Abstract
Objective
IQ domain GTPase-activating protein 1 (IQGAP1) contributes to cytoskeletal network regulation in epithelial cells by its scaffolding properties and by binding the Rho GTPase Rac1 to maintain its activity. The functions of IQGAP1 in endothelial cells beyond angiogenesis remain unclear. We hypothesized that IQGAP1 participates in the regulation of endothelial barrier function.
Methods/Results
Silencing IQGAP1 in human microvascular ECs (HMVECs) resulted in a disruption of adherens junctions, formation of inter-endothelial gaps, and a reduction in barrier function. Furthermore, silencing of IQGAP1 abrogated Angiopoietin-1's (Angpt-1) barrier enhancement effect and abolished the barrier-stabilizing effect of Angpt-1 on thrombin-stimulated cells. Co-immunoprecipitation detected binding of endogenous IQGAP1 with Rac1 at baseline that was stronger when Rac1 was activated and weaker when deactivated. Measurement of GTP-bound Rac1 revealed that Angpt-1 not only failed to activate Rac1 if IQGAP1 was silenced, but also if cells were transfected with a mutant disabled in Rac1 binding (T1050AX2). Furthermore, a dominant-active Rac1 was sufficient to completely reverse the morphological and functional changes induced by reduction in IQGAP1.
Conclusions
These experiments are the first demonstration of IQGAP1 regulating barrier function in any cell type. Further, our data show that Angpt-1 requires IQGAP1 as an indispensable activator of Rac1.
Keywords: IQGAP1, Rac1, Angiopoietin, endothelium, barrier function, permeability
Introduction
The inner layer of blood vessels is covered with endothelial cells (ECs), which provide the primary physical barrier between the blood and underlying tissues. Intercellular junctions and the actin-cytoskeleton (determining cell architecture) are important regulators of paracellular permeability, which enables both normal physiological processes (e.g. immune surveillance and antigen recognition)1, 2 and inflammation (classic electron microscopy experiments by George Palade).3 Dysregulation of normal permeability characterizes acute systemic inflammatory diseases, in which vascular leakage initiates a vicious cycle of tissue edema and hypoxia that culminates in multiple organ failure and even death.4
Angiopoietin-1 (Angpt-1) is a 498-amino acid secreted glycoprotein that was first described in 1996 as a ligand for the endothelial tyrosine kinase receptor Tie2.5 Earlier studies had already established an essential role of Tie2 in developmental angiogenesis.6, 7 The observation of Tie2 signaling in non-angiogenic adult tissues suggested an additional role in mature vasculature. Thurston et al. reported about a decade ago that Angpt-1 overexpressing mice were resistant to VEGF-induced vascular leakage.8, 9 Since then, Angpt-1 has been shown to counteract the permeability induced by a wide spectrum of mediators – e.g. bradykinin, TNFα, thrombin, LPS 10, 11 – suggesting induction of a downstream barrier defense mechanism in ECs upon which these diverse permeability mediators converge. Cytoskeletal rearrangements, including the formation of actin stress fibers and reduction in junctional localization of adhesion proteins, may be one such mechanism.12, 13 The balance between the mutually inhibiting small GTPases RhoA and Rac1 has been implicated as a gatekeeper of cytoskeletal forces and adherens junction integrity.14-16 We and others have shown that Angpt-1 is a strong PI3K-dependent activator of Rac1 and deactivator of RhoA, promoting endothelial barrier function in vitro and in vivo.13, 17 However, the mechanisms by which Angpt-1 signals an accumulation of GTP-bound Rac1 are not fully understood.
IQ domain GTPase-activating protein 1 (IQGAP1) is a 189-kD scaffolding protein connecting an array of intracellular signaling and structural molecules with the cytoskeletal actin filaments.18-23 IQGAP1 is expressed in virtually all tissues and it appears to play a pivotal role in the control of cell adhesion, polarization and migration,20, 24, 25 particularly as these properties relate to neoplastic cells,26 where IQGAP1 has been shown to bind GTP-Rac1 and stabilize it in this active form.20 Comparatively less is known about IQGAP1's functions in quiescent non-cancerous epithelial cells, and even less is known about its role(s) in ECs.
Expression of IQGAP1 in ECs has only recently been shown 27 where initial studies have implicated this protein in angiogenesis.28, 29 Given the signaling characteristics of IQGAP1 in malignant epithelial cells and the critical importance of Angpt-1-induced activation of Rac1, we hypothesized that IQGAP1 may be involved in the regulation of barrier function in ECs. Furthermore, we speculated that Angpt-1's effect on Rac1 activation – or more generally, in protecting endothelial barrier function – may require the interaction between IQGAP1 and Rac1.
Methods
Reagents
Antibodies were purchased from the following manufacturers: IQGAP1 (H-109) and actin (C-11) (Santa Cruz), VE-cadherin (BD Bioscience), and monoclonal Rac1 (Cytoskeleton). Fluorophore- or horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Invitrogen as well as phalloidin and DAPI (4,6-diamidino-2-phenylindole). Recombinant human Angpt-1 (R&D systems) was used at a concentration of 300ng/ml. A cell-permeable pyrimidine compound that specifically inhibits Rac1 GDP/GTP exchange activity (NSC 23677, Calbiochem) was used to keep Rac1 in a GDP-bound state.30 A pool of 3 IQGAP1-specific siRNAs (Santa Cruz, sc-35700, see Supplemental Table I for sequences) was transfected in a Fast Forward fashion (HiPerFect, Qiagen). An oligonucleotide without homology to any known mammalian gene was used as control siRNA. The IQGAP1 mutant T1050AX2 was a kind gift from Dr. Kaibuchi's laboratory.31 Plasmid Transfection was done using XFect® (Clontech).
Human Microvascular EC Culture
Passage 3-6 human microvascular endothelial cells (HMVECs) from dermis (Lonza) were cultured on collagen I (Advanced BioMatrix Inc.) in EBM-2 media (Lonza) supplemented with 5% FBS and growth factors.
Quantitative PCR
RNA was extracted using TRIzol (Invitrogen) followed by clean-up using the RNeasy Mini Kit (Qiagen). RNA was then reverse transcribed to cDNA followed by SYBR Green real-time PCR in the ABI Prism 7500 Sequence Detection System (Applied Biosystems).
Packaging Cells Expressing Retroviruses Encoding DA L61Rac1
Dominant active L61Rac1 (DA Rac1) mutant cDNAs were subcloned into BamHI sites in a modified retrovirus vector pLCNX2/IRES-EGFP as described earlier.32 PT67 retroviral packaging cells (Clontech), which express the 10A1 viral envelope for production of amphotropic virus, were transfected with pLCNX2/IRES-EGFP vector containing active L61Rac1 (DA Rac1) or vector without insert (GFP control).
Retroviral Transduction
HMVECs at passage 3 were transduced with retroviruses according to a previously established method.33 The transduction procedure was repeated 3 times before subjecting cells to selection (G418, 500 μg/mL).
Western Immunoblot (IB)
HMVEC lysates (RIPA) were sonicated and centrifuged at 4°C, and supernatants were collected. A mixture of lysate, NuPAGE reducing agent, and NuPAGE sample buffer were electrophoresed in NuPAGE 4%–12% Novex Bis-Tris Gels (all from Invitrogen), transferred and immunoblotted with specific primary antibodies and detected via HRP-conjugated secondary antibodies (Amersham Pharmacia Biotech).
Immunoprecipitation (IP)
HMVECs were lysed in Brij99 buffer. 200 μg of protein was incubated with IQGAP1 antibody for 2 h. Magnetic Dynabeads conjugated with protein G (Invitrogen) were added to precipitate the antigen-antibody complex. After washing the beads, proteins were eluted by heating in LDS-sample & eluting buffer and detected by Western blot analysis with monoclonal Rac1 and IQGAP1 antibodies, respectively.
Confocal Studies
Cells were fixed in paraformaldehyde, permeabilized in Triton X-10 and blocked overnight. Primary antibodies were incubated for 12 h, followed by secondary Alexa-antibody and phalloidin. All images were taken by a Zeiss LSM510 META confocal system at 63× and were obtained with the same laser power, gain, and offset conditions.
Measurement of GTP-Bound Form of Rac1 (G-LISA)
Forty-eight hours after siRNA transfection or 24 h after transfection of the IQGAP1 mutant (T1050AX2), HMVECs were treated with Angpt-1 (300 ng/mL) to activate Rac1 or with the Rac1 GEF NSC 23677 to deactivate Rac1. Activated GTP-bound Rac1 was analyzed with a G-LISA activation assay biochemistry kit (Cytoskeleton) according to the manufacturer's instructions.
Transendothelial Electrical Resistance (TER)
TER was measured using an electrical cell-substrate impedance sensing system (ECIS) (Applied BioPhysics Inc.). Values were pooled at discrete time points and either plotted versus time or reported as bar graphs at the time-point of maximal response to a given stimulus as described elsewhere in detail.34 Each condition's endpoint resistance was divided by its starting resistance to give the normalized TER. Confluency was determined by the monolayer achieving manufacturer-recommended electrical criteria (resistance > 1800 ohms and capacitance < 10 nF).
To perform washout experiments without disturbing the underlying cellular monolayer, 50% of media was removed and replaced with an equal volume of fresh, compound- (or vehicle-) free media. This was repeated five times, achieving a final dilution of compound (and vehicle) to 1.5% of the original.
Statistical Analysis
Results are presented as mean +/- SEM. Statistical significance was evaluated by two-sided, unpaired t-test unless otherwise noted. A paired t-test was used for the comparison between longitudinal measurements from TER experiments. Analysis and graph generation was performed in GraphPad Prism 5.0 (La Jolla).
Results
Reduction of IQGAP1 is Sufficient to Disrupt Basal Endothelial Barrier Function
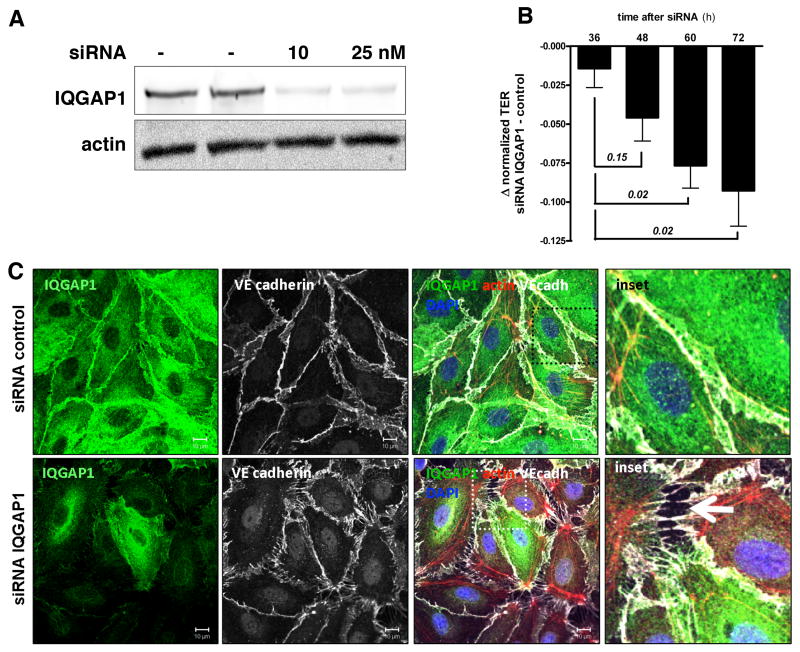
We first confirmed the expression of IQGAP1 and related isoforms in various endothelial cell types (Supplemental Figure I.A, I.G). Based on our interest in barrier regulation, we focused on human microvascular ECs (HMVECs). We then used RNAi to test the effect of reduced IQGAP1 abundance on EC morphology and function. The siRNA transfection resulted in approximately 75% protein reduction over the course of 72 hours (Figure 1A and Supplemental Figure I.B), with no change in proliferation rate or cell viability (Supplemental Figure I.C-F), a finding in agreement with two previous studies of IQGAP1-silencing in unstimulated ECs.27, 28 Fluorescent immunocytochemistry for IQGAP1, the adherens junction protein VE-cadherin and the cytoskeletal component F-actin not only verified the efficient knockdown of IQGAP1, but also uncovered profound changes in HMVEC morphology (Figure 1B), including attenuation of junctional VE-cadherin and development of paracellular gaps. These effects were specific enough that neighboring cells – that did not incorporate siRNA in their transcription machinery and therefore had normal IQGAP1 expression (i.e., “green cells” in the merged image, 2nd panel) – showed intact junctions to adjacent cells. To quantify changes in barrier function, we performed transendothelial electrical resistance (TER) measurements in IQGAP1-silenced and control HMVECs. Consistent with the observed change in EC morphology, suppression of IQGAP1 resulted in a time-dependent reduction in TER compared to controls (Figure 1C). Analogous results were obtained in an albumin flux assay (Supplemental Figure I.H). These experiments show that the presence of IQGAP1 in ECs is necessary for basal barrier integrity. This effect is independent of changes in cell proliferation, but is associated with permeability-promoting cytoskeletal and junctional rearrangements.
Figure 1.
Reduction of IQ domain GTPase-activating protein 1 (IQGAP1) is sufficient to disrupt basal endothelial barrier function. A, Immunoblot for IQGAP1 72 hours after control or IQGAP1 small interfering RNA (siRNA) transfection in human microvascular endothelial cells (HMVECs). B, Immunocytochemistry 72 hours after control or IQGAP1 siRNA transfection in HMVECs. White arrow (merged inset) highlights severe gap formation between adjacent ECs (representative of 4 independent experiments) (scale bars=10 μm). DAPI indicates 4,6-diamidino-2-phenylindole. C, Bar graph ± SEM showing the Δ between normalized transendothelial electrical resistance (TER) in IQGAP1-silenced and control silenced HMVECs at multiple time points after siRNA transfection (n=4 per condition). TER normalization refers to the start point of the experiment for each condition (ie, 32 hours after siRNA transfection, when cells reached 100% confluence).
Angiopoietin-1 Cannot Augment Endothelial Barrier Function in the Absence of IQGAP1
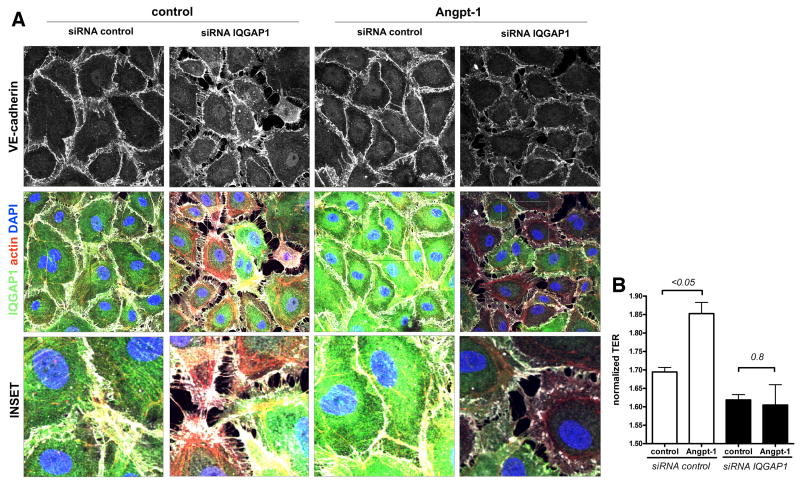
We next asked whether Angpt-1 could counteract the morphologic changes and barrier dysfunction induced by IQGAP1 silencing. Angpt-1 treatment could not prevent the disruption of inter-endothelial junctions nor reduce gap formation (Figure 2A). TER showed an overall higher resistance in Angpt-1 treated control cells, a response that was lost when IQGAP1 was silenced (Figure 2B).
Figure 2.
Angiopoietin-1 (Angpt-1) cannot augment endothelial barrier function in the absence of IQ domain GTPase-activating protein 1 (IQGAP1). A, Immunocytochemistry for IQGAP1, VE-cadherin, actin, and 4,6-diamidino-2-phenylindole (DAPI) 72 hours after control or IQGAP1 small interfering RNA (siRNA). There was no difference in gap formation when cells were incubated with Angpt-1 (300 ng/mL) or control vehicle (PBS) for 72 hours (representative of 4 experiments) (scale bar=10 μm). B, Bar graph±SEM showing normalized transendothelial electrical resistance (TER) in IQGAP1-silenced and control-silenced human microvascular endothelial cells (HMVECs) after siRNA transfection+Angpt-1 (300 ng/mL) or vehicle treatment for 72 hours (n=4 per condition). TER normalization refers to the start point of the experiment for each condition (ie, 36 hours after siRNA transfection, when cells reached 100% confluence).
Angpt-1 Requires IQGAP1 to Prevent Acute Mediator-Induced Endothelial Permeability
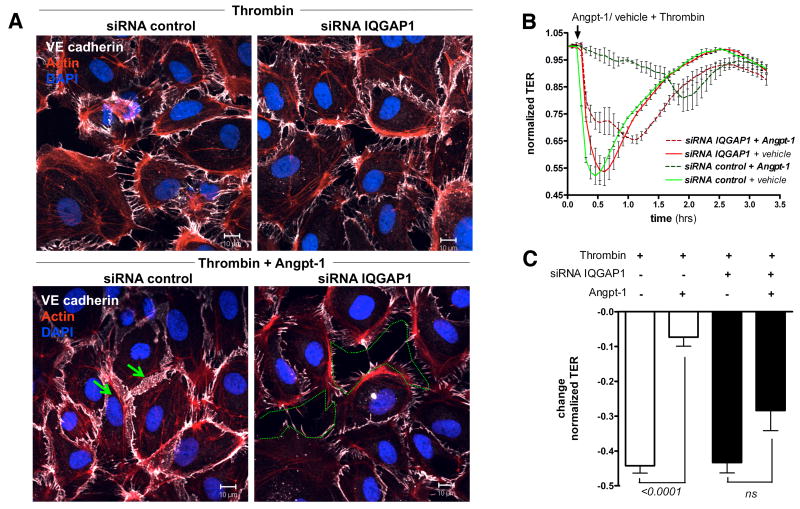
Angpt-1 is recognized to protect against acute increases in vascular permeability signaled by numerous mediators.10 In these experiments, we used thrombin, a canonical and well-studied inducer of permeability, to test whether Angpt-1's barrier-enhancing properties require IQGAP1. Treatment of HMVECs with thrombin resulted in an acute, but self-limited state of endothelial hyper-permeability (Supplemental Figure II.A). Simultaneous incubation of HMVECs with thrombin and Angpt-1 prevented inter-endothelial gap formation (Figure 3A and Supplemental Figure II.B) and the thrombin-induced drop in TER (Figure 3B and C). Both the structural and functional “protection” conferred by Angpt-1 was abolished by IQGAP1 knockdown. These experiments establish an additional role of IQGAP1 not only in regulating basal endothelial barrier function but also in its dynamic regulation by Angpt-1.
Figure 3.
Angiopoietin-1 (Angpt-1) requires IQ domain GTPase-activating protein 1 (IQGAP1) to rescue acute mediator-induced endothelial permeability. A, Immunocytochemistry for VE-cadherin, actin, and 4,6-diamidino-2-phenylindole (DAPI) 72 hours after control or IQGAP1 small interfering RNA (siRNA) transfection. Cells were challenged for 15 minutes with thrombin (1 U/mL) (a and b) or coincubated with thrombin and Angpt-1 (300 ng/mL) (c and d). In control-silenced cells, Angpt-1 abolished the paracellular gaps (green arrows) but completely failed to do so if IQGAP1 was silenced (green dotted line) (representative of 3 experiments; scale bar 10 μm). B, Human microvascular endothelial cells (HMVECs) were transfected with control or IQGAP1 siRNA. After 72 hours, thrombin (1 U/mL)+Angpt-1 (300 ng/mL) or vehicle was applied. Angpt-1 was able to rescue thrombin-induced drop in TER only in the presence of IQGAP1 (n=2 per condition). C, Bar graph±SEM representing the change in normalized TER in response to thrombin for above conditions at the time of maximal response (n=4 per condition). TER normalization refers to the start point of the experiment for each condition (ie, 72 hours after siRNA transfection, when cells reached 100% confluence).
IQGAP1 Binds Endothelial Rac1 in its Active GTP-Bound Form
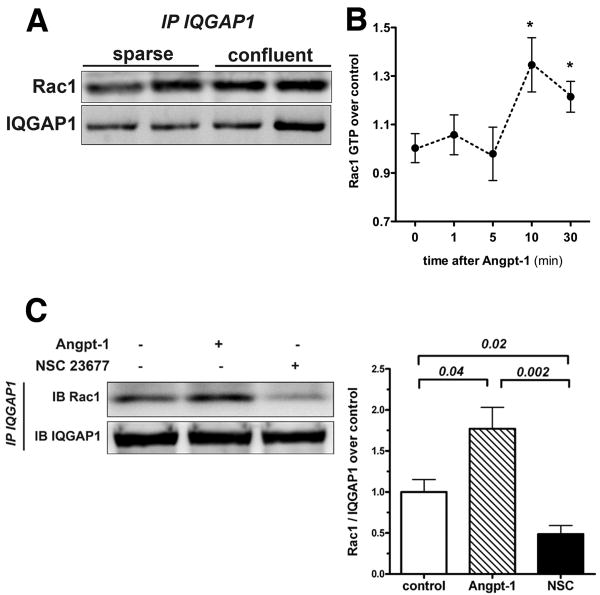
Angpt-1's barrier-protective effect against Gram-negative endotoxin is mediated through Rac1 activation.17 Since IQGAP1 has been reported to interact with Rac1 in epithelial cells,20 we asked whether IQGAP1 binds Rac1 in ECs, and if so, how this putative interaction is regulated. Co-immunoprecipitation experiments demonstrated a baseline interaction between endogenous IQGAP1 and Rac1 in sparse and confluent HMVECs (Figure 4A). Next, we confirmed that Angpt-1 is able to induce Rac1 activation in a time-dependent fashion (Figure 4B). Based on these results, we chose the 15 min time-point for all further Angpt-1 induction experiments. We used a previously established small molecule (NSC 23677) to deactivate Rac1.30 The binding of endogenous endothelial IQGAP1 to Rac1 was approximately 1.75-fold stronger when Rac1 was in an activated, GTP-bound state and much weaker when it was in a deactivated, GDP-bound state (Figure 4C). These experiments show that endogenous IQGAP1 binds Rac1 in ECs preferentially in an active state. Deactivation of Rac1 leads to a dissociation of the Rac1 - IQGAP1 complex.
Figure 4.
IQ domain GTPase-activating protein 1 (IQGAP1) binds endothelial Rac1 in its active GTP-bound form. A, Representative immunoprecipitation (IP) for IQGAP1 and immunoblot for Rac1/IQGAP1 from sparse and confluent human microvascular endothelial cell (HMVEC) lysates showing the baseline colocalization of both endogenous proteins. B, Rac1 activation assay from confluent HMVEC incubated with angiopoietin-1 (Angpt-1) (300 ng/mL) over 1, 5, 10, and 30 minutes (n=4-5 per condition, *P[lt]0.05 compared with 0 hours). C, Representative IP for IQGAP1 and immunoblot (IB) for Rac1/IQGAP1 from confluent HMVEC lysates. Cells were treated with control vehicle, 15 minutes of Angpt-1 (300 ng/mL), or NSC 23677 (25 mmol/L) for 2 hours. Bar graph±SEM shows densitometry for n=4 independent experiments.
The IQGAP1–Rac1 Interaction is Required for Angpt-1 to Activate Rac1
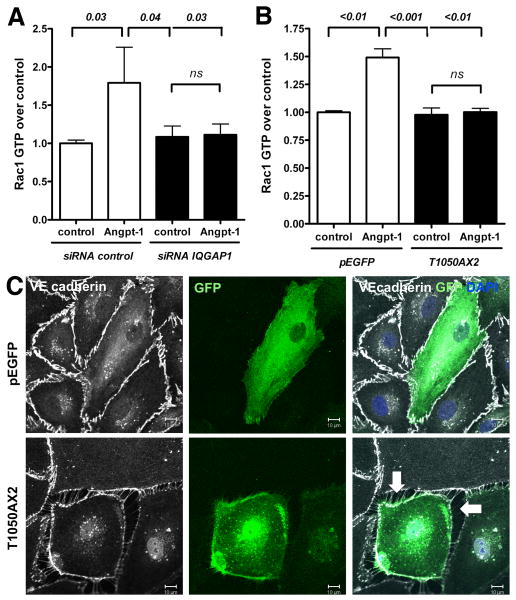
Given the physical interaction between IQGAP1 and Rac1 (Figure 4A), and the fact that Angpt-1 requires IQGAP1 for endothelial barrier protection (Figure 3B), we hypothesized that IQGAP1 may be important for the activation of Rac1 in this cell type. Hence we measured the GTP-bound form of Rac1 by G-LISA after stimulation with Angpt-1 in control or IQGAP1 siRNA-treated HMVECs. Angpt-1 induced the GTP-bound form reliably by ∼1.75-fold, an effect that mirrored the level of Rac1 - IQGAP1 binding induced by Angpt-1 (Figure 4C), and was completely absent in IQGAP1-silenced ECs (Figure 5A). To further test the necessity of IQGAP1-Rac1 interaction for Angpt-1 induced Rac1 activation, we transfected HMVECs with an IQGAP1 point-mutant lacking the Rac1 binding site (T1050AX2).31 In the presence of T1050AX2, Angpt-1 could not activate Rac1 (Figure 5B). Further, in a confluent HMVEC monolayer, cells transfected with the T1050AX2 mutant (as indicated by GFP positivity) consistently lose contact to their adjacent cells without any further stimulation (Figure 5C). These experiments establish in two different ways that IQGAP1 is required for Angpt-1 to activate Rac1 and thereby mediate its barrier protective properties.
Figure 5.
The IQ domain GTPase-activating protein 1 (IQGAP1)–Rac1 interaction is required for angiopoietin-1 (Angpt-1) to activate Rac1. A, Rac1 activation assay from confluent human microvascular endothelial cell (HMVEC) lysates transfected with control or IQGAP1 small interfering RNA (siRNA). Seventy-two hours after transfection, starved HMVECs were treated with Angpt-1 (300 ng/mL, 15 minutes) or vehicle. Only control-silenced cells were able to increase the amount of Rac1-GTP as a result of Angpt-1 treatment (n=6 per condition). B, Rac1 activation assay from confluent HMVEC lysates transfected with either a dominant negative IQGAP1-mutant disabled in Rac1 binding (T1050AX2) or with the corresponding backbone control vector (pEGFP). Seventy-two hours after transfection, starved HMVECs were treated with Angpt-1 (300 ng/mL, 15 minutes) or vehicle. ECs expressing T1050AX2 were not able to increase their endogenous Rac1-GTP abundance after Angpt-1 treatment (n=8 per condition). C, Immunocytochemistry for green fluorescent protein (GFP), VE-cadherin, and 4,6-diamidino-2-phenylindole (DAPI) 72 hours after transfection with either T1050AX2 IQGAP1-mutant or the corresponding backbone control vector (pEGFP). HMVECs expressing a high amount of T1050AX2 IQGAP1 (as indicated by extensive GFP signal) lost contact with adjacent ECs and formed paracellular gaps similar to those seen before in IQGAP1-silenced cells (representative of 3 experiments, scale bar=10 μm).
Rac1 Deactivation Results in Barrier Breakdown that is Not Aggravated by Lack of IQGAP1
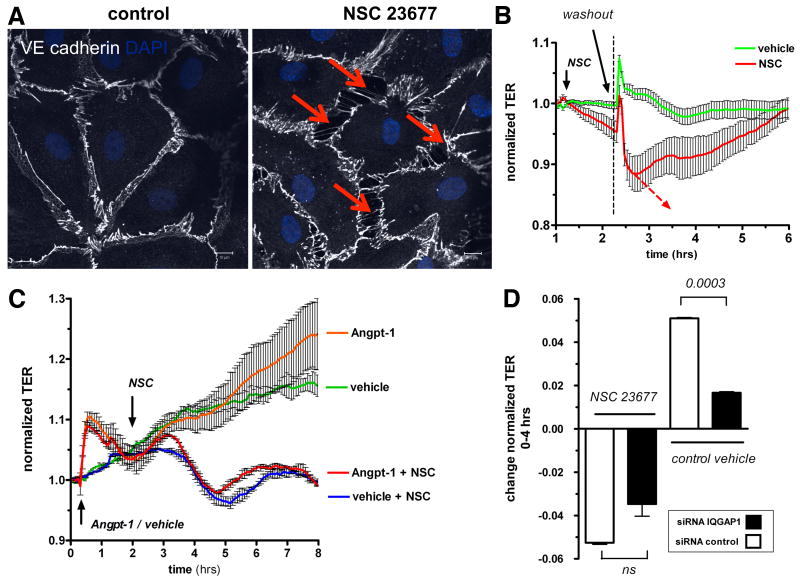
Having established that reducing one partner of this interaction (i.e. IQGAP1) is sufficient to induce barrier dysfunction, we tested if deactivation of the 2nd partner – i.e. Rac1 – is similarly sufficient to induce endothelial permeability. Inhibition of Rac1 resulted in a dose-dependent barrier breakdown (Supplemental Figure III), accompanied by similar changes in cell morphology as seen earlier in IQGAP1-silenced ECs (Figure 6A). NSC 23677's effect was fully reversed after 2 hours by washout (Figure 6B). Analogous to our findings in IQGAP1-silenced cells, Angpt-1 was not able to prevent barrier dysfunction arising from Rac1 deactivation (Figure 6C). We then measured the effect of Rac1 deactivation in cells treated with control or IQGAP1 siRNA to ask whether the barrier effects of inhibiting both partners were additive. Inhibition of Rac1 had a comparable permeability effect on control-/ and IQGAP1-silenced cells at 2 hours (Figure 6D) as well as over the course of 20 hours (Supplemental Figure IV). In other words, knockdown of IQGAP1 did not further weaken barrier function when Rac1 was already inhibited. These results argue that IQGAP1 is not downstream or parallel to Rac1, but rather, may be exerting its barrier-promoting effect through Rac1.
Figure 6.
Rac1 deactivation results in barrier breakdown that is not aggravated by lack of IQ domain GTPase-activating protein 1 (IQGAP1). A, Immunocytochemistry for VE-cadherin and 4,6-diamidino-2-phenylindole (DAPI) from confluent human microvascular endothelial cells (HMVECs) 2 hours after treatment with NSC 23677 (25 mmol/L) or vehicle. Red arrows highlight severe paracellular gap formation similar to IQGAP1-silenced cells (representative of 3 experiments, scale bars=10 μm). B, Normalized transendothelial electrical resistance (TER) was measured in real time after treatment with NSC 23677 or control vehicle. After 2 hours—when TER was noted to drop continuously—medium was carefully changed to test for reversibility of NSC-induced permeability (n=4 per condition). TER normalization refers to the start point of the experiment for each condition (ie, 1 hour before NSC treatment). C, TER was recorded in real time similarly to the method described in B. At the start of the experiment, HMVECs were treated with angiopoietin-1 (Angpt-1) (300 ng/mL, orange and red) or control vehicle (green and blue). One hour later, NSC 23677 (blue and red) or control vehicle was added, and TER followed for another 6 hours. NSC-induced permeability was notable after 2 hours and was also not reversed by pretreatment with Angpt-1 (n=2 per condition). TER normalization refers to the start point of the experiment for each condition (ie, 30 minutes before Angpt-1 treatment). D, TER 2 hours after treatment with NSC 23677 or control vehicle in control or IQGAP1-silenced (48 hours) HMVECs. NSC-induced permeability was not aggravated by silencing of IQGAP1 (n=2 per condition). TER normalization refers to the start point of the experiment for each condition (ie, 36 hours after small interfering RNA [lsqb]siRNA[rsqb] transfection). ns indicates not significant.
Dominant-Active Rac1 Abolishes the Effects of Silencing IQGAP1
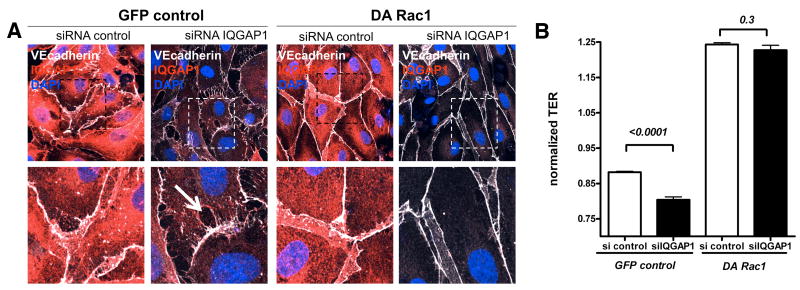
Since IQGAP1 was necessary for Angpt-1 driven Rac1 activation (Figure 5A) and barrier enhancement, we used a retrovirally-encoded dominant active (DA) Rac1 (L61Rac1)32 to test whether IQGAP1 regulates barrier function through Rac1. If IQGAP1 is upstream of Rac1, a constitutively active Rac1 should protect ECs from the barrier disruption induced by IQGAP1-silencing. Consecutive antibiotic selection of HMVECs transfected with DA Rac1 or a GFP-control retrovirus resulted in nearly 100% transfection efficiency (data not shown). Stable transfected HMVECs were then either treated with control or IQGAP1 siRNA. The earlier described changes in EC morphology following IQGAP1 knockdown were replicated in GFP-control virus-treated cells, but DA Rac1 prevented the disruption of junctional proteins and the formation of inter-endothelial gaps (Figure 7A and Supplemental Figure V). DA Rac1 expressing HMVECs had higher TER, and DA Rac1 prevented the barrier dysfunction induced by IQGAP1-silencing (Figure 7B).
Figure 7.
Dominant activation of Rac1 abolishes the effects of silencing IQ domain GTPase-activating protein 1 (IQGAP1). A, Immunocytochemistry for IQGAP1, VE-cadherin, and 4,6-diamidino-2-phenylindole (DAPI) from human microvascular endothelial cells (HMVECs) stably expressing green fluorescent protein (GFP) control or dominant-active (DA) Rac1 protein (100% transduction efficiency, see GFP channel in Supplemental Figure V). Confocal images were captured 72 hours after control or IQGAP1 small interfering RNA (siRNA) transfection. (Representative for 3 experiments; scale bars=10 μm (top row) or 5 μm (bottom row, representing insets). B, Bar graph±SEM shows normalized TER measurements 48 hours after siRNA transfection (n=2 per group×12 readings per hour) for the above-described conditions. TER normalization refers to the start point of the experiment for each condition (ie, 32 hours after siRNA transfection).
Discussion
The present study describes a novel role of IQGAP1 in endothelial cells and places it in a signaling pathway critical to a core function of microvascular ECs. To our knowledge, these experiments suggest for the first time that IQGAP1 acts through Rac1 and is thereby an essential regulator of endothelial permeability. Inhibition of either Rac1 or IQGAP1 in otherwise unstimulated ECs is sufficient to induce profound structural and functional changes, neither of which could be rescued by Angpt-1. While the endothelial barrier defense action of Rac1 is well-established,35 the role of IQGAP1 has not previously been studied in this context. The present experiments strongly suggest that the physical interaction of IQGAP1 with Rac1 is required for activation of Rac1, with critical consequences to basal barrier function and its dynamic regulation.
In states of acute mediator-induced vascular leakage, the endothelial-stabilizing factor Angpt-1 is known to prevent hyper-permeability induced by a wide-ranging spectrum of agents.8, 10, 11 The barrier defense action of Angpt-1 appears to rely critically on its ability to activate Rac1.17, 32, 36 In turn, the mechanism of Rac1 activation appears to be both Tie-2 and PI3-Kinase-dependent.17, 36 However, in the absence of IQGAP1, this anti-permeability effect of Angpt-1 is lost. We found that endogenous IQGAP1 and Rac1 physically interact in unstimulated ECs and increase this interaction following Angpt-1 application. Furthermore, two independent approaches suggest that the presence of endogenous IQGAP1 (siRNA experiments), and, even more specifically, the binding itself (T1050AX2 mutant experiments) are fundamentally important for Rac1 activation. Since IQGAP1 appears to bind activated Rac1 and, yet, Rac1, cannot be maximally activated in the absence of IQGAP1, we speculate that IQGAP1 is important for the stabilization of activated Rac1, thus sustaining the signal to fortify barrier function (Figure 8).
Figure 8.
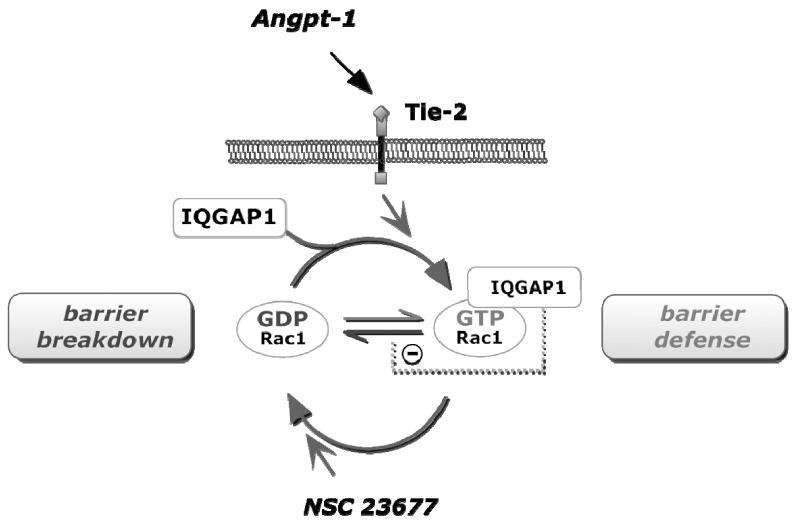
IQ domain GTPase-activating protein 1 (IQGAP1) barrier signaling in endothelial cells. The Tie-2 ligand angiopoietin-1 (Angpt-1) increases the binding of intracellular IQGAP1 to Rac1-GTP. This binding further sustains Rac1 in an active, GTP-bound state, thereby promoting endothelial barrier defense (upper half of the circle). Deactivation of Rac1 using a specific inhibitor of the Rac1 GDP/GTP exchange activity leads to IQGAP1-Rac1 complex dissociation (lower half of the circle) and endothelial barrier breakdown.
The signaling cascade was further explored by rescue experiments using DA Rac1 transduced ECs. The phenotype induced by lack of IQGAP1 could be reversed in DA Rac1-transfected ECs, demonstrating that Rac1 functions as a downstream effector of IQGAP1's barrier effects. This finding is somewhat unexpected, as others have proposed the opposite in epithelial cells, namely that IQGAP1 is a downstream effector of Rac1. In this regard, Kaibuchi's early work was primarily focused on IQGAP1's scaffolding properties between IQGAP1 and adherens junctions rather than signaling events involving small GTPases.21, 22, 24 One of their chief findings in epithelial cells was that Rac1 stabilizes adherens junctions by sequestering IQGAP1, which impedes “free” IQGAP1 from binding to—and thereby disassembling—the cadherin-catenin complex. By focusing more on IQGAP1's signaling properties, rather than its scaffolding actions, our work suggests a novel mechanism of IQGAP1-mediated barrier enhancement.
Comparatively few studies have examined the endothelial actions of IQGAP1,27, 29, 37-39 and no prior experiments have established a link between IQGAP1 and endothelial barrier function. Yamaoka-Tojo, et al., elegantly showed how IQGAP1 functions as a VEGFR2-associated scaffold protein that enables VEGF-induced migration and proliferation.27 The same group also found that IQGAP1 recruits VEGFR1 to adherens junctions and thereby facilitates VEGF-mediated VE-cadherin phosphorylation to initiate angiogenesis.39 Analogous to their findings, IQGAP1 may also be responsible for recruiting other receptor tyrosine kinases, such as Tie-2, to adherens junctions.40, 41 Such “fine-tuning” of tyrosine kinase recruitment to junctions by IQGAP1 could help explain why two growth factors, VEGF and Angpt-1, have similar effects on EC survival and proliferation but opposed effects on barrier function. Stated differently, IQGAP1 may be recruited into unique signaling complexes or physical compartments based upon the extracellular ligands that signal into the cell, which in turn, dictate whether its scaffolding or signaling functions predominate.
Some questions regarding the interaction between IQGAP1 and Rac1 remain unanswered from our experiments. First, it has been shown in cell-free assays and epithelial cells that IQGAP1 can bind and activate Rac1,20, 22 results supported by our findings in ECs. However, the exact underlying mechanisms are undetermined. One possibility is that IQGAP1 actually possesses anti-GAP activity.18 The Rac1-activating effect of IQGAP1 may also be indirect, for example, by activating endothelial Akt phosphorylation, which would in turn induce Rac1.27 Second, we show that activation or deactivation of Rac1 changes its ability to bind IQGAP1. It is unknown how the affinity is regulated and which role the numerous other IQGAP1 binding partners might play. Third, our findings implicate a signaling action of IQGAP1 for the maintenance of basal barrier function and the induction of Angpt-1-mediated barrier defense. Future studies should also examine how IQGAP1's scaffolding function between adherens junctions and the cytoskeleton impacts barrier regulation, for example, by using mutant that abolishes IQGAP1's binding of actin. Finally, little is known about other IQGAP isoforms in ECs. The lack of an in vivo confirmation of the role of IQGAP1 in endothelial barrier function represents a major limitation of our current work.
We conclude from these experiments that IQGAP1 has a fundamental role in modulating baseline endothelial permeability as well as endothelial responsiveness to Angpt-1. The barrier-maintaining effect of IQGAP1 in ECs relies on its direct binding to Rac1-GTP and the inhibition of its intrinsic GTPase function. Given that DA Rac1 abolishes the permeability changes induced by IQGAP1 silencing, we propose a novel barrier-defense signaling pathway in ECs in which Angpt-1 induces Rac1 via IQGAP1.
Supplementary Material
Acknowledgments
(a) We thank Drs. Senger and Hong for providing the DA Rac1 (L61Rac1) retrovirus, Drs. Kaibuchi and Watanabe for the T1050AX2 plasmid, Kate Spokes for primary mouse endothelial cells and Dr. Nucera for advice.
(b) SMP is supported by NIH grants DK096316 and HL093234. SD is supported by the German Research Foundation (DA 1209/1-1).
(c) The authors have no relationships that could be perceived as real or apparent conflicts of interest.
References
- 1.Aghajanian A, Wittchen ES, Allingham MJ, Garrett TA, Burridge K. Endothelial cell junctions and the regulation of vascular permeability and leukocyte transmigration. Journal of thrombosis and haemostasis : JTH. 2008;6:1453–1460. doi: 10.1111/j.1538-7836.2008.03087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 3.Majno G, Palade GE. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: An electron microscopic study. The Journal of biophysical and biochemical cytology. 1961;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 5.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the tie2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 6.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stratmann A, Risau W, Plate KH. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. The American Journal of Pathology. 1998;153:1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 9.Thurston G, Rudge JS, Ioffe E, Zhou H, Ross L, Croll SD, Glazer N, Holash J, McDonald DM, Yancopoulos GD. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 10.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, Rudge J, Yancopoulos G, Vadas MA. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 11.Pizurki L, Zhou Z, Glynos K, Roussos C, Papapetropoulos A. Angiopoietin-1 inhibits endothelial permeability, neutrophil adherence and il-8 production. Br J Pharmacol. 2003;139:329–336. doi: 10.1038/sj.bjp.0705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res. 2008;76:202–207. doi: 10.1016/j.mvr.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Csortos C, Kolosova I, Verin AD. Regulation of vascular endothelial cell barrier function and cytoskeleton structure by protein phosphatases of the ppp family. Am J Physiol Lung Cell Mol Physiol. 2007;293:L843–854. doi: 10.1152/ajplung.00120.2007. [DOI] [PubMed] [Google Scholar]
- 14.Lee MJ, Thangada S, Claffey KP, Ancellin N, Liu CH, Kluk M, Volpi M, Sha'afi RI, Hla T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- 15.Burridge K, Wennerberg K. Rho and rac take center stage. Cell. 2004;116:167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 16.Wildenberg GA, Dohn MR, Carnahan RH, Davis MA, Lobdell NA, Settleman J, Reynolds AB. P120-catenin and p190rhogap regulate cell-cell adhesion by coordinating antagonism between rac and rho. Cell. 2006;127:1027–1039. doi: 10.1016/j.cell.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, Ingber DE, Sukhatme VP. Angiopoietin-1 requires p190 rhogap to protect against vascular leakage in vivo. The Journal of biological chemistry. 2007;282:23910–23918. doi: 10.1074/jbc.M702169200. [DOI] [PubMed] [Google Scholar]
- 18.Briggs MW, Sacks DB. Iqgap proteins are integral components of cytoskeletal regulation. EMBO Rep. 2003;4:571–574. doi: 10.1038/sj.embor.embor867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. Iqgap1: A key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–2092. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K. Interaction with iqgap1 links apc to rac1, cdc42, and actin filaments during cell polarization and migration. Dev Cell. 2004;7:871–883. doi: 10.1016/j.devcel.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 21.Fukata M, Kuroda S, Fujii K, Nakamura T, Shoji I, Matsuura Y, Okawa K, Iwamatsu A, Kikuchi A, Kaibuchi K. Regulation of cross-linking of actin filament by iqgap1, a target for cdc42. The Journal of biological chemistry. 1997;272:29579–29583. doi: 10.1074/jbc.272.47.29579. [DOI] [PubMed] [Google Scholar]
- 22.Kuroda S, Fukata M, Nakagawa M, Fujii K, Nakamura T, Ookubo T, Izawa I, Nagase T, Nomura N, Tani H, Shoji I, Matsuura Y, Yonehara S, Kaibuchi K. Role of iqgap1, a target of the small gtpases cdc42 and rac1, in regulation of e-cadherin- mediated cell-cell adhesion. Science. 1998;281:832–835. doi: 10.1126/science.281.5378.832. [DOI] [PubMed] [Google Scholar]
- 23.Briggs MW, Sacks DB. Iqgap1 as signal integrator: Ca2+, calmodulin, cdc42 and the cytoskeleton. FEBS Lett. 2003;542:7–11. doi: 10.1016/s0014-5793(03)00333-8. [DOI] [PubMed] [Google Scholar]
- 24.Noritake J, Fukata M, Sato K, Nakagawa M, Watanabe T, Izumi N, Wang S, Fukata Y, Kaibuchi K. Positive role of iqgap1, an effector of rac1, in actin-meshwork formation at sites of cell-cell contact. Mol Biol Cell. 2004;15:1065–1076. doi: 10.1091/mbc.E03-08-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mataraza JM, Briggs MW, Li Z, Entwistle A, Ridley AJ, Sacks DB. Iqgap1 promotes cell motility and invasion. The Journal of biological chemistry. 2003;278:41237–41245. doi: 10.1074/jbc.M304838200. [DOI] [PubMed] [Google Scholar]
- 26.White CD, Brown MD, Sacks DB. Iqgaps in cancer: A family of scaffold proteins underlying tumorigenesis. FEBS Lett. 2009;583:1817–1824. doi: 10.1016/j.febslet.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaoka-Tojo M, Ushio-Fukai M, Hilenski L, Dikalov SI, Chen YE, Tojo T, Fukai T, Fujimoto M, Patrushev NA, Wang N, Kontos CD, Bloom GS, Alexander RW. Iqgap1, a novel vascular endothelial growth factor receptor binding protein, is involved in reactive oxygen species--dependent endothelial migration and proliferation. Circ Res. 2004;95:276–283. doi: 10.1161/01.RES.0000136522.58649.60. [DOI] [PubMed] [Google Scholar]
- 28.Meyer RD, Sacks DB, Rahimi N. Iqgap1-dependent signaling pathway regulates endothelial cell proliferation and angiogenesis. PLoS ONE. 2008;3:e3848. doi: 10.1371/journal.pone.0003848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ikeda S, Yamaoka-Tojo M, Hilenski L, Patrushev NA, Anwar GM, Quinn MT, Ushio-Fukai M. Iqgap1 regulates reactive oxygen species-dependent endothelial cell migration through interacting with nox2. Arterioscler Thromb Vasc Biol. 2005;25:2295–2300. doi: 10.1161/01.ATV.0000187472.55437.af. [DOI] [PubMed] [Google Scholar]
- 30.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a rac gtpase-specific small molecule inhibitor. Proc Natl Acad Sci U S A. 2004;101:7618–7623. doi: 10.1073/pnas.0307512101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and cdc42 capture microtubules through iqgap1 and clip-170. Cell. 2002;109:873–885. doi: 10.1016/s0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 32.Hoang MV, Nagy JA, Senger DR. Active rac1 improves pathologic vegf neovessel architecture and reduces vascular leak: Mechanistic similarities with angiopoietin-1. Blood. 2011;117:1751–1760. doi: 10.1182/blood-2010-05-286831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le Doux JM, Landazuri N, Yarmush ML, Morgan JR. Complexation of retrovirus with cationic and anionic polymers increases the efficiency of gene transfer. Hum Gene Ther. 2001;12:1611–1621. doi: 10.1089/10430340152528110. [DOI] [PubMed] [Google Scholar]
- 34.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by edg-dependent cytoskeletal rearrangement. The Journal of clinical investigation. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spindler V, Schlegel N, Waschke J. Role of gtpases in control of microvascular permeability. Cardiovasc Res. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 36.Harfouche R, Malak NA, Brandes RP, Karsan A, Irani K, Hussain SN. Roles of reactive oxygen species in angiopoietin-1/tie-2 receptor signaling. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19:1728–1730. doi: 10.1096/fj.04-3621fje. [DOI] [PubMed] [Google Scholar]
- 37.Nakhaei-Nejad M, Zhang QX, Murray AG. Endothelial iqgap1 regulates efficient lymphocyte transendothelial migration. Eur J Immunol. 2010;40:204–213. doi: 10.1002/eji.200839214. [DOI] [PubMed] [Google Scholar]
- 38.Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, Natarajan V. Phospholipase d-mediated activation of iqgap1 through rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. The Journal of biological chemistry. 2009;284:15339–15352. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaoka-Tojo M, Tojo T, Kim HW, Hilenski L, Patrushev NA, Zhang L, Fukai T, Ushio-Fukai M. Iqgap1 mediates ve-cadherin-based cell-cell contacts and vegf signaling at adherence junctions linked to angiogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1991–1997. doi: 10.1161/01.ATV.0000231524.14873.e7. [DOI] [PubMed] [Google Scholar]
- 40.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, Shibuya M, Takakura N, Koh GY, Mochizuki N. Differential function of tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 41.Saharinen P, Eklund L, Miettinen J, Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D, Deutsch U, Koh GY, Olsen BR, Alitalo K. Angiopoietins assemble distinct tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat Cell Biol. 2008;10:527–537. doi: 10.1038/ncb1715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.