SUMMARY
The diminution in certain aspects of cognitive function that is reported to occur in some patients during or after adjuvant cancer chemotherapy is variously known as ‘chemo-fog’, ‘chemo-brain’ or other such term. In addition to reported deficits in attention, concentration and other functions, most, if not all, of the studies report deficits involving visual-spatial function or visual memory. Since the visual system is part of the nervous system, it seems reasonable to ask if it is susceptible to some of the deleterious effects produced by adjuvant chemotherapeutic drugs. We propose here the possibility that some portion of the vision-related aspects of the ‘chemo-fog’ spectrum of cognitive deficits results from a direct action of the adjuvant drugs on the visual system or from drug / drug or site / site interaction between effects on the visual system and other critical brain regions.
Keywords: adverse effects, cancer chemotherapy, chemo-brain, chemo-fog, cognition, eye
INTRODUCTION
During or following adjuvant cancer chemotherapy, transient or permanent self-reported or observed cognitive impairment is noted to occur in a subgroup of patients (see previous review in this journal by Raffa et al. (1) and references therein). This deficit in cognitive functioning is called ‘chemo-fog’, ‘chemo-brain’ or similar term and it has been reported to occur in 4–75% of treated patients. Several domains of cognition are characteristically impacted more than others in these patients, including effects on attention, concentration, language skills or executive functions such as multitasking and ability to organize information. Also commonly included in the list of deficits are ‘visuo-spatial’ (e.g. refs 2, 3) or ‘visual-motor’ (e.g. ref 4) functioning or ‘visual memory’ (e.g. ref 5). To date, it has been explicitly or tacitly assumed that the anatomic locale of these latter deficits is at the level of cognitive processing and not the eye or visual processing pathways. But the extent to which visual impairments might contribute to ‘chemo-fog’ is unknown and is worthy of further exploration. The rational basis for such exploration is the fact that the retina of the eye is a thin layer of nervous tissue that is connected to the optic nerve; essentially it is a part of the brain. This anatomical fact suggests that certain defects in vision may accompany and be indicators of impairment of brain activity.
We here propose the possibility that chemotherapeutic drug-induced toxicity to the visual system (overt or subclinical) might contribute – alone, additively or synergistically with other toxicities – to some of the cognitive functioning deficits described in ‘chemo-fog’.
VISUAL-RELATED DEFICITS REPORTED IN ‘CHEMO-FOG’
Several reports of adjuvant cancer chemotherapy-induced cognitive deficits describe impaired functioning that might actually be secondary manifestations of toxicity to the visual system (sensory input) rather than to direct effect or sole effect on cognitive functioning (processing). A sampling of such deficits from studies highlighted in the prior review by Raffa et al. (1) are described in more detail than previously below.
Wieneke and Dienst (2) used a broad battery of neuropsychological tests to evaluate the current cognitive functioning of breast cancer (stages I and II) patients (n = 28; aged 28–54, mean 42 years; 82% Caucasian, 18% African-American, Asian or Hispanic) who had received conventional (no high-dose) adjuvant chemotherapy 2 weeks to 1 year prior to the evaluation. The test battery was designed to detect mild / subtle cognitive impairments and the tests were age-, education level- and gender-adjusted. The specific tests are described and referenced in (6) and (7). The patients had been treated with a cyclophosphamide / methotrexate / 5-fluorouracil (CMF) regimen (86%) some of which (26%) also received cyclophosphamide / adriamycin / 5-fluorouracil (CAF), CAF alone (14%) or tamoxifen at the time of testing (39%). Visuo-spatial functioning was assessed using three measures: Rey CFT-direct copy (Z-score); block design (WAIS-R) (T-score) and Digit symbol (WAIS-R) (T-score). The patients displayed significant impairment (P < 0.001) in Rey CFT-direct copy test. Memory deficits were also detected using other measures. Interestingly, it was not verbal memory, but visual memory that was significantly impaired.
van Dam et al. (5) assessed the prevalence of cognitive deficits in breast cancer (stages II and III) patients (n = 70, plus 34 controls) younger than 55 years (mean age 45–49 years) who had been administered adjuvant chemotherapy plus tamoxifen 1.5–2 years prior to the evaluation. Neuropsychological status was assessed using a standard battery of 13 tests (yielding 19 test indices). The patients received a regimen of fluorouracil / epidoxorubicin / cyclophosphamide plus tamoxifen or the same regimen plus high-dose cyclophosphamide, thiotepa and carboplatin. Measures involving visual ability included the Complex Figure test copy and recall, Trailmaking A & B and the D2 test (details in ref 5). The largest deficits compared to controls were reported for Trailmaking B and, as in the above study (2), for visual memory.
Schagen et al. (4) used neuropsychological tests and interviews to evaluate cognitive function, compared with controls (age-matched axillary lymph node negative breast carcinoma) in breast cancer patients who had been treated with adjuvant CMF alone (n = 19) or followed (n = 20) by 3 years of tamoxifen a median of 2.4 years prior to the evaluation. The battery of tests included several already mentioned above, plus the Fepsy visual reaction and visual searching tests and the visual reproduction of the Wechsler memory scale (WMS), revised: immediate and delayed recall (further description of these tests can be found in ref 4). Of these measures, there was a significant deficit in performance (P < 0.05) in the trailmaking (B), Fepsy visual reaction, complex figure recall and WMS immediate and delayed recall tests.
Brezden et al. (3) assessed cognitive function of breast cancer (stages I or II) patients (aged 24–70, median 41.5–49.0 years) who were at the time receiving standard-dose adjuvant chemotherapy (either cyclophosphamide / epirubicin / 5-fluorouracil or CMF) (n = 31), had completed adjuvant chemotherapy (n = 4) a median of 2 years earlier and healthy controls (n = 36). Assessment was made using the High Sensitivity Cognitive Screen and the profile of Mood States (described further in 3). Visual-motor function was significantly impaired (P < 0.05) in the chemotherapy-treated patients compared to controls.
There are more such studies, but these serve as examples and allow a consistent conclusion to be reached: some of the cognitive domains evaluated in patients who have been administered cancer chemotherapeutic agents reveal significant deficits in domains that require normal visual function. Cancer chemotherapy agents appear to be implicated as causative of these deficits. Do such agents possess ocular or other visual-related toxicities?
VISUAL-RELATED TOXICITY OF TREATMENT AGENTS
The ocular complications / toxicities associated with cancer chemotherapeutic drugs have been reviewed at least since 1983 (8) and in at least three major systematic comprehensive reviews over the past 20 years (9–11). Because of their inherent and intended cytotoxic properties, it is not surprising that these agents have untoward effects on the visual system. A recent report on a large cohort of patients (12) found that ocular toxicity during cancer chemo / adjuvant therapy is a common side-effect (appearing in 538 / 4948 patients (11%) for the regimen studied). A summary of these toxicities is presented in Table 1, which integrates the information in the three reviews. The toxicities of individual agents implicated in the ‘chemo-fog’ literature, cited in the previous section, follow (summarized directly from 9–11).
Table 1.
Targets of ocular toxicity of selected cancer chemotherapeutic agents
| Agent | Conjunctiva | Cornea | Retina | Optic nerve | Other |
|---|---|---|---|---|---|
| Busulfan | |||||
| Carboplatin | |||||
| Carmustine | |||||
| Chlorambucil | |||||
| Cis-platinum | |||||
| Cyclophosphamide | |||||
| Cytosine arabinoside | |||||
| Deoxycoformycin | |||||
| Docetaxel | |||||
| Doxorubicin | |||||
| 5-Fluorouracil | |||||
| Ifosfamide | |||||
| Mechlorethamine | |||||
| Methotrexate | |||||
| Mitomysin C | |||||
| Paclitaxel | |||||
| Tamoxifen |
Cyclophosphamide
Ocular toxicity associated with the use of the nitrogen mustard derivative alkylating agent cyclophosphamide includes blurred vision, keratoconjunctivitis sicca in as many as 50% of patients (8), blepharoconjunctivitis, pinpoint pupils and others (13–16).
Docetaxel
Canalicular and nasalacrimal duct obstruction, possibly due to stromal fibrosis (17, 18), are rare ocular side-effects of the taxane mitototic inhibitor doxetaxel.
Doxorubicin
The anthracycline antibiotic doxorubicin produces excessive lacrimation and conjunctivitis in about 25% of patients (10, 19) when given alone. Serious ocular toxicity has been noted when desferrioxamine, a substance with its own ocular toxicity (20), has been co-administered to increase doxorubicin anti-tumour activity (21).
5-fluorouracil
The pyrimidine analogue antimetabolite (inhibitor of thymidine synthetase) 5-FU causes ocular toxicity in an estimated 25–38% of patients treated with the drug either alone or in a combination-regimen. Ocular toxicities include usually mild-to-moderate blurred vision, ocular pain, photophobia, excessive lacrimation, eye irritation, conjunctivitis, circumorbital edema, ectropion, keratitis (8, 22), inhibition of mitosis of retinal pigment epithelial cells and fibrocytes (23) and other effects (24–29).
Methotrexate
Up to 25% of patients undergoing high-dose i.v. therapy with the folic acid antagonist antimetabolite (inhibitor of dihydrofolate reductase) methotrexate develop ocular toxicity within 2–7 days after initiation of therapy. The major observed toxicities include periorbital oedema, ocular pain, blurred vision, photophobia, conjunctivitis, blepharitis and decreased reflex tear secretion (10), possibly the result of the drug’s antimiotic effect in the rapidly dividing cells of the cornea and conjunctival epithelium (16). Intrathecal methotrexate can produce optic neuropathy and internuclear opthalmoplegia, which can be potentiated by concurrent cranial irradiation (30, 31). A severe case of meningeal metastasis of breast carcinoma that was treated with an intraventricular combination of methotrexate with cytosine arabinoside developed multiple foci of axonal degeneration and demyelination in the optic nerve and the chiasm (32).
Although each of the reviews summarized above (9–11) suggest that combinations of cancer chemotherapeutic drugs might produce greater toxicity on the visual system than individual drugs given alone, to our knowledge this has never been systematically investigated in patients or studied in biological models. However, based on synergistic interactions among these drugs on other endpoints (e.g. 33–38), synergistic toxic effects – between drugs or between the visual and other brain systems – would not be surprising.
In the review by Raffa et al. (1), the authors commented that the best way to address the question of putative cognitive deficits attributed to cancer chemotherapy agents, independent of other complications, is to test the agents in animal models. Such testing is underway at Temple University with E. A. Walker, PhD, R. Clark-Vetri, PharmD, and S. Nagar, PhD and incorporates novel quantitative methods (summarized below) to analyse the drug combination data. The contribution of visual impairments that might contribute to chemo-fog, as reviewed here, suggest that a second path for investigation might also benefit from the use of animal models, and this is being explored as a possible additional pursuit in the authors’ laboratories. In that regard one very interesting model is The Royal College of Surgeons rat (e.g. see 39). This is a widely studied animal model of retinal degeneration in which the inability of the retinal pigment epithelium to phagocytize shed photoreceptor outer segments leads to a progressive loss of rod and cone photoreceptors.
CONCEPTS IN DRUG–DRUG OR SITE–SITE TOXICITY OF DRUGS
Well-defined quantitative methods for studying drug effects are available (40–42). In most of these methods one obtains dose–effect data from which the potency and efficacy (or toxicity) of a drug can be measured. When combinations of drugs are used (as in cancer treatments) very powerful newer methods have been developed to determine unusual interactions between the drugs and the consequent exaggerated effects that these produce (43–45). Hence, impairments of visual function can be the end point (effect) in these studies using both naїve and drug-treated animals. A brief description of the methodology used in drug combination studies is presented below with the aim of emphasizing the importance of using this kind of quantitative approach in assessing visual and related brain impairment that may contribute to the chemo-fog phenomenon.
Quantitative methods for drug interaction: isobole theory
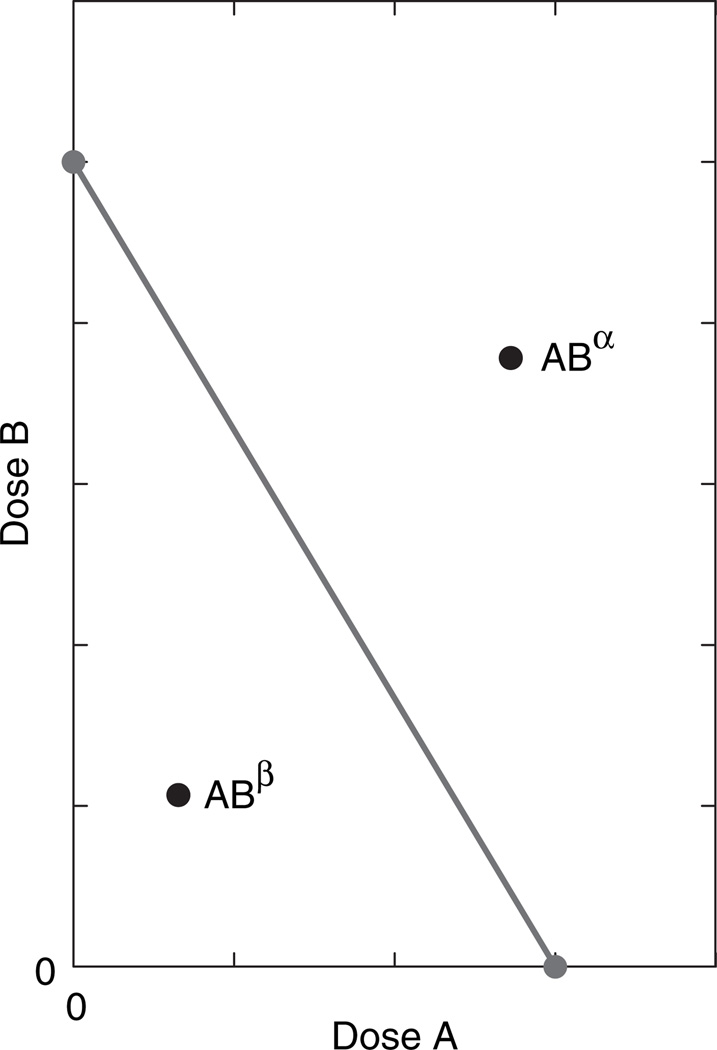
When two different drugs produce overtly similar effects (whether therapeutic or toxic) there is interest in assessing the effect of a combination. Especially important is the question, does concomitant dosing produce an exaggerated effect, i.e. an effect that is greater than the expected effect? This expected effect is calculated from the individual drug potencies (43). Potency refers to the quantity of a drug that is needed to attain a specified effect level, very often the level chosen is half the maximum. We shall here denote these quantities as A for drug A and B for drug B. In the simplest case the ratio A / B is the same for all effect levels and this leads to construction of a linear plot of doses in Cartesian coordinates in which the intercepts are A and B (Fig. 1). This plot, whose theoretical basis is contained in the above-cited references, is called an isobole. The isobole represents all dose pairs that are expected, from their individual potencies, to yield the same specified effect magnitude. It is seen that the isobole slope is negative, a fact consistent with the idea that increasing the dose of one of the agents means a decrease in the dose of the other. This predictive line, made from the dose–effect relation of each drug acting alone, is then used along with experimentally determined combinations that yield the selected effect. Those experimental combinations are plotted as points (dose pairs) on the graph containing the linear isobole. If experiment shows that the desired effect is attained with doses that plot as a point below the isobole (illustrated in the figure as point ABβ) this means that the dose combination is lower than that predicted and, therefore, an exaggerated interaction (synergism) has occurred. In contrast, an experimental point (dose pair) that lies above the isobole (shown as point ABα) means that greater quantities of the combination are required; thus, this denotes an antagonism that is often called ‘subadditive’.
Fig. 1.
Illustration of an isobologram. Drug A alone achieves the specified effect with dose A, whereas drug B requires dose B. These values, shown as the intercepts, define the isobole, which represents all combination dose pairs that are expected to give this effect. An experimental point such as point ABα represents a combination dose pair that is above the line and, hence, doses greater than expected. This interaction is subadditive. Point ABβ, on the other hand, shows that a lesser dose combination was needed to get the effect; this is synergism. This kind of analysis is applicable to both therapeutic and toxic effects.
The isobole methodology is not restricted to two different drugs. It is also applicable to a single agent given at two different routes or sites, e.g. intravenous and oral; spinal and supra-spinal, etc. For example, Raffa et al. (46) administered the analgesic paracetamol to mice by two different routes (spinal and supra-spinal) and applied this methodology to demonstrate site–site synergism. Each site yields its own dose–effect relation (hence potency) and the individual potencies represent the terms denoted A and B that were previously described in the two-drug case. All other aspects of the two-drug case apply to the site–site use as well.
SUMMARY AND PERSPECTIVE
We previously published a review on ‘chemo-fog’ / ’chemo-brain’ in patients who had received cancer chemotherapeutic agents as part of their treatment regimen (1). That publication invited extended discussion and prompted certain additional questions related to the chemo-fog mechanism. Of particular interest was the question (S.J. Harmelin, personal communication) on the possible role of visual impairment as a contributor to chemo-fog. In other words, might some of the effects on cognitive domains be manifestations of, or exacerbations of chemotherapeutic agent-induced toxic effects on visual function? We found, and present here, substantial evidence that many of the commonly used agents do produce such toxicities. Whether typical clinical exposures to individual agents are sufficient to produce overt toxicity is open to further study. However, it seems possible that combinations of these agents might give rise to synergistic visual toxicity (just as they do to synergistic cytotoxic therapeutic efficacy). The contribution of visual system toxicity to the cognitive deficits reported in ‘chemo-fog’ / ’chemo-brain’ deserve further, and more direct, investigation.
ACKNOWLEDGEMENTS
Although this work preceded NIH award CA-129092 to study the possible cognitive deficits in mice of chemotherapeutic agents administered alone or in combination, the positive reviews and notice of that award (E. A. Walker, PI), along with readers’ comments on our previous review, provided an added stimulus to consider the visual system toxicity that is discussed here.
REFERENCES
- 1.Raffa RB, Duong PV, Finney J, et al. Is ‘chemofog’ /’chemo-brain’ caused by cancer chemotherapy? Journal of Clinical Pharmacy and Therapeutics. 2006;31:129–138. doi: 10.1111/j.1365-2710.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 2.Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psycho-Oncology. 1995;4:61–66. [Google Scholar]
- 3.Brezden CB, Phillips K-A, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. Journal of Clinical Oncology. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 4.Schagen SB, van Dam FSAM, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.van Dam FSAM, Schagen SB, Muller MJ, Boogerd W, Wall Evd, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose vs. standard-dose chemotherapy. Journal of the National Cancer Institute. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- 6.Lezak M. Neuropsychological Assessment. 2nd edn. New York: Oxford University Press; 1993. [Google Scholar]
- 7.Delis DC, Kramer JH, Kaplan E, Ober BA. CVLT Research Edition Manual. New York: The Psychological Association; 1987. [Google Scholar]
- 8.Fraunfelder FT, Meyer SM. Ocular toxicity of anineoplastic agents. Ophthalmology. 1983;90:1–3. doi: 10.1016/s0161-6420(83)34600-5. [DOI] [PubMed] [Google Scholar]
- 9.Imperia PS, Lazarus HM, Lass JH. Ocular complications of systemic cancer chemotherapy. Survey of Ophthalmology. 1989;34:209–230. doi: 10.1016/0039-6257(89)90105-7. [DOI] [PubMed] [Google Scholar]
- 10.AL-Tweigeri T, Nabholtz J-M, Mackey JR. Ocular toxicity and cancer chemotherapy. Cancer. 1996;78:1359–1373. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1359::AID-CNCR1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 11.Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Survey of Ophthalmology. 2006;51:19–40. doi: 10.1016/j.survophthal.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Gianni L, Panzini I, Li S, et al. International Breast Cancer Study Group (IBCSG) Ocular toxicity during adjuvant chemoendocrine therapy for early breast cancer: results from International Breast Cancer Study Group trials. Cancer. 2006;106:505–513. doi: 10.1002/cncr.21651. [DOI] [PubMed] [Google Scholar]
- 13.Jack MK, Hicks JD. Ocular complications in high-dose chemoradiotherapy and marrow transplantation. Annals of Ophthalmology. 1981;13:709–711. [PubMed] [Google Scholar]
- 14.Kende G, Sirkin SR, Thomas PR, Freeman AI. Blurring of vision: a previously undescribed complication of cyclophosphamide therapy. Cancer. 1979;44:69–71. doi: 10.1002/1097-0142(197907)44:1<69::aid-cncr2820440113>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Stevens A, Spooner D. Lacrimal duct stenosis and other ocular toxicity associated with adjuvant cyclophosphamide, methotrexate and 5-fluorouracil combination chemotherapy for early stage breast cancer. Clinical Oncology. 2001;13:438–440. doi: 10.1053/clon.2001.9308. [DOI] [PubMed] [Google Scholar]
- 16.Lee V, Bentley CR, Olver JM. Sclerosing canaliculitis after 5-fluorouracil breast cancer chemotherapy. Eye. 1998;12:343–349. doi: 10.1038/eye.1998.83. [DOI] [PubMed] [Google Scholar]
- 17.Esmaeli B, Burnstine MA, Ahmadi MA, Prieto VG. Docetaxel-induced histologic changes in the lacrimal sac and the nasal mucosa. Ophthalmic Plastic and Reconstructive Surgery. 2003;19:305–308. doi: 10.1097/01.IOP.0000075016.29682.E0. [DOI] [PubMed] [Google Scholar]
- 18.Esmaeli B, Hidaji L, Adinin RB, et al. Blockage of the lacrimal drainage apparatus as a side effect of docetaxel therapy. Cancer. 2003;98:504–507. doi: 10.1002/cncr.11527. [DOI] [PubMed] [Google Scholar]
- 19.Vizel M, Oster MW. Ocular side effects of cancer chemotherapy. Cancer. 1982;49:1999–2002. doi: 10.1002/1097-0142(19820515)49:10<1999::aid-cncr2820491009>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 20.Rahl AHS, Hungerford JL, Ahmed AI. Ocular toxicity of desferrioxamine: light microscopic histochemical and ultrastructural findings. British Journal of Ophthalmology. 1988;70:373–381. doi: 10.1136/bjo.70.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voest EE, Neijt JP, Keunen JE, et al. Phase I study using desferrioxamine and iron sorbitol citrate in an attempt to modulate the iron status of tumor cells to enhance doxorubicin activity. Cancer Chemotherapy and Pharmacology. 1993;31:357–362. doi: 10.1007/BF00686148. [DOI] [PubMed] [Google Scholar]
- 22.Khaw PT, Sherwood MB, MacKay SL, Rossi MJ, Schultz G. Five-minute treatments with fluorouracil, floxuridine, and mitomycin have long-term effects on human Tenon’s capsule fibroblasts. Archives of Ophthalmology. 1992;110:1150–1154. doi: 10.1001/archopht.1992.01080200130040. [DOI] [PubMed] [Google Scholar]
- 23.Stern WH, Guerin CJ, Erickson PA, Lewis GP, Anderson DH, Fisher SK. Ocular toxicity of fluorouracil after vitrectomy. American Journal of Ophthalmology. 1983;96:43–51. doi: 10.1016/0002-9394(83)90453-1. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal MR, Esmaeli B, Burnstine MA. squamous metaplasia of the canaliculi associated with 5-fluorouracil: a clinicopathologic case report. Ophthalmology. 2002;109:2359–2361. doi: 10.1016/s0161-6420(02)01290-3. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro MS, Thoft RA, Friend J, Parrish RK, Gressel MG. 5-fluorouracil toxicity to the ocular surface epithelium. Investigative Ophthalmology and Visual Science. 1985;26:580–583. [PubMed] [Google Scholar]
- 26.Brink HM, Beex LV. Punctal and canalicular stenosis associated with systemic fluorouracil therapy. Documenta Ophthalmologica. 1995;90:1–6. doi: 10.1007/BF01203288. [DOI] [PubMed] [Google Scholar]
- 27.Prasad S, Kamath GG, Phillips RP. Lacrimal canalicularstenosisassociatedwithsystemic5-fluorouracil therapy. ActaOphthalmologicaScandinavica. 2000;78:110–113. [Google Scholar]
- 28.Bixenman WW, Nicholls JVV, Warwick OH. Oculomotor disturbances associated with 5-fluorouracil chemotherapy. American Journal of Ophthalmology. 1968;83:604–608. doi: 10.1016/0002-9394(77)90904-7. [DOI] [PubMed] [Google Scholar]
- 29.Sato Y, Morita M, Takahashi HO, Watanabe N, Kirikae I. Combined surgery, radiotherapy, and regional chemotherapy in carcinoma of the paranasal sinuses. Cancer. 1970;25:571–579. doi: 10.1002/1097-0142(197003)25:3<571::aid-cncr2820250312>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Fishman ML, Bean SC, Cogan DG. Optic atrophy following prophylactic chemotherapy and cranial radiation for acute lymphocytic leukemia. American Journal of Ophthalmology. 1976;82:571–576. doi: 10.1016/0002-9394(76)90544-4. [DOI] [PubMed] [Google Scholar]
- 31.Margileth DA, Poplack DG, Pizzo PA, Leventhal BG. Blindness during remission in two patients with acute lymphoblastic leukemia: a possible complication of multimodality therapy. Cancer. 1977;39:58–61. doi: 10.1002/1097-0142(197701)39:1<58::aid-cncr2820390111>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Boogerd W, Moffie D, Smets LA. Early blindness and coma during intrathecal chemotherapy for meningeal carcinomatosis. Cancer. 1990;65:452–457. doi: 10.1002/1097-0142(19900201)65:3<452::aid-cncr2820650313>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 33.Carter WH, Jr, Wampler GL, Stablein DM, Campbell ED. Drug activity and therapeutic synergism in cancer treatment. Cancer Research. 1982;42:2963–2971. [PubMed] [Google Scholar]
- 34.Greco WR, Park HS, Rustum YM. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-β-D-arabinofuranosylcytosine. Cancer Research. 1990;50:5318–5327. [PubMed] [Google Scholar]
- 35.Raymond E, Djelloul S, Buquet-Fagot C, Mester J, Gespach C. Synergy between the non-classical thymidylate synthase inhibitor AG337 (Thymitaq) and cisplatin in human colon and ovarian cancer cells. Anti-Cancer Drugs. 1996;7:752–757. doi: 10.1097/00001813-199609000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh TC, Wu JM. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen receptor-positive MCF-7 breast cancer cells. International Journal of Oncology. 2008;33:851–859. [PubMed] [Google Scholar]
- 37.Shuhendler AJ, O’Brien PJ, Rauth AM, Wu XY. On the synergistic effect of doxorubicin and mitomycin C against breast cancer cells. Drug Metabolism and Drug Interactions. 2007;22:201–233. doi: 10.1515/dmdi.2007.22.4.201. [DOI] [PubMed] [Google Scholar]
- 38.Pentheroudakis G, Razis E, Athanassiadis A, Pavlidis N, Fountzilas G. Paclitaxel–carboplatin combination chemotherapy in advanced breast cancer: accumulating evidence for synergy, efficacy, and safety. Medical Oncology. 2006;23:147–160. doi: 10.1385/MO:23:2:147. [DOI] [PubMed] [Google Scholar]
- 39.McGill TJ, Douglas RM, Lund RD, Prusky GT. Quantification of spatial vision in the Royal College of Surgeons rat. Investigative Ophthalmology and Visual Science. 2004;45:932–936. doi: 10.1167/iovs.03-0964. [DOI] [PubMed] [Google Scholar]
- 40.Tallarida RJ, Jacob LS. The Dose–Response Relation in Pharmacology. New York: Springer-Verlag; 1979. [Google Scholar]
- 41.Tallarida RJ, Murray RB. Manual of Pharmacologic Calculations with Computer Programs. 2nd edn. New York: Springer-Verlag; 1987. [Google Scholar]
- 42.Tallarida RJ, Raffa RB, McGonigle P. Principles in General Pharmacology. New York: Springer-Verlag; 1988. [Google Scholar]
- 43.Tallarida RJ. DrugSynergism and Dose–Effect Data Analysis. Boca Raton, FL: CRC/Chapman-Hall; 2000. [Google Scholar]
- 44.Tallarida RJ. Perspectives in pharmacology: an overview of drug combination analysis with isobolograms. Journal of Pharmacology and Experimental Therapeutics. 2006;319:1–7. doi: 10.1124/jpet.106.104117. [DOI] [PubMed] [Google Scholar]
- 45.Tallarida RJ. Interactions between drugs and occupied receptors. Pharmacology and Therapeutics. 2007;113:197–209. doi: 10.1016/j.pharmthera.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raffa RB, Stone DJ, Tallarida RJ. Discovery of self-synergistic spinal/supraspinal antinociception produced by acetaminophen (paracetamol) Journal of Pharmacology and Experimental Therapeutics. 2000;295:291–294. [PubMed] [Google Scholar]