Abstract
Aims
In newly diagnosed type 2 diabetes mellitus (T2DM) patients, short-term insulin therapy might improve β-cell function and glycemic control. This study aimed to compare the effects of basal insulin monotherapy with continuous subcutaneous insulin infusion (CSII) treatment.
Methods
Fifty-nine cases of newly diagnosed T2DM patients with fasting plasma glucose of 9.0–16.7 mmol/L were recruited into this study. They were hospitalized and randomly assigned to a basal insulin monotherapy group (n=27) or a CSII group (n=32). Insulin dosage was titrated according to fasting capillary blood glucose levels, and treatment was stopped after 2 weeks. Intravenous glucose tolerance tests were performed, and blood glucose, insulin, C-peptide, and lipid profiles were measured before therapy and 2 days after therapy withdrawal.
Results
Both treatments reduced fasting and postprandial blood glucose levels (after treatment vs. baseline, both P<0.05). Fasting glycemic control target was achieved in 52 cases (88.14%) with 2 weeks of insulin treatment, and there were no significant differences between the glargine and CSII groups (P=0.059). The time to achieve fasting glycemic target in the CSII group was shorter than that in the glargine group (P<0.01). Plasma lipid profiles such as triglycerides and total cholesterol also decreased significantly after the intervention. Overall β-cell function improved significantly after insulin intervention (P<0.01). Variation did not differ between two groups, nor did the effects on insulin and C-peptide secretion (P>0.05).
Conclusions
The effect of basal insulin monotherapy was similar to that of CSII, and thus basal insulin monotherapy might be a reasonable alternative to CSII for initial insulin therapy in newly diagnosed T2DM patients.
Introduction
Insulin resistance and progressive β-cell deterioration are the two main pathophysiological mechanisms of type 2 diabetes mellitus (T2DM).1,2 The UK Prospective Diabetes Study has shown that β-cell function begins to deteriorate before type 2 diabetes is diagnosed and often occurs years before patients demonstrate clinical symptoms.3,4 In most T2DM patients, metabolic control and β-cell function progressively deteriorate as the duration of diabetes increases, and this deterioration can be explained by decreased insulin secretion by β-cells.1,2,4 As β-cell function declines, most patients will ultimately need insulin therapy, and insulin treatment could be of benefit as it provides relative “β-cell rest.” At present, insulin is the most important drug available to achieve tight glycemic control; however, when and how to initiate insulin treatment in T2DM still remain controversial.
Because the loss of acute insulin response (AIR) is a quite common defect in the pathogenesis of newly diagnosed T2DM, it may require specific therapeutic intervention.3 Several clinical trials in different ethnic population have suggested that early insulin treatment could preserve endogenous insulin secretion and improve metabolic control.5–9 Our previous study has also shown that short-term intensive insulin therapy can induce long-term glycemic control in newly diagnosed T2DM patients with severe hyperglycemia. The improvement of β-cell function, especially the restoration of first-phase insulin secretion, could be responsible for the remission.10,11
However, insulin is often withheld from people with T2DM until they are unresponsive to a combination of lifestyle changes and one or more oral antidiabetes drugs (OADs). This is partly due to physicians' and patients' psychological resistance to initiation of insulin,12 the risk of hypoglycemia, and concern about insulin injection or restrictions of lifestyle. With T2DM becoming a major chronic disease and a global health disaster, it forces us to come up with an ideal strategy to cope with it. The latest national survey in China showed the prevalence of total diabetes was 9.7%, which amounts to nearly 92.4 million Chinese adults suffering from diabetes.13 Although clinical guidelines recommend target glycosylated hemoglobin (HbA1c) values of<7% (European Association for the Study of Diabetes criterion) for persons with diabetes, unfortunately less than one-third of patients globally can achieve this target.14,15 Failure to reach glycemic target may be partly explained by two reasons: one is the progressive deterioration in the natural course of β-cell function,1,2 and the other is a delay in initiating insulin therapy.15
Moreover, in the previous studies, continuous subcutaneous insulin infusion (CSII) or multiple daily insulin injections were used as initial insulin treatment protocols, which are expensive and complex and could not be widely adopted in clinical practice, especially for outpatients. Therefore, we performed this randomized, open-label, parallel-group trial of two short-term insulin therapy programs with basal insulin monotherapy or CSII treatment. The aim of this observational study was to compare the effects of two insulin therapy programs on glycemic control and β-cell function in patients with newly diagnosed T2DM.
Subjects and Methods
Patients
Fifty-nine patients (30 men and 29 women) at The Third Affiliated Hospital of Sun Yat-sen University (Guangzhou, Guangdong, China) hospitalized with newly diagnosed T2DM (fasting plasma glucose [FPG], 9.0–16.7 mmol/L) from November 2007 to December 2008 were recruited into the study. Diabetes mellitus was diagnosed according to the criteria published by the World Health Organization in 1999. The patients had no history of using any antihyperglycemic drugs and were negative for anti–glutamic acid decarboxylase antibodies. Patients were excluded from the trial if they were unlikely to adhere to the protocol and if they had acute or severe chronic diabetes complications or severe intercurrent illness or were pregnant. This work was approved by the ethical committees of the Third Affiliated Hospital of Sun Yat-sen University, and all subjects gave informed consent.
Study design and treatment
After 2 weeks of diet control and run-in, patients were hospitalized and assigned to a basal insulin monotherapy group (n=27) or a CSII group (n=32) according to the random number method. Patients in the basal insulin monotherapy group received insulin glargine (Lantus®, Sanofi-Aventis, Paris, France) injection once daily at bedtime. Patients in the CSII group received rapid-acting insulin analog (insulin Aspart®, Novo Nordisk, Bagsværd, Denmark) as basal and prandial insulin with an insulin pump (Paradigm® 712, Medtronic, Minneapolis, MN). Total initial insulin doses were 0.3 IU/kg for the basal insulin group and 0.5 IU/kg in the CSII group (including basal and boluses insulin, as usual). The doses were titrated every day in order to achieve the fasting glycemic target, which was defined as fasting capillary blood glucose varying between 4.4 mmol/L and 6.1 mmol/L, regardless of the patients' postprandial blood glucose levels. During the intervention, their capillary blood glucose was monitored by nurses using a blood glucometer (OneTouch® Ultra®, LifeScan, Milpitas, CA) at least seven times per day, including fasting, before lunch/dinner, 2 h postprandial (after breakfast, lunch, and supper), and at bedtime. A continuous glucose monitoring system (CGMS® Gold™, Medtronic) was used in 18 cases (eight cases in the basal insulin group and 10 cases in the CSII group). Intervention was stopped after 2 weeks. After interventions were stopped, patients were instructed to continue diet and physical exercise or received OAD treatment according to their plasma glucose levels. Hypoglycemia was defined as capillary blood glucose ≤3.9 mmol/L with or without clinical symptoms. The primary end point was the time when fasting capillary blood glucose reached the target; the second end point was the effect of different insulin interventions on β-cell function and glycemic control in these patients.
Assessments
Before and after treatment, fasting blood samples were obtained after an overnight fasting for measurement of glucose, HbA1c, and lipid levels. Before intervention and 2 days after insulin cessation, all patients received an intravenous glucose tolerance test (IVGTT) using 25 g of glucose (50 mL of 50% glucose), and serum samples were obtained prior to and 1, 2, 4, 6, and 10 min after the intravenous glucose load to measure insulin and C-peptide concentrations.
Routine clinical biochemical analyses were performed in the hospital laboratory. FPG was measured by the glucose oxidase method. Plasma triglycerides, total cholesterol, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol were measured by the oxides method with an automatic biochemistry instrument. HbA1c was assayed using the Bio-Rad (Hercules, CA) Variant HbA1c assay. Plasma insulin and C-peptide levels were measured using a chemiluminescence enzyme-linked immunosorbent assay kit (Bioekon Inc., Beijing, China). The first-phase β-cell insulin secretion, which was calculated as the incremental trapezoidal area under the curve (AUC) for insulin and C-peptide during the first 10 min of the IVGTT, was used to assess the AIR. β-Cell function and insulin resistance were assessed by homeostasis model (HOMA-B and HOMA-IR, respectively) according to the following equations: HOMA-B=(20×fasting insulin)/(FPG−3.5) and HOMA-IR=(FPG×fasting insulin)/22.5.
Statistical analyses
Normally distributed and quantitative data were expressed as mean±SD values. The experimental variables (triglycerides, very-low-density lipoprotein-cholesterol, insulin, and C-peptide) that were not normally distributed were expressed as median±interquartile range values and log-transformed to improve data normality. Quantitative variable differences between the two groups were compared by Student's t tests; the differences before and after treatment were analyzed with sample-paired Student's t test. The χ2 test was performed to compare for discrete variables. All statistical analyses were conducted by using the SPSS version 13.0 statistical package (SPSS, Inc., Chicago, IL). A value of P<0.05 was considered statistically significant.
Results
Patients studied
A total of 59 patients completed this research. None dropped out, and no serious adverse effects were observed during the intervention. Demographic and metabolic baseline characteristics of the two groups are summarized in Table 1. The two groups were well balanced, with HbA1c averaging 10.85% and FPG averaging 12.9 mmol/L. There were no significant differences in age, sex, or plasma lipid profiles between the two groups.
Table 1.
Demographic and Metabolic Baseline Characteristics of the Two Patient Groups
| Variable | Basal insulin group | CSII group | Total |
|---|---|---|---|
| Male/female (n) | 12/15 | 18/14 | 30/29 |
| Age (years) | 52.72±8.54 | 48.90±10.67 | 50.37±9.82 |
| BMI (kg/m2) | 25.11±2.54 | 25.73±3.91 | 25.46±3.12 |
| FPG (mmol/L) | 13.27±3.80 | 12.47±3.7 | 12.88±3.76 |
| HbA1c (%) | 10.78±2.57 | 10.93±2.23 | 10.85±2.40 |
| Triglycerides (mmol/L)a | 3.07±1.78 | 2.92±1.16 | 3.04±1.21 |
| Total cholesterol (mmol/L) | 5.89±1.03 | 5.65±1.08 | 5.76±0.94 |
| LDL-C (mmol/L) | 3.57±0.94 | 3.55±0.93 | 3.56±0.94 |
| HDL-C (mmol/L) | 1.09±0.26 | 1.15±0.62 | 1.12±0.45 |
| AIR (μIU/mL/min)a | 0.13±2.96 | −1.09±4.46 | −0.76±2.52 |
| AUCIns (μIU/mL/min)a | 104.75±44.98 | 114.89±54.42 | 108.69±50.27 |
| AUCCpep (pmol/mL/min)a | 6.22±1.65 | 6.96±2.72 | 6.67±2.13 |
| HOMA-Ba | 26.7±19.0 | 30.4±18.7 | 28.72±18.86 |
| HOMA-IRa | 6.02±2.37 | 6.68±2.97 | 6.38±2.65 |
Data are mean±SD unless otherwise indicated.
Data are median±IQR values. P>0.05 compared with two groups.
AIR, acute insulin response; AUCIns and AUCCpep, area under the curve for insulin and C-peptide, respectively; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HOMA-B and HOMA-IR, β-cell function and insulin resistance, respectively, which were assessed by homeostasis model according to the equations HOMA-B=(20×fasting insulin)/(FPG−3.5) and HOMA-IR=(FPG×fasting insulin)/22.5; LDL-C, low-density lipoprotein cholesterol.
Glycemic control and plasma lipid improvement
Table 2 depicts the variations in glycemic and plasma lipid profiles after two different insulin treatments. Among these patients, 52 cases achieved the glycemic control target, and there was no statistical difference between the two groups (88.89% in the basal insulin monotherapy group and 87.50% in the CSII group, P=0.059). The time to achieve glycemic target in the CSII group was shorter than that in the basal insulin group (3.8±1.9 days vs. 5.4±1.4 days), and the difference was statistically significant (P<0.01). The lower rate of fasting glucose levels was similar between the two groups (7.61±3.72 vs. 6.37±3.93 mmol/L, P>0.05). There was a great decrease in 2-h postprandial capillary blood glucose in both groups (before vs. after treatment, P<0.001), but slightly more in the CSII group (11.1±3.7 mmol/L vs. 9.1±3.4 mmol/L, P=0.049). Figure 1 depicts dynamic changes in fasting and daily average 2-h postprandial capillary blood glucose. From Figure 1 we can see that 2 weeks of insulin therapy could significantly decrease fasting and postprandial blood glucose. In addition, there was no difference in fasting capillary glucose levels after insulin treatment (5.66±1.09 vs. 5.89±1.22 mmol/L, P>0.05), but patients in the CSII group had lower postprandial blood glucose levels (6.2±0.9 vs. 10.2±2.7 mmol/L, P<0.001). HbA1c decreased nearly 1% in 2 weeks, and there was no significant difference between the two groups.
Table 2.
Data After Treatment
| Basal insulin group | CSII group | P value | |
|---|---|---|---|
| n | 27 | 32 | NS |
| FBG (mmol/L) | |||
| After treatment | 5.66±1.09** | 5.89±1.22** | 0.157 |
| Change | −7.61±3.72 | −6.37±3.93 | 0.222 |
| 2-h PBG (mmol/L) | |||
| After treatment | 10.2±2.7* | 6.2±0.9** | <0.001 |
| Change | −9.1±3.4 | −11.1±3.7 | 0.049 |
| HbA1c (%) | |||
| After treatment | 9.91±1.95* | 10.03±1.91* | 0.554 |
| Change | −1.06±0.66 | −0.84±0.57 | 0.196 |
| Triglycerides (mmol/L)a | |||
| After treatment | 5.14±0.92* | 4.79±0.75** | 0.137 |
| Change | −0.76±0.82 | −0.66±0.62 | 0.638 |
| Total cholesterol (mmol/L) | |||
| After treatment | 1.74±0.83** | 1.81±0.65** | 0.725 |
| Change | −1.33±1.57 | −0.80±0.97 | 0.143 |
| LDL-C (mmol/L) | |||
| After treatment | 3.42±0.68 | 3.09±0.78* | 0.198 |
| Change | −0.12±0.61 | −0.47±0.76 | 0.035 |
| HDL-C (mmol/L) | |||
| After treatment | 1.17±0.27 | 1.19±0.59 | 0.886 |
| Change | −0.07±0.22 | −0.04±0.20 | 0.624 |
| AIR (μIU/mL/min)a | |||
| After treatment | 6.75±5.85 | 7.18±12.94 | 0.202 |
| Change | 6.62±6.78 | 8.27±13.55 | 0.08 |
| AUCIns (μIU/mL/min)a | |||
| After treatment | 205.22±89.23** | 205.30±143.98** | 0.998 |
| Change | 100.47±91.66 | 90.41±122.87 | 0.733 |
| AUCCpep (pmol/mL/min)a | |||
| After treatment | 6.93±1.84* | 8.01±2.72* | 0.092 |
| Change | 0.71±1.60 | 1.06±1.18 | 0.350 |
| HOMA-Ba | |||
| After treatment | 164.3±90.9** | 141.9±113.1** | 0.412 |
| Change | 137.63±87.38 | 101.52±118.62 | 0.196 |
| HOMA-IRa | |||
| After treatment | 3.59±1.66** | 3.85±2.09** | 0.593 |
| Change | −2.44±2.82 | −2.83±2.82 | 0.597 |
| Days of achieving euglycemia (days) | 5.4±1.4 | 3.8±1.9 | 0.002 |
Data are mean±SD values unless otherwise indicated.
Data are median±interquartile values.
Compared with baseline, *P<0.05, **P<0.01.
Compared between the two groups, P values are in boldface if there was a statistical difference.
AIR, acute insulin response; AUCIns and AUCCpep, area under the curve for insulin and C-peptide, respectively; BMI, body mass index; CSII, continuous subcutaneous insulin infusion; FPG, fasting plasma glucose; HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; HOMA-B and HOMA-IR, β-cell function and insulin resistance, respectively, which were assessed by homeostasis model according to the equations HOMA-B=(20×fasting insulin)/(FPG−3.5) and HOMA-IR=(FPG×fasting insulin)/22.5; LDL-C, low-density lipoprotein cholesterol; NS, not significant; 2-h PBG, 2-h postprandial blood glucose.
FIG. 1.
Dynamic changes of capillary blood glucose with time in two insulin treatment schemes: (A) fasting blood glucose (FBG) and (B) 2-h postprandial blood glucose (PBG). FBG levels compared between the two groups were not significantly different (P>0.05); 2-h PBG levels compared between the two groups were significantly different (*P<0.05, **P<0.01). CSII, continuous subcutaneous insulin infusion.
Plasma lipid profiles and triglyceride and total cholesterol levels were decreased greatly after insulin treatment (P<0.01) in both groups, and there was no significant difference between the two groups. Plasma low-density lipoprotein-cholesterol levels were significantly lowered in the CSII group (3.55±0.93 vs. 3.09±0.78 mmol/L, P<0.05), whereas in the glargine group they were lowered, but without statistical difference (3.57±0.94 vs. 3.42±0.68 mmol/L, P>0.05). Plasma high-density lipoprotein-cholesterol levels increased somewhat, but no significant difference was found before and after insulin treatment.
Effects on β-cell function
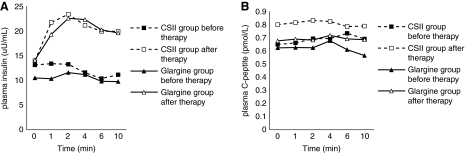
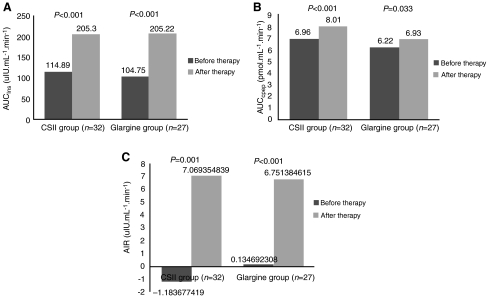
Figure 2 depicts the variations of plasma insulin and C-peptide changes during IVGTT before and after insulin treatment. Both were elevated after insulin treatment (P<0.05), but there was no statistical difference between the two groups (P>0.05). From Figure 3 we can see that the AIR, represented by AUC for insulin and AUC for C-peptide, was low in all patients before treatment (Fig. 3C). After 2 weeks of insulin therapy, AIR increased significantly (P<0.001) (Fig. 3), indicating that β-cell function was partially recovered in most patients. HOMA-B improved greatly after insulin therapy, and HOMA-IR declined significantly in both groups (P<0.001) (Table 2). No differences were found between the two groups before and after insulin treatment.
FIG. 2.
Changes in (A) plasma fasting insulin and (B) C-peptide during intravenous glucose tolerance test before and after insulin treatment. Both groups' plasma fasting insulin and C-peptide levels were elevated after insulin treatment, compared with before (P<0.05), and there was no statistical difference between the two groups (P>0.05). CSII, continuous subcutaneous insulin infusion.
FIG. 3.
(A) Area under the curve for insulin (AUCIns), (B) area under the curve for C-peptide (AUCCpep), and (C) acute insulin response (AIR) in the two groups before and after insulin therapy. P<0.01 before versus after insulin therapy in each group, P>0.05 between the two groups both before and after insulin therapy.
Insulin dosing
The daily dose of insulin was recorded every day. The maximum dose and final daily dose of insulin mean, respectively, the maximum dosing of insulin during the intervention and the final dose of insulin before the intervention stopped. These data are shown in Table 3. From these data, we can see both the maximum and final daily insulin doses were less in the basal insulin monotherapy group than in the CSII group.
Table 3.
Insulin Dose in Each Group
| Insulin dose | Basal insulin group | CSII group | P value |
|---|---|---|---|
| Maximum daily | |||
| IU | 42.7±10.2 | 59.3±17.1 | 0.001 |
| IU/kg | 0.66±0.15 | 0.89±0.26 | 0.003 |
| Final daily | |||
| IU | 35.5±10.9 | 48.1±17.5 | 0.009 |
| IU/kg | 0.55±0.21 | 0.72±0.31 | 0.025 |
| Total | |||
| IU daily | 38.6±10.5 | 54.7±17.3 | 0.002 |
| IU/kg | 0.59±0.18 | 0.81±0.29 | 0.007 |
CSII, continuous subcutaneous insulin infusion.
Adverse side effects
During the insulin treatment period, no serious adverse side effects were observed in either group. There were no changes in liver and renal function before and after treatment. The most commonly reported adverse events were hypoglycemic episodes. No severe hypoglycemic episodes, which were defined as an event requiring the assistance of another person, occurred during the intervention. A higher prevalence of minor hypoglycemic episodes or classical symptoms of hypoglycemia was observed in the CSII group (31.25% in CSII vs. 22.22% in the basal insulin group, P<0.05), but the basal insulin group had a higher incidence of nocturnal hypoglycemia (11.11% vs. 3.13%, P<0.01). About 56.25% of hypoglycemic episodes were asymptomatic and were detected by CGMS (60% in CSII vs. 50% in the basal insulin group). No obvious weight gain was observed in either group after insulin therapy. Mild injection site reactions (erythema, edema, and pain) were observed, but there was no significant difference between the two groups.
Discussion
In the present study, we compared the effects of basal insulin monotherapy and CSII therapy in newly diagnosed T2DM patients. We found that after 2 weeks of short-term insulin therapy, 88.14% of patients achieved fasting glycemic control target, which was defined as fasting capillary blood glucose varying from 4.4 mmol/L to 6.0 mmol/L. The average postprandial plasma glucose concentrations also declined greatly in both groups, and HbA1c decreased nearly 1%, with no significant difference between the two groups. Compared with those in the CSII group, patients in the basal insulin group achieved the same glycemic control target and β-cell function restoration with a lower insulin dose. Moreover, their β-cell function, especially AIR, was greatly improved, irrespective of the use of CSII or basal insulin monotherapy. HOMA-B and HOMA-IR were also improved after short-term insulin intervention. These results are consistent with our previous study.11
Basal insulin therapy is recommended to initiate insulin treatment in patients with T2DM whose glycemia is inadequately controlled by diet/exercise and OADs.15,16 Insulin glargine is a long-acting human insulin analog with a longer time–action profile and no pronounced peak of action compared with neutral protamine Hagedorn (NPH) insulin.17,18 In light of these advantages, insulin glargine has taken the place of NPH insulin for use as basal insulin. In this trial, we used it as monotherapy in newly diagnosed T2DM patients and compared its effect with CSII as the initial insulin therapy schema. We found basal insulin monotherapy and CSII had the same effects on fasting glycemic control and β-cell function improvement. We also found that the dose of insulin to reach the same end point was lower in basal insulin monotherapy. Moreover, it is more convenient to use—especially in outpatients, who have more psychological resistance to insulin (which is the greatest obstacle to the initiation of insulin).12,19
The advantage of insulin treatment on glycemic control and β-cell function improvement in diabetes has been fully confirmed.7–11,20 All those trials used CSII or multiple daily insulin injections as initial insulin intervention and attributed the recovery of β-cell function to insulin replacement allowing β-cell rest. Considering the importance of insulin treatment and FPG, each of which has a close relationship with the first-phase insulin secretion, in the present study we used only basal insulin as supplement monotherapy and only controlled fasting blood glucose levels. We found the same effect on the improvement of β-cell function and glycemic control. This observation suggests again that the damage to β-cell function in newly diagnosed T2DM patients can be reversible through any kind of early glycemic control by insulin, whether it is CSII, multiple daily insulin injections, or basal insulin monotherapy. It is notable that the plasma lipid profile and HOMA-IR were also improved after short-term insulin therapy without using lipid-adjusting agents. Glucolipotoxicity generated by hyperglycemia and related pathogenesis factors can damage β-cell function. By eliminating these damaging effects, early insulin therapy could reduce β-cell stress, improve endogenous insulin processing, and restore the injured β-cells' function.2,3,21 Obviously, the metabolic effect of any basal insulin preparation on fasting blood glucose level is supported by endogenous insulin secretion in individuals with T2DM. In addition, insulin glargine reduced β-cell stress more effectively because of its longer-lasting pharmacodynamic profile.22 Other insulin effects such as reducing inflammation and decreasing ectopic lipid deposition might also contribute to the recovery of overall β-cell function in T2DM patients.23,24
In addition, our results indicated that fasting blood glucose level was the main risk factor affecting β-cell function. In vitro experiments have shown that the first-phase insulin secretion of β-cells was damaged or vanished when the fasting blood glucose level was above 97 or 100 mg/dL, respectively.25,26 With the increasing of fasting blood glucose levels, β-cell mass decreases.27 Tfayli et al.28 observed a total of 223 youths with FPG<126 mg/dL who underwent evaluation of first- and second-phase insulin secretion during a 2-h hyperglycemic clamp and found that the impairment in β-cell function relative to insulin sensitivity was apparent even within the FPG range of those without diabetes; at the current cutoff of 100 mg/dL for impaired fasting glucose, there is an approximately 49% decline in the glucose disposition index.28 Subsequently, some experts have suggested that the target of fasting blood glucose control should be less than 100 mg/dL.
The present study does, however, have some limitations that need to be discussed. First, this was a short-term open-label study, and the favorable clinical benefits observed in this trial should be tested through long-term follow-up studies. Second, this was an observational study that had relatively straightforward aims; in this trial, we primarily paid attention to the patients' fasting blood glucose and less so to their postprandial blood glucose levels, whereas strict clinical guidelines would stipulate giving equal attention to both. Third, the results only apply to lean patients with T2DM with poor baseline glycemic control because the β-cell function of these patients exhibits obvious deterioration and insulin therapy can provide β-cell rest. However, in obese and mild T2DM patients, the most important concern is insulin resistance, so drugs that can improve insulin resistance, such as metformin, might be more suitable for them. Despite all these limitations, the significance of this study should not be ignored. The results suggest that basal insulin monotherapy could have similar effects to CSII. And, considering its importance and limitations, the results of this study should be reproducible in the vast majority of T2DM patients with similar characteristics.
In summary, compared with CSII, basal insulin monotherapy had a similar effect on glycemic control and β-cell function improvement in newly diagnosed T2DM patients. Therefore basal insulin monotherapy might be a reasonable alternative to CSII for initial insulin therapy in newly diagnosed T2DM patients.
Acknowledgments
This work was funded by the Social Development Foundation of Guangdong Province, China (grant number 2009B030801167). The authors are grateful to all the patients, without whom this study could not have been conducted. Specifically, we thank Prof. Jianping Weng for his constructive suggestions. We also acknowledge the efforts of staff members of our hospital for their efforts in this study.
Author Disclosure Statement
All authors disclose that there are no institutional or commercial affiliations that might pose a conflict of interest regarding the publication of this manuscript.
References
- 1.Reaven GM. HOMA-beta in the UKPDS and ADOPT. Is the natural history of type 2 diabetes characterized by a progressive and inexorable loss of insulin secretory function? Maybe? Maybe not? Diabtes Vasc Dis Res. 2009;6:133–138. doi: 10.1177/1479164109336038. [DOI] [PubMed] [Google Scholar]
- 2.Wajchenberg BL. Clinical approaches to preserve beta-cell function in diabetes. Adv Exp Med Biol. 2010;654:515–535. doi: 10.1007/978-90-481-3271-3_23. [DOI] [PubMed] [Google Scholar]
- 3.Wajchenberg BL. β-Cell failure in diabetes and preservation by clinical treatment. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- 4.Stratton IM. Adler AI. Neil HA. Matthews DR. Manley SE. Cull CA. Hadden D. Turner RC. Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35) BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS33) UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;52:837–853. Erratum in Lancet 1999;354:602. [PubMed] [Google Scholar]
- 6.Holman RR. Paul SK. Bethel MA. Neil HA. Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–1576. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 7.Ryan EA. Imes S. Wallace C. Short-term intensive insulin therapy in newly diagnosed type 2 diabetes. Diabetes Care. 2004;27:1028–1032. doi: 10.2337/diacare.27.5.1028. [DOI] [PubMed] [Google Scholar]
- 8.Ilkova H. Glaser B. Tunckale A. Bagriaçik N. Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- 9.McFalane SI. Chaiken RL. Hirsch S. Harrington P. Lebovitz HE. Banerji MA. Near-normoglycaemic remission in African-Americans with type 2 diabetes mellitus is associated with recovery of beta cell function. Diabet Med. 2001;18:10–16. doi: 10.1046/j.1464-5491.2001.00395.x. [DOI] [PubMed] [Google Scholar]
- 10.Li Y. Xu W. Liao Z. Yao B. Chen X. Huang Z. Hu G. Weng J. Induction of long-term glycaemic control in newly diagnosed type 2 diabetic patients is associated with improvement of beta-cell function. Diabetes Care. 2004;27:2597–2602. doi: 10.2337/diacare.27.11.2597. [DOI] [PubMed] [Google Scholar]
- 11.Weng J. Li Y. Xu W. Shi L. Zhang Q. Zhu D. Hu Y. Zhou Z. Yan X. Tian H. Ran X. Luo Z. Xian J. Yan L. Li F. Zeng L. Chen Y. Yang L. Yan S. Liu J. Li M. Fu Z. Cheng H. Effect of intensive insulin therapy on β-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomized parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- 12.Brod M. Kongsø JH. Lessard S. Christensen TL. Psychological insulin resistance: patient beliefs and implications for diabetes management. Qual Life Res. 2009;18:23–32. doi: 10.1007/s11136-008-9419-1. [DOI] [PubMed] [Google Scholar]
- 13.Yang W. Lu J. Weng J. Jia W. Ji L. Xiao J. Shan Z. Liu J. Tian H. Ji Q. Zhu D. Ge J. Lin L. Chen L. Guo X. Zhao Z. Li Q. Zhou Z. Shan G. He J. China National Diabetes and Metabolic Disorders Study Group:Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362:1090–1101. doi: 10.1056/NEJMoa0908292. [DOI] [PubMed] [Google Scholar]
- 14.Saydah SH. Fradkin J. Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 15.Nathan DM. Buse JB. Davidson MB. Ferrannini E. Holman RR. Sherwin R. Zinman B. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203. doi: 10.2337/dc08-9025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan DM. Buse JB. Davidson MB. Heine RJ. Holman RR. Sherwin R. Zinman B. Management of hyperglycaemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. Diabetes Care. 2006;29:1963–1972. doi: 10.2337/dc06-9912. [DOI] [PubMed] [Google Scholar]
- 17.Lepore M. Pampanelli S. Fanelli C. Porcellati F. Bartocci L. Di Vincenzo A. Cordoni C. Costa E. Brunetti P. Bolli GB. Pharmacokinetics and pharmacodynamics of subcutaneous injection of long-acting human insulin analog glargine, NPH insulin, and ultralente human insulin and continuous subcutaneous infusion of insulin lispro. Diabetes. 2000;49:2142–2148. doi: 10.2337/diabetes.49.12.2142. [DOI] [PubMed] [Google Scholar]
- 18.Porcellati F. Bolli GB. Fanelli CG. Pharmacokinetics and pharmacodynamics of basal insulins. Diabetes Technol Ther. 2011;13(Suppl 1):S15–S24. doi: 10.1089/dia.2011.0038. [DOI] [PubMed] [Google Scholar]
- 19.Nam S. Chesla C. Stotts NA. Kroon L. Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33:1747–1749. doi: 10.2337/dc10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Retnakaran R. Yakubovich N. Qi Y. Opsteen C. Zinman B. The response to short-term intensive insulin therapy in type 2 diabetes. Diabetes Obes Metab. 2010;12:65–71. doi: 10.1111/j.1463-1326.2009.01129.x. [DOI] [PubMed] [Google Scholar]
- 21.Poitout V. Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forst T. Larbig M. Hohberg C. Forst S. Diessel S. Borchert M. Roth W. Pfützner A. Adding insulin glargine vs. NPH insulin to metformin results in a more efficient postprandial beta-cell protection in individuals with type 2 diabetes. Diabetes Obes Metab. 2010;12:437–441. doi: 10.1111/j.1463-1326.2010.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dandona P. Aljada A. Mohanty P. Ghanim H. Hamouda W. Assian E. Ahmad S. Insulin inhibits intranuclear nuclear factor κB and stimulates IκB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? J Clin Endocrinol Metab. 2001;86:3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 24.Chen X. Yu QQ. Zhu YH. Bi Y. Sun WP. Liang H. Cai MY. He XY. Weng JP. Insulin therapy stimulates lipid synthesis and improves endocrine functions of adipocytes in dietary obese C57BL/6 mice. Acta Pharmacol Sin. 2010;31:341–346. doi: 10.1038/aps.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brunzell JD. Robertson RP. Lerner RL. Hazzard WR. Ensinck JW. Bierman EL. Porte D., Jr Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976;42:222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- 26.Godsland IF. Jeffs JA. Johnston DG. Loss of beta cell function as fasting glucose increases in the non-diabetic range. Diabetologia. 2004;47:1157–1166. doi: 10.1007/s00125-004-1454-z. [DOI] [PubMed] [Google Scholar]
- 27.Ritzel RA. Butler AE. Rizza RA. Veldhuis JD. Butler PC. Relationship between β-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29:717–718. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 28.Tfayli H. Lee S. Arslanian S. Declining beta-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care. 2010;33:2024–2030. doi: 10.2337/dc09-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]