each sample. Three parallels of five different concentrations of the standards were used in each run throughout the study and each sample was analyzed in triplicate. The exact mtDNA copy number per cell was obtained by multiplying the mtDNA/nDNA ratio by two, as there are two nDNA copies present in each cell.
2.7 Statistical analysis
Data were represented as mean ± SEM of five clones. All experiments were performed at least three times. Statistical significance between any two groups was determined by the two-tailed Student’s t-test, P values less than 0.05 were considered to be significant.
3. Results
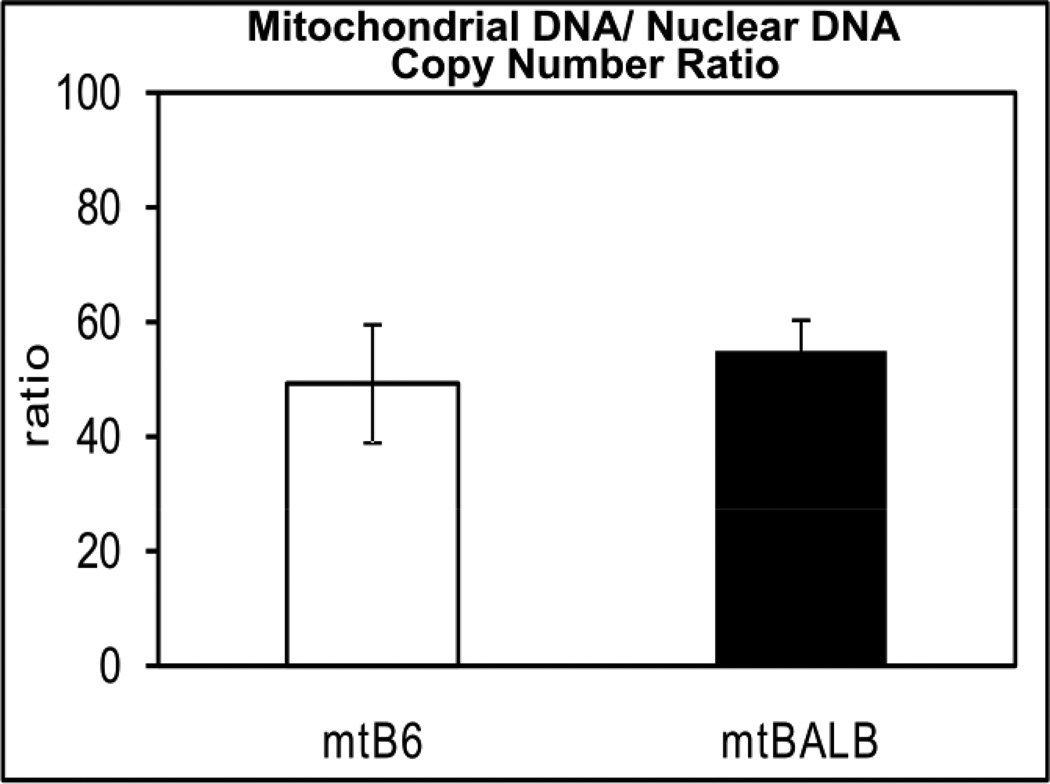
3.1 Mitochondrial DNA/ Nuclear DNA ratio are not significantly different in mtBALB or mtB6 cybrid cells
We characterized both LMEB3(mtBALB) and LMEB3(mtB6) cells for nuclear/mitochondrial DNA ratio (Figure 1) and we observed no significant differences between multiple clones of these two cybrid lines. We selected a set of cybrid cell clones for further study that contained similar mtDNA copy numbers, insuring that the cell lines should not behave differently based on the mtDNA copy number.
Figure 1. Mitochondrial DNA/ Nuclear DNA ratio are not significantly different in mtBALB or mtB6 cybrid cells.
Mitochondrial DNA to nuclear DNA copy number was measured by quantitative real time PCR. Values represent the mean ± SEM of three independent experiments of five clones.
3.2 mtBALB cybrid cells have higher proliferative rate than mtB6 cybrids
We observed a striking change in cellular proliferative activity between mtB6 and mtBALB cybrid cells. The media containing the mtBALB cells showed a quicker color change indicating a higher rate of acidity production than was seen in the mtB6 cells where the media remained at a more constant pH over a greater time. As indicated by Figure 2a, there was a similar growth rate between both cybrid lines after 24 h incubation in high glucose media but after 48 h and 72 h incubation, there was significantly enhanced proliferative activity of mtBALB cells compared to mtB6. Figure 2b indicates that Mitomycin C is sufficient to mitotically inactivate the cybrids what has to be considered in further migratory experiments where the phenotype of enhanced proliferation in mtBALB cybrids should be avoided. Additionally, we explored the proliferative behavior using galactose-containing medium as an alternative sugar source to glucose. Overall, the proliferation for each of the cybrid types was reduced in galactose medium compared to glucose, although this effect was more profound in the mtBALB cybrids. At 48 h and 72 h after plating, the mtBALB cybrids had significantly increased proliferation over the mtB6 cybrids when grown in galactose, a result that mirrors what is seen in glucose medium (Figure 2c).
Figure 2. mtBALB cybrid cells have higher proliferative rate than mtB6.
(a) 1 × 105 cybrid cells were incubated in high glucose Dulbecco’s modified Eagle’s medium (DMEM) media for the times indicated and viable cells shown. (b) To mitotically inactivate the cells, they were incubated in high glucose DMEM media in the presence of 10μg/ml Mito C. (c) Cybrid cells incubated in either high glucose DMEM or DMEM supplemented with 4.5 g/L galactose. Data are mean ± SEM of three independent experiments of three clones. Asterisks indicate statistical differences between the cybrid cells (*P value< .05, **P value< .005).
3.3 ROS levels are higher in mtBALB cybrid cells
One theory that may explain the increased proliferative activity of the mtBALB cybrid cells compared to the mtB6 cells would be an increased level of ROS. While high levels of ROS are known to be toxic to cells, low levels of ROS have been proposed as possible mitogenic signals for cell growth (11–17). We measured baseline and hydrogen peroxide-stimulated ROS levels using fluorescent 2’, 7-dichlorodihydrofluorescein diacetate (DCFH2-DA) analysis. The results revealed significantly higher levels of ROS at the baseline (Figure 3a) in the mtBALB cells compared to mtB6 cybrids indicating a higher level of baseline oxidative stress. When the cells were stimulated with H2O2, there was a profound increase in ROS levels in cybrids of both genotypes, with a significantly higher level observed in the mtBALB cells compared to the mtB6 cybrids (Figure 3b).
Figure 3. ROS levels are higher in mtBALB cybrid cells.
(a) ROS levels at rest or (b) in the presence of 2μM hydrogen peroxide. Data are mean ± SEM of three independent experiments of five clones. Asterisks indicate statistical difference between mtB6 and mtBALB cybrid cells (*P value < .05; **P value< .005).
3.4 Proliferation rate of mtBALB cybrids is significantly decreased by antioxidants in comparison with mtB6 cybrids
We treated the cybrid cells with the antioxidant compounds N-acetyl-L-cysteine (NAC) or vitamin E (vit E) to determine if scavenging ROS would diminish the proliferative capabilities of mtBALB cybrid cells selectively. We found that the addition of NAC selectively reduced the proliferative capacity of the mtBALB cybrids while having no significant antiproliferative effect on the mtB6 cybrids. Vit E caused a greater reduction in proliferation of the cybrids than did NAC, and the effect was not selective as was seen with NAC as the reduction in proliferation was significant for both mtBALB and mtB6 cybrids compared to their untreated controls (Figure 4a). Additionally, we explored whether a 7 d pre-treatment with NAC in medium supplemented with either glucose or galactose (conditions used in Moreno-Loshuertos), would have the same or even more profound effect on growing ability of cybrid cells (5). We observed that NAC did selectively slow down the growth of the mtBALB cybrids but had no significant antiproliferative effect on the mtB6 cybrids when growing in glucose (Figure 4b). The same effect was seen when cybrid cells were growing in galactose medium (Figure 4c). The ability of NAC to selectively inhibit the proliferation of mtBALB over mtB6 cybrids (an effect not seen by vit E) may reflect the differences in oxygen scavenging activity of the two compounds for specific radicals.
Figure 4. Proliferation rate of mtBALB cybrids is significantly decreased by antioxidants in comparison with mtB6.
(a) 1.5 × 105 cybrid cells were incubated in high glucose DMEM and treated with 200μM vit E or 10mM NAC after the cells were seded. (b) cybrid cells were grown for 7 d in the presence of 10mM NAC (Sigma) and then were plated in 6-well plates and incubated in high glucose DMEM or (c) DMEM supplemented with 4.5 g/L galactose and additionally treated with 10mM NAC for the 24h, 48h and 72h as indicated. Viable cells are shown. Data are mean ± SEM of three independent experiments of three clones. Asterisks indicate statistical differences between the cybrid cells (*P value< .05, **P value< .005).
3.5 mtBALB cybrids resist UVA-induced apoptosis compared to mtB6 cybrids
As the mt-Tr somatic change from an 8A homopolymeric tract to a 9A tract at 9821 was associated with UV-induced tumor formation, we sought to explore whether the mtBALB haplotype would be more resistant to apoptosis, a feature seen in many tumors. We examined UV-induced apoptosis in the cybrids by exposing the cultured cells to UVA followed by measuring fluorescent markers of apoptosis and necrosis. Figure 5b indicates that mtBALB cells were significantly less sensitive to UVA-induced apoptotic cell death than mtB6. There was a significant lower number of mtBALB cybrids positively stained with Annexin V entering apoptosis. These results demonstrated that the phenotype of resistance to apoptosis that is seen in some UV-induced tumors can be conferred to cybrid cells by the transfer of mtDNA containing mt-Tr 9821insA.
Figure 5. mtBALB cybrids resist UVA-induced apoptosis compared to mtB6 cybrids.
Cells were either (a) mock treated (0J UVA) or (b) irradiated with 15J of UVA to induce cell death. Annexin V-positive cells are shown as apoptotic and 7-AAD-positive cells are shown as necrotic. Values represent the mean ± SEM of three independent experiments of five clones. Asterisk indicates statistical difference between mtB6 and mtBALB cybrid cells (*P value < .05).
3.6 Migration and invasion capabilities of mtBALB cybrids are enhanced compared to mtB6 cybrids
Another behavior of cells that is associated with tumors is their ability to migrate and invade (18–20). We sought to examine whether the mtBALB haplotype would be associated with higher levels of cell migration and invasivness compared to mtB6 haplotype cells. We conducted transwell migration and invasion assays to determine the migratory and invasive potential of the various cybrids. mtBALB cybrids demonstrated 3-fold higher migration through uncoated inserts (Figure 6a, b) than mtB6 cybrids. In order to eliminate the possibility that higher numbers of the mtBALB cybrids might be caused by an initial migration followed by an increase in proliferation of the cells, we also executed parallel transwell migration experiments with the addition of 10 μg/ml Mitomycin C (Mito C) (Figure 6c, d) to mitotically inactivate the cells. These experiments excluded enhanced mtBALB proliferation as an explanation for the increased numbers of cells on the distal aspect of the transwell insert as all of the cybrid cells displayed similar motility characteristics after treatment with Mito C, although they were rendered incapable of further cell division (Figure 2b). As indicated by Figure 6e, f mtBALB cells also showed significantly increased (2.5-fold) invasion ability through matrigel coated inserts in comparison to mtB6 cybrids. This behavior of increased invasiveness is a feature that is associated with a phenotype of malignant cells (18–20).
Figure 6. Migration and invasion capabilities of mtBALB cybrids are enhanced compared to mtB6 and are modulated by ROS levels.
Migration transwell assays were performed using uncoated inserts and invasion assays were conducted on inserts coated with matrigel. The bar graph shows the number of cybrid cells migrated through uncoated inserts in the absence (a) or presence (c) of Mito C. Representative microscopic pictures of stained cells that migrated through the pores of the uncoated inserts in the absence (b) or presence (d) of Mito C. (e) The bar graph shows the number of invading cells through the matrigel-coated inserts. (f) Representative microscopic pictures of cells that invaded through the pores of the matrigel-coated inserts. The number of cells migrated through uncoated inserts in the presence of vit E (g) or NAC (h). Asterisk indicates statistical difference in migration or invasion between mtB6 and mtBALB cybrid cells (*P value < .005). Data are mean ± SEM of three independent experiments of three clones.
3.7 Antioxidants significantly decreased migration and invasion capabilities of mtBALB cybrids
As was seen in the experiments on proliferation, the antioxidant compounds vit E and NAC were also able to diminish the migratory capacity of the cybrid cells in transwell assays (Figure 6g, h). The addition of NAC resulted in the significant reduction in the migration of the mtBALB cybrids which caused them to migrate at a level that was similar to the mtB6 cybrid cells. The addition of vitE to the transwell assays resulted in a significant decrease in migration for both mtB6 cybrids and mtBALB cybrids, with the mtBALB cybrids showing levels of migration that are similar to untreated mtB6 cybrids. The similar reduction of migration seen in mtBALB cells treated with NAC or vit E are suggestive of an effect potentially mediated through hydroxyl radical (·OH) that is scavenged by both compounds (21).
4. Discussion
The biochemical differences between the cybrids suggest that energetic metabolism may be directly linked to neoplastic behavior. This notion of glycolytic metabolism as a key component of cellular transformation was proposed more than 50 years ago (22). More recently, the discovery of mutations in complex II subunits causing familial tumors syndromes involving paraganglioma tumors (23), and mutations in fumarate hydratase causing familial leiomyomas and renal cell carcinomas (24–27) have implicated enzymes involved in mitochondrial energy production as dual-role proteins, involved in both metabolism and tumor suppression. mtDNA alterations such as seen in the mtBALB haplotype that result in diminished levels of complex I protein, lower levels of baseline oxygen consumption, lower cellular ATP production and enhanced CII substrate sensitivity (4) may provide a biochemical environment that promotes cellular hyperproliferation as was seen in the mtBALB cybrids. Additionally, increased levels of endogenous ROS may provide a prolonged mitogenic cell signal, and is capable of direct nuclear DNA and mtDNA mutagenesis. Increased levels of ROS may also set up a cycle of oxidative damage to mtDNA which will further perturb OXPHOS activity and inhibit electron flow through the respiratory chain, with resulting further increases in ROS liberation.
Our results confirm the data of Moreno-Loshuertos et al. who generated similar transmitochondrial cybrids and showed decreased proliferation of mtBALB cybrids by NAC as well as diminished proliferation of mtB6 L929 cybrid cells when exposed to NAC (5). We did observe certain differences in the behavior of our cybrid cell lines in proliferation rate and the selectivity of the antiproliferative effects of specific antioxidants on mtBALB cybrids. Nuclear background as well as observed differences in the levels of measured ROS in their cybrids may explain the differences between their experimental findings and these presented in this paper. The differences in observed responses to antioxidants by the cell lines likely reflect the specific differences in oxygen scavenging activity of different compounds for the specific radicals generated by the various mtDNA haplotypes.
For the first time, we demonstrate that mtDNA changes such as seen in mtBALB haplotype can produce significant alterations in migration and invasion capabilities of cybrid cells. We have also shown that there are mtDNA-driven differences in ROS production which could also have profound influence on migration abilities since antioxidants NAC and vit E were able to diminish the migratory capacity of the mtBALB cells and caused them to migrate at a level that was similar to the mtB6 cells. Obtained data support a role of ROS as specific second messengers in signaling cascades involved not only in cell proliferation and differentiation but in migration as well (11–15, 17).
Changes in mtDNA associated with somatic mutation and subsequent allelic segregation may have broad consequences including differences in cellular metabolism and more complex phenotypes such as cell growth, inducible resistance to cell death stimuli and proliferation, migration and invasion. These results strongly support a potential contribution of mtDNA changes in cancer progression. The profound differences in cellular physiology displayed by the cybrids may explain some of the varying predispositions to developing tissue specific malignancies in some mouse strains (28). Of the three nucleotide changes between the two mtDNA types, the 9821insA in mt-Tr locus together with 9348G to A base change are likely to be the most important contributors to these diverse phenotypes since the 9461T to C change is a neutral polymorphism coding the same amino acid (Methionine) in both haplotypes. Given the highly pleomorphic consequences of inherited tRNA mutations in human mitochondrial disease, it is not surprising that these minimal changes in the mtDNA may be linked to such diverse cellular and organismal changes such as learning, hearing, metabolism, and neoplasia. The insA nucleotide change occurs in dihydrouridine-loop region of the tRNA molecule and alters the length of the loop between the two D-stem segments. Alteration in this region can produce profound and highly pleomorphic phenotypes as evidenced by the A3243G mutation in tRNALeu (29–30) occurring in mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes (MELAS) (31–32), maternally inherited diabetes with or without deafness (DMDF) (33–35) and mitochondrial myopathy (MM). The wide spectrum of clinical phenotypes is likely caused by changes in heteroplasmy levels in different tissues as well as differing tissuespecific energetic requirements. In the case of neoplasia associated with somatic mtDNA mutation, a mechanism of “loss of mtDNA heteroplasmy” coupled with transforming nuclear DNA mutations may allow for the expression of a tumor promoting biochemical phenotype such as that seen in the mitochondrial mtBALB cybrids.
While the role of mtDNA mutations in the development of keratinocyte neoplasia is not fully understood, the investigation of the role of mtDNA changes in the development of skin tumors might give us the necessary clues to better understand the complex and multistep process of skin carcinogenesis and detection of mtDNA mutations can potentially be used as an early screening tool for squamous cell carcinoma. Moreover, modulation of ROS mediated cell signaling would be a prime target based on our observations. In conclusion, further understanding of the role of mtDNA mutations in skin cancer as well as other malignancies may help to identify new molecular targets for cancer therapies. An interesting corollary to somatic mtDNA mutations supporting the phenotypes seen in tumor cells is that altering mitochondrial function in skin may have distinct and desired advantages in certain disease status. In chronic wounds, when increased cellular motility is desired or in atrophic conditions of the skin, when increased cellular proliferation would correct the pathological process, altered mitochondrial function may be advantageous. Topical medications modulating the downstream effects of ROS signaling would be a natural therapeutic target in these diseases status.
Research Highlights.
Cybrids are useful models to study the role of mtDNA changes in cancer development.
Somatic mutations in mtDNA produce changes in cellular growth and motility.
There are mtDNA-driven differences in production of intracellular ROS.
Antioxidants were able to diminish the tumorigenic phenotypes of mutant cybrids.
Mutations in mtDNA play important roles in the development of neoplasia.
Acknowledgements
This work was supported by an NCI Cancer Center Support Grant P30 CA023074 (CCSG) and R01 AR 0501552 to JS, by the VA Advanced Career Development Award and VA Merit Award to JS and by the Vanderbilt Skin Diseases Research Center Core Grant (NIH P30AR41943).
Abbreviations
- mtDNA
mitochondrial DNA
- UV
Ultraviolet
- ROS
Reactive Oxidative Species
- DCFH2-DA
2’, 7-dichlorodihydrofluorescein diacetate
- NAC
N-acetyl-L-cysteine
- Vit E
vitamin E
- Mito C
Mitomycin C
- ATP
Adenosine Triphosphate
- OXPHOS
oxidative phosphorylation
- SNP
single nucleotide polymorphism
- MELAS
mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes
- DMDF
maternally inherited diabetes with or without deafness
- MM
mitochondrial myopathy
- DMEM
Dulbecco's Modified Eagle’s Medium
- FBS
Fetal Bovine Serum
- BSA
bovine serum albumin
- DPBS
Dulbecco's Phosphate Buffered Saline
- HBSS
Hanks' Balanced Salt Solution
- 7-AAD
7-amino-actinomycin D
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copeland WC, Wachsman JT, Johnson FM, Penta JS. Mitochondrial DNA alterations in cancer. Cancer Invest. 2002;20:557–569. doi: 10.1081/cnv-120002155. [DOI] [PubMed] [Google Scholar]
- 2.Verma M, Kumar D. Application of mitochondrial genome information in cancer epidemiology. Clin Chim Acta. 2007;383:41–50. doi: 10.1016/j.cca.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 3.Wallace DC, Brown MD, Lott MT. Mitochondrial DNA variation in human evolution and disease. Gene. 1999;238:211–230. doi: 10.1016/s0378-1119(99)00295-4. [DOI] [PubMed] [Google Scholar]
- 4.Jandova J, Shi M, Norman KG, Stricklin GP, Sligh JE. Identification of a mtDNA mutation hotspot in UV-induced mouse skin tumors producing altered cellular biochemistry. Journal of Invest Derm. 2011 doi: 10.1038/jid.2011.320. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, de Cordoba SR, Gallardo ME, Enriquez JA. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nature Genetics. 2006;38:1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- 6.Bayona-Bafaluy MP, Acin-Perez R, Mullikin JC, Park JS, Moreno-Loshuertos R, Hu P, Perez-Martos A, Fernandez-Silva P, Bai Y, Enriquez JA. Revisiting the mouse mitochondrial DNA sequence. Nucleic Acids Res. 2003;31:5349–5355. doi: 10.1093/nar/gkg739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson KR, Zheng QY, Bykhovskaya Y, Spirina O, Fischel-Ghodsian N. A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nat Genet. 2001;27:191–194. doi: 10.1038/84831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roubertoux PL, Sluyter F, Carlier M, Marcet B, Maarouf-Veray F, Cherif C, Marican C, Arrechi P, Godin F, Jamon M, Verrier B, Cohen-Salmon C. Mitochondrial DNA modifies cognition in interaction with the nuclear genome and age in mice. Nat Genet. 2003;35:65–69. doi: 10.1038/ng1230. [DOI] [PubMed] [Google Scholar]
- 9.Trounce I, Schmiedel J, Yen HC, Hosseini S, Brown MD, Olson JJ, Wallace DC. Cloning of neuronal mtDNA variants in cultured cells by synaptosome fusion with mtDNA-less cells. Nucleic Acids Res. 2000;28:2164–2170. doi: 10.1093/nar/28.10.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norman KG, Canter JA, Shi M, Milne GL, Morrow JD, Sligh JE. Cyclosporine A suppresses keratinocyte cell death through MPTP inhibition in a model for skin cancer in organ transplant recipients. Mitochondrion. 2009:94–101. doi: 10.1016/j.mito.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burdon RH. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic Biol Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 12.Irani K, Xia Y, Zweier JL, Sollott SJ, Der CJ, Fearon ER, Sundaresan M, Finkel T, GoldschmidtClermont PJ. Mitogenic signaling mediated by oxidants in ras-transformed fibroblasts. Science. 1997;275:1649–1652. doi: 10.1126/science.275.5306.1649. [DOI] [PubMed] [Google Scholar]
- 13.Liu SL, Lin X, Shi DY, Cheng J, Wu CQ, Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch Biochem Biophys. 2002;406:173–182. doi: 10.1016/s0003-9861(02)00430-7. [DOI] [PubMed] [Google Scholar]
- 14.Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, Marsh EE, Nowak RA. Reactive Oxygen Species Mediate Mitogenic Growth Factor Signaling Pathways in Human Leiomyoma Smooth Muscle Cells. Biol Reprod. 2009:341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer H, Wartenberg M, Hescheler J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell Physiol Biochem. 2001;11:173–186. doi: 10.1159/000047804. [DOI] [PubMed] [Google Scholar]
- 16.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008;266:53–59. doi: 10.1016/j.canlet.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 18.Makrilia N, Kollias A, Manolopoulos L, Syrigos K. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest. 2009;27:1023–1037. doi: 10.3109/07357900902769749. [DOI] [PubMed] [Google Scholar]
- 19.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nature Reviews Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 20.Etienne-Manneville S. Polarity proteins in migration and invasion. Oncogene. 2008;27:6970–6980. doi: 10.1038/onc.2008.347. [DOI] [PubMed] [Google Scholar]
- 21.Wu SJ, Ng LT, Lin CC. Effects of vitamin E on the cinnamaldehyde-induced apoptotic mechanism in human PLC/PRF/5 cells. Clin Exp Pharmacol Physiol. 2004;31:770–776. doi: 10.1111/j.1440-1681.2004.04091.x. [DOI] [PubMed] [Google Scholar]
- 22.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 23.Baysal BE, Ferrell RE, Willett-Brozick JE, Lawrence EC, Myssiorek D, Bosch A, van der Mey A, Taschner PE, Rubinstein WS, Myers EN, Richard CW, 3rd, Cornelisse CJ, Devilee P, Devlin B. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science. 2000;287:848–851. doi: 10.1126/science.287.5454.848. [DOI] [PubMed] [Google Scholar]
- 24.Barker KT, Bevan S, Wang R, Lu YJ, Flanagan AM, Bridge JA, Fisher C, Finlayson CJ, Shipley J, Houlston RS. Low frequency of somatic mutations in the FH/multiple cutaneous leiomyomatosis gene in sporadic leiomyosarcomas and uterine leiomyomas. Br J Cancer. 2002;87:446–448. doi: 10.1038/sj.bjc.6600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiuru M, Lehtonen R, Arola J, Salovaara R, Jarvinen H, Aittomaki K, Sjoberg J, Visakorpi T, Knuutila S, Isola J, Delahunt B, Herva R, Launonen V, Karhu A, Aaltonen LA. Few FH mutations in sporadic counterparts of tumor types observed in hereditary leiomyomatosis and renal cell cancer families. Cancer Res. 2002;62:4554–4557. [PubMed] [Google Scholar]
- 26.Tomlinson IP, Alam NA, Rowan AJ, Barclay E, Jaeger EE, Kelsell D, Leigh I, Gorman P, Lamlum H, Rahman S, Roylance RR, Olpin S, Bevan S, Barker K, Hearle N, Houlston RS, Kiuru M, Lehtonen R, Karhu A, Vilkki S, Laiho P, Eklund C, Vierimaa O, Aittomaki K, Hietala M, Sistonen P, Paetau A, Salovaara R, Herva R, Launonen V, Aaltonen LA. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 27.Toro JR, Nickerson ML, Wei MH, Warren MB, Glenn GM, Turner ML, Stewart L, Duray P, Tourre O, Sharma N, Choyke P, Stratton P, Merino M, Walther MM, Linehan WM, Schmidt LS, Zbar B. Mutations in the fumarate hydratase gene cause hereditary leiomyomatosis and renal cell cancer in families in North America. Am J Hum Genet. 2003;73:95–106. doi: 10.1086/376435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krupke DM, Begley DA, Sundberg JP, Bult CJ, Eppig JT. The mouse tumor biology database. Nature Reviews Cancer. 2008;8:459–465. doi: 10.1038/nrc2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finsterer J. Genetic, pathogenetic, and phenotypic implications of the mitochondrial A3243G tRNALeu(UUR) mutation. Acta Neurol Scand. 2007;116:1–14. doi: 10.1111/j.1600-0404.2007.00836.x. [DOI] [PubMed] [Google Scholar]
- 30.Schapira AHV. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]
- 31.Goto Y, Nonaka I, Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990;348:651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- 32.Goto Y. Clinical features of MELAS and mitochondrial DNA mutations. Muscle Nerve. 1995;3:S107–S112. doi: 10.1002/mus.880181422. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki S, Hinokio Y, Hirai S, Onoda M, Matsumoto M, Ohtomo M, Kawasaki H, Satoh Y, Akai H, Abe K, et al. Diabetes with mitochondrial gene tRNALYS mutation. Diabetes Care. 1994;17:1428–1432. doi: 10.2337/diacare.17.12.1428. [DOI] [PubMed] [Google Scholar]
- 34.van den Ouweland JM, Lemkes HH, Ruitenbeek W, Sandkuijl LA, de Vijlder MF, Struyvenberg PA, van de Kamp JJ, Maassen JA. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992;1:368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]
- 35.van den Ouweland JM, Lemkes HH, Trembath RC, Ross R, Velho G, Cohen D, Froguel P, Maassen JA. Maternally inherited diabetes and deafness is a distinct subtype of diabetes and associates with a single point mutation in the mitochondrial tRNA(Leu(UUR)) gene. Diabetes. 1994;43:746–751. doi: 10.2337/diab.43.6.746. [DOI] [PubMed] [Google Scholar]