Abstract
The antioxidant activity and contents of various polyphenol classes in the seeds of seven soybean varieties of different seed color and one yellow seed cultivar, representing a reference genotype, were evaluated. Total polyphenols and tannins were determined after extraction of plant material with 70% aqueous acetone, and total flavonoids were extracted with methanol and acetic acid, whereas anthocyanins were extracted with 20% aqueous ethanol. In addition, isoflavone content and composition were determined using high-performance liquid chromatography analysis. Antioxidant activity of seed extracts was evaluated by the 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity assay. A positive linear correlation between antioxidant activity and contents of total polyphenols and anthocyanins was established. The highest antioxidant activity was observed in the extracts of black and brown varieties, which also showed high levels of all polyphenol classes examined. Yellow seed had the highest total isoflavone content (3.62 mg/g of dry material). The highest concentration of total daidzein was determined in black seeds (>2.0 mg/g of dry material), and the highest total glycitein and genistein contents occurred in the yellow cultivar (0.53 and 1.49 mg/g of dry material, respectively). According to our results, varieties of black and brown seeds could be of special interest not only for their large content of total polyphenols, ranging from 4.94 to 6.22 mg of gallic acid equivalents/g of dry material, but also for their high content of natural antioxidants such as anthocyanins.
Key Words: anthocyanins; 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity; flavonoids; isoflavones; tannins
Introduction
Naturally occurring plant polyphenols include several groups of compounds that have health-promoting properties. Polyphenols may act as antioxidants, thereby reducing the risk of atherosclerosis and coronary heart disease. Dietary intake of polyphenols has been associated with reduced risk of chronic diseases, such as heart disease and cancer, probably because of their antioxidant properties.1 Polyphenols play also an important role in plant resistance and defense against microbial infections that are intimately connected with reactive oxygen species.2–4 Many phenolic compounds found in plant tissues (in addition to tocopherols) are potential antioxidants: flavonoids, tannins, and lignin precursors may all work as scavenging compounds for reactive oxygen species. Antioxidants act as a cooperative network, using a series of different redox reactions. To prevent oxidation of fats and oils, antioxidants are widely used in foods and cosmetics. Because of possible toxicity of the widely used synthetic antioxidants such as butylated hydroxytoluene and butylated hydroxyanisole, together with the consumers` preference for “natural” products, much research on natural antioxidants has been undertaken in recent years.5
Soybean [Glycine max (L.) Merrill] seeds are one of the most important sources of protein and oil in the world. Soybean can be classified into food beans and oil beans, based on its usage in the East and West. In Western countries, the oil beans are normally harvested as dried seeds and further processed into some non-food applications and many soyfoods, such as soybean oil, defatted soybean meal, and soy flour. In the Far East, black soybean has been used as food and medicinal material in Korea and China since ancient times.6 The beans were used as whole beans at the mature dried stage for the production of natto, soy sprouts, tofu, soy milk, miso, and tampeh or as immature seeds as a vegetable.7 There is much evidence suggesting that compounds present in soybean may be beneficial to human health. Soybean has the potential of playing a role in the prevention and treatment of chronic diseases, most notably cancer, atherosclerosis, osteoporosis, and coronary heart disease.8,9 Its potential for cancer prevention and suppression is due to the high content of the isoflavone genistein (a phytoestrogen), a naturally occurring inhibitor of tyrosine-specific protein kinases. Soybean also contains other components, such as saponin, protease inhibitors, phytic acid, and fiber. Therefore, the soybean could be regarded as a functional food.7
Commercially grown soybean varieties have yellow seed (common soybean). Most cultivated soybean varieties are homozygous for a dominant form of the I (inhibitor) gene, which has four alleles that determine the absence or presence of anthocyanin pigments. Some soybean varieties are self-colored and accumulate anthocyanins within the epidermal layer of the seed coat. However, spontaneous mutations from yellow seed to dark-colored seed with the i/i genotype arise frequently within highly inbred soybean varieties.10,11 The anthocyanins (anthocyanidin glycosides) of the mature, black seed coat have been identified as cyanidin-3-monoglucoside and delphinidin-3-monoglucoside.12 Furthermore, petunidin-3-glucoside13 and pelargonidin-3-O-glucoside, with several minor anthocyanins—catechin-cyanidin-3-O-glucoside, delphinidin-3-O-galactoside, cyanidin-3-O-galactoside, and peonidin-3-O-glucoside—were detected and identified based on fragmentation patterns of high-performance liquid chromatography (HPLC)–diode array detector–electrospray ionization/mass spectrometry analysis.14 Black soybean has long been consumed in the Far East and Southeast Asia as an important source of natural antioxidants because of the rich anthocyanin content in its seed coat.15,16
Despite the possible health benefits of colored soybeans, there is only limited information related to chemical constituents of pharmacological and nutritional importance. These literature references come from Asia and the United States, but none of them from Europe. To the best of our knowledge, the present study is the first report on polyphenols with antioxidant properties in different varieties of colored soybean grown in central Europe. Because of the widespread use of soy products, knowing the amount and kind of polyphenols in them is of increasing importance. Dietetic supplements based on soybean extracts have been adopted as a natural alternative to hormone replacement therapy in menopause due to high amounts of phytoestrogens.17 Therefore, the aim of this study was to investigate the presence and content of major polyphenols constituents in different soybean varieties of colored seeds. The data obtained should enable the selection of soybean genotypes rich in biologically active compounds that could further be processed into either functional food or pharmaceutical raw material.
Materials and Methods
Plant material
One specimen of yellow seeds (cultivar Meli) and seven advanced experimental varieties in the Institute of Field and Vegetable Crops, Novi Sad, Serbia, breeding program were chosen for the experiment. Varieties were selected based on their seed color (black, brown, ocher, green, and reddish). Plant materials were grown on experimental fields, near Novi Sad. Seeds for the in vitro experiments were collected in the stage of full maturity.
Extraction procedure
Plant material (1 g of whole seeds per sample) was ground in a mill, reduced to a fine powder, and extracted with 70% aqueous acetone (50 mL) by sonication for 20 minutes in an ultrasonic bath at ambient temperature. The extracts were rapidly vacuum-filtered through a sintered glass funnel and kept refrigerated before assay.
Determination of total polyphenol contents
Total polyphenols were determined by the Folin–Ciocalteu method.18 The amount of total polyphenols was calculated as a gallic acid equivalent (GAE) from the calibration curve of gallic acid standard solutions (covering the concentration range between 0.1 and 1.0 mg/mL) and expressed as milligrams of GAE per gram of dry plant material (DM). All measurements were done in triplicate.
Determination of tannins
Total tannin content was determined by the Folin–Ciocalteu procedure,19 after removal of tannins by their adsorption on insoluble matrix (polyvinylpolypyrrolidone). Calculated values were subtracted from total polyphenol contents, and total tannin contents were expressed as milligrams of GAE per gram of DM. All measurements were done in triplicate.
Determination of flavonoids
Total flavonoids were determined after extraction of plant material (1 g of whole seeds) with 20 mL of extracting solvent methanol–water–acetic acid (140:50:10 by volume), for 60 minutes, according to the procedure of Marckam.20 The amount of flavonoids was calculated as a rutin equivalent from the calibration curve of rutin standard solutions and expressed as milligrams of rutin per gram of DM.
Determination of anthocyanins
Anthocyanins were determined using the pH differential method.21 Anthocyanins were extracted after soaking in 20% (vol/vol) ethanol solution at a 1:10 ratio (wt/wt) at 25°C for up to 10 days. Samples of the extracts were filtered through Whatman (Maidstone, United Kingdom) No. 1 filter paper. The filtered extracts in 5-mL aliquots were diluted either with 0.2 M KCl/0.2 M HCl (25:67, vol/vol) buffer to 100 mL and adjusted to pH 1.0 or with 1.0 M NaCH3COO/1.0 M HCl/water (10:6:9 by volume) to 50 mL and adjusted to pH 4.5. The absorbance values at 520 nm of these diluted solutions were measured spectrophotometrically. Absorbance readings were converted to total amount of anthocyanins as a cyanidin-3-glucoside equivalent using a molar extinction coefficient of 2.96×104, a molecular weight of 484, and absorbances at pH 1.0 and 4.5 and expressed as milligrams of cyanidin-3-glucoside per gram of DM.
Determination of isoflavones (phytoestrogens)
Sample preparation
All the samples were harvested on the same day and were stored for 2 months in a storage room, without removal of the seed coat. The moisture content of seeds, determined by heating at 105°C to constant weight, was 5.5%. For further analysis, whole seeds were ground using a coffee mill. A powdered portion of 500 mg of soybean seed was defatted by n-hexane extraction (2×10 mL for 30 minutes and subsequent centrifugation for 30 minutes at 1,096 g).22 Defatted and powdered soybean seed (100 mg) was then extracted for 2 hours with 5 mL of acetone–water (7:3, vol/vol) and centrifuged (30 minutes at 1,096 g). The extracts obtained were evaporated on a rotary evaporator at 70–75°C and redissolved in 2 mL of methanol. Prior to HPLC injection each extract was passed through a membrane filter (pore size, 0.45 μm).
HPLC analysis
An Agilent (Palo Alto, CA, USA) model 1100 HPLC instrument equipped with a binary pump, degasser, autosampler, and diode array detector was used to separate, identify, and quantify isoflavones. Separation of these compounds was achieved using a 5-μm (film thickness) Zorbax SB C18 reversed-phase HPLC column (150×4.6 mm) with a Zorbax SB C18 guard column. Mobile phase gradients were formed between two degassed solvents: solvent A was 1% (vol/vol) acetic acid in water, and solvent B was 100% acetonitrile. Gradient conditions were as follows: 0–5 minutes, 15% B; 5–44 minutes, from 15% to 35% B; 44–45 minutes, from 35% to 15% B; and 45–50 minutes, 15% B. A post-time period of 20 minutes was used. The column temperature was 25°C, the solvent flow rate was 0.6 mL/minute, and the injection volume was 10 μL. The spectra were collected between 240 and 400 nm by diode array detector, and components in the eluate were detected at 270 nm. Isoflavones were identified by retention times, by comparison of ultraviolet spectra with those of standard compounds, and from literature data.22,23
Aglycones were quantified from three five-point regression curves (R≥0.9998) obtained using the corresponding standards (daidzein, glycitein, and genistein). Actual concentrations of isoflavones in glycoside forms were calculated from the regression curve of the corresponding aglycones.24
Measurement of 2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
The possible antioxidant activity of the test samples was assessed based on scavenging activity of the 70% aqueous acetone seed extracts of the stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical.25 The absorbance was measured at 515 nm, and the 50% inhibitory concentration (IC50) values were calculated. The IC50 value denotes the concentration of sample that is required to scavenge 50% of the DPPH free radicals. A decreased value for IC50 indicates an increase in DPPH radical scavenging activity.
Statistical analysis
Results of this study represent data from three different experiments. Values were expressed as means of determinations made in triplicates. Statistical significance was tested by analysis of variance followed by comparisons of means by Duncan's multiple range test (P<.05) calculated using STATISTICA for Windows version 9.0 (StatSoft, Tulsa, OK, USA). Stepwise multiple regression analyses were used to determine the correlation among variables.
Results and Discussion
Screening for polyphenols in soybean varieties of different seed color showed the presence of compounds with phenolic structure in all specimens examined, regardless of their seed color (Table 1). Amounts of total polyphenols ranged from 2.68 (in the yellow variety) to 6.22 (in the black 2 variety) mg of GAE/g of DM. Comparison between contents of polyphenols in yellow and colored seeds showed that varieties with black and brown seeds possessed higher levels of these substances. Their content, on average, was 2.5-fold higher compared with yellow samples. In the ocher, green, and reddish samples, polyphenol levels were lower, and somewhat uniform, but also higher compared with the control (yellow seeds). Total tannin content was the lowest in total polyphenol-rich varieties (i.e., black and brown) (0.71–0.89 mg of GAE/g of DM), whereas the green seed variety had the highest content (1.55 mg of GAE/g of DM). The relative portion of tannins in black and brown seeds may be considered low, which is of importance to soybean features as an industrial and protein plant. In physiological terms tannins may be toxic to bacteria and fungi and act as feeding deterrents to herbivores and insects. Compared with some other industrial crops, such as maize,26 soybean varieties of colored seeds were poor in flavonoids, but compared with commercially grown soybean hybrids of yellow seeds,27 they were flavonoid rich. Their contents, except for the ocher shine variety, were up to 300–450% higher compared with the yellow seed specimen, with contents of 1.49 and 2.19 mg of rutin/g of DM in the ocher opaque and black 1 variety, respectively. Flavonoid content in the varieties black 2, brown, and green was rather uniform, ranging from 1.07 to 1.16 mg of rutin/g of DM. In the reddish variety, these compounds were present only in traces, which suggests that their color may originate from some other plant pigments, such as carotenoids. Except for in the reddish specimen, anthocyanins, being flavonoids themselves, were also determined in the rest of the soybean varieties examined. The highest content of anthocyanins was recorded in the black and brown varieties, being the highest in black 1 and brown (0.65 and 0.62 mg of cynidin-3-O-glucoside/g of DM, respectively). Their presence has not been established in the yellow seed cultivar Meli and the reddish variety.
Table 1.
Polyphenol Contents and Antioxidant Activity of Extracts of Soybean Seeds of Different Color
| Sample number (variety) | Total polyphenolsa | Tanninsa | Flavonoidsb | Anthocyaninsc | IC50d |
|---|---|---|---|---|---|
| 1. Yellow | 2.68±0.47 | 0.93±0.20 | 0.48±0.02 | 0 | 1.29±0.19 |
| 2. Ocher shine | 3.57±0.32 | 1.42±0.14 | 0.47±0.10 | 0.29±0.13 | 1.09±0.09 |
| 3. Ocher opaque | 3.61±0.10 | 1.50±0.13 | 1.49±0.35 | 0.54±0.12 | 1.01±0.15 |
| 4. Black 1 | 6.13±0.12 | 0.74±0.03 | 2.19±0.46 | 0.65±0.46 | 0.09±0.01 |
| 5. Black 2 | 6.22±0.68 | 0.89±0.03 | 1.16±0.29 | 0.57±0.22 | 0.13±0.10 |
| 6. Brown | 4.94±0.10 | 0.71±0.14 | 1.16±0.15 | 0.62±0.35 | 0.22±0.11 |
| 7. Green | 3.46±0.09 | 1.55±0.36 | 1.07±0.40 | 0.41±0.28 | 1.29±0.05 |
| 8. Reddish | 3.26±0.17 | 1.40±0.16 | 0 | 0 | 1.16±0.07 |
Data are mean±SE values.
Expressed as mg of gallic acid equivalents/g of dry material.
Expressed as mg of rutin/g of dry material.
Expressed as mg of cyanidin-3-glucoside/g of dry material.
50% inhibitory concentration (IC50), expressed as mg/mL.
Plants consumed by humans may contain thousands of different phenolic compounds. The effects of dietary phenolics are of great current interest due to their antioxidative and possible anticarcinogenic activities. A popular belief is that dietary phenolics are anticarcinogens because they are antioxidants, but there is no direct evidence.28 Epidemiological studies concerning consumption of phenolics and human cancer risk suggest the protective effects of certain food items and phenolics, but more studies are needed to reach clear-cut conclusions.29 According to their color and their total polyphenol content, our investigated varieties could be divided into three groups: (1) black and brown seeds, with the highest contents of total polyphenols and anthocyanins and the lowest portion of tannins; (2) ocher and green seeds, with moderate amounts of total polyphenols, flavonoids, and anthocyanins and a high amount of tannins; and (3) yellow and reddish seeds, which lacked seed anthocyanins and had a lower content of total polyphenols. Thus, it seems that varieties belonging to the first group are of special interest not only for their considerable content of total polyphenols but also for their high content of natural antioxidants such as anthocyanins.
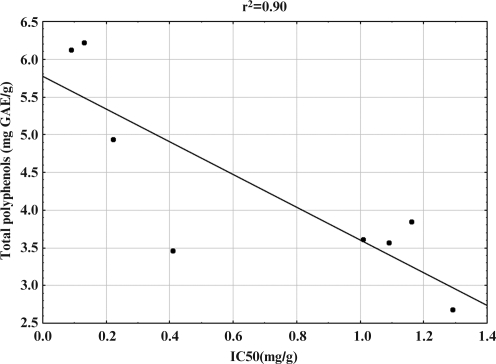
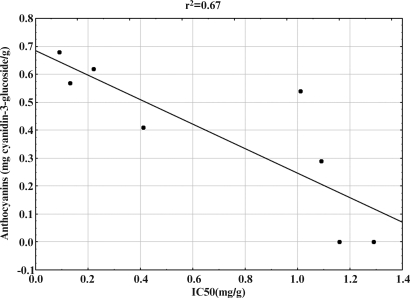
The particular polyphenol classes were further tested for their antioxidant capability using the DPPH free radical assay. The IC50 values for the extracts investigated varied in a range between 0.09 and 1.29 mg/mL (Table 1). DPPH radical scavenging activity is a measure of nonenzymatic antioxidant activity. Higher levels of DPPH activity have been correlated with tolerance to different stress conditions,30 but they also point out the presence of biologically active biomolecules with pronounced antioxidant activity.27 Hence, we correlated the polyphenol contents and antioxidant activity by regression analysis. Antioxidant activity increased proportionally to the polyphenol content: a linear relationship between IC50 values and contents of total polyphenols (Fig. 1) and anthocyanins (Fig. 2) was established. At the same time, there was no correlation between DPPH values and flavonoid and tannin contents. Containing relatively high levels of all polyphenol classes investigated, seeds of black and brown color expressed the most significant antioxidant activities among the varieties tested.
FIG. 1.
Correlation of 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity expressed as IC50 (in mg/g) and total polyphenol content (mg of gallic acid equivalents/g of dry plant material) in extracts of soybean seeds of different color.
FIG. 2.
Correlation of 2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity expressed as IC50 (mg/g) and anthocyanin content (mg of cyanidin-3-glucoside/g of dry plant material) in extracts of soybean seeds of different color.
All soybean varieties investigated contained phytochemicals of isoflavone structure (i.e., phytoestrogens). Isoflavones are considered as simple and metabolically inexpensive defense chemicals in plants. Soybean isoflavones have been also able to reduce the risk of various cancers. Isoflavones exhibit a multitude of medicinal effects that influence cell growth and regulation, which may have a potential value in treatment of cancer. Isoflavone content of colored soybean varieties in our study was determined by HPLC analysis, and the results are presented in Table 2.
Table 2.
Isoflavone Contents in Extracts of Soybean Seeds of Different Color
| Sample number (variety) | Total daidzein | Total glycitein | Total genistein | Total isoflavone |
|---|---|---|---|---|
| 1. Yellow | 1.60±0.02 | 0.53±0.05 | 1.49±0.01 | 3.62±0.06 |
| 2. Ocher shine | 1.56±0.01 | 0.29±0.04 | 0.77±0.02 | 2.62±0.05 |
| 3. Ocher opaque | 1.64±0.02 | 0.28±0.03 | 0.80±0.01 | 2.72±0.09 |
| 4. Black 1 | 2.55±0.03 | 0.27±0.04 | 0.76±0.06 | 3.58±0.04 |
| 5. Black 2 | 2.06±0.33 | 0.21±0.17 | 0.89±0.02 | 3.16±0.17 |
| 6. Brown | 1.63±0.01 | 0.17±0.02 | 0.64±0.02 | 2.44±0.01 |
| 7. Green | 1.92±0.14 | 0.44±0.05 | 1.03±0.01 | 3.39±0.20 |
| 8. Reddish | 1.39±0.04 | 0.40±0.05 | 0.79±0.01 | 2.58±0.11 |
Data are mean±SE values, expressed as mg/g of dry plant material.
The total isoflavone content in the eight soybean samples analyzed ranged from 2.44 mg/g (in the brown variety) to 3.62 mg/g (in the yellow cultivar) of soybean seed (Table 2). Average isoflavone content among all analyzed cultivars was 2.97 mg/g, which corresponds to previous results obtained from 20 cultivars of yellow seed for which average total isoflavone content was 3.25 mg/g.31 The average value of isoflavones obtained is considerably higher than those reported in a study performed in the United States (1.86 mg/g)32 or in India (1.05 mg/g).6 Black and green seed varieties showed higher content of total isoflavones (3.16–3.58 mg/g) compared with other colored seeds, although the yellow variety had the highest total isoflavone content. No significant differences were observed for individual forms of isoflavones and total isoflavone contents across the seed coat color (Table 2). These results are consistent with values obtained in recent studies in Minnesota (United States)32 and the Madhya Pradesh (India) region,6 which showed that black soybeans did not have higher isoflavone content compared with different varieties of yellow seed and that values of total isoflavones in black and yellow seeds were in the same range.
Daidzein with glycosides and total genistein were the dominant forms of isoflavones in all samples analyzed. The concentration of glycitein with corresponding glycosides was the lowest in all samples compared with the other isoflavone forms. This agrees with previous observations that soybeans and soy foods usually contain similar amounts of genistein and daidzein and a much lower amount of glycitein.33,34 The percentage of total daidzein in all specimens varied from 44% to 71% of total isoflavone content. The highest content of total daidzein was found in the two varieties of black seeds (2.55 and 2.06 mg/g), and also in these varieties the percentage of total daidzein compared with total isoflavone content was the highest (65.2% and 71.2%). The highest total glycitein and genistein content was determined in the yellow cultivar (0.53 mg/g and 1.49 mg/g, respectively) and in green seed (0.44 mg/g and 1.03 mg/g, respectively) (Table 2).
Regression analysis for the isoflavone content and IC50 values showed low correlation, which is in agreement with previous findings.31,35 This points out the primary role of isoflavones in plants, being predominantly hormonal substances and exhibiting less potent antioxidant activity compared with some other components.
Conclusions
The results presented here showed that all soybean varieties accumulate polyphenols and isoflavones independently of the color of their seeds. Yellow seed soybean accumulates flavonoids but does not accumulate anthocyanins. Black, brown, and ocher seeds contain anthocyanins, whereas the reddish variety does not. The highest antioxidant activity was observed in the extracts of black and brown varieties, which also showed high levels of all polyphenol classes examined. Black soybean seeds showed a high level of daidzein and antioxidant activity, but the variety with yellow seed coat showed the highest amount of total isoflavones, especially genistein.
Black and brown varieties showed combined characteristics of favorable features, having both strong antioxidant capacities and higher contents of anthocyanins and isoflavones. In addition, we correlated the polyphenol contents and antioxidant activities by regression analysis. Antioxidant activity increased proportionally to levels of most of the polyphenol classes: a linear relationship between IC50 values and contents of total polyphenols and anthocyanins was established. In parallel, there was no positive correlation between IC50 values and tannin content. Regression analysis for the isoflavone content and DPPH scavenging activity showed low correlation, which is in agreement with previous findings. This points out the primary role of isoflavones in plants, being predominantly hormonal substances and exhibiting low antioxidant activity.
It might be concluded that varieties with black and brown seeds may act as a better source of natural antioxidants compared with the common soybean grown in Europe (i.e., the yellow seed coat soybean). Data on all the polyphenol classes investigated as well as the antioxidant activity of extracts of soybean varieties of different seed color could be valuable to the pharmaceutical and food industries for selection of cultivars suitable for production of dietary supplements rich in polyphenols and isoflavones and could also be valuable to soybean breeding programs in order to increase the biological value of the commercial products.
Acknowledgment
This study was carried out within a project (grant TR-31022) of the Ministry of Science and Technological Development of the Republic of Serbia.
Author Disclosure Statement
All authors declare no competing financial interests exist.
References
- 1.Scaron;avikin K. Zdunić G. Janković T. Tasić S. Menković N. Stević T. đorđević B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum Nutr. 2009;64:212–217. doi: 10.1007/s11130-009-0123-2. [DOI] [PubMed] [Google Scholar]
- 2.Emmons CL. Peterson DM. Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci. 2001;41:1676–1681. [Google Scholar]
- 3.Grassmann J. Hippeli S. Elstner EF. Plant's defence and its benefits for animals and medicine: role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiol Biochem. 2002;40:471–478. [Google Scholar]
- 4.Malenčić Dj. Maksimović Z. Popović M. Miladinović J. Polyphenol contents and antioxidant activity of soybean seed extracts. Bioresour Technol. 2008;99:6688–6691. doi: 10.1016/j.biortech.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 5.Cuvelier ME. Richard H. Berset C. Antioxidative activity and phenolic composition of pilot-plant and commercial extracts of sage and rosemary. J Am Oil Chem Soc. 1996;73:645–652. [Google Scholar]
- 6.Kumar V. Rani A. Kumar Dixit A. Pratap D. Bhatnagar D. A comparative assessment of total phenolic content, ferric reducing-anti-oxidative power, free radical-scavenging activity, vitamin C and isoflavones content in soybean with varying seed coat colour. Food Res Int. 2010;43:323–328. [Google Scholar]
- 7.Simonne AH. Weaver DB. Wei C. Immature soybean seeds as a vegetable or snack food: acceptability by American consumers. Innov Food Sci Emerg Technol. 2001;1:289–296. [Google Scholar]
- 8.Kim YH. Yun HT. Chung WK. Park KY. Investigation of biological effect in black colored soybean; New Directions for a Diverse Planet: Proceedings of the 4th International Crop Science Congress; [Dec 2;2010 ]. [Google Scholar]
- 9.Takahata Y. Ohnishi-Kameyama M. Furuta S. Takahashi M. Suda I. Highly polymerized procyanidins in brown soybean seed coat with a high radical-scavenging activity. J Agric Food Chem. 2001;49:5843–5847. doi: 10.1021/jf010307x. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox JR. Performance and use of seedcoat mutants in soybean. Crop Sci. 1988;28:30–32. [Google Scholar]
- 11.Todd JJ. Vodkin LO. Pigmented soybean (Glycine max) seed coats accumulate proanthocyanidins during development. Plant Physiol. 1993;102:663–670. doi: 10.1104/pp.102.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buzzell RI. Buttery BR. MacTavish DC. Biochemical genetics of black pigmentation of soybean seed. J Hered. 1987;78:53–54. [Google Scholar]
- 13.Choung MG. Baek IY. Kang ST. Han WY. Shin DC. Moon HP. Kang KH. Isolation and determination of anthocyanins in seed coats of black soybean (Glycine max (L.) Merr.) J Agric Food Chem. 2001;49:5848–5851. doi: 10.1021/jf010550w. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH. Kang NS. Shin S-O. Shin S-H. Lim S-G. Suh D-Y. Baek I-Y. Park K-Y. Ha TJ. Characterisation of anthocyanins in the black soybean (Glycine max L.) by HPLC-DAD-ESI/MS analysis. Food Chem. 2009;112:226–231. [Google Scholar]
- 15.Astadi IR. Astuti M. Santoso U. Nugraheni PS. In vitro antioxidant activity of anthocyanins of black soybean seed coat in human low density lipoprotein (LDL) Food Chem. 2009;112:659–663. [Google Scholar]
- 16.Xu J. Zhang M. Liu X. Zhang R. Sun L. Quui L. Correlation between antioxidation and the content of total phenolics and anthocyanin in black soybean accessions. Agric Sci China. 2007;6:150–158. [Google Scholar]
- 17.Williamson-Hughes PS. Flickinger BD. Messina MJ. Empie MW. Isoflavone supplements containing predominantly genistein reduce hot flush symptoms: a critical review of published studies. Menopause. 2006;13:831–839. doi: 10.1097/01.gme.0000227330.49081.9e. [DOI] [PubMed] [Google Scholar]
- 18.Kroyer GT. Red clover extract as antioxidant active and functional food ingredient. Innov Food Sci Emerg Technol. 2003;5:101–105. [Google Scholar]
- 19.Hagermann A. Harvey-Mueller I. Makkar HPS. FAO/IAEA Working Document. International Atomic Energy Agency; Vienna: 2000. Quantification of Tannins in Tree Foliage—A Laboratory Manual; pp. 4–7. [Google Scholar]
- 20.Marckam KR. Methods in Plant Biochemistry. Academic Press; London: 1989. pp. 48–56. [Google Scholar]
- 21.Shen SC. Tseng KC. Chao FT. Wu SB. Color quality of rose liquer. J Food Qual. 2007;30:202–217. [Google Scholar]
- 22.Andlauer W. Martena MJ. Furst P. Determination of selected phytochemicals by reversed-phase high-performance liquid chromatography combined with ultraviolet and mass spectrometric detection. J Chromatogr. 1999;849:341–348. doi: 10.1016/s0021-9673(99)00597-x. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH. Renita M. Fioritto RJ. Martin SK. Schwartz SJ. Vodovotz Y. Isoflavone characterization and antioxidant activity of Ohio soybeans. J Agric Food Chem. 2004;52:2647–2651. doi: 10.1021/jf035426m. [DOI] [PubMed] [Google Scholar]
- 24.Romani A. Vignolini P. Galardi C. Aroldi C. Vazzana C. Heimler D. Polyphenolic content in different plant parts of soy cultivars grown under natural conditions. J Agric Food Chem. 2003;51:5301–5306. doi: 10.1021/jf0212136. [DOI] [PubMed] [Google Scholar]
- 25.Brand-Wiliams W. Cuvelier ME. Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995;28:25–30. [Google Scholar]
- 26.Maksimović Z. Malenčić D. Kovačević N. Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol. 2005;96:873–877. doi: 10.1016/j.biortech.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Malenčić D. Popović M. Miladinović J. Phenolic content and antioxidant properties of soybean (Glycine max (L.) Merr.) seeds. Molecules. 2007;12:576–581. doi: 10.3390/12030576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang CS. Landau JM. Huang MT. Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi R. Ohmori R. Kiyose C. Momiyama Y. Ohsuzu F. Kondo K. Antioxidant activities of black and yellow soybeans against low density lipoprotein oxidation. J Agric Food Chem. 2005;53:4578–4582. doi: 10.1021/jf048062m. [DOI] [PubMed] [Google Scholar]
- 30.Kang HM. Saltveit ME. Reduced chilling tolerance in elongating cucumber seedling radicals is related to their reduced antioxidant enzyme and DPPH-radical scavenging activity. Physiol Plant. 2002;115:244–250. doi: 10.1034/j.1399-3054.2002.1150210.x. [DOI] [PubMed] [Google Scholar]
- 31.Tepavčević V. Atanacković M. Miladinović J. Malenčić D. Popović J. Cvejić J. Isoflavone composition, total polyphenolic content, and antioxidant activity in soybeans of different origin. J Med Food. 2010;13:1–8. doi: 10.1089/jmf.2009.0050. [DOI] [PubMed] [Google Scholar]
- 32.Xu BJ. Chang SKC. Characterization of phenolic substances and antioxidant properties of food soybeans grown in the North Dakota–Minnesota region. J Agric Food Chem. 2008;56:9102–9113. doi: 10.1021/jf801451k. [DOI] [PubMed] [Google Scholar]
- 33.Wang HJ. Murphy PA. Isoflavone composition of American and Japanese soybeans in Iowa: effects of variety crop, year and location. J Agric Food Chem. 1994;42:1674–1677. [Google Scholar]
- 34.Hoeck JA. Fehr WR. Murphy PA. Welke GA. Influence of genotype and environment on isoflavone contents of soybean. Crop Sci. 2000;40:48–51. [Google Scholar]
- 35.Riedl KM. Lee JH. Renita M. Martin SK. Schwartz SJ. Vodovotz Y. Isoflavone profiles, phenol content, and antioxidant activity of soybean seeds as influenced by cultivar and growing location in Ohio. J Sci Food Agric. 2007;87:1197–1206. [Google Scholar]