Abstract
The current model of antigen assembly with major histocompatibility complex (MHC) class I molecules posits that interactions between the tapasin N-terminal immunoglobulin (Ig)-like domain and the MHC class I peptide-binding groove permit tapasin to regulate antigen selection. Much less is known regarding interactions that might involve the tapasin C-terminal Ig-like domain. Additionally, the tapasin transmembrane/cytoplasmic region enables tapasin to bridge the MHC class I molecule to the transporter associated with antigen processing (TAP). In this investigation, we made use of two tapasin mutants to determine the relative contribution of the tapasin C-terminal Ig-like domain and the tapasin transmembrane/cytoplasmic region to the assembly of MHC class I molecules. Deletion of a loop within the tapasin C-terminal Ig-like domain (Δ334-342) prevented tapasin association with the MHC class I molecule Kd. Although tapasin Δ334-342 did not increase the efficiency of Kd folding, Kd surface expression was enhanced on cells expressing this mutant relative to tapasin-deficient cells. In contrast to tapasin Δ334-342, a soluble tapasin mutant lacking the transmembrane/cytoplasmic region retained the ability to bind to Kd molecules, but did not facilitate Kd surface expression. Furthermore, when soluble tapasin and tapasin Δ334-342 were co-expressed, soluble tapasin had a dominant negative effect on the folding and surface expression of not only Kd, but also Db and Kb. In addition, our molecular modeling of the MHC class I-tapasin interface revealed novel potential interactions involving tapasin residues 334-342. Together, these findings demonstrate that the tapasin C-terminal and transmembrane/cytoplasmic regions are critical to tapasin's capacity to associate effectively with the MHC class I molecule.
Keywords: antigen processing and presentation, Kd, MHC class I, peptide-loading complex, tapasin, transporter associated with antigen processing
1. Introduction
Antigen presentation by MHC class I molecules enables cytolytic T lymphocytes to recognize intracellular abnormalities arising from infection or cancer. Before trafficking to the cell surface, MHC class I molecules are integrated into the peptide-loading complex, which assists in antigen selection. Within the peptide-loading complex, tapasin participates in several intermolecular interactions, including ones which bridge the MHC class I molecule to TAP (Garbi et al., 2003; Raghavan et al., 2008; Sadasivan et al., 1996). Of these interactions, the molecular nature of the MHC class I-tapasin interface remains a particularly important question. The tapasin N-terminal domain is believed to associate with the MHC class I peptide-binding groove in a manner that stabilizes the MHC groove in a receptive conformation (Chen and Bouvier, 2007; Praveen et al., 2010; Van Hateren et al., 2010). Perhaps as a necessary consequence of tapasin's function (since the MHC class I molecule must be released from tapasin once a stable peptide has been bound), the in vitro affinity between tapasin and the MHC class I molecule is weak (Chen and Bouvier, 2007; Wearsch and Cresswell, 2007).
Our lab has previously characterized a tapasin mutant that lacks a short sequence (residues 334-342) within the tapasin C-terminal Ig-like domain (Turnquist et al., 2001; Turnquist et al., 2002; Turnquist et al., 2004). The tapasin mutant lacking amino acids 334-342 (tapasin Δ334-342) is unable to associate with the murine MHC class I molecule Ld (Turnquist et al., 2001; Turnquist et al., 2004). However, due to the presence of the transmembrane/cytosolic domains, tapasin Δ334-342 retains the ability to stabilize TAP, and thereby permits Ld surface expression (Turnquist et al., 2001; Turnquist et al., 2004). As shown in Fig. 1A, based on the x-ray crystallographic structure of tapasin (Dong et al., 2009), tapasin residues 334-342 comprise an exposed loop that extends upwards towards the N-terminal Ig-like domain. Moreover, the tapasin 334-342 sequence lies on the same face of tapasin as does a conserved patch of residues shown by Dong et al. (2009) to be important for tapasin-MHC class I association (Fig 1A). These observations support the suggestion that tapasin residues 334-342 directly contact the MHC class I α3 domain (Bouvier, 2003; Turnquist et al., 2002). As such, amino acids E222 and D227 within the MHC class I α3 domain have been shown to be necessary for efficient tapasin association (Carreno et al., 1995; Harris et al., 2001; Suh et al., 1999).
Figure 1. Location of tapasin residues involved in MHC class I association.
(A) Tapasin residues 334-342 (shown in orange) lie on a loop within the C-terminal Ig-like domain. Also, shown in blue are residues within the N-terminal Ig-like domain which were proposed to comprise a binding site for the MHC class I α2-1 helix (Dong et al., 2009). The ribbon diagram was generated from an x-ray crystallographic structure of the tapasin/ERp57 heterodimer (Dong et al., 2009) (PDB code 3F8U) using PyMOL (Schrödinger, 2010). (B) Deletion of residues 334-342 is not predicted to alter the overall tapasin structure. Residues 334-342 were removed from the tapasin crystal structure, and the resulting structure was subjected to energy minimization as described in the Materials and methods. Amino acid residues involved in MHC class I binding are colored as in (A). Residues 333 and 343, flanking the deleted sequence, are shown in orange.
The tapasin transmembrane/cytoplasmic region also plays a critical role within the peptide-loading complex. For instance, the ability of the tapasin transmembrane/cytoplasmic domains to stabilize TAP was first appreciated as a result of studies utilizing a soluble version of tapasin (Lehner et al., 1998). Despite the lack of TAP stabilization, soluble tapasin restores the surface expression of human leukocyte antigen (HLA) molecules, albeit at a reduced level as compared to the extent of HLA surface expression on cells expressing wild type tapasin (Everett and Edidin, 2007; Lehner et al., 1998; Rizvi and Raghavan, 2010). Furthermore, the HLA molecules that are assembled in the presence of soluble tapasin are less stable than those assembled in the presence of wild type tapasin (Tan et al., 2002). In striking contrast, we have previously reported that both soluble mouse tapasin and soluble human tapasin do not enable the cell surface expression of murine MHC class I molecules (Simone et al., 2009a; Simone et al., 2009b). Rather, soluble tapasin suppressed murine MHC class I surface expression below the level present on cells without tapasin, suggesting that soluble tapasin impedes murine MHC class I assembly (Simone et al., 2009b). Thus, there is a clear distinction on the reliance of human versus mouse MHC class I molecules on the tapasin transmembrane/cytoplasmic region.
Understanding the mechanisms influencing murine MHC class I assembly is necessary for the interpretation of mouse models of MHC class I-mediated immunity. Furthermore, in order to translate research performed in mice to human subjects successfully, it is necessary to understand the fundamental differences between the murine and human immune systems. For these reasons, we sought to resolve the mechanism by which soluble tapasin inhibits murine MHC class I surface expression. In this study, we detected the binding of soluble tapasin to the folded form of the mouse MHC class I molecule Kd. Despite the soluble tapasin-Kd association, soluble tapasin was unable to increase the proportion of folded Kd molecules present within cells and on the cell surface. On the other hand, tapasin Δ334-342 did not enhance the efficiency of intracellular Kd folding, but did allow a partial restoration of Kd surface expression. Even so, expression of tapasin Δ334-342 was unable to overcome the influence of soluble tapasin on MHC class I assembly and surface expression. In addition, we used molecular modeling to predict interactions involving tapasin residues 334-342 with the MHC class I molecule. This analysis also revealed novel potential interactions involving tapasin N-terminal domain residues. Collectively, our analysis of tapasin Δ334-342 supports the hypothesis that interactions involving the tapasin C-terminal and N-terminal domains cooperate to stabilize the intrinsically weak association between tapasin and the MHC class I molecule.
2. Materials and methods
2.1 Cell lines
The tapasin knockout mouse fibroblast (MF) cell line was generated by Drs. A. Grandea and L. Van Kaer and coworkers (Vanderbilt University, Nashville, TN) (Grandea et al., 2000). The MF cells are of the H2b haplotype and so express endogenous Db and Kb molecules. The Kd cDNA (a gift from Dr. T. Hansen, Washington University, St. Louis, MO) was cloned into the pMIN retroviral vector and transduced into MF cells using the 293E packaging cell line. The cDNA encoded a Kd molecule that contained an epitope tag such that open, peptide-free Kd molecules could be detected using the 64-3-7 monoclonal antibody (mAb). Incorporation of this epitope tag does not influence MHC class I assembly (Harris et al., 2001; Lybarger et al., 2001; Myers et al., 2000; Yu et al., 1999a). The wild type mouse tapasin cDNA was a kind gift from Dr. P. Wang (Barts and London School of Medicine). The generation of soluble mouse tapasin and mouse tapasin Δ334-342 has been previously described (Simone et al., 2009b; Turnquist et al., 2004). Wild type and mutant tapasin cDNAs were cloned into the pMIN or pLXSH retroviral vectors and transduced into MF cells. MF cells transduced with Kd and/or tapasin were sorted and cloned for stable Kd and tapasin expression. All cells were cultured at 37°C in 5% CO2 in Dulbecco's Modified Eagle Medium containing 10% fetal bovine serum, 4 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 mM L-glutamine, 100 U/ml penicillin, 100 U/ml streptomycin, and 4×10-5 M 2-mercaptoethanol. The fetal bovine serum was purchased from Atlanta Biologicals, and all other media reagents were acquired from Invitrogen.
2.2 Antibodies
The 34-1-2 mAb recognizes an epitope on the α1 domain of folded H2d molecules, and displays additional cross-reactivity with the b, s, r, q, and p MHC class I haplotypes (Evans et al., 1982; Nieto et al., 1989; Ozato et al., 1982; Solheim et al., 1995; Straus et al., 1985; Stroynowski et al., 1985). The 64-3-7 mAb recognizes an epitope found on certain open, peptide-free MHC class I molecules. The epitope for 64-3-7 occurs naturally on Ld and Lq molecules (Lie et al., 1991), and can be transferred to other MHC class I molecules by site-directed mutagenesis (Myers et al., 2000; Yu et al., 1999a). The B22/249 and Y3 mAbs recognize folded Db and Kb molecules, respectively (Allen et al., 1984; Allen et al., 1986; Hammerling et al., 1982; Lemke et al., 1979). The following antibodies were gifts from Dr. T. Hansen (Washington University, St. Louis, MO), and were used to detect proteins on western blots: 64-3-7 (to detect denatured Kd molecules), hamster anti-mouse tapasin mAb, rabbit anti-mouse ERp57 serum, and rabbit anti-Kb cytoplasmic tail serum. The rabbit anti-Db serum used to detect denatured Db on western blots was a kind gift from Dr. K.P. Kane (University of Alberta, Edmonton, Canada). Rabbit α-mouse TAP1 serum (#503) (a gift from Dr. T. Hansen) was used to immunoprecipitate mouse TAP1, and goat anti-mouse TAP1 Ab (Santa Cruz Biotechnology) was used for western blot analysis. A goat anti-mouse tapasin Ab (Santa Cruz Biotechnology) was used to immunoprecipitate murine tapasin. The rabbit anti-calreticulin serum was purchased from Stressgen, and the mouse anti-actin mAb was purchased from Sigma-Aldrich.
2.3 Immunoprecipitation and western blotting
Before immunoprecipitation, equivalent numbers of cells were aliquoted and washed three times in phosphate-buffered saline (PBS) containing 20 mM iodoacetamide (Sigma-Aldrich). After washing, the cells were lysed in a solution consisting of 1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS) (Roche), 20 mM iodoacetamide (Sigma-Aldrich), and 0.2 mM phenylmethanesulfonyl fluoride in Tris-buffered saline (pH 7.4). Excess antibody was added directly to the lysis buffer before the buffer was applied to the cells. After 1 h of incubation on ice, the lysates were centrifuged to remove the cell nuclei, and the supernatants were applied to Protein A-Sepharose beads (Amersham Biosciences). The lysates were incubated with the beads for 45 min on ice with intermittent mixing. The beads were then washed four times with a buffer containing 0.1% CHAPS (Roche) and 20 mM iodoacetamide (Sigma-Aldrich) in Tris-buffered saline (pH 7.4). After the final wash, proteins were eluted from the Protein A-Sepharose beads by boiling in 1x NuPAGE lithium dodecyl sulfate sample buffer (pH 8.4) (Invitrogen). For immunoprecipitation of ERp57 molecules, the cells were treated with methyl methanethiosulfonate (indicated in the relevant figure legend) as previously described (Simone et al., 2010).
The eluted proteins were electrophoresed on precast polyacrylamide Tris-glycine gels (Invitrogen), and the proteins were transferred to Immobilon-P membranes (Millipore). The membranes were blocked overnight in 10% dry milk (prepared in 0.05% Tween 20/PBS). The membranes were then incubated with primary antibody diluted in 10% dry milk (prepared in 0.05% Tween 20/PBS) for 1 h. Following three washes with 0.05% Tween 20/PBS, the membranes were incubated for 1 h with the appropriate secondary antibody (diluted in 0.05% Tween 20/PBS). The secondary antibody was conjugated to biotin or to horseradish peroxidase. All of the secondary antibodies (goat anti-mouse IgG [light chain specific], mouse anti-rabbit IgG [light chain specific], goat anti-Armenian hamster IgG [heavy and light chain specific]), and bovine anti-goat [heavy and light chain specific]) were purchased from Jackson ImmunoResearch. In experiments in which biotin-conjugated secondary antibodies were used, the membranes were washed three times with 0.05% Tween 20/PBS and then incubated for 1 h with horseradish peroxidase-conjugated streptavidin (Invitrogen) diluted in 0.05% Tween 20/PBS. Following incubation with either horseradish peroxidase-conjugated streptavidin or with horseradish peroxidase-conjugated secondary antibody, the membranes were washed three times with 0.3% Tween 20/PBS and treated with Enhanced Chemiluminescent Western Blotting Substrate (Pierce). To visualize the proteins, the membranes were exposed to Kodak BioMax film. Protein bands were quantified using the Molecular Imager ChemiDoc XRS system with Quantity One 1-D Analysis Software (Bio-Rad).
For western blots of proteins from cell lysates without immunoprecipitation, aliquots of cell equivalents were first washed three times with PBS containing 20 mM iodoacetamide (Sigma-Aldrich). Next, the cells were lysed in 150 mM NaCl/20 mM Tris (pH 7.5)/0.5% Triton X-100/5 mM ethylenediaminetetraacetic acid (EDTA)/0.2 mM phenylmethanesulfonyl fluoride. The lysates were subjected to a freeze-thaw cycle by overnight storage at -80°C followed by thawing on ice. Next, the lysates were centrifuged, and an aliquot of the supernatant was combined with 4x NuPAGE lithium dodecyl sulfate sample buffer (pH 8.4) (Invitrogen) containing 8% 2-mercaptoethanol, such that the final concentrations of the sample buffer and 2-mercaptoethanol were 1x and 2%, respectively. Finally, the samples were boiled for 5 min. Electrophoresis and western blotting were performed as described above.
For cycloheximide (CHX) treatment prior to western blot analysis, cells were incubated for 0, 4, or 8 h in the presence of 10 μg CHX (Sigma-Aldrich) per ml of cell culture media at 37°C in 5% CO2. Following CHX treatment, equivalent numbers of cells were aliquoted, washed three times with PBS containing 20 mM iodoacetamide, and lysed as described above.
2.4 Flow cytometry
In preparation for flow cytometric analysis, cells were washed twice with 0.5% bovine serum albumin/2 mM EDTA/PBS. Fifty thousand cells per well were aliquoted into a 96-well plate and excess mAb or 0.5% bovine serum albumin/2 mM EDTA/PBS (as a control) was added. The cells were incubated for 30 min on ice and then washed three times with 0.5% bovine serum albumin/2 mM EDTA/PBS. After washing, the cells were incubated for 30 min on ice with a phycoerythrin-conjugated, Fc-specific F(ab')2 portion of goat anti-mouse IgG (Jackson ImmunoResearch). Finally, the cells were washed three times with 0.5% bovine serum albumin/2 mM EDTA/PBS and analyzed on a FACSCalibur flow cytometer (BD Biosciences). Statistical analyses were done with the Cell Quest software (BD Biosciences).
2.5 Molecular modeling and structure analysis
To analyze the interactions of open, peptide-free Kd and tapasin, we used an approach similar to that taken by van Hateren and colleagues (2010). First, the folded, peptide-bound structure of Kd (PDB code 2FWO (Mitaksov and Fremont, 2006)) was modified as follows. Residues 136-153, containing the short α2-1 helix, were rotated by 45° about a vector from the α-carbon of A153 to the α-carbon of A136 using MOLEMAN2 (Kleywegt, 1997), as postulated by Elliott (1997). The peptide chain was removed from the resulting PDB file, and the energy of the structure was minimized with Tinker (version 6.0) (Ponder, 2001) using the GBSA implicit solvent model (Qiu et al., 1997) and the all-atom OPLS (OPLS-AA) force field (Chopra et al., 2008; Jorgensen et al., 1996). As compared to molecular dynamics in explicit solvent, Chopra et al. found that energy minimization with GBSA implicit solvation performed better at moving perturbed protein structures back to their native states. Therefore, we performed our energy minimizations using the same parameters as Chopra et al. (2008). Tinker uses a modified L-BFGS minimization algorithm in Cartesian coordinates. The target root mean square (RMS) gradient value for energy minimization was 0.01; our energy minimization of Kd had a final RMS gradient value of 0.0096 achieved in 5596 steps. In the energy minimized structure, the α2-1 helix was positioned intermediately between the original, peptide-bound structure and the fully rotated structure. To prepare the output PDB file for docking, the hydrogen atoms were removed using a custom AWK script and PDBCUR in CCP4i (Collaborative Computational Project, Number 4, 1994). Lastly, the occupancies and B-factors from the input PDB file were added back to the output file using a custom AWK script. The modified Kd structure was docked with the x-ray crystallographic structure of human tapasin (PDB code 3F8U (Dong et al., 2009), chain B) using the HADDOCK web server (de Vries et al., 2010). HADDOCK performs an initial rigid-body energy minimization, followed by a semi-flexible refinement in torsion angle space, and finally refinement in explicit solvent. Furthermore, during the docking, HADDOCK permits conformational changes of the peptide side chains and backbones, thereby omitting the need for molecular dynamics of the final docked structure. The following amino acids were entered as active residues for the docking: MHC residues 128-136 (Yu et al., 1999b), 222 (Harris et al., 2001; Suh et al., 1999), and 227 (Carreno et al., 1995; Harris et al., 2001); tapasin residues 185, 187, 189, 261, 334, and 335 (Dong et al., 2009; Turnquist et al., 2004, and this report). Figures were generated with PyMOL (Schrödinger, 2010) and LigPlot+ (Wallace et al., 1995).
The tapasin Δ334-342 mutant was modeled by first deleting the ATOM entries for these residues from the PDB file of wild type tapasin (PDB code 3F8U (Dong et al., 2009), chain B) and connecting the carbon and nitrogen atoms of R333 and S343, respectively, with CONECT entries. The energy of the structure was then minimized as above. The final RMS gradient value was 0.0097 achieved in 3783 steps.
3. Results
3.1 Expression and stability of wild type, Δ334-342, and soluble tapasin in MF-Kd cells
In this current study we first sought to determine the relative importance of the tapasin-TAP and the tapasin-MHC class I interactions for MHC class I surface expression. Soluble tapasin does not bind to TAP (Lehner et al., 1998), and fails to facilitate stable MHC class I folding and surface expression (Simone et al., 2009b; Tan et al., 2002). We reasoned that if deficient peptide transport was the major contributing factor to the soluble tapasin phenotype, then the added expression of tapasin Δ334-342, which enhances TAP expression (Turnquist et al., 2001; Turnquist et al., 2004), should restore murine MHC class I expression at the plasma membrane. To this end, we utilized tapasin-knockout mouse fibroblasts (MFs) which expressed Kd alone (MF-Kd), or Kd along with wild type tapasin (MF-Kd-WT), soluble tapasin (MF-Kd-SOL), tapasin Δ334-342 (MF-Kd-ΔTsn), or both soluble tapasin and tapasin Δ334-342 (MF-Kd-SOL-ΔTsn). As shown in Fig. 2A, the levels of Kd expression among the cell lines were roughly equal, although the MF-Kd and the MF-Kd-ΔTsn cells had lower expression of Kd compared to the other cell lines. Additionally, the levels of tapasin Δ334-342 and soluble tapasin were lower in the MF-Kd-ΔTsn and MF-Kd-SOL-ΔTsn cell lines, respectively (Fig. 2A). In a separate analysis of these cell lines, the levels of Kd and tapasin were again found to be similar (Supplementary Fig. 1A).
Figure 2. Expression of Kd and wild type or mutant tapasin in MF cell lines.
(A) The level of Kd and tapasin expressed in the indicated MF cell lines was determined by western blot analysis. Samples of cell lysates were electrophoresed on a 4→20% acrylamide Tris-glycine gel (for Kd) or on 10% acrylamide Tris-glycine gels (for tapasin and actin), transferred to membranes, and probed with the 64-3-7 mAb (for Kd), hamster anti-mouse tapasin mAb, or mouse anti-actin mAb. In the MF-Kd-SOL-ΔTsn cell line, soluble tapasin and tapasin Δ334-342 can be distinguished due to the smaller size of soluble tapasin. Noncontiguous lanes from the same gel are indicated by a dashed line. Data are representative of two experiments. (B) Stability of wild type and mutant tapasin. The indicated cell lines were incubated in the presence of 10 μg CHX/ml for 0, 4, or 8 h prior to lysis. Samples of the cell lysates were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane and probed with hamster anti-mouse tapasin mAb (upper panel). Proteins were then stripped, and the membrane was reprobed with mouse anti-actin mAb (lower panel). (C) Quantification of tapasin stability. Densitometry was performed on four different exposures with similar intensities of the tapasin and actin blots shown in (B). For each exposure, the relative signal of each band was determined by setting the band with the greatest signal to 100 in order to correct for the different intensities between exposures. Next, the average relative signal of each band from the four exposures was calculated. The average relative signal of tapasin was then normalized to the average relative signal of actin. Finally, the tapasin/actin ratio of cells treated with CHX for 0 h was set to 100% and used to calculate the percent tapasin/actin remaining at each time point. Error bars represent the standard deviation associated with the average relative signals from the four film exposures. Similar results were obtained on a separate SDS-PAGE/western blot of the samples.
The deletion of residues 334-342 within the tapasin C-terminal domain could potentially affect tapasin folding and/or stability. However, our molecular modeling of the tapasin Δ334-342 mutation predicts that removal of this loop does not grossly affect the tapasin folded structure (compare Figs. 1A and 1B). Furthermore, when expressed in the MF cells, the stability of wild type tapasin and tapasin Δ334-342 were similar (Fig. 2B). Notably, soluble tapasin was highly stable (Fig. 2B), consistent with a previous finding that soluble tapasin mutants were expressed at higher levels than full-length forms of tapasin (Vigneron et al., 2009). As suggested by Vigneron et al. (2009), positively charged residues within the tapasin transmembrane region (e.g. K408 (Petersen et al., 2005)) likely contribute to the destabilization of wild type tapasin and tapasin Δ334-342 as compared to soluble tapasin. Overall, these data support the conclusion that both tapasin Δ334-342 and soluble tapasin are stably folded when expressed in MF cells.
3.2 Soluble tapasin has a dominant negative effect over tapasin Δ334-342 on Kd surface expression
First, we investigated the ability of wild type and mutant tapasin to restore the surface expression of Kd on the MF cell lines. Kd surface levels were quantified by flow cytometry using the 64-3-7 mAb to detect the open forms of Kd and the 34-1-2 mAb to detect folded Kd molecules. Open, peptide-free forms of MHC class I molecules can be serologically detected by the 64-3-7 antibody if the epitope is either naturally present (as in Ld and Lq) or introduced by mutagenesis (as in Kd, Kb, and other MHC class I molecules) (Lie et al., 1991; Myers et al., 2000; Yu et al., 1999a). On the other hand, the 34-1-2 mAb binds to the α1-helix of folded MHC class I molecules (Nieto et al., 1989; Ozato et al., 1982; Solheim et al., 1995). We found that, as compared to wild type tapasin, tapasin Δ334-342 facilitated a partial restoration of folded Kd surface expression (Fig. 3A). This observation was consistent across multiple flow cytometric analyses as well as in the analysis of a separate MF-Kd-ΔTsn cell line (Supplementary Fig. 1B and data not shown). Although the MF-Kd-ΔTsn cell line expressed slightly less Kd and tapasin than did the MF-Kd-WT cells (Fig. 2A), this cannot account for the ~3-fold difference in Kd surface expression on MF-Kd-WT as compared to MF-Kd-ΔTsn cells (Fig. 3A-B).
Figure 3. Expression of tapasin Δ334-342 partially restores surface expression of Kd molecules, whereas expression of soluble tapasin, alone or with tapasin Δ334-342, does not improve Kd levels at the plasma membrane.
(A) Cell surface expression of Kd on the indicated cell lines was determined by flow cytometry using the 64-3-7 and 34-1-2 mAbs. (B) Cell surface expression of Kd molecules on MF-Kd-SOL and MF-Kd-SOL-ΔTsn cell lines, as determined by the flow cytometric analysis performed in (A), but here shown in expanded detail. (C) The ratio of open to folded Kd at the cell surface was calculated by dividing the mean fluorescence units obtained with the 64-3-7 mAb by the mean fluorescence units obtained with the 34-1-2 mAb. Data are representative of 5 independent flow cytometric analyses.
The ratio of open (64-3-7+) to folded (34-1-2+) MHC class I molecules present at the plasma membrane is a representation of the quality of the assembled molecules. On the MF cell surface, the ratio of open:folded Kd molecules was substantially greater in the absence of tapasin than in the presence of wild type tapasin (Fig. 3C), as previously observed for Ld (Turnquist et al., 2004) and consistent with findings by Myers et al. (2000) with transfected human cells. As with Ld (Turnquist et al., 2004), tapasin Δ334-342 improved (lowered) the ratio of open:folded Kd at the plasma membrane relative to no tapasin, though not as much as wild type tapasin (Fig. 3C). As we have observed previously (Simone et al., 2009b), the expression of soluble tapasin did not raise Kd surface expression above the level seen on cells with no tapasin (Fig. 3A), and did not improve the cell surface ratio of open:folded Kd (Fig. 3C). Surprisingly, the expression of tapasin Δ334-342 along with soluble tapasin caused only a very minor, but reproducible, increase in folded Kd surface expression (Fig. 3B and Supplementary Fig. 1C). Accordingly, in the presence of soluble tapasin, tapasin Δ334-342 slightly improved the cell surface ratio of open:folded Kd molecules as compared to MF cells with no tapasin (Fig. 3C). Overall, these findings indicate that soluble tapasin has a dominant negative influence on Kd assembly.
3.3 Soluble tapasin suppresses the ability of tapasin Δ334-342 to stabilize TAP
The ability of our tapasin constructs to facilitate Kd surface expression may be linked to TAP stabilization. To this end, we assessed the levels of TAP in each of the MF cell lines by immunoprecipitation and western blot analysis of TAP1 (Fig. 4A). Little to no TAP1 was detected in MF cells without tapasin, whereas the presence of wild type tapasin greatly enhanced mouse TAP1 (Fig. 4A compare lanes 1-2 with lane 3). This is consistent with the previous observation that tapasin increased TAP expression 100-fold in mouse cells (Garbi et al., 2003). Although not as much as wild type tapasin, tapasin Δ334-342 also enhanced TAP1 levels (Fig. 4A compare lanes 3 and 4). Thus, the lower level of Kd surface expression on MF-Kd-ΔTsn cells (as compared to MF-Kd-WT cells) (Fig. 3) is likely due (at least in part) to lower TAP1 levels in these cells. As expected, deletion of the transmembrane/cytosolic domains prevented soluble tapasin from restoring TAP1 levels (Fig. 4A lane 5). Interestingly, very little TAP1 was detected in MF-Kd cells that co-expressed tapasin Δ334-342 and soluble tapasin (Fig. 4A lane 6). Apparently, soluble murine tapasin prevents tapasin Δ334-342 from stabilizing TAP1.
Figure 4. Intermolecular interactions among peptide-loading complex proteins.
(A) Mouse TAP1 was immunoprecipitated from cell equivalents with rabbit anti-mouse TAP1 serum (#503). The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed on a western blot with goat anti-mouse TAP1 Ab. (B) Kd molecules were immunoprecipitated from cell equivalents using the 34-1-2 mAb or the 64-3-7 mAb. The immunoprecipitated proteins were electrophoresed on a 4→20% acrylamide Tris-glycine gel (for Kd) or on a 10% acrylamide Tris-glycine gel (for tapasin, calreticulin, and ERp57). The proteins were then transferred to membranes and probed on western blots with the 64-3-7 mAb (for Kd), hamster anti-mouse tapasin mAb, rabbit anti-calreticulin polyclonal antibody, or rabbit anti-ERp57 serum. On the Kd blot, a background band occurring upon immunoprecipitation with the 34-1-2 mAb (more visible in cells with Kd) is marked with an asterisk. Noncontiguous lanes from the same gel are indicated by a dashed line. Results shown here are representative of the results from two experiments. (C) Tapasin molecules were immunoprecipitated from cell equivalents with a goat anti-mouse tapasin antibody. The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel (for tapasin) or on a 4→20% acrylamide Tris-glycine gel (for Kd), transferred to membranes and probed on western blots with hamster anti-mouse tapasin mAb or with the 64-3-7 mAb (for Kd).
3.4 Soluble tapasin associates with 34-1-2+Kd molecules, but does not support Kd folding
In order to better understand the influence of soluble tapasin and tapasin Δ334-342 on murine MHC class I assembly, we assessed Kd intracellular folding. Kd molecules were immunoprecipitated with either the 34-1-2 or 64-3-7 mAb and probed on a western blot to reveal the Kd heavy chains (Fig. 4B). Variation in the amount of Kd expressed among the cell lines (Fig. 2A) prevented us from directly comparing the levels of folded and open Kd molecules. Nevertheless, we could qualitatively compare the levels of immunoprecipitated folded (34-1-2+) Kd relative to the levels of open (64-3-7+) Kd in each cell line. In this analysis, only wild type tapasin substantially augmented the level of folded Kd molecules and simultaneously lowered the amount of open Kd (Fig. 4B). In MF cells expressing soluble tapasin alone, there were similar or slightly more 34-1-2+ forms of Kd relative to 64-3-7+ forms (Fig. 4B, and Supplementary Fig. 2A compare lanes 5 and 11); however, these MHC class I molecules are likely unstable as very few of them are present at the cell surface (Fig. 2A). In cells with tapasin Δ334-342, with or without soluble tapasin, the ratio of open:folded Kd was ~1 (Fig. 4B). An interesting conclusion from this finding is that, although tapasin Δ334-342 permits Kd surface expression, it does so with little or no increase in the efficiency of Kd folding.
The physical association between tapasin and the MHC class I molecule permits tapasin to optimize peptide loading (Bangia et al., 1999; Lewis et al., 1996; Peace-Brewer et al., 1996; Yu et al., 1999b). Our lab has recently discovered that tapasin preferentially associates with 34-1-2+, rather than 64-3-7+, Kd (Simone et al., 2009b; Wang et al., 2009). This prompted us to evaluate the ability of soluble tapasin to associate with 34-1-2+, as well as with 64-3-7+, Kd. As shown in Fig. 4B (lane 3), wild type tapasin associates strongly with 34-1-2+ Kd. Surprisingly, soluble tapasin was also found to associate, to a lesser extent, with 34-1-2+ Kd (Fig. 4B, lanes 5 and 6). As compared to 34-1-2+ Kd, very little open, 64-3-7+ Kd exists in cells expressing full length tapasin; nevertheless, we could detect some weak binding of wild type tapasin to 64-3-7+ Kd (Fig. 4B, lane 9). In contrast, although there was a greater amount of 64-3-7+ Kd immunoprecipitated in the presence of soluble tapasin (Fig. 4B, lane 11), soluble tapasin was only weakly detected in association with the open form of Kd upon very prolonged film exposure (data not shown). This is consistent with previous findings from our lab demonstrating that soluble tapasin is not readily detectable in association with open, peptide-free Kd, Ld, or Kb molecules (Simone et al., 2009b). Thus, it appears that tapasin C-terminal truncation does not completely abrogate the tapasin-Kd association, but may hamper the binding of tapasin to a specific form of Kd, or simply reduce binding to Kd to the point that the interaction between open Kd and soluble tapasin is negligible. As expected, tapasin Δ334-342 did not readily associate with either 34-1-2+ or 64-3-7+ Kd (Fig. 4B, lanes 4 and 10). However, in some experiments, tapasin Δ334-342 could be weakly visualized in association with open and folded Kd upon very long film exposure (data not shown). Therefore, transient binding of tapasin Δ334-342 to Kd potentially contributes to Kd surface expression in the MF-Kd-ΔTsn cells (Fig. 3).
In order to confirm our findings regarding the tapasin-Kd association, wild type and mutant tapasin were immunoprecipitated from each of the MF cell lines and probed on a western blot for co-immunoprecipitated Kd (Fig. 4C). We again observed Kd molecules in association with wild type and soluble tapasin, but not with tapasin Δ334-342. In this experiment, Kd molecules were weakly associated with wild type tapasin, but more strongly associated with soluble tapasin (in both MF-Kd-SOL and MF-Kd-SOL-ΔTsn cells) (Fig. 4C compare lanes 3, 5 and 6). Because of the strong association between the 34-1-2+ form of Kd and tapasin (Fig. 4B lane 3), we were surprised by the relatively low level of Kd molecules co-immunoprecipitating with wild type tapasin (Fig. 4C lane 3). One possible explanation is that the association between wild type tapasin and the 34-1-2+ forms of Kd masks the epitope recognized by the anti-tapasin Ab used for the immunoprecipitation shown in Fig. 4C. If so, then the Kd molecules that co-immunoprecipitated with tapasin in our experiment would represent the small amount of open, 64-3-7+ Kd molecules that associate with wild type tapasin (Fig. 4B lane 9). Additionally, this hypothesis suggests that the mode of interaction between soluble tapasin and Kd differs from the wild type tapasin-Kd interface (since Kd readily co-immunoprecipitated with soluble tapasin [Fig. 4C lanes 5-6], but soluble tapasin did not readily associate with open, 64-3-7+ Kd [Fig. 4B lanes 11-12]).
We also assessed the ability of tapasin to promote the interaction of calreticulin and ERp57 with both 34-1-2+ and 64-3-7+ Kd (Fig. 4B). In general, the ability of calreticulin and ERp57 to associate with Kd correlated with the binding of tapasin to Kd, consistent with the cooperative nature of peptide-loading complex protein interactions. In these analyses, we were unable to detect an association between ERp57 and 34-1-2+ Kd in the presence of tapasin Δ334-342 and/or soluble tapasin (Fig. 4B, lanes 4-6). Additionally, calreticulin and ERp57 were not seen in association with 64-3-7+ Kd in the presence of wild type or mutant tapasin (Fig. 4B, lanes 9-12). Thus, these interactions are likely to fall below the detection threshold of our assay.
An important implication of these data is that binding of tapasin to the MHC class I molecule does not always support peptide loading. A prior investigation found that the disulfide-bonded tapasin/ERp57 heterodimer, but not tapasin alone, facilitated MHC class I assembly (Wearsch and Cresswell, 2007). In order to test whether tapasin truncation affected tapasin conjugation to ERp57, we immunoprecipitated ERp57 in the presence of the thiol-reactive agent methyl methanethiosulfonate, which has been shown to preserve the tapasin-ERp57 disulfide bond (Peaper et al., 2005). Under these conditions, both soluble tapasin and wild type tapasin were found to associate with ERp57 (Supplementary Fig. 2B), consistent with previous studies of soluble human tapasin (Rizvi and Raghavan, 2010; Vigneron et al., 2009). Therefore, the inability of soluble tapasin to facilitate murine MHC class I surface expression cannot be explained by a lack of tapasin/ERp57 heterodimerization.
3.5 Soluble tapasin fails to promote the folding and surface expression of endogenously expressed mouse MHC class I molecules
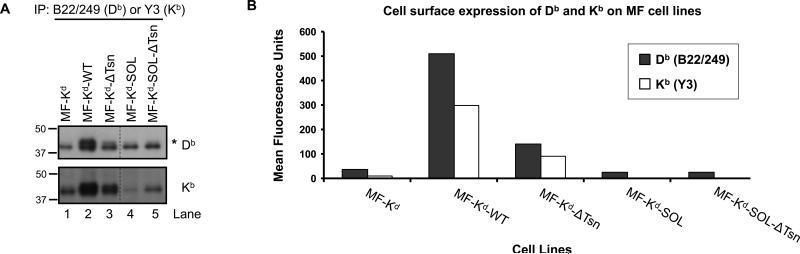
In order to extend our analysis beyond the Kd molecule, we assessed the influence of wild type tapasin, tapasin Δ334-342, and soluble tapasin on the folding and surface expression of the Db and Kb molecules endogenously expressed by the MF cells. The folded forms of Db and Kb were immunoprecipitated using the B22/249 and Y3 mAbs, respectively (Fig. 5A). Folded Db and Kb molecules were apparent in the absence of tapasin (Fig. 5A). However, these MHC class I molecules did not efficiently traffic beyond the cis-Golgi, as is evidenced by the absence of an upper band corresponding to the mature glycoprotein. Wild type tapasin, and to a lesser extent tapasin Δ334-342, caused an increase in the level of folded Db and Kb molecules, as well as the appearance of a slower migrating form of Db and Kb, which likely represents the mature glycoprotein (Fig. 5A). Soluble tapasin (expressed alone or in the context of tapasin Δ334-342) did not raise the levels of folded Db and Kb above those found in the absence of tapasin, although in some experiments, soluble tapasin alone caused the folding of some Db molecules (Fig. 5A and Supplementary Fig. 2C).
Figure 5. Tapasin Δ334-342, but not soluble tapasin, enhances the surface expression of Db and Kb molecules.
(A) Folded Db and Kb molecules were immunoprecipitated from cell equivalents with the B22/249 and Y3 mAbs, respectively. The immunoprecipitated proteins were electrophoresed on 4→20% acrylamide Tris-glycine gels, transferred to membranes, and probed on western blots with rabbit anti-Kb serum or rabbit anti-Db serum. In the Db blot, an additional band (marked with an asterisk) with slower migration is found in the MF-Kd-WT and MF-Kd-ΔTsn cell lines. This band presumably represents MHC class I molecules that are mature glycoproteins. Mature Kb molecules are also likely to be present in the MF-Kd-WT and MF-Kd-ΔTsn cell lines; however, the Kb bands did not resolve as well. Noncontiguous lanes from the same gel are indicated by a dashed line. The finding of less folded Db and Kb in MF-Kd-SOL-ΔTsn than in MF-Kd-WT, as shown, is representative of data from two experiments. (B) Cell surface expression of folded Db and Kb molecules on the indicated cell lines was determined by flow cytometry using the B22/249 and Y3 mAbs, respectively.
We additionally assessed the impact of tapasin mutation on Db and Kb surface expression. As shown in Fig. 5B, tapasin Δ334-342 enabled an increase in the level of folded Db and Kb at the plasma membrane as compared to cells with no tapasin. However, as with Kd (Fig. 3A), the level of cell surface folded Db and Kb achieved by tapasin Δ334-342 was significantly reduced as compared to wild type tapasin. In contrast to wild type tapasin and tapasin Δ334-342, soluble tapasin did not facilitate Db and Kb surface expression (Fig. 5B). Furthermore, when expressed in combination with tapasin Δ334-342, soluble tapasin exerted a dominant negative impact on the ability of folded Db and Kb to reach the plasma membrane (Fig. 5B). Overall, the impact of tapasin Δ334-342 and soluble tapasin on the surface levels of folded Db and Kb is similar to the impact of these tapasin mutants on folded Kd surface expression (compare Figs. 3A and 5B). Thus, the influence of soluble tapasin and tapasin Δ334-342 on murine MHC class I surface expression is consistent across multiple MHC class I alloforms, with soluble tapasin exerting a dominant negative influence on murine MHC class I folding and surface expression.
3.6 Molecular modeling of the MHC class I-tapasin interface
In the absence of x-ray crystallographic data concerning the MHC class I-tapasin interface, molecular modeling can be used to predict potential modes of interaction. Therefore, we employed the protein-protein docking web server HADDOCK to predict, at the structural level, how tapasin residues 334-342 might contribute to tapasin's association with MHC class I. Previous studies have found that the tapasin N-terminal domain contains a large MHC-interaction surface (Fig. 1A) (Dong et al., 2009), and that multiple residues within the MHC class I α2 domain loop (residues 128-136) are important for tapasin association (Lewis et al., 1996; Peace-Brewer et al., 1996; Yu et al., 1999b). Together, these findings suggest that the MHC class I-tapasin interface is likely to be characterized by multiple interactions involving the tapasin N-terminal domain and the MHC class I α1/α2 domains, and so we sought to generate a model that recapitulated these prior observations.
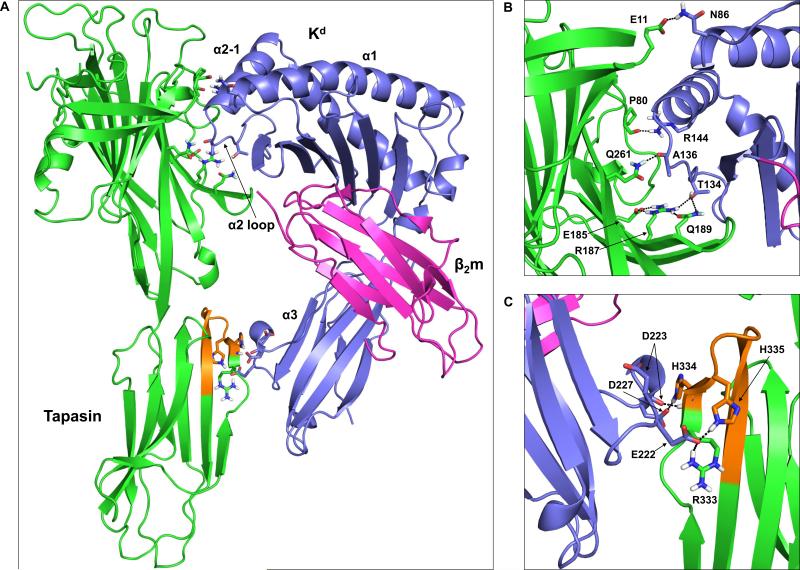
First, using the crystal structure of peptide-bound Kd, we generated an open, peptide-free Kd conformation (as described in the Materials and methods). This open, peptide-free Kd structure was then docked with the tapasin crystal structure using amino acids determined through mutagenesis to be important for MHC class I-tapasin association (see the Materials and methods). Although we used the crystal structure of human tapasin solved by Dong, et al. (2009), each of the tapasin residues that we found to interact with Kd is conserved between mice and humans. Through this analysis, we were able to identify several novel potential interactions involving both the tapasin N-terminal and C-terminal domains (Table I). In our model, the MHC class I α2 domain loop (residues 128-136) interacts with the tapasin TN6 region that was identified by Dong et al. (2009). Specifically, MHC class I T134 forms two hydrogen bonds with tapasin: one with tapasin R187, and one with tapasin Q189 (Fig 6A and B). Notably, an intramolecular salt bridge between tapasin E185 and tapasin R187 (also noted by van Hateren et al. (2010)), as well as a hydrogen bond between tapasin Q189 and tapasin R187, likely stabilize tapasin R187 in a favorable orientation for association with MHC class I T134 (Fig 6B). Also involving the MHC class I α2 domain loop is a hydrogen bond between the main chain carbonyl oxygen of MHC class I A136 and the side chain amide of tapasin Q261 (also part of the TN6 cluster) (Fig. 6B).
Table I.
Potential bonding interactions between Kd and tapasin
| Kd | Tapasin | Distance between atoms (Å) | Type of interaction |
|---|---|---|---|
| ND2-N86 (α1 helix)a | OE1-E11 | 2.70 | hydrogen bond |
| OG1-T134 (α2 loop) | NH1-R187 | 3.07 | hydrogen bond |
| OG1-T134 (α2 loop) | NE2-Q189 | 3.37 | hydrogen bond |
| O-A136 (α2 loop) | NE2-Q261 | 2.99 | hydrogen bond |
| NH1-R144 (α2-1 helix) | O-P80 | 2.68 | hydrogen bond |
| NH2-R144 (α2-1 helix) | O-P80 | 3.33 | hydrogen bond |
| OE2-E222 (α3) | NH1-R333 | 2.69 | salt bridge |
| OE2-E222 (α3) | NE2-H335 | 2.70 | salt bridge |
| O-D223 (α3) | N-H334 | 2.75 | hydrogen bond |
| OD2-D227 (α3) | NE2-H334 | 2.79 | salt bridge |
MHC class I domain structure
Figure 6. Molecular model of the tapasin-Kd interaction reveals possible intermolecular interactions involving the tapasin N-terminal Ig-like domain, as well as tapasin residues within the 334-342 loop.
(A) The tapasin crystal structure (PDB code 3F8U) (Dong et al., 2009) was docked with the open, peptide-free form of Kd (generated from the crystal structure of peptide-bound Kd, PDB code 2FWO (Mitaksov and Fremont, 2006)) as described in the Materials and methods. Tapasin is shown in green with residues 334-342 in orange. The Kd heavy chain is shown in purple, and β2m is in magenta. Also labeled on the Kd structure are the α1 helix, the α2 domain loop, the short α2-1 helix, and the α3 domain. (B) Interactions involving residues within the MHC class I α1/α2 domains and the tapasin N-terminal Ig-like domain. The amino acids that are involved in hydrogen bonds/salt bridges (see also Table I) are shown as sticks and labeled. In addition to these intermolecular interactions, an intramolecular hydrogen bond between tapasin R187 and Q189, and an intramolecular salt bridge between tapasin R187 and E185 are shown. (C) Interactions involving residues within the MHC class I α3 domain and the tapasin C-terminal Ig-like domain are indicated. The orientation shown in (C) is rotated 180° from that shown in (A).
In addition to the MHC class I α2 domain loop, both the MHC class I α2-1 helix and α1 helix were found to interact with tapasin in our model. MHC class I R144 (which extends outward from the α2-1 helix) forms a hydrogen bond with tapasin P80 (Fig. 6B). Within the α1 helix, MHC class I N86 hydrogen bonds with tapasin E11 (part of the TN4 region identified by Dong et al. (2009)) (Fig. 6B). Importantly, the N-linked glycosylation that occurs at N86 on both human and mouse MHC class I molecules is not expected to interfere with this potential hydrogen bond (i.e. the ND2 atom of N86 still has a hydrogen free to interact with tapasin E11 even after N-linked glycosylation). Overall, our model is consistent with previous studies suggesting that the concave surface within the tapasin N-terminal domain accommodates a portion of the MHC class I peptide-binding groove. In addition, our analysis identified previously unrecognized amino acids (including MHC N86 and R144, and tapasin P80) that may be important for MHC class I-tapasin association.
Relevant to our findings with the tapasin Δ334-342 mutant, our molecular model also revealed potential interactions linking the MHC class I α3 domain and the tapasin 334-342 loop. In particular, tapasin H334 was found to form a salt bridge with MHC class I D227, and a hydrogen bond with the MHC class I D223 carbonyl oxygen (Fig. 6C). In addition to the potential interaction between MHC class I E222 and tapasin R333 (first predicted by van Hateren, et al. (2010)), we identified a second salt bridge between MHC class I E222 and tapasin H335 (Fig. 6C). Thus, it is likely that tapasin residues R333, H334, and H335 constitute the MHC class I α3 domain interaction surface.
4. Discussion
We have shown that soluble tapasin is unable to associate successfully with the open, 64-3-7+ forms of murine MHC class I molecules (Fig. 4A) (Simone et al., 2009b). However, in this study, we found that soluble tapasin associates with the 34-1-2+ form of Kd molecules (Fig. 4A). Therefore, our data demonstrate that the interaction of soluble tapasin with a folded, rather than an open, peptide-free, form of MHC class I cannot facilitate effective peptide loading that leads to surface expression. Furthermore, these data indicate that a crucial function of the tapasin transmembrane/cytoplasmic region is to strengthen the association between tapasin and its substrate (open, peptide-free MHC class I molecules) (Fig. 4B).
The observation that soluble tapasin hinders murine MHC class I surface expression, even in the presence of tapasin Δ334-342 (Figs. 3 and 5C), is intriguing. This finding suggests that soluble tapasin plays a direct role in preventing MHC class I trafficking to the cell surface. It is likely that the lack of TAP stabilization in cells co-expressing soluble and Δ334-342 tapasin leaves the MHC class I molecules devoid of peptide and unable to fold. Future studies should reveal the mechanism by which soluble tapasin prevents tapasin Δ334-342-mediated TAP stabilization. One interesting possibility is that soluble tapasin out-competes tapasin Δ334-342 for TAP interaction (implying that residues outside of the tapasin transmembrane region associate with TAP), but because the soluble tapasin-MHC class I interaction is unproductive, the complex dissociates and TAP is degraded.
Additionally, the ability of soluble tapasin to associate with the 34-1-2+ form of Kd may contribute to soluble tapasin's dominant negative effect. For instance, soluble tapasin may prevent murine MHC class I surface expression by augmenting the recycling of MHC class I molecules from the cis-Golgi to the ER. Several investigations have shown that sub-optimally loaded MHC molecules are retrieved from the Golgi by chaperones including tapasin and calreticulin (Garstka et al., 2007; Howe et al., 2009; Paulsson et al., 2006); however, many questions remain regarding this phenomenon. In studies using the 721.220 B lymphoblastoid cell line, soluble tapasin progressed through the secretory pathway (Lehner et al., 1998; Tan et al., 2002). In contrast, findings with HeLa cells and a melanoma cell line indicated that soluble tapasin did not traffic beyond the cis-Golgi (Everett and Edidin, 2007; Rizvi and Raghavan, 2010). Thus, it is possible that, despite lacking a KDEL sequence, soluble tapasin may be retained in the ER via ER-Golgi recycling. As such, soluble tapasin may coordinate with calreticulin to recognize misfolded MHC class I molecules in the cis-Golgi and return them to the ER. Because tapasin Δ334-342 does not associate to an appreciable extent with MHC class I, this quality control mechanism would not be expected to operate in cells expressing tapasin Δ334-342 alone. However, when soluble tapasin and tapasin Δ334-342 are co-expressed, soluble tapasin could bring sub-optimally loaded MHC class I molecules back to the ER, thereby explaining the dominant negative phenotype observed in Figs. 3 and 5B. Moreover, if this hypothesis is found to be true, then MF cells that express soluble tapasin could be used as a model to investigate the pathway of ER-Golgi recycling.
Our model of the MHC class I-tapasin interface (Fig. 6) both corroborates existing evidence and yields novel insights as to potential intramolecular interactions. It has been suggested that tapasin associates with the MHC class I α2 domain loop (residues 128-136) and/or the short α2-1 helix (residues 138-149), and that this interaction enables tapasin to facilitate peptide editing (Dong et al., 2009; Elliott, 1997). In docking HLA-B*0801 with tapasin, van Hateren et al. (2010) identified a potential hydrogen bond between MHC class I T134 and tapasin R187, and a salt bridge between MHC class I E222 and tapasin R333. We too observed these interactions, and also identified several other associations between tapasin and MHC class I. Notably, our analysis suggests that tapasin may bond with amino acid side chains located on the exposed side of the MHC class I α2-1 helix (e.g., MHC class I R144). Thus, as previously proposed by Elliott (1997), by directly binding to the α2-1 helix, tapasin may facilitate widening of the MHC class I peptide-binding groove. Intriguingly, Fig. 6B shows that tapasin may also associate with the MHC class I α1 helix via tapasin E11, which is located on an extended loop. Given that residues 12-17/19 were disordered in the tapasin crystal structure (Dong et al., 2009), it is likely that additional intermolecular interactions involving tapasin residues 11-19 exist that were not captured in our analysis. For instance, our model suggests that tapasin residues 11-19 may be in position to interact with the glycan moiety attached to N86 on the MHC class I molecule. Perhaps the greatest limitation to molecular modeling of the MHC class I-tapasin interface is the lack of knowledge regarding the precise nature of open, peptide-free MHC class I molecules, which biophysical studies have shown to be conformationally flexible and unstable (Bouvier and Wiley, 1998; Zacharias and Springer, 2004). Thus, we predict the occurrence of additional intermolecular bonds beyond those proposed by the model presented here. In particular, it is highly likely that, in addition to T134, other amino acid side chains in the MHC class I α2 domain loop directly interact with tapasin (Yu et al., 1999b).
In regards to the tapasin 334-342 loop, our model suggests that residues R333, H334, and H335 may be particularly important for stabilizing tapasin's association with MHC class I. In a previous study, we found that the tapasin H334F/H335Y mutant maintained association with Ld (Turnquist et al., 2004). Therefore, we modeled these amino acid substitutions into the structure shown in Fig. 6A, and found that tyrosine at position 335 maintained the ability to hydrogen bond with MHC class I E222 (data not shown). Thus, our model offers a molecular explanation as to the ability of tapasin H334F/H335Y to associate with Ld, which lends added support to the validity of our model. According to our model, negatively charged amino acid residues at tapasin positions 334/335 are expected to repel MHC class I residues E222 and D227. Indeed, we have found that when tapasin H334/H335 are mutated to aspartic acid residues (H334D/H335D), interaction with Ld is lost (data not shown). Our lab has also previously reported that tapasin D337A and S341R/L342T mutations abrogate MHC class I binding (Turnquist et al., 2004). To explain these findings, we assessed the positioning of these residues in the tapasin crystal structure. In doing so, we found that tapasin D337 forms intramolecular bonds with tapasin K270 and H299, possibly stabilizing the loop/β-sheet containing R333, H334, and H335. Similarly, the S341R/L342T substitutions may distort the tapasin 334-342 loop. Tapasin L342 is in a hydrophobic pocket, so the presence of the hydrophilic –OH group in the tyrosine substitution may be destabilizing. Also, placement of an arginine at tapasin position 341 may repel other nearby positively charged residues (i.e. R333, H335, H299), and/or introduce steric clashes due to its bulky side chain, forcing neighboring residues into different conformations. Thus, the tapasin D337A and S341R/L342T point mutations likely disrupt MHC class I association by indirectly preventing interactions with tapasin residues 333-345.
A greater understanding of the tapasin 334-342 loop at the structural level emphasizes the importance of understanding the functions associated with the tapasin C-terminal Ig-like domain. Interestingly, although tapasin Δ334-342 permits surface expression of 34-1-2+ Kd molecules (Fig. 3), the level of intracellular folded, 34-1-2+ Kd molecules was virtually unaffected by tapasin Δ334-342. (Compare the level of 34-1-2+ Kd molecules immunoprecipitated from cells expressing no tapasin [Fig. 4B lane 2] to that found in cells expressing tapasin Δ334-342 [Fig. 4B lane 4]. Also, note the validity of this comparison since the MF-Kd and MF-Kd-ΔTsn cell lines express similar levels of Kd [Fig. 2A lanes 2 and 4]). Thus, tapasin Δ334-342 has little influence on the ratio of intracellular folded:open Kd molecules. Tapasin Δ334-342 does not associate appreciably with Kd (Fig. 4B lanes 4 and 10); thus, peptide loading in the MF-Kd-ΔTsn cells presumably proceeds through a tapasin-independent mechanism. Given that the ratio of 34-1-2+ Kd to 64-3-7+ Kd is a measure of MHC class I folding efficiency, our studies indicate that tapasin-independent peptide loading of Kd is highly inefficient. Since tapasin Δ334-342 does not enhance Kd folding efficiency, an alternative mechanism must enable Kd surface expression. Therefore, it is likely that tapasin Δ334-342 facilitates MHC class I surface expression by augmenting the ER peptide pool, which increases the probability that MHC class I molecules will acquire stabilizing peptide.
Additionally, the ability of tapasin Δ334-342 to promote Kd surface expression may be pertinent to our recent finding that the 34-1-2 mAb recognizes an MHC class I conformation that may be intermediately folded (34-1-INT), as well as binding to the fully folded conformation (Simone et al. submitted). It is reasonable to hypothesize that, in the absence of tapasin-induced peptide editing, a greater proportion of the Kd molecules in the MF-Kd-ΔTsn cell line will be in a sub-optimally folded, 34-1-2-INT conformation. If these 34-1-2-INT are permitted to leave the ER, the quality of cell surface MHC class I molecules is likely to be compromised.
As well as providing molecular insights into tapasin function, studies involving tapasin mutants may eventually lead to the understanding of naturally occurring tapasin variants. Recent findings suggest that tapasin may undergo alternative splicing (reviewed in Belicha-Villanueva et al., 2010a). For instance, a mutation found within a tapasin sequence derived from a cDNA database has been found to result in alternative splicing, which generates a soluble form of tapasin (Gao et al., 2004). Another recently discovered tapasin alternative splice form lacks a portion of the N-terminal domain and does not associate with MHC class I molecules (Belicha-Villanueva et al., 2010b). Future studies are likely to uncover additional tapasin splice variants in mice and humans, underscoring the relevance of studies involving alternative forms of tapasin. Collectively, our studies extend our current knowledge regarding the function and cooperation of specific tapasin regions during MHC class I assembly.
Supplementary Material
Supplementary Figure 1. Independent analysis of total Kd and tapasin expression, and Kdsurface expression. (A) The level of Kd and tapasin expressed in the indicated MF cell lines was determined as in Fig. 2A, using independently generated cell lysates. Samples of cell lysates were electrophoresed on a 4→20% acrylamide Tris-glycine gel (for Kd) or on 10% acrylamide Tris-glycine gels (for tapasin and actin), transferred to membranes, and probed with the 64-3-7 mAb (for Kd), hamster anti-mouse tapasin mAb, or mouse anti-actin mAb. (B) The cell surface expression of Kd on the indicated cell lines was determined as in Fig. 3A in an independent flow cytometric analysis. Samples of each cell line were prepared for flow cytometric analysis in triplicate. Kd surface expression is shown as the average mean fluorescence units +/- standard deviation.
Supplementary Figure 2. Independent analysis of Kd, Db, and Kb intracellular folding, and intermolecular interactions involving Kd, tapasin, and ERp57. (A) Kd molecules were immunoprecipitated from cell equivalents using the 34-1-2 mAb or the 64-3-7 mAb. The immunoprecipitated proteins were electrophoresed on a 4→20% acrylamide Tris-glycine gel (for Kd) or on a 10% acrylamide Tris-glycine gel (for tapasin, calreticulin, and ERp57). The proteins were then transferred to membranes and probed on western blots with the 64-3-7 mAb (for Kd), hamster anti-mouse tapasin mAb, or rabbit anti-ERp57 serum. On the Kd blot, a background band occurring upon immunoprecipitation with the 34-1-2 and 64-3-7 mAbs (which was present in MF cells without Kd) is marked with an asterisk. (B) Cell equivalents were treated with methyl methanethiosulfonate to preserve intermolecular disulfide bonds, and immunoprecipitated with rabbit anti-mouse ERp57 serum. The immunoprecipitated proteins were electrophoresed under non-denaturing conditions, transferred to a membrane, and probed on a western blot with hamster anti-mouse tapasin mAb. (C) Folded Db and Kb molecules were immunoprecipitated from cell equivalents with the B22/249 and Y3 mAbs, respectively. The immunoprecipitated proteins were electrophoresed on 4→20% acrylamide Tris-glycine gels, transferred to membranes, and probed on western blots with rabbit anti-Db serum or rabbit anti-Kb serum.
> Tapasin Δ334-342, but not soluble tapasin, facilitates murine MHC class I surface expression.
> Soluble tapasin has a dominant negative effect over tapasin Δ334-342 on MHC class I cell surface levels.
> Soluble tapasin actively retains murine MHC class I molecules in the endoplasmic reticulum.
> Molecular modeling of the MHC class I-tapasin interface reveals novel potential interactions involving the tapasin N-terminal and C-terminal domains.
Acknowledgements
This work was supported by NIH Grants R01 GM057428 and R01 GM057428-09S1 and a Nebraska Department of Health and Human Services LB506 grant (to J.C.S.). Support for this work was also provided by a UNMC Graduate Studies Fellowship (to L.C.S.), an NIH/NCI Training Grant T32 CA009476 Fellowship (to L.C.S.), and a Student Research Stipend from the UNMC College of Medicine (to C.J.G). We gratefully acknowledge the personnel of the UNMC Cell Analysis Core Facility and Monoclonal Antibody Core Facility (funded in part from National Cancer Institute Cancer Center Support Grant P30 CA036727). We thank Drs. T. Hansen, A. Grandea, L. Van Kaer, P. Wang, and K.P. Kane for generously providing antibodies, cDNA, and cell lines.
Abbreviations
- CHAPS
3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate
- Δ
deletion
- EDTA
ethylenediaminetetraacetic acid
- ER
endoplasmic reticulum
- HLA
human leukocyte antigen
- IP
immunoprecipitation
- mAb
monoclonal antibody
- MF
tapasin knockout mouse fibroblast
- MHC
major histocompatibility complex
- PAS
Protein A-Sepharose
- PBS
phosphate-buffered saline
- RMS
root mean square
- SOL
soluble tapasin
- TAP
transporter associated with antigen processing
- Tsn
tapasin
- WT
wild type tapasin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allen H, Wraith D, Pala P, Askonas B, Flavell RA. Domain interactions of H-2 class I antigens alter cytotoxic T-cell recognition sites. Nature. 1984;309:279–281. doi: 10.1038/309279a0. [DOI] [PubMed] [Google Scholar]
- Allen H, Fraser J, Flyer D, Calvin S, Flavell R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc. Natl. Acad. Sci. U. S. A. 1986;83:7447–7451. doi: 10.1073/pnas.83.19.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangia N, Lehner PJ, Hughes EA, Surman M, Cresswell P. The N-terminal region of tapasin is required to stabilize the MHC class I loading complex. Eur. J. Immunol. 1999;29:1858–1870. doi: 10.1002/(SICI)1521-4141(199906)29:06<1858::AID-IMMU1858>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Belicha-Villanueva A, Blickwedehl J, McEvoy S, Golding M, Gollnick SO, Bangia N. What is the role of alternate splicing in antigen presentation by major histocompatibility complex class I molecules? Immunol. Res. 2010a;46:32–44. doi: 10.1007/s12026-009-8123-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belicha-Villanueva A, Golding M, McEvoy S, Sarvaiya N, Cresswell P, Gollnick SO, Bangia N. Identification of an alternate splice form of tapasin in human melanoma. Hum. Immunol. 2010b;71:1018–1026. doi: 10.1016/j.humimm.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier M, Wiley DC. Structural characterization of a soluble and partially folded class I major histocompatibility heavy chain/beta 2m heterodimer. Nat. Struct. Biol. 1998;5:377–384. doi: 10.1038/nsb0598-377. [DOI] [PubMed] [Google Scholar]
- Bouvier M. Accessory proteins and the assembly of human class I MHC molecules: A molecular and structural perspective. Mol. Immunol. 2003;39:697–706. doi: 10.1016/s0161-5890(02)00261-4. [DOI] [PubMed] [Google Scholar]
- Carreno BM, Solheim JC, Harris M, Stroynowski I, Connolly JM, Hansen TH. TAP associates with a unique class I conformation, whereas calnexin associates with multiple class I forms in mouse and man. J. Immunol. 1995;155:4726–4733. [PubMed] [Google Scholar]
- Chen M, Bouvier M. Analysis of interactions in a tapasin/class I complex provides a mechanism for peptide selection. EMBO J. 2007;26:1681–1690. doi: 10.1038/sj.emboj.7601624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra G, Summa CM, Levitt M. Solvent dramatically affects protein structure refinement. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20239–20244. doi: 10.1073/pnas.0810818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- de Vries SJ, van Dijk M, Bonvin AM. The HADDOCK web server for data-driven biomolecular docking. Nat. Protoc. 2010;5:883–897. doi: 10.1038/nprot.2010.32. [DOI] [PubMed] [Google Scholar]
- Dong G, Wearsch PA, Peaper DR, Cresswell P, Reinisch KM. Insights into MHC class I peptide loading from the structure of the tapasin-ERp57 thiol oxidoreductase heterodimer. Immunity. 2009;30:21–32. doi: 10.1016/j.immuni.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T. How does TAP associate with MHC class I molecules? Immunol. Today. 1997;18:375–379. doi: 10.1016/s0167-5699(97)01097-9. [DOI] [PubMed] [Google Scholar]
- Evans GA, Margulies DH, Shykind B, Seidman JG, Ozato K. Exon shuffling: Mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982;300:755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Everett MW, Edidin M. Tapasin increases efficiency of MHC I assembly in the endoplasmic reticulum but does not affect MHC I stability at the cell surface. J. Immunol. 2007;179:7646–7652. doi: 10.4049/jimmunol.179.11.7646. [DOI] [PubMed] [Google Scholar]
- Gao B, Williams A, Sewell A, Elliott T. Generation of a functional, soluble tapasin protein from an alternatively spliced mRNA. Genes Immun. 2004;5:101–108. doi: 10.1038/sj.gene.6364043. [DOI] [PubMed] [Google Scholar]
- Garbi N, Tiwari N, Momburg F, Hammerling GJ. A major role for tapasin as a stabilizer of the TAP peptide transporter and consequences for MHC class I expression. Eur. J. Immunol. 2003;33:264–273. doi: 10.1002/immu.200390029. [DOI] [PubMed] [Google Scholar]
- Garstka M, Borchert B, Al-Balushi M, Praveen PV, Kuhl N, Majoul I, Duden R, Springer S. Peptide-receptive major histocompatibility complex class I molecules cycle between endoplasmic reticulum and cis-Golgi in wild-type lymphocytes. J. Biol. Chem. 2007;282:30680–30690. doi: 10.1074/jbc.M701721200. [DOI] [PubMed] [Google Scholar]
- Grandea AG, 3rd, Golovina TN, Hamilton SE, Sriram V, Spies T, Brutkiewicz RR, Harty JT, Eisenlohr LC, Van Kaer L. Impaired assembly yet normal trafficking of MHC class I molecules in tapasin mutant mice. Immunity. 2000;13:213–222. doi: 10.1016/s1074-7613(00)00021-2. [DOI] [PubMed] [Google Scholar]
- Hammerling GJ, Rusch E, Tada N, Kimura S, Hammerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc. Natl. Acad. Sci. U. S. A. 1982;79:4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MR, Lybarger L, Myers NB, Hilbert C, Solheim JC, Hansen TH, Yu YY. Interactions of HLA-B27 with the peptide loading complex as revealed by heavy chain mutations. Int. Immunol. 2001;13:1275–1282. doi: 10.1093/intimm/13.10.1275. [DOI] [PubMed] [Google Scholar]
- Howe C, Garstka M, Al-Balushi M, Ghanem E, Antoniou AN, Fritzsche S, Jankevicius G, Kontouli N, Schneeweiss C, Williams A, Elliott T, Springer S. Calreticulin-dependent recycling in the early secretory pathway mediates optimal peptide loading of MHC class I molecules. EMBO J. 2009;28:3730–3744. doi: 10.1038/emboj.2009.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen WL, Maxwell DS, Tirado-Rives J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 1996;118:11225–11236. [Google Scholar]
- Kleywegt GJ. Validation of protein models from calpha coordinates alone. J. Mol. Biol. 1997;273:371–376. doi: 10.1006/jmbi.1997.1309. [DOI] [PubMed] [Google Scholar]
- Lehner PJ, Surman MJ, Cresswell P. Soluble tapasin restores MHC class I expression and function in the tapasin-negative cell line .220. Immunity. 1998;8:221–231. doi: 10.1016/s1074-7613(00)80474-4. [DOI] [PubMed] [Google Scholar]
- Lemke H, Hammerling GJ, Hammerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol. Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Neisig A, Neefjes J, Elliott T. Point mutations in the alpha 2 domain of HLA-A2.1 define a functionally relevant interaction with TAP. Curr. Biol. 1996;6:873–883. doi: 10.1016/s0960-9822(02)00611-5. [DOI] [PubMed] [Google Scholar]
- Lie WR, Myers NB, Connolly JM, Gorka J, Lee DR, Hansen TH. The specific binding of peptide ligand to ld class I major histocompatibility complex molecules determines their antigenic structure. J. Exp. Med. 1991;173:449–459. doi: 10.1084/jem.173.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lybarger L, Yu YY, Chun T, Wang CR, Grandea AG, 3rd, Van Kaer L, Hansen TH. Tapasin enhances peptide-induced expression of H2-M3 molecules, but is not required for the retention of open conformers. J. Immunol. 2001;167:2097–2105. doi: 10.4049/jimmunol.167.4.2097. [DOI] [PubMed] [Google Scholar]
- Mitaksov V, Fremont DH. Structural definition of the H-2Kd peptide-binding motif. J. Biol. Chem. 2006;281:10618–10625. doi: 10.1074/jbc.M510511200. [DOI] [PubMed] [Google Scholar]
- Myers NB, Harris MR, Connolly JM, Lybarger L, Yu YY, Hansen TH. Kb, Kd, and Ld molecules share common tapasin dependencies as determined using a novel epitope tag. J. Immunol. 2000;165:5656–5663. doi: 10.4049/jimmunol.165.10.5656. [DOI] [PubMed] [Google Scholar]
- Nieto MC, Song ES, McKinney D, McMillan M, Goodenow RS. The association of H-2Ld with human beta-2 microglobulin induces localized conformational changes in the alpha-1 and -2 superdomain. Immunogenetics. 1989;30:361–369. doi: 10.1007/BF02425276. [DOI] [PubMed] [Google Scholar]
- Ozato K, Mayer NM, Sachs DH. Monoclonal antibodies to mouse major histocompatibility complex antigens. Transplantation. 1982;34:113–120. doi: 10.1097/00007890-198209000-00001. [DOI] [PubMed] [Google Scholar]
- Paulsson KM, Jevon M, Wang JW, Li S, Wang P. The double lysine motif of tapasin is a retrieval signal for retention of unstable MHC class I molecules in the endoplasmic reticulum. J. Immunol. 2006;176:7482–7488. doi: 10.4049/jimmunol.176.12.7482. [DOI] [PubMed] [Google Scholar]
- Peace-Brewer AL, Tussey LG, Matsui M, Li G, Quinn DG, Frelinger JA. A point mutation in HLA-A*0201 results in failure to bind the TAP complex and to present virus-derived peptides to CTL. Immunity. 1996;4:505–514. doi: 10.1016/s1074-7613(00)80416-1. [DOI] [PubMed] [Google Scholar]
- Peaper DR, Wearsch PA, Cresswell P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 2005;24:3613–3623. doi: 10.1038/sj.emboj.7600814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen JL, Hickman-Miller HD, McIlhaney MM, Vargas SE, Purcell AW, Hildebrand WH, Solheim JC. A charged amino acid residue in the transmembrane/cytoplasmic region of tapasin influences MHC class I assembly and maturation. J. Immunol. 2005;174:962–969. doi: 10.4049/jimmunol.174.2.962. [DOI] [PubMed] [Google Scholar]
- Ponder JW. TINKER: Software tools for molecular design, version 6.0. 2001. [DOI] [PMC free article] [PubMed]
- Praveen PV, Yaneva R, Kalbacher H, Springer S. Tapasin edits peptides on MHC class I molecules by accelerating peptide exchange. Eur. J. Immunol. 2010;40:214–224. doi: 10.1002/eji.200939342. [DOI] [PubMed] [Google Scholar]
- Qiu D, Shenkin PS, Hollinger FP, Still WC. The GB/SA continuum model for solvation. A fast analytical method for the calculation of approximate born radii. The Journal of Physical Chemistry A. 1997;101:3005–3014. [Google Scholar]
- Raghavan M, Del Cid N, Rizvi SM, Peters LR. MHC class I assembly: Out and about. Trends Immunol. 2008;29:436–443. doi: 10.1016/j.it.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizvi SM, Raghavan M. Mechanisms of function of tapasin, a critical major histocompatibility complex class I assembly factor. Traffic. 2010;11:332–347. doi: 10.1111/j.1600-0854.2009.01025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5:103–114. doi: 10.1016/s1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- Schrödinger LLC. The PyMOL molecular graphics system, version 1.3. 2010.
- Simone LC, Wang X, Solheim JC. A transmembrane tail: Interaction of tapasin with TAP and the MHC class I molecule. Mol. Immunol. 2009a;46:2147–2150. doi: 10.1016/j.molimm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone LC, Wang X, Tuli A, McIlhaney MM, Solheim JC. Influence of the tapasin C terminus on the assembly of MHC class I allotypes. Immunogenetics. 2009b;61:43–54. doi: 10.1007/s00251-008-0335-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone LC, Wang X, Tuli A, Solheim JC. Effect of a tapasin mutant on the assembly of the mouse MHC class I molecule H2-Kd. Immunol. Cell Biol. 2010;88:57–62. doi: 10.1038/icb.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solheim JC, Carreno BM, Myers NB, Lee DR, Hansen TH. Peptide-induced rescue of serologic epitopes on class I MHC molecules. J. Immunol. 1995;154:1188–1197. [PubMed] [Google Scholar]
- Straus DS, Stroynowski I, Schiffer SG, Hood L. Expression of hybrid class I genes of the major histocompatibility complex in mouse L cells. Proc. Natl. Acad. Sci. U. S. A. 1985;82:6245–6249. doi: 10.1073/pnas.82.18.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroynowski I, Forman J, Goodenow RS, Schiffer SG, McMillan M, Sharrow SO, Sachs DH, Hood L. Expression and T cell recognition of hybrid antigens with amino-terminal domains encoded by Qa-2 region of major histocompatibility complex and carboxyl termini of transplantation antigens. J. Exp. Med. 1985;161:935–952. doi: 10.1084/jem.161.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh WK, Derby MA, Cohen-Doyle MF, Schoenhals GJ, Fruh K, Berzofsky JA, Williams DB. Interaction of murine MHC class I molecules with tapasin and TAP enhances peptide loading and involves the heavy chain alpha3 domain. J. Immunol. 1999;162:1530–1540. [PubMed] [Google Scholar]
- Tan P, Kropshofer H, Mandelboim O, Bulbuc N, Hammerling GJ, Momburg F. Recruitment of MHC class I molecules by tapasin into the transporter associated with antigen processing-associated complex is essential for optimal peptide loading. J. Immunol. 2002;168:1950–1960. doi: 10.4049/jimmunol.168.4.1950. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Vargas SE, Reber AJ, McIlhaney MM, Li S, Wang P, Sanderson SD, Gubler B, van Endert P, Solheim JC. A region of tapasin that affects Ld binding and assembly. J. Immunol. 2001;167:4443–4449. doi: 10.4049/jimmunol.167.8.4443. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Vargas SE, Schenk EL, McIlhaney MM, Reber AJ, Solheim JC. The interface between tapasin and MHC class I: Identification of amino acid residues in both proteins that influence their interaction. Immunol. Res. 2002;25:261–269. doi: 10.1385/ir:25:3:261. [DOI] [PubMed] [Google Scholar]
- Turnquist HR, Petersen JL, Vargas SE, McIlhaney MM, Bedows E, Mayer WE, Grandea AG, 3rd, Van Kaer L, Solheim JC. The Ig-like domain of tapasin influences intermolecular interactions. J. Immunol. 2004;172:2976–2984. doi: 10.4049/jimmunol.172.5.2976. [DOI] [PubMed] [Google Scholar]
- Van Hateren A, James E, Bailey A, Phillips A, Dalchau N, Elliott T. The cell biology of major histocompatibility complex class I assembly: Towards a molecular understanding. Tissue Antigens. 2010;76:259–275. doi: 10.1111/j.1399-0039.2010.01550.x. [DOI] [PubMed] [Google Scholar]
- Vigneron N, Peaper DR, Leonhardt RM, Cresswell P. Functional significance of tapasin membrane association and disulfide linkage to ERp57 in MHC class I presentation. Eur. J. Immunol. 2009;39:2371–2376. doi: 10.1002/eji.200939536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- Wang X, Simone LC, Tuli A, Solheim JC. Comparative analysis of the impact of a free cysteine in tapasin on the maturation and surface expression of murine MHC class I allotypes. Int. J. Immunogenet. 2009;36:183–187. doi: 10.1111/j.1744-313X.2009.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wearsch PA, Cresswell P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat. Immunol. 2007;8:873–881. doi: 10.1038/ni1485. [DOI] [PubMed] [Google Scholar]
- Yu YY, Myers NB, Hilbert CM, Harris MR, Balendiran GK, Hansen TH. Definition and transfer of a serological epitope specific for peptide-empty forms of MHC class I. Int. Immunol. 1999a;11:1897–1906. doi: 10.1093/intimm/11.12.1897. [DOI] [PubMed] [Google Scholar]
- Yu YY, Turnquist HR, Myers NB, Balendiran GK, Hansen TH, Solheim JC. An extensive region of an MHC class I alpha 2 domain loop influences interaction with the assembly complex. J. Immunol. 1999b;163:4427–4433. [PubMed] [Google Scholar]
- Zacharias M, Springer S. Conformational flexibility of the MHC class I alpha1-alpha2 domain in peptide bound and free states: A molecular dynamics simulation study. Biophys. J. 2004;87:2203–2214. doi: 10.1529/biophysj.104.044743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Independent analysis of total Kd and tapasin expression, and Kdsurface expression. (A) The level of Kd and tapasin expressed in the indicated MF cell lines was determined as in Fig. 2A, using independently generated cell lysates. Samples of cell lysates were electrophoresed on a 4→20% acrylamide Tris-glycine gel (for Kd) or on 10% acrylamide Tris-glycine gels (for tapasin and actin), transferred to membranes, and probed with the 64-3-7 mAb (for Kd), hamster anti-mouse tapasin mAb, or mouse anti-actin mAb. (B) The cell surface expression of Kd on the indicated cell lines was determined as in Fig. 3A in an independent flow cytometric analysis. Samples of each cell line were prepared for flow cytometric analysis in triplicate. Kd surface expression is shown as the average mean fluorescence units +/- standard deviation.
Supplementary Figure 2. Independent analysis of Kd, Db, and Kb intracellular folding, and intermolecular interactions involving Kd, tapasin, and ERp57. (A) Kd molecules were immunoprecipitated from cell equivalents using the 34-1-2 mAb or the 64-3-7 mAb. The immunoprecipitated proteins were electrophoresed on a 4→20% acrylamide Tris-glycine gel (for Kd) or on a 10% acrylamide Tris-glycine gel (for tapasin, calreticulin, and ERp57). The proteins were then transferred to membranes and probed on western blots with the 64-3-7 mAb (for Kd), hamster anti-mouse tapasin mAb, or rabbit anti-ERp57 serum. On the Kd blot, a background band occurring upon immunoprecipitation with the 34-1-2 and 64-3-7 mAbs (which was present in MF cells without Kd) is marked with an asterisk. (B) Cell equivalents were treated with methyl methanethiosulfonate to preserve intermolecular disulfide bonds, and immunoprecipitated with rabbit anti-mouse ERp57 serum. The immunoprecipitated proteins were electrophoresed under non-denaturing conditions, transferred to a membrane, and probed on a western blot with hamster anti-mouse tapasin mAb. (C) Folded Db and Kb molecules were immunoprecipitated from cell equivalents with the B22/249 and Y3 mAbs, respectively. The immunoprecipitated proteins were electrophoresed on 4→20% acrylamide Tris-glycine gels, transferred to membranes, and probed on western blots with rabbit anti-Db serum or rabbit anti-Kb serum.