Abstract
Purpose
Mortality following bariatric surgery is a rare event in contemporary series, making it difficult for any single center to draw meaningful conclusions as to cause of death. Nevertheless, much of the published mortality data come from single center case series and reviews of administrative databases. These sources tend to produce lower mortality estimates than those obtained from controlled clinical trials. Furthermore, information about the causes of death and how they were determined is not always available. The aim of the present report is to describe in detail all deaths occurring within 30-days of surgery in the Longitudinal Assessment of Bariatric Surgery (LABS).
Methods
LABS is a 10-center observational cohort study of bariatric surgical outcomes. Data were collected prospectively for bariatric surgeries performed between March 2005 and April 2009. All deaths occurring within 30-days of surgery were identified, and cause of death assigned by an independent Adjudication Subcommittee, blinded to operating surgeon and site.
Results
6118 patients underwent primary bariatric surgery. 18 deaths (0.3%) occurred within 30-days of surgery. The most common cause of death was sepsis (33% of deaths), followed by cardiac causes (28%) and pulmonary embolism (17%). For one patient cause of death could not be determined despite examination of all available information.
Conclusions
This study confirms the low 30-day mortality rate following bariatric surgery. The recognized complications of anastomotic leak, cardiac events, and pulmonary emboli accounted for the majority of 30-day deaths.
Keywords: Mortality, Cause of Death, Bariatric Surgery, Gastric Bypass
INTRODUCTION
The prevalence of obesity has increased to epidemic proportions in the United States over the past four decades [1, 2] with similar trends now being observed worldwide [3, 4, 5]. In 2008 more than 14% of the U.S. population had a body mass index (BMI) greater than 35 kg/m2, with 5.7% having a BMI greater than 40 kg/m2 [6]. This epidemic has major implications for society with increasing BMI and other measures of excess adiposity strongly associated with increased risk of mortality and disease [7, 8].
In response to this epidemic, bariatric surgery has been reported to be the most reliable way to achieve sustained weight loss in severe obesity [9, 10] and to have a major impact on many co-morbidities, especially type 2 diabetes [11, 12]. Bariatric surgery has also been shown to result in increased survival for morbidly obese patients when compared to non-surgical control groups [13, 14].
These factors have led to a dramatic increase in the number of bariatric surgical procedures performed, from 10,000 per year in the US in 1997-98 [15] up to 220,000 in North America in 2008 [16]. Over 90% of bariatric operations are now being performed laparoscopically, with Roux-en-Y gastric bypass (RYGB) and laparoscopic adjustable gastric banding (LAGB) the most commonly performed operations [16].
While the benefits of successful bariatric surgery are widely accepted, bariatric surgical patients are virtually by definition high risk surgical candidates. Nevertheless, although the rates of complications, including mortality, from bariatric surgery in early reports were appreciable and a cause for concern, reported mortality rates in contemporary series are low. In one study, overall surgery-associated inpatient mortality declined by 79% (from 0.89% to 0.19%) between 1998 and 2004 [17] and a systematic review in 2005 found mortality rates to vary between 0.2% to 1.9% for RYGB and 0.0% to 2.1% for LAGB [18]. In 2009 the Longitudinal Assessment of Bariatric Surgery (LABS) reported that overall thirty day mortality among 4172 patient undergoing either laparoscopic Roux-en-y gastric bypass (RYGB) or laparoscopic adjustable gastric banding (LAGB) in the LABS-1 study was 0.3% [19]. In 2010 the Bariatric Outcomes Longitudinal Database (BOLD) was used to report on the outcomes of nearly 58,000 bariatric surgical operations with a 30-day mortality rate of 0.09% [20]. In addition to operation type, mortality rates have been reported to vary by surgical approach [21, 22], setting [23], and patient characteristics [19, 24-26].
Mortality following bariatric surgery is now such a rare event that it is difficult for any single center to accrue enough cases to draw statistically meaningful conclusions as to factors associated with particular causes of death. Nevertheless, much of the published mortality data come from single center case series and reviews of administrative databases. These sources tend to produce lower mortality estimates than those obtained from controlled clinical trials [18]. Furthermore, information about the causes of death and how they were determined is not always available [22, 27, 28].
The Longitudinal Assessment of Bariatric Surgery (LABS) is a 10-center consortium funded by the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK) in the National Institute of Health (NIH) that conducts observational cohort studies of bariatric surgical outcomes. These involve largely prospective, standardized and comprehensive collection of clinical data. LABS-1 collected 30-day outcome data in consecutive patients, aged 18-years or older, undergoing primary bariatric surgery. LABS-2 comprises more detailed and ongoing data collection in a selected cohort of patients, restricted to those who had not had prior bariatric surgery. The present report describes in detail all deaths occurring within 30-days of surgery in LABS.
METHODS
Patients
Patients were recruited by LABS into either of two studies, designated LABS-1 and LABS-2, at one of the ten participating centers: University of Pittsburgh Medical Center (Pennsylvania), New York-Presbyterian Hospital [Columbia-Presbyterian or Valley Hospitals, or Weill-Cornell Medical College] (New York and New Jersey), East Carolina Medical Center (North Carolina), the MeritCare Health Systems through the Neuropsychiatric Research Institute (North Dakota), Sacramento Bariatric (California), University of Washington Medical Center or Virginia Mason Medical Center (Washington), and Oregon Health and Sciences University or Legacy Good Samaritan Hospital (Oregon). The LABS protocols and consent forms were approved by the Institutional Review Board at each institution.
Protocols
LABS-1, a study of 30 day outcomes, included all consecutive patients at least 18 years of age who consented to participate. LABS-2 involves long term follow-up and data collection. Accordingly, in contrast to LABS-1, non-consecutive patients who would be able to undertake the required follow-up were selected for recruitment, excluding anyone who had undergone bariatric surgery prior to enrolling in LABS-2. LABS inclusion criteria and data collection have previously been described in detail [29]. Data were collected for bariatric surgeries performed between March 2005 and April 2009 and sent to the Data Coordinating Center (DCC) at the University of Pittsburgh, Graduate School of Public Health.
Determination of Causes of Death
The causes of all deaths occurring in LABS participants were determined by an Adjudication Subcommittee. All available data including principal investigators’ reports, hospital and procedural reports, death certificates and coroners’ reports were masked with respect to patient and medical staff and sent to the DCC. The DCC forwarded the information to two independent reviewers from the LABS Adjudication Subcommittee, neither of whom was from the site that recruited the participant. Each reviewer was asked independently to assign a cause of death from one of 14 common causes, (bleeding, sepsis from anastomotic leak, sepsis from other abdominal source, pulmonary embolus, cardiac failure, myocardial infarction, cerebrovascular accident, bowel obstruction, evisceration, pneumonia, respiratory failure, accident, suicide, other) and a level of certainty (definite, probable, less than probable). When the two reviewers could not agree on cause of death, or the level of certainty was less than probable, the reviewers attempted to resolve the discrepancy by means of discussion. If the discrepancy could not be resolved by discussion, the case was reviewed by three other members of the Adjudication Subcommittee, again without participation from the recruiting site, until consensus was achieved.
Statistical analysis
The number of events, with percentages and proportions, are used to describe deaths following surgery. Despite the large number of surgeries involved, the numbers of deaths are insufficient for meaningful statistical comparisons. Nevertheless, the information available is of interest. Accordingly, characteristics of each participant who died, including cause of death, are listed. Medians and ranges are used to describe the age and BMI distribution of these cases, while time to death is depicted using a histogram.
RESULTS
Of the 6383 patients (5051 enrolled in LABS-1, 1332 enrolled in LABS-2 after LABS-1 ended), 6118 had primary bariatric surgery in LABS. Thirty-day follow-up was available for 6114 of these patients, with the remaining four patients having a last known vital status recorded less than 30-days after surgery. There were 18 deaths within 30-days of these 6118 primary surgeries (0.3%).
The clinical data from the patients who died are presented in Table 1. Of the 18 patients who died, nine were male and 10 had undergone open surgery. Their median age was 47 years (range 30-61) and median BMI was 58.8 kg/m2 (range 42.7-89.7). Sixteen of the patients died following RYGB; no deaths occurred after LAGB. Two patients, with BMIs of 51.7 and 62.9 kg/m2, died following sleeve gastrectomy. In both cases the sleeve gastrectomy had been planned as the first stage of a two-stage surgical approach.
Table 1.
Clinical Data for Patients Who Died After Bariatric Surgery
| LABS 1/2 |
Age | Sex | Weight | BMI | Surgery | Days | Cause |
|---|---|---|---|---|---|---|---|
| LABS-1 | 58 | M | 351 | 50.4 | Lap RYGB | 6 | Sepsis from anastomotic leak |
| LABS-1 | 41 | M | 358 | 56.1 | Lap RYGB | 9 | Sepsis from anastomotic leak |
| LABS-1 | 30 | M | 425 | 53.1 | Lap to Open RYGB |
24 | Sepsis from anastomotic leak |
| LABS-1 | 41 | F | 409 | 60.4 | Open RYGB | 6 | Sepsis from anastomotic leak |
| LABS-1 | 37 | F | 556 | 89.7 | Open RYGB | 13 | Sepsis from other abdominal source |
| LABS-2 | 57 | M | 462 | 60.9 | Open RYGB | 14 | Sepsis from other abdominal source |
| LABS-1 | 55 | F | 247 | 43.7 | Lap RYGB | 17 | Myocardial infarction |
| LABS-1 | 52 | M | 363 | 49.2 | Open RYGB | 10 | Myocardial infarction |
| LABS-1 | 41 | F | 355 | 57.3 | Lap RYGB | 13 | Cardiac arrhythmia |
| LABS-1 | 57 | M | 340 | 51.7 | Lap Sleeve | 0 | Cardiac arrhythmia |
| LABS-1 | 61 | F | 356 | 69.5 | Open RYGB | 9 | Cardiac failure |
| LABS-1 | 55 | M | 397 | 60.4 | Open RYGB | 16 | Pulmonary embolism |
| LABS-1 | 39 | F | 504 | 76.6 | Open RYGB | 6 | Pulmonary embolism |
| LABS-1 | 32 | F | 420 | 60.3 | Open RYGB | 30 | Pulmonary embolism |
| LABS-1 | 53 | F | 335 | 61.3 | Lap RYGB | 12 | Aspiration |
| LABS-2 | 43 | M | 414 | 62.9 | Lap Sleeve | 8 | Hemorrhage |
| LABS-1 | 42 | M | 312 | 46.1 | Lap RYGB | 2 | Loss of airway |
| LABS-1 | 51 | F | 241 | 42.7 | Open RYGB | 22 | Indeterminate |
A specific cause of death was identified for 17 patients, although autopsy reports were available in only 5 cases. Sepsis was the most common cause of death (n=6, 33% of deaths) and was associated with anastomotic leak in 4 of the 6 cases. Cardiac causes were the next most common cause of death (n=5, 28%), followed by pulmonary embolism (n=3, 17%). All three patients who died of pulmonary embolism received unfractionated heparin and sequential calf compression devices at the time of their surgery.
The cause of death could not be determined to the standard required by the Adjudication Subcommittee in one case. This death occurred following discharge from hospital with probable arteriosclerotic cardiovascular disease listed on the death certificate; no reports of associated symptoms were available and no autopsy was performed.
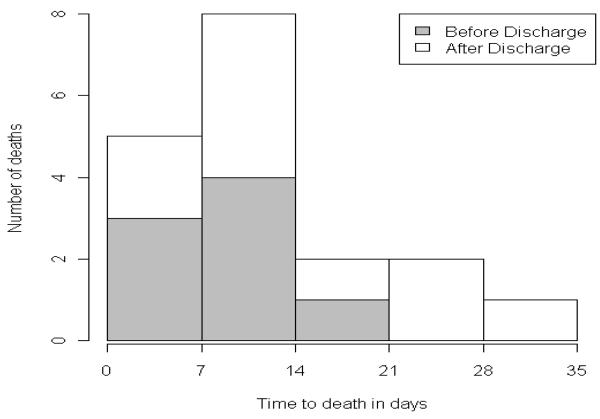
The distribution of the time between surgery and death is presented in Figure 1. Ten of the deaths occurred after the patient had been discharged from hospital.
Figure 1.
Post-operative distribution of Deaths
DISCUSSION
Although there is a considerable and quite variable literature on the mortality rate after bariatric surgery, few reports provide detailed information on the actual causes of death. Studies that do report specifically on cause of death include a multi-center review of prospective databases, conducted to validate the obesity surgery mortality risk score [30]. This study reported on 33 deaths from 4431 operations (0.7% mortality) and reported that 30% of the deaths were due to pulmonary embolism, 27% due to cardiac causes, and 21% due to anastomotic leak. The cause of death could not be determined in 15% of cases. The authors did not report how cause of death was determined. Another multi-center study was conducted using the Italian Society of Obesity Surgery National Registry [31]. These authors reported on 34 deaths occurring after 13,871 operations (0.2% mortality). Cause of death was determined by reviewing the complete clinical record. Pulmonary embolism was the most common cause of death (38%), followed by cardiac failure (18%), anastomotic leak (18%) and respiratory failure (12%).
A different approach was taken by Goldfeder et al. [32] who reported on all deaths attributable to complications of bariatric surgery investigated by the New York City Office of the Chief Medical Examiner. They identified 97 deaths attributable to bariatric surgery between 1997 and 2005. Sixty three percent of these deaths occurred within 30-days of the initial bariatric surgery, 40% occurred after hospital discharge. Anastomotic leak was the most common underlying cause of death (36% of deaths), followed by pulmonary embolism (12%) and cardiac causes (9%). As the number of bariatric surgeries conducted in New York City over this same period was not known, the authors were unable to estimate an overall mortality rate.
In keeping with these and other contemporary series, 30-day mortality was rare in the Longitudinal Assessment of Bariatric Surgery cohort. The 0.3% of patients dying within 30 days of surgery is more consistent with rates reported by prospective clinical trials and higher than reported in many case series [18]. This is likely to reflect the rigorous pursuit of information regarding vital status used by LABS, resulting in 99.93% 30-day followup, and the inclusion of consecutive, unselected patients in LABS-1. Deaths in this cohort were most commonly due to sepsis, cardiac causes and pulmonary embolism. A specific cause of death could not always be determined despite the detailed adjudication process. These findings are similar to those of other large, multi-institutional series’ [30, 31].
The strengths of this study include the high quality, essentially complete, data collection, and the adjudication of causes of death by adjudicators who were masked with respect to the site and surgeon. In particular, independent formal adjudication was not reported by any of the other major studies examining mortality following bariatric surgery. The adjudication process improves the assessment of the cause of death, and decreases the possibility of speculation as inadequate information results in an “indeterminate” cause of death by the adjudicators. Unfortunately, while the adjudication process minimizes error, it was limited by the available information in some cases. However, this is no different from the information available in other studies of mortality following bariatric surgery. In most cases adjudication was performed according to the clinical record, without access to autopsy reports. In the one case for which cause of death could not be determined, there was insufficient information available to determine cause of death.
A potential criticism of this study is the lack of comparative analysis to determine risk factors for mortality. While the low number of deaths overall reflects well on the safety of modern bariatric surgery, it also limits the power to perform meaningful statistical analysis. When LABS-1 was originally designed, it was determined that 11,588 cases would be required to have at least 90% power to detect a doubling in the relative risk of 30-day mortality (hypothesized to be 1% in the lower risk group) between men and women. Even 80% power would have required 8,484 participants. For this reason, and because the final cohort included fewer than 7,000 participants, we have avoided comparative statistical analysis, although clinical factors associated with risk of a composite adverse endpoint in LABS-1 have been identified and published previously [19, 33].
This study confirms the low mortality risk associated with contemporary bariatric surgery. In particular, the low rate of death due to pulmonary embolism, in contrast to previous studies [30, 31], suggests that this risk is manageable even in the bariatric population. The most common cause of death in this study was sepsis, highlighting that even in experienced hands, diagnosis and management of intra-abdominal infection is challenging in morbidly obese patients [34, 35].
Though small in number, cardiac causes are among the most frequent causes of death suggesting that thorough cardiac risk assessment should be performed in patients presenting for bariatric surgery. In addition to arteriosclerotic cardiovascular disease, obesity cardiomyopathy, which pre-disposes to congestive heart failure and arrhythmias, should be considered in evaluating candidates for bariatric surgery [36]. Guidelines from the American Heart Association advocate clinical risk assessment and chest X-rays for all obese patients, with additional cardiac imaging for patients with decreased function capacity [37]. It is important to remember non-surgical management options for morbid obesity when surgical risks are judged to be prohibitively high.
This study also highlights the difficulties with statistical power that occur when examining rare endpoints regardless of their clinical significance. One solution is to increase the numbers of study participants, but this comes with added cost, and for some endpoints may be impractical even for multi-center studies. Another approach is to combine several infrequent endpoints in to a composite endpoint, such as has previously been reported for LABS-1 [19, 33]. While this increases the ability to draw statistically valid conclusions, this advantage may be accompanied by a loss of specificity.
In conclusion, mortality within 30-days of surgery was rare in LABS. The recognized complications of anastomotic leak, cardiac events, and pulmonary emboli accounted for the majority of 30-day deaths. Importantly, although most deaths occurred within the first two weeks, most also occurred after hospital discharge, indicating that information regarding post-surgical mortality requires active followup of patients for at least 30-days after their discharge from the hospital.
LABS Study Acknowledgments
This clinical study was a cooperative agreement funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Grant numbers: DCC -U01 DK066557; Columbia - U01-DK66667 (in collaboration with Cornell University Medical Center CTSC, Grant UL1- RR024996); University of Washington - U01-DK66568 (in collaboration with CTRC, Grant M01RR-00037); Neuropsychiatric Research Institute - U01-DK66471; East Carolina University – U01-DK66526; University of Pittsburgh Medical Center – U01-DK66585 (in collaboration with CTRC, Grant UL1-RR024153); Oregon Health & Science University – U01-DK66555.
Footnotes
LABS personnel contributing to the study include:
Columbia University Medical Center, New York, NY: Paul D. Berk, MD, Marc Bessler, MD, Amna Daud, Harrison Lobdell IV, Jemela Mwelu, Beth Schrope, MD, PhD, Akuezunkpa Ude, MD Cornell University Medical Center, New York, NY: Michelle Capasso, BA, Ricardo Costa, BS, Greg Dakin, MD, Faith Ebel RD, MPH, Michel Gagner, MD, Jane Hsieh BS, Alfons Pomp, MD, Gladys Strain, PhD Mt. Sinai Medical Center, New York, NY: W. Barry Inabnet, MD East Carolina Medical Center, Greenville, NC: Rita Bowden, RN, William Chapman, MD, FACS, Lynis Dohm, PhD, John Pender MD, Walter Pories, MD, FACS Neuropsychiatric Research Institute, Fargo, ND: Jennifer Barker, MBA, Michael Howell, MD, Luis Garcia, MD, FACS, MBA, Kathy Lancaster, BA, Erika Lovaas, BS, James E. Mitchell, MD, Tim Monson, MD, Oregon Health & Science University: Chelsea Cassady, BS, Clifford Deveney, MD, Katherine Elder, PhD, Andrew Fredette, BA, Stefanie Greene, Jonathan Purnell, MD, Robert O’Rourke, Lynette Rogers, MD, Chad Sorenson, Bruce M. Wolfe, MD, Legacy Good Samaritan Hospital, Portland, OR: Emma Patterson, MD, Mark Smith, MD, William Raum, MD, Lisa VanDerWerff, PAC, Jason Kwiatkowski, PAC, Jamie Laut Med Sacramento Bariatric Medical Associates, Sacramento, CA: Iselin Austrheim-Smith, CCRP, Laura Machado, MD University of Pittsburgh Medical Center, Pittsburgh, PA: Chris Costa, BA Anita P. Courcoulas, MD, MPH, FACS, Jessie Eagleton , BS, George Eid, MD, William Gourash, MSN, CRNP, Lewis H. Kuller, MD, DrPH, Carol A. McCloskey, MD, Ramesh Ramanathan, MD, Rebecca Search, MPH, Eleanor Shirley, MA University of Washington, Seattle, WA: David E. Cummings, MD, E. Patchen Dellinger, MD, Hallie Ericson, BA, David R. Flum, MD, MPH, Katrina Golub, MPH, CCRC, Brant Oelschlager, MD, Skye Steptoe, MS, CCRC, Tomio Tran, Andrew Wright, MD Virginia Mason Medical Center, Seattle, WA: Lily Chang, MD, Stephen Geary, RN, Jeffrey Hunter, MD, Anne MacDougall, BA Ravi Moonka, MD, Olivia A. Seibenick, CCRC, Richard Thirlby, MD Data Coordinating Center, Graduate School of Public Health at the University of Pittsburgh, Pittsburgh, PA: Abi Adenijii, MS, Steven H. Belle, PhD, MScHyg, Lily (Jia-Yuh) Chen, MS, Michelle Fouse, BS, Jesse Hsu, MS, Wendy C. King, PhD, Kevin Kip, PhD, Kira Leishear, BS, Laurie Iacono, MFA, Debbie Martin, BA, Rocco Mercurio, MBA, Faith Selzer, PhD, Abdus Wahed, PhD National Institute of Diabetes and Digestive and Kidney Diseases: Mary Evans, Ph.D, Mary Horlick, MD, Carolyn W. Miles, PhD, Myrlene A. Staten, MD, Susan Z. Yanovski, MD National Cancer Institute: David E. Kleiner, MD, PhD
Conflicts of Interest Mark D. Smith, Abdus S. Wahed, Steven H. Belle, Paul D. Berk, Anita P. Courcoulas, Gregory F. Dakin, Laura Machado, James E. Mitchell, John Pender, Alfons Pomp, Ramesh Ramanathan, Beth Schrope, Myrlene Staten, and Akuezunkpa Ude have no relevant financial interests to disclose. Emma Patterson discloses that she is an owner and co-founder of Doctors of Weight Loss, and receives consulting fees from Transenterix, Allergan Health and Reshape Medical. David R. Flum discloses that he receives research grant support from Covidien and Sanofi Aventis. Walter Pories discloses that he receives research grant support from Ethicon and GlaxoSmithKline. Bruce M. Wolfe discloses that he receives consulting fees from EnteroMedics and Crospon/Wellcome.
References
- 1.Flegal KM, Carroll MD, Kuczmarski RJ, Johnson CL. Overweight and obesity in the United States: prevalence and trends, 1960-1994. Int J Obes Relat Metab Disord. 1998;22:39–47. doi: 10.1038/sj.ijo.0800541. [DOI] [PubMed] [Google Scholar]
- 2.Kuczmarski RJ, Flegal KM, Campbell SM, Johnson CL. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994;272:205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Ford ES, Mokdad AH. Epidemiology of obesity in the Western Hemisphere. J Clin Endocrinol Metab. 2008;93:S1–8. doi: 10.1210/jc.2008-1356. [DOI] [PubMed] [Google Scholar]
- 4.Low S, Chin MC, Deurenberg-Yap M. Review on epidemic of obesity. Ann Acad Med Singapore. 2009;38:57–59. [PubMed] [Google Scholar]
- 5.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- 6.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 7.de Gonzalez A Berrington, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobs EJ, Newton CC, Wang Y, et al. Waist circumference and all-cause mortality in a large US cohort. Arch Intern Med. 2010;170:1293–1301. doi: 10.1001/archinternmed.2010.201. [DOI] [PubMed] [Google Scholar]
- 9. [on April 4, 2011];Rationale for the surgical treatment of morbid obesity. Accessed online at http://www.asbs.org/Newsite07/patients/resources/asbs_rationale.htm.
- 10.Brolin RE. Gastric bypass. Surg Clin North Am. 2001;81:1077–1095. doi: 10.1016/s0039-6109(05)70185-7. [DOI] [PubMed] [Google Scholar]
- 11.Pories WJ, Swanson MS, MacDonald KG, et al. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–50. doi: 10.1097/00000658-199509000-00011. discussion 350-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–559. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 14.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 15.Livingston EH. Procedure incidence and in-hospital complication rates of bariatric surgery in the United States. Am J Surg. 2004;188:105–110. doi: 10.1016/j.amjsurg.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Buchwald H, Oien DM. Metabolic/bariatric surgery Worldwide 2008. Obes Surg. 2009;19:1605–1611. doi: 10.1007/s11695-009-0014-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Encinosa W. Bariatric Surgery Utilization and Outcomes in 1998 and 2004. 2007. [PubMed]
- 18.Maggard MA, Shugarman LR, Suttorp M, et al. Meta-analysis: surgical treatment of obesity. Ann Intern Med. 2005;142:547–559. doi: 10.7326/0003-4819-142-7-200504050-00013. [DOI] [PubMed] [Google Scholar]
- 19.Flum DR, Belle SH, King WC, et al. Perioperative safety in the longitudinal assessment of bariatric surgery. N Engl J Med. 2009;361:445–454. doi: 10.1056/NEJMoa0901836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeMaria EJ, Pate V, Warthen M, Winegar DA. Baseline data from American Society for Metabolic and Bariatric Surgery-designated Bariatric Surgery Centers of Excellence using the Bariatric Outcomes Longitudinal Database. Surg Obes Relat Dis. 2010;6:347–355. doi: 10.1016/j.soard.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Sekhar N, Torquati A, Youssef Y, Wright JK, Richards WO. A comparison of 399 open and 568 laparoscopic gastric bypasses performed during a 4-year period. Surg Endosc. 2007;21:665–668. doi: 10.1007/s00464-006-9151-2. [DOI] [PubMed] [Google Scholar]
- 22.Efthimiou E, Court O, Sampalis J, Christou N. Validation of Obesity Surgery Mortality Risk Score in patients undergoing gastric bypass in a Canadian center. Surg Obes Relat Dis. 2009;5:643–647. doi: 10.1016/j.soard.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Parker M, Loewen M, Sullivan T, et al. Predictors of outcome after obesity surgery in New York state from 1991 to 2003. Surg Endosc. 2007;21:1482–1486. doi: 10.1007/s00464-007-9245-5. [DOI] [PubMed] [Google Scholar]
- 24.Varela JE, Wilson SE, Nguyen NT. Outcomes of bariatric surgery in the elderly. Am Surg. 2006;72:865–869. doi: 10.1177/000313480607201005. [DOI] [PubMed] [Google Scholar]
- 25.Dunkle-Blatter SE, St Jean MR, Whitehead C, et al. Outcomes among elderly bariatric patients at a high-volume center. Surg Obes Relat Dis. 2007;3:163–9. doi: 10.1016/j.soard.2006.12.004. discussion 169-70. [DOI] [PubMed] [Google Scholar]
- 26.Modanlou KA, Muthyala U, Xiao H, et al. Bariatric surgery among kidney transplant candidates and recipients: analysis of the United States renal data system and literature review. Transplantation. 2009;87:1167–1173. doi: 10.1097/TP.0b013e31819e3f14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sundbom M, Karlson BM. Low mortality in bariatric surgery 1995 through 2005 in Sweden, in spite of a shift to more complex procedures. Obes Surg. 2009;19:1697–1701. doi: 10.1007/s11695-008-9684-7. [DOI] [PubMed] [Google Scholar]
- 28.Lancaster RT, Hutter MM. Bands and bypasses: 30-day morbidity and mortality of bariatric surgical procedures as assessed by prospective, multi-center, risk-adjusted ACS-NSQIP data. Surg Endosc. 2008;22:2554–2563. doi: 10.1007/s00464-008-0074-y. [DOI] [PubMed] [Google Scholar]
- 29.Belle SH, Berk PD, Courcoulas AP, et al. Safety and efficacy of bariatric surgery: Longitudinal Assessment of Bariatric Surgery. Surg Obes Relat Dis. 2007;3:116–126. doi: 10.1016/j.soard.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeMaria EJ, Murr M, Byrne TK, et al. Validation of the obesity surgery mortality risk score in a multicenter study proves it stratifies mortality risk in patients undergoing gastric bypass for morbid obesity. Ann Surg. 2007;246:578–82. doi: 10.1097/SLA.0b013e318157206e. discussion 583-4. [DOI] [PubMed] [Google Scholar]
- 31.Morino M, Toppino M, Forestieri P, Angrisani L, Allaix ME, Scopinaro N. Mortality after bariatric surgery: analysis of 13,871 morbidly obese patients from a national registry. Ann Surg. 2007;246:1002–7. doi: 10.1097/SLA.0b013e31815c404e. discussion 1007-9. [DOI] [PubMed] [Google Scholar]
- 32.Goldfeder LB, Ren CJ, Gill JR. Fatal complications of bariatric surgery. Obes Surg. 2006;16:1050–1056. doi: 10.1381/096089206778026325. [DOI] [PubMed] [Google Scholar]
- 33.Smith MD, Patterson E, Wahed AS, et al. Relationship between surgeon volume and adverse outcomes after RYGB in Longitudinal Assessment of Bariatric Surgery (LABS) study. Surg Obes Relat Dis. 2010;6:118–125. doi: 10.1016/j.soard.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Solh A. Morbid Obesity in the Medical ICU. Chest. 2001;120:1989–1997. doi: 10.1378/chest.120.6.1989. [DOI] [PubMed] [Google Scholar]
- 35.Kermarrec N, Marmuse J-P, Faivre J, et al. High Mortality Rate for Patients Requiring Intensive Care After Surgical Revision Following Bariatric Surgery. Obes Surg. 2008;18:171–178. doi: 10.1007/s11695-007-9301-1. [DOI] [PubMed] [Google Scholar]
- 36.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 37.Poirier P, Alpert MA, Fleisher LA, et al. Cardiovascular evaluation and management of severely obese patients undergoing surgery: a science advisory from the American Heart Association. Circulation. 2009;120:86–95. doi: 10.1161/CIRCULATIONAHA.109.192575. [DOI] [PubMed] [Google Scholar]