Abstract
Here we used a within-subject design to evaluate hypothalamic-pituitary-adrenal (HPA) activity following replacement of low and high physiological levels of testosterone (T) to adult, gonadally-suppressed, male rhesus macaques, and replacement with sex-specific low and high physiological doses of dihydrotestosterone (DHT) in the same adult males as well as in adult, gonadally-suppressed, female rhesus macaques. As indexes of HPA axis activation following T and DHT replacement, serum levels of cortisol (CORT) were measured before and following dexamethasone (DEX) inhibition, and corticotrophin-releasing factor (CRF) induced activation. Female monkeys were assessed for differences in response associated with dominant (DOM) and subordinate (SUB) social status. Data show that the high physiological dose of DHT significantly decreased basal CORT in both male and female monkeys irrespective of social status, but reduced CRF-stimulated CORT only in males. SUB female monkeys showed a trend towards increased CRF-stimulated CORT release under high-dose DHT replacement compared to DOM females or males given the same treatment, indicating that androgens likely have no influence on reducing HPA activation under chronic psychosocial stress in females. The normal circadian rhythm of CORT release was absent in placebo-replaced SUB and DOM females and was restored with low-dose DHT replacement. These results indicate that DHT significantly reduces CRF-stimulated CORT release only in male monkeys, and plays a role in maintaining circadian changes in CORT release in female monkeys.
Keywords: HPA, stress, androgen, sex-differences, cortisol, monkey
1. INTRODUCTION
It has been established that gonadal hormones exert substantial modulatory effects on the activity of the hypothalamic-pituitary-adrenal (HPA) axis. Females show enhanced stress responsiveness in several species and this has been attributed to the activational effects of estrogens in females and the inhibitory effect of testosterone (T) in males (Broadbear et al., 2005a; Broadbear et al., 2005b; Handa et al., 1994; Handa et al., 2009; Iwasaki-Sekino et al., 2009; McCormick et al., 2002; Ogilvie and Rivier, 1997; Patchev and Almeida, 1996; Sanchez et al.; Wilson et al., 2005). To date, almost all studies examining the effect of androgens on the HPA axis have been done in rats. For example, male rats show reduced glucocorticoid and adrenocorticotrophin responses to stress compared to their female conspecifics (Viau, 2002), and the magnitude of this decrease has been related to individual testosterone levels within males (Viau and Meaney, 1996). Moreover, castration increases both corticosterone (the major glucocorticoid in rodents) and adrenocorticotrophin in rats and this is normalized by replacement with testosterone or the non-aromatizable metabolite of testosterone: dihydrotestosterone (DHT), which is a more potent ligand to the androgen receptor than T (Seale et al., 2004). This was taken to indicate that the inhibition of HPA reactivity in males is predominately due to the effect of androgen receptor activation rather than to the action of the estrogenic metabolites of T.
The mechanisms whereby androgens curb HPA activation in rats are related to the inhibition of arginine vasopressin-releasing neurons in the parvocellular portion of the hypothalamic paraventricular nucleus. Arginine vasopressin released by these neurons into the median eminence portal circulation potentiates the effect of CRF at the pituitary corticotropes, stimulating the release of adrenocorticotrophin. In the rat, T appears to act on upstream limbic circuits to constrain arginine vasopressin synthesis within the parvocellular paraventricular nucleus (Viau and Meaney, 1996; Viau et al., 2001; Williamson et al., 2005; Williamson and Viau, 2008).
We have previously evaluated the effect of estrogen and progesterone on HPA activity in female macaques (Wilson et al, 2005). That study showed enhanced CORT secretion with estrogen replacement following feedback inhibition and subsequent to CRF-induced HPA activation. In addition, that same study reported decreased feedback inhibition of CORT under estrogen replacement that was more pronounced in socially subordinate female monkeys compared to dominant female monkeys (Wilson et al, 2005), supporting previous observations that the psychosocial stressor of subordination produces a dysregulation of the HPA axis in rhesus females (Brooke et al., 1994; Riddick et al., 2009; Shively, 1998).
The purpose of this present study is to begin to evaluate the effect of androgens on indices of HPA activation in a primate model. To that end we used a within-subject design to compare the effect of low- and high- physiological levels of T and DHT in male rhesus monkeys, and sex-specific low- and high- physiological doses of DHT in female rhesus monkeys. All animals were socially housed, with females distributed throughout the dominance hierarch in one group and males the alpha member of one of several small social groups. Basal levels of CORT, as well as feedback inhibition, and subsequent CRF-induced activation of the CORT release were assessed under the different androgen replacement regimes. The use of DHT instead of T was implemented in female monkeys because we had already assessed the effect of estrogen in female macaques and wished to examine a more androgenic effect. DHT was used in males in order to parse out any estrogen receptor alpha effects of aromatized testosterone in male monkeys. It is important to note that DHT has been recently shown to activate estrogen receptor β through one of its neuroactive metabolites (5α-androstane- 3β-diol) (Handa et al., 2008) and thus we cannot eliminate all estrogenic effects on the HPA axis by the use of DHT.
Female monkeys are included in this study for a two-fold purpose. First, even though the ovaries in virtually all mammal species, including women, produce a significant quantity of androgens (Nelson, 2005), as do the adrenals in both sexes (Longcope, 1986), and the female brain expresses substantial levels of androgen receptors (Balthazart et al., 1996; Fink et al., 1999; Gahr, 2001; Handa et al., 1986; Handa et al., 1988), the study of sex differences in HPA function has largely focused on the effect of estrogens in females and androgens in males (although see: (McCormick et al., 2002). Thus, it is important to assess the effect of androgens separately from that of estrogens on HPA activity in female animals in order to test the hypotheses that sex differences in HPA activation could, in part, be explained by differences in androgen-mediated effects. Second, because socially housed rhesus female monkeys form hierarchies in which SUB females are subjected to prolonged psychosocial stress (for reviews see (Shively and Kaplan, 1984):(Bernstein, 1970; Bernstein et al., 1974; Bernstein, 1976; Bernstein, 1980), it affords us the opportunity to examine the effect of DHT on HPA parameters in female monkeys exposed to chronic stress.
We hypothesized that, as is the case with rodents, T and DHT will reduce the CORT release following CRF stimulation in male monkeys compared to female monkeys. Further, we predicted that chronic psychogenic stress due to subordinate social status would produce impaired feedback inhibition and exaggerated CRF-induced CORT irrespective of DHT treatment in subordinate female compared to dominant female and male monkeys.
We found that the high physiological dose of DHT alone reduced basal CORT in male as well as female monkeys irrespective of social status. However, CRF-stimulated CORT was reduced in male but not female monkeys at the high-dose of DHT, suggesting a marked inhibitory effect of androgen receptor activation on HPA reactivity in males alone. In addition, we found a flattening of normal circadian CORT release in gonadally-suppressed female, but not male, monkeys that was restored with low physiological levels of DHT. Additionally we found that intact feedback inhibition to CORT was apparent in females of regardless of social status under both DHT treatment regimes. Several mechanisms are discussed as possible mediators of these findings.
2. RESULTS
2.1 Serum Levels of T and DHT
Hormone assays done on samples taken from males and females between experiment day 10 and 14 are shown in Table 1. Results indicate that lupron treatment was effective in suppressing gonadal androgen release, and that the high-dose of T produced significantly higher plasma T levels than the low-dose T in male monkeys, and that high-dose DHT produced significantly higher plasma levels than the low-dose DHT in both male and female monkeys.
TABLE 1.
Shows hormone assays for testosterone and dihydrotestosterone from samples taken from males and females between experiment day 10 and 14.
| Treatment | Low-dose Testosterone | High-dose Testosterone | Placebo | Low-dose Dihydro-testosterone | High-dose Dihydro-testosterone | Placebo |
|---|---|---|---|---|---|---|
| Males | 3.60 ± 0.60*# ng/ml | 10.36±3.26*# ng/ml | 0.95±0.12 ng/ml | 2.25 ± .11*# ng/ml | 3.40±0.16*# ng/ml | 0.80 ± 0.15 ng/ml |
| Females | N/A | N/A | N/A | 0.93 ± .03*# ng/ml | 1.38 ±.02*# ng/ml | 0.65 ± 0.12 ng/ml |
values significantly differ from placebo.
values significantly different between low and high doses.
2.2 Basal CORT
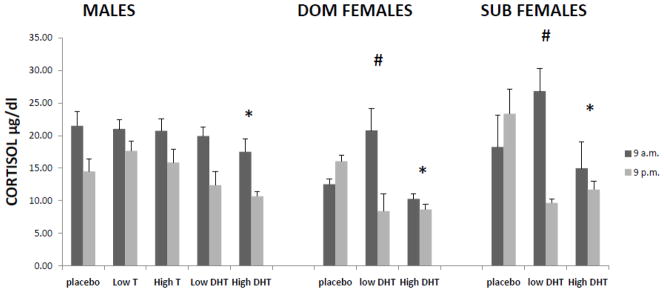
Results depicted in Figure 1 show the effect of androgen treatment on basal levels of CORT in male and DOM and SUB female monkeys. Direct comparison of each treatment between males and DOM and SUB females found no significant group differences.
Figure 1.
Basal levels of CORT in gonadally-suppressed male and dominant (DOM) and subordinate (SUB) female rhesus monkeys replaced with vehicle (placebo), dihydro-testosterone (DHT), and testosterone (T) (in males only).* indicates a significant overall difference compared to the placebo replacement condition within each group, # indicates a significant change in circadian CORT release compared to the placebo replacement condition in females.
A separate analysis examining the effect of DHT doses compared individually to placebo on basal CORT in SUB female monkeys found that there was a significant treatment by time effect on diurnal cortisol comparing the low-dose DHT with placebo (F (1, 2)=101.632, p=0.010) indicating a reversal in levels from 9 a.m. to 9 p.m. and therefore a restoration of normal CORT circadian rhythm with low DHT treatment(see:(Plant, 1981; Quabbe et al., 1982). High DHT in subordinates significantly lowered basal CORT (F (1, 2)=30.541, p=0.031).
A separate analysis examining the effect of DHT doses compared individually to placebo on basal CORT in DOM female monkeys found that there was also a significant treatment by time effect on diurnal cortisol comparing the low-dose DHT with placebo (F (1, 3)=250.845, p=0.001) indicating a reversal in levels from 9 a.m. to 9 p.m. and a restoration of normal CORT circadian rhythm (see:(Plant, 1981; Quabbe et al., 1982) with low DHT treatment. High-dose DHT also restored circadian rhythm (F (1, 3)=95.531, p=0.002) and significantly lowered basal CORT (F (1, 3)=115.927, p=0.002).
A separate analysis examining the effect of T and DHT doses compared individually to placebo on basal CORT within male monkeys found that CORT was significantly lower with high-dose DHT replacement (F(1,6)=6.561 p=0.043), compared with the placebo condition, indicating high-dose DHT reduces basal CORT in males monkeys.
2.3 Feedback inhibition of CORT
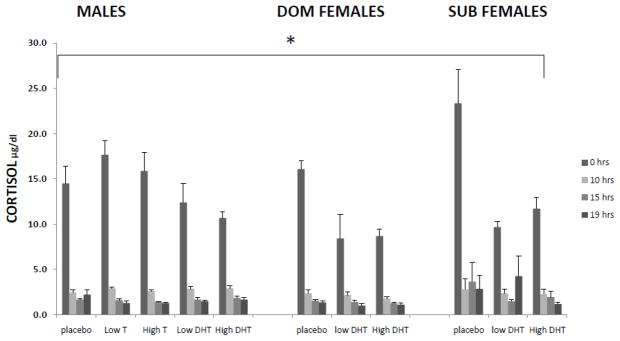
Results depicted in Figure 2 show the effect of each androgen treatment on feedback inhibition of CORT. Values are shown at 0 hr, immediately before DEX injection, and at 10,15 and 19 hrs after DEX injection, in male and DOM and SUB female monkeys. Holding basal CORT values as a covariate showed there was no significant within-subject effect of time but there was a significant between group difference in pre-DEX basal CORT values (F(1,38)=9.592, p=0.004). Looking at the effect of each androgen treatment between males and DOM and SUB females; there were no significant group difference under placebo treatment, and no group differences in pre-DEX basal values, there was a trend towards a group difference under low-dose DHT treatment (F(2,10)=3.524, p=0.069), as well as a trend towards differences in pre-DEX basal values (F(1,10)=3.570, p=0.088), there were no significant group difference under high-dose DHT treatment, and no differences in pre-DEX basal values.
Figure 2.
Feedback inhibition of CORT at 0, 10, 15 and 19 hours after DEX injection, in gonadally-suppressed male and dominant (DOM) and subordinate (SUB) female rhesus monkeys with vehicle (placebo), dihydro-testosterone (DHT), and testosterone (T) (in males only), replacement. 0 hrs is immediately prior to DEX injection.* indicates a significant difference in pre-DEX basal values
2.4 CRF stimulation of CORT
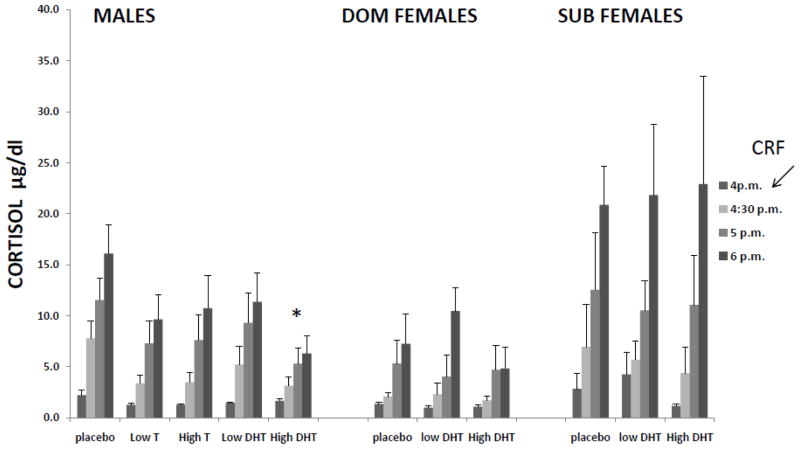
Results depicted in Figure 3 show the effect of androgen treatment on CRF-induced stimulation of CORT in male and DOM and SUB female monkeys. Direct comparison of each treatment between males and DOM and SUB females showed a significant within-subject effect of time for all treatments: placebo:(F(2,22)=42.564, p<0.001), low-dose DHT:(F(2,22)=31.284, p<0.001)High-dose DHT:(F(2,22)=31.284, p<0.001) and found a statistical trend towards a difference at the high-dose of DHT (F (2,11) =2.840, P=0.10) with SUB females displaying the largest CORT response to CRF.
Figure 3.
CRF stimulation of CORT in gonadally-suppressed male and dominant (DOM) and subordinate (SUB) female rhesus monkeys replaced with vehicle (placebo), dihydro-testosterone (DHT), and testosterone (T) (in males only).* indicates a significant difference compared to placebo for CORT in males. CRF=corticotrophin-releasing factor, arrow indicates the time point at which CRF was injected.
A separate analysis within SUB and a separate analysis within DOM females was done examining the effect of DHT doses compared individually to placebo on CRF-stimulation of CORT found no effect on either low or high-dose DHT on CFR stimulated CORT release in either SUB or DOM females.
A separate analysis examining the effect of T and DHT doses compared individually to placebo on CRF-stimulation of CORT in male monkeys found a significant decrease in CRF-stimulated CORT under high-dose DHT treatment (F(1,6)=11.898, p=0.014), and a strong statistical trend towards decreased CORT with both low and high-dose T (F(1,6) =4.575, p=0.076; F(1,6) =4.651, p=0.074, respectively), suggesting that androgens limit the release of CORT after CRF stimulation in male rhesus monkeys.
3. DISCUSSION
Results show that DHT constrains the release of CORT under basal conditions in both male and female rhesus monkeys, and reduces CRF-stimulated CORT in male alone. Moreover, data show that the circadian rhythm of basal CORT release (Plant, 1981; Quabbe et al., 1982) is flattened in placebo-replaced DOM and SUB female monkeys and is restored with a low physiological dose of DHT in both.
The high physiological dose of DHT significantly reduced basal CORT as well as the CORT response following CRF injection in male monkeys, This is consistent with the literature in rats showing that androgens, including DHT, constrain corticosterone release following stress or pharmacological stimulation (Viau, 2002). It should be noted that both doses of T produced a statistical trend toward a reduced CRF-induced CORT response in male monkeys. It may be that the sample size was simply inadequate here and that T alone would have an inhibitory effect on CORT if a few more male monkeys had been included in this study.
It has been shown that the DHT metabolite 5α-androstane- 3β-diol can act on ERβ in rodents, (Handa et al., 2008), and that activation of ERβ is anxiolytic and reduces corticosterone release after exposure of female rats to the elevated plus maze (Weiser et al.). Although we did not observe a significant reduction of CORT release compared to placebo following CRF injection in female monkeys, high-dose DHT replacement did lower basal levels of CORT compared to the placebo-replaced condition in both DOM and SUB females. ERβ is present in temporal, midbrain and hypothalamic regions of the female macaque brain (Gundlah et al., 2000). To our knowledge ERβ distribution has not been characterized in the male brain of any macaque species, although it is present in the male brain in human beings (Gonzalez et al., 2007; Ishunina et al., 2000; Ishunina et al., 2003), rodents (Frye et al., 2008; Sakuma et al., 2009), and other species (Hileman et al., 1999). Thus, the most parsimonious interpretation of the present data is that activation of ERβ by 5α-androstane- 3β-diol may play a role in reducing basal CORT in both male and female monkeys, but conclusive proof would require more specific experimentation using 5α-androstane- 3β-diol treatment itself.
Results suggest that social status may be a factor in the effect of androgens on CRF-stimulated CORT release in female monkeys. SUB females show a trend towards an exaggerated CORT response compared to both DOM females and males following CRF injection under high-dose DHT replacement. Because all of the males used in this study were the most dominant members of each of their groups, we could not assess the effects of status in males.
Although the CRF-stimulated CORT response of DOM females under high-dose DHT replacement appeared restrained compared to SUB females on the same dose DHT, it was not significantly lower than when these same DOM females were on placebo replacement. Thus, there may be no effect of DHT on CRF-stimulated CORT in DOM females.
With respect to the trend towards hyper-responsivity to CRF under high-dose DHT replacement in SUB females, it has been shown in rats that the corticosterone response to acute stress can be facilitated by experiencing a previous stressor (Akana et al., 1992), suggesting that there are mechanisms which can offset or bypass central inhibitory control under prolonged or chronic stress (see (Jankord and Herman, 2008) for possible central sites for these mechanisms). In addition, it has been demonstrated in rat models that uncontrollable or unpredictable stress is particularly pernicious and results in a dysregulation of the HPA axis and a number of behavioral and physiological phenotypes that resemble those seen in several human psychopathologies (Kim et al., 2003; Maier and Watkins, 2005; McKinney, 1984; Miller and McEwen, 2006; Roman et al., 2004; Sandi et al., 2008). Social subordination in female rhesus monkeys is both chronic and unpredictable in that while SUB females attempt to offset aggression and harassment from DOM females by displaying submissive behaviors, there is no guarantee that these attempts will be successful (Chamove and Bowman, 1976; Cheney and Seyfarth, 1990; Estrada, 1977; Riddick et al., 2009). Hence, this social structure might produce a situation in which similar types of HPA dysregulation as those observed in human psychopathology may occur.
An unexpected result of this study were data showing that the normal circadian rhythm of basal CORT release was not present in both DOM and SUB female monkeys in the absence of DHT. Although, It has been shown that androgens are important at puberty for calibrating the regulation of circadian cycles related to changes in activity level in male rodents, and in adolescent boys (Hagenauer et al.; Hagenauer et al., 2009), and that the diurnal rhythm of CORT is absent following long-term castration in male macaques (Smith and Norman, 1987b), this is the first study in which the presence of androgens appears to be important in regulating circadian CORT release in female monkeys. Unlike the study mentioned above showing that castration disrupts circadian CORT release in male macaques (Smith and Norman, 1987b), we found an intact diurnal release of CORT in out gonadally-suppressed male monkeys. This difference may be due to the comparatively short length of time that our males were without androgens, and suggests that long-term absence of gonadal hormones may have to occur before a disruption of circadian CORT release develops in male, as opposed to female, monkeys. A study looking at the effect of ovariectomy on circadian CORT in female macaques showed that although absolute levels of CORT were lower in OVX females, there was no disruption in the circadian CORT rhythm (Smith and Norman, 1987a). However, this study was confounded by a rise in mid-day CORT which may have been caused by the feeding or room-cleaning that took place at noon throughout the experiment. This rise in CORT may have activated negative–feedback mechanisms that would have produced a decline in evening levels of CORT which may have obscured accurate detection of basal CORT changes. Results from our study suggest that androgens are particularly important in CORT circadian release in female macaques, in that the short-term absence of androgens disrupts diurnal CORT pattern and short-term DHT replacement restores the normal diurnal rhythm. It is also important to note that changes in circadian rhythms are often observed in depressive illnesses (Bao et al., 2008; Rubin et al., 1987; Swaab et al., 2005), which disproportionately affect women (Solomon and Herman, 2009; Steiner et al., 2003). Thus, the regulation of circadian CORT by androgens in female Rhesus macaques may have important clinical relevance.
It has previously been shown using cynomolgus monkeys that SUB female monkeys are less sensitive to glucocorticoid feedback inhibition than DOM females (Shively et al., 1997), and we have previously shown, using the same group of female monkeys as in the present study, that estrogen mediates a reduction of negative feedback, which is exaggerated in SUB females (Wilson et al., 2005). In female rats estrogen has also been shown to inhibit glucocorticoid negative-feedback through an estrogen receptor alpha (ERα) mediated mechanism (Weiser and Handa, 2009). Our results do not indicate a reduction in feedback inhibition under any androgen treatment regime that is associated with gender or social status. Although data here suggest that placebo-replaced SUB females do not show disrupted feedback inhibition of CORT following DEX, this was confounded by the flattening of circadian CORT levels in the placebo-replaced SUB females. This flattening of CORT rhythm may have produced an endogenous drop from the previous night even in the absence of DEX treatment. Thus, it is difficult to disentangle the direct effect of feedback inhibition from the effects of the disruption of circadian CORT secretion. The same is true for determining feedback inhibition in DOM females in the placebo-replaced state.
In summary, results indicate that DHT, but not testosterone, significantly reduces basal CORT in male rhesus monkeys. DHT also significantly reduces basal CORT in female rhesus monkeys regardless of social status. CRF-stimulated CORT release is reduced only in male monkeys. In addition, DHT plays a role in maintaining circadian changes in CORT release in female monkeys. This study opens the door towards further investigation of the mechanisms underlying the effects of androgens on the HPA axis of rhesus monkeys, especially sex differences in androgen control of HPA axis that may inform the study of human psychiatric illnesses. In addition, this study suggests that androgens are particularly important in daily basal CORT secretion in female monkeys, which may be important in psychopathologies, like clinical depression, which show a dysregulation of circadian rhythms, and are more prevalent in women than in men.
4. Experimental Procedures
4.1 Subjects
Seven adult, gonadally intact, male and seven gonadally-intact female rhesus monkeys (Macaca mulatta), housed outside in a social group at the Yerkes National Primate Research Center Field Station were used for these studies. The seven males were housed in one of seven small groups containing adult females and their offspring. The subject males were the alpha animals in each of their groups. The seven female subjects were members of one large breeding group (n = 140 animals) that contained 2 adult males, 30 additional adult females, and their juvenile and infant offspring. The seven female subjects (see below) were chosen to span the dominance hierarchy in their group. Body weight did not significantly differ between dominant (mean body weight 7.59+/− 0.58 kg) and subordinate (mean body weight 7.4 +/− 0.82 kg) during the course of these experiments Data on the effects of estradiol and progesterone on HPA activity in these females has been reported previously (Wilson et al 2005).
Social ranks were determined from the outcome of dyadic interactions between the subject females themselves and other group members (Kaplan and Manuck, 1999). Behavior was recorded in two 1- h sessions each week randomly distributed between the morning and afternoon throughout the study (including both treatment and washout periods). Using a standard rhesus monkey ethogram (Jarrell et al., 2008), an animal was categorized as subordinate (SUB) if she emitted unequivocal submission gestures to another animal, including avoidance to an approach, flee from a chase, grimace from an approach or stare, and squeal from a threat. Females were selected as subjects to represent a range of dominance ranks. Excluding infants, subjects were ranked 3, 18, 23, 54, 74, 76, and 100 out of 115 possible animals following previously established protocols (Kaplan and Manuck, 1999). Those subjects whose rank was in the upper half of the dominance hierarchy (n = 4) were considered dominant (DOM) and those in the bottom half (n = 3) SUB. Thus, the subject pool contained both male and female dominant animals but only female subordinate animals. Because all of the males used in this study were the most dominant members of each of their groups, we could not assess the effects of status in males.
Animals were fed commercial monkey chow (Lab Diet, number 5038, Purina Mills International) twice daily and, but food was available ad libitum, and all animals received a daily supplement of fresh fruit and vegetables. Animals were trained for conscious venipuncture as described previously (Walker et al., 1982). Animals readily habituate and show minimal arousal to blood drawing procedures (Blank et al., 1983). It has been well demonstrated that no adverse consequences on reproductive parameters or neuroendocrine responsivity result from these test procedures (Wilson et al., 2003). The protocol was approved by the Emory University Institutional Animal Care and Use Committee in accordance with the Animal Welfare Act and the US Department of Health and Human Services “Guide for Care and Use of Laboratory Animals.”
4.2 Gonadal hormone suppression and androgen replacement
In order to assess the effects of DHT on HPA responsivity, the pituitary–gonadal axis was suppressed by treating all animals every 28 days with the long-acting GnRH agonist, Lupron Depot (Tap Pharmaceuticals, Deerfield IL) at a dose (injected sc 200 μg/kg/28 d) that completely arrests ovarian function in women (Berman et al., 1997), precocious puberty in girls (Periti et al., 2002) and spontaneous puberty in female monkeys (Wilson et al 2006). Treatment efficacy was verified by hormonal analyses for T, and DHT. Lupron Depot treatments were begun 6 wk prior to the initiation of the hormone replacement to ensure the pituitary–gonadal axis was completely suppressed. The HPA axis was assessed in male and female monkeys under placebo (sham-implantation:no steroid treatment), testosterone for males only (low-dose: 180 μg/kg/day high-dose: 360 μg/kg/day) and sex-specific doses of DHT: Low-dose DHT (15 μg/kg/day for females, 180 μg/kg/day for males) and high-dose DHT (30 μg/kg/day for females, 360 μg/kg/day for males). Androgen replacement doses were used to produce T levels in the low and high physiological range (Schlatt et al., 2008). DHT was applied at the same dose to produce similar receptor activation. The doses of DHT for females were adjusted to produce low and high plasma DHT levels under those shown to elicit male-like behavioral changes in female macaques(Graves and Wallen, 2006). Steroid hormone replacement was accomplished by subcutaneous placement of 21-d, sustained-release pellets (Innovative Research of America, Sarasota FL). Levels of testosterone and DHT were assessed on day 10 of the treatment period. Each 21-d steroid treatment was separated by a 21-d washout period. The order of androgen treatment was randomly determined.
4.3 The dexamethasone (DEX)-CRF challenge test
A combined dexamethasone (DEX) suppression test–CRF stimulation test was performed day 10 and 11 of each treatment period in order to test HPA responsivity. Following the serum sample collected at 2100 h, monkeys received dexamethasone (DEX) at a dose of 0.50 mg/kg, im and serum samples were obtained at 10, 15, and 19 h thereafter. Immediately following the +19 h sample, monkeys received CRF at a dose of 1.0 μg/kg, iv and samples were collected at +30, +60, and +120 min thereafter. DEX at this dose has been used previously to assess glucocorticoid negative feedback in monkeys (Shively et al., 1997). The dose of CRF stimulates ACTH release in people (Thomas et al., 1991). All samples were assayed for CORT.
4.4 Hormone assays
Assays were performed in the Biomarkers Core Laboratory at the Yerkes National Primate Research Center. These assays have been used extensively to assay hormens in rhesus monkeys. Serum T and DHT were determined using a modification (Pazol et al., 2004)of a commercially available radioimmunoassay [Diagnostic Products Corporation (DPC), Los Angeles CA]. Prior to assay, samples (250 μl) were extracted twice with 5 mL of anesthesia grade ether. Following evaporation of the solvent, samples were reconstituted with 250 μl of zero calibrator and 100 μL aliquots were assayed in duplicate. The assay has a sensitivity of 5 pg/mL using 100 μL of extracted serum, with an intra- and interassay coefficient of variation (CV) of 5.8% and 11.7%, respectively. Sample values of DHT were corrected for extraction efficiency, which exceeded 95%. Serum cortisol was determined by radioimmunoassay of 25 μL duplicates using commercially available reagents (Diagnostic Systems Laboratory). The assay has a sensitivity of 0.02 μg/dL and an intra- and interassay CV of 3.1% and 7.6%, respectively. Serum ACTH was determined by radioimmunoassay of 100 μL duplicates using commercially available reagents (DiaSorin, Stillwater, MN). The assay has a sensitivity of 4.5 pg/mL and an intra- and interassay CV of 7.1% and 12.4%, respectively.
4.5 Statistical Analysis
CORT data were analyzed with analysis of variance models for repeated measures with group (males, dominant females and subordinate females) as the between subject factors and androgen treatment and time as within-subject factors. We did separate analysis to compare effects on basal, feedback inhibition and CRF-induced CORT. In addition, within-subject drug (DEX or CRF) and time effects were analyzed separately for males, DOM and SUB females for basal and CRF-stimulated CORT secretion.
The effect of androgen treatment between and within experimental groups was assessed by examining each time period as described below: Basal CORT was evaluated by comparing combined hormone values collected at 9 a.m. and 9 p.m. on treatment day 10 (TD 10) before any pharmacological manipulation of the HPA axis was initiated. The effect of treatment condition between and within experimental groups on glucocorticoid negative feedback was evaluated by comparing the hormone values between males, DOM and SUB females from samples collected at minus 0, 10, 15 and 19 h following DEX on TD 10 and 11 holding pre-DEX basal values for each group (0 hr) as a covariate. The effect of treatment condition on the response to CRF was evaluated both between and within experimental groups by comparing hormone values from samples collected at 30, 60 and 120 min following CRF injection (given immediately after the 4 p.m. sample on treatment TD 11).
Statistical tests were performed with SPSS (v18) and results with p < 0.05 were considered significant. Post-hoc analysis was done where appropriate using LSD analysis.
Highlights.
Dihydrotestosterone significantly reduces basal cortisol secretion in male and female rhesus monkeys
Stimulated cortisol release is reduced by dihydrotestosterone only in male monkeys
Dihydrotestosterone plays a role in maintaining circadian changes in cortisol release in female monkeys.
Acknowledgments
We acknowledge the expert technical assistance of Kathy Chikazawa and Ariadne Legendre. This work was supported by, HD46501, MH081816, and, in part RR00165. The YNPRC is fully accredited by AAALAC, International.
Abbreviations
- HPA
hypothalamic pituitary adrenal axis
- CORT
cortisol
- DOM
Dominant social rank
- SUB
subordinate social rank
- DEX
dexamethosone
- T
testosterone
- DHT
dihydrotestosterone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology. 1992;131:57–68. doi: 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Tlemcani O, Ball GF. Do sex differences in the brain explain sex differences in the hormonal induction of reproductive behavior? What 25 years of research on the Japanese quail tells us. Horm Behav. 1996;30:627–61. doi: 10.1006/hbeh.1996.0066. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–53. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Berman KF, Schmidt PJ, Rubinow DR, Danaceau MA, Van Horn JD, Esposito G, Ostrem JL, Weinberger DR. Modulation of cognition-specific cortical activity by gonadal steroids: a positron-emission tomography study in women. Proc Natl Acad Sci U S A. 1997;94:8836–41. doi: 10.1073/pnas.94.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein IS. Activity patterns in pigtail monkey groups. Folia Primatol (Basel) 1970;12:187–98. doi: 10.1159/000155288. [DOI] [PubMed] [Google Scholar]
- Bernstein IS, Gordon TP, Rose RM. Aggression and social controls in rhesus monkey (Macaca mulatta) groups revealed in group formation studies. Folia Primatol (Basel) 1974;21:81–107. doi: 10.1159/000155607. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Dominance, aggression and reproduction in primate societies. J Theor Biol. 1976;60:459–72. doi: 10.1016/0022-5193(76)90072-2. [DOI] [PubMed] [Google Scholar]
- Bernstein IS. Activity patterns in a stumptail macaque group (Macaca arctoides) Folia Primatol (Basel) 1980;33:20–45. doi: 10.1159/000155926. [DOI] [PubMed] [Google Scholar]
- Blank MS, Gordon TP, Wilson ME. Effects of capture and venipuncture on serum levels of prolactin, growth hormone and cortisol in outdoor compound-housed female rhesus monkeys (Macaca mulatta) Acta Endocrinol (Copenh) 1983;102:190–5. doi: 10.1530/acta.0.1020190. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Pierce BN, Clarke IJ, Canny BJ. Role of sex and sex steroids in mediating pituitary-adrenal responses to acute buspirone treatment in sheep. J Neuroendocrinol. 2005a;17:804–10. doi: 10.1111/j.1365-2826.2005.01368.x. [DOI] [PubMed] [Google Scholar]
- Broadbear JH, Winger G, Woods JH. Self-administration of methohexital, midazolam and ethanol: effects on the pituitary-adrenal axis in rhesus monkeys. Psychopharmacology (Berl) 2005b;178:83–91. doi: 10.1007/s00213-004-1986-4. [DOI] [PubMed] [Google Scholar]
- Brooke SM, de Haas-Johnson AM, Kaplan JR, Manuck SB, Sapolsky RM. Dexamethasone resistance among nonhuman primates associated with a selective decrease of glucocorticoid receptors in the hippocampus and a history of social instability. Neuroendocrinology. 1994;60:134–40. doi: 10.1159/000126743. [DOI] [PubMed] [Google Scholar]
- Chamove AS, Bowman RE. Rank, rhesus social behavior, and stress. Folia Primatol (Basel) 1976;26:57–66. doi: 10.1159/000155730. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. The representation of social relations by monkeys. Cognition. 1990;37:167–96. doi: 10.1016/0010-0277(90)90022-c. [DOI] [PubMed] [Google Scholar]
- Estrada A. A study of the social relationships in a free-ranging troop of stumptail macaques (Macaca arctoides) Bol Estud Med Biol. 1977;29:313–94. [PubMed] [Google Scholar]
- Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105:53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Koonce CJ, Edinger KL, Osborne DM, Walf AA. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm Behav. 2008;54:726–34. doi: 10.1016/j.yhbeh.2008.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M. Distribution of sex steroid hormone receptors in the avian brain: functional implications for neural sex differences and sexual behaviors. Microsc Res Tech. 2001;55:1–11. doi: 10.1002/jemt.1151. [DOI] [PubMed] [Google Scholar]
- Gonzalez M, Cabrera-Socorro A, Perez-Garcia CG, Fraser JD, Lopez FJ, Alonso R, Meyer G. Distribution patterns of estrogen receptor alpha and beta in the human cortex and hippocampus during development and adulthood. J Comp Neurol. 2007;503:790–802. doi: 10.1002/cne.21419. [DOI] [PubMed] [Google Scholar]
- Graves FC, Wallen K. Androgen-induced yawning in rhesus monkey females is reversed with a nonsteroidal anti-androgen. Horm Behav. 2006;49:233–6. doi: 10.1016/j.yhbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- Hagenauer MH, Ku JH, Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Horm Behav. 60:37–45. doi: 10.1016/j.yhbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Perryman JI, Lee TM, Carskadon MA. Adolescent changes in the homeostatic and circadian regulation of sleep. Dev Neurosci. 2009;31:276–84. doi: 10.1159/000216538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Reid DL, Resko JA. Androgen receptors in brain and pituitary of female rats: cyclic changes and comparisons with the male. Biol Reprod. 1986;34:293–303. doi: 10.1095/biolreprod34.2.293. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Roselli CE, Resko JA. Distribution of androgen receptor in microdissected brain areas of the female baboon (Papio cynocephalus) Brain Res. 1988;445:111–6. doi: 10.1016/0006-8993(88)91079-7. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Nunley KM, Lorens SA, Louie JP, McGivern RF, Bollnow MR. Androgen regulation of adrenocorticotropin and corticosterone secretion in the male rat following novelty and foot shock stressors. Physiol Behav. 1994;55:117–24. doi: 10.1016/0031-9384(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Horm Behav. 2008;53:741–52. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta,17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J Neuroendocrinol. 2009;21:351–8. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hileman SM, Handa RJ, Jackson GL. Distribution of estrogen receptor-beta messenger ribonucleic acid in the male sheep hypothalamus. Biol Reprod. 1999;60:1279–84. doi: 10.1095/biolreprod60.6.1279. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Kruijver FP, Balesar R, Swaab DF. Differential expression of estrogen receptor alpha and beta immunoreactivity in the human supraoptic nucleus in relation to sex and aging. J Clin Endocrinol Metab. 2000;85:3283–91. doi: 10.1210/jcem.85.9.6826. [DOI] [PubMed] [Google Scholar]
- Ishunina TA, Kamphorst W, Swaab DF. Changes in metabolic activity and estrogen receptors in the human medial mamillary nucleus: relation to sex, aging and Alzheimer’s disease. Neurobiol Aging. 2003;24:817–28. doi: 10.1016/s0197-4580(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Iwasaki-Sekino A, Mano-Otagiri A, Ohata H, Yamauchi N, Shibasaki T. Gender differences in corticotropin and corticosterone secretion and corticotropin-releasing factor mRNA expression in the paraventricular nucleus of the hypothalamus and the central nucleus of the amygdala in response to footshock stress or psychological stress in rats. Psychoneuroendocrinology. 2009;34:226–37. doi: 10.1016/j.psyneuen.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell H, Hoffman JB, Kaplan JR, Berga S, Kinkead B, Wilson ME. Polymorphisms in the serotonin reuptake transporter gene modify the consequences of social status on metabolic health in female rhesus monkeys. Physiol Behav. 2008;93:807–19. doi: 10.1016/j.physbeh.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Status, stress, and atherosclerosis: the role of environment and individual behavior. Ann N Y Acad Sci. 1999;896:145–61. doi: 10.1111/j.1749-6632.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Whang WW, Kim HT, Pyun KH, Cho SY, Hahm DH, Lee HJ, Shim I. Expression of neuropeptide Y and cholecystokinin in the rat brain by chronic mild stress. Brain Res. 2003;983:201–8. doi: 10.1016/s0006-8993(03)03087-7. [DOI] [PubMed] [Google Scholar]
- Longcope C. Adrenal and gonadal androgen secretion in normal females. Clin Endocrinol Metab. 1986;15:213–28. doi: 10.1016/s0300-595x(86)80021-4. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Linkroum W, Sallinen BJ, Miller NW. Peripheral and central sex steroids have differential effects on the HPA axis of male and female rats. Stress. 2002;5:235–47. doi: 10.1080/1025389021000061165. [DOI] [PubMed] [Google Scholar]
- McKinney WT. Animal models of depression: an overview. Psychiatr Dev. 1984;2:77–96. [PubMed] [Google Scholar]
- Miller MM, McEwen BS. Establishing an agenda for translational research on PTSD. Ann N Y Acad Sci. 2006;1071:294–312. doi: 10.1196/annals.1364.023. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. An introduction to behavioral endocrinology. Sinauer Associates; Sunderland, Mass: 2005. p. xvii.p. 822.p. 111. [Google Scholar]
- Ogilvie KM, Rivier C. Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in the rat: activational role of gonadal steroids. Brain Res. 1997;766:19–28. doi: 10.1016/s0006-8993(97)00525-8. [DOI] [PubMed] [Google Scholar]
- Patchev VK, Almeida OF. Gonadal steroids exert facilitating and “buffering” effects on glucocorticoid-mediated transcriptional regulation of corticotropin-releasing hormone and corticosteroid receptor genes in rat brain. J Neurosci. 1996;16:7077–84. doi: 10.1523/JNEUROSCI.16-21-07077.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazol K, Kaplan JR, Abbott D, Appt SE, Wilson ME. Practical measurement of total and bioavailable estradiol in female macaques. Clin Chim Acta. 2004;340:117–26. doi: 10.1016/j.cccn.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Periti P, Mazzei T, Mini E. Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet. 2002;41:485–504. doi: 10.2165/00003088-200241070-00003. [DOI] [PubMed] [Google Scholar]
- Plant TM. Time courses of concentrations of circulating gonadotropin, prolactin, testosterone, and cortisol in adult male rhesus monkeys (Macaca mulatta) throughout the 24 h light-dark cycle. Biol Reprod. 1981;25:244–52. doi: 10.1095/biolreprod25.2.244. [DOI] [PubMed] [Google Scholar]
- Quabbe HJ, Gregor M, Bumke-Vogt C, Hardel C. Pattern of plasma cortisol during the 24-hour sleep/wake cycle in the rhesus monkey. Endocrinology. 1982;110:1641–6. doi: 10.1210/endo-110-5-1641. [DOI] [PubMed] [Google Scholar]
- Riddick NV, Czoty PW, Gage HD, Kaplan JR, Nader SH, Icenhower M, Pierre PJ, Bennett A, Garg PK, Garg S, Nader MA. Behavioral and neurobiological characteristics influencing social hierarchy formation in female cynomolgus monkeys. Neuroscience. 2009;158:1257–65. doi: 10.1016/j.neuroscience.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman O, Seres J, Pometlova M, Jurcovicova J. Neuroendocrine or behavioral effects of acute or chronic emotional stress in Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats. Endocr Regul. 2004;38:151–5. [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM, Winston RA, Blodgett AL. Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Arch Gen Psychiatry. 1987;44:328–36. doi: 10.1001/archpsyc.1987.01800160032006. [DOI] [PubMed] [Google Scholar]
- Sakuma S, Tokuhara D, Hattori H, Matsuoka O, Yamano T. Expression of estrogen receptor alpha and beta in reactive astrocytes at the male rat hippocampus after status epilepticus. Neuropathology. 2009;29:55–62. doi: 10.1111/j.1440-1789.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack K, Grand AP, Fulks R, Graff A, Maestripieri D. Effects of sex and early maternal abuse on adrenocorticotropin hormone and cortisol responses to the corticotropin-releasing hormone challenge during the first 3 years of life in group-living rhesus monkeys. Dev Psychopathol. 22:45–53. doi: 10.1017/S0954579409990253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Cordero MI, Ugolini A, Varea E, Caberlotto L, Large CH. Chronic stress-induced alterations in amygdala responsiveness and behavior--modulation by trait anxiety and corticotropin-releasing factor systems. Eur J Neurosci. 2008;28:1836–48. doi: 10.1111/j.1460-9568.2008.06451.x. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Pohl CR, Ehmcke J, Ramaswamy S. Age-related changes in diurnal rhythms and levels of gonadotropins, testosterone, and inhibin B in male rhesus monkeys (Macaca mulatta) Biol Reprod. 2008;79:93–9. doi: 10.1095/biolreprod.107.066126. [DOI] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Harbuz MS, Lightman SL. Gonadal steroid replacement reverses gonadectomy-induced changes in the corticosterone pulse profile and stress-induced hypothalamic-pituitary-adrenal axis activity of male and female rats. J Neuroendocrinol. 2004;16:989–98. doi: 10.1111/j.1365-2826.2004.01258.x. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology of Macaca fascicularis. Physiol Behav. 1984;33:777–82. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–82. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior, and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–91. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Norman RL. Influence of the gonads on cortisol secretion in female rhesus macaques. Endocrinology. 1987a;121:2192–8. doi: 10.1210/endo-121-6-2192. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Norman RL. Circadian periodicity in circulating cortisol is absent after orchidectomy in rhesus macaques. Endocrinology. 1987b;121:2186–91. doi: 10.1210/endo-121-6-2186. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Herman JP. Sex differences in psychopathology: of gonads, adrenals and mental illness. Physiol Behav. 2009;97:250–8. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner M, Dunn E, Born L. Hormones and mood: from menarche to menopause and beyond. J Affect Disord. 2003;74:67–83. doi: 10.1016/s0165-0327(02)00432-9. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–94. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Thomas MA, Rebar RW, LaBarbera AR, Pennington EJ, Liu JH. Dose-response effects of exogenous pulsatile human corticotropin-releasing hormone on adrenocorticotropin, cortisol, and gonadotropin concentrations in agonadal women. J Clin Endocrinol Metab. 1991;72:1249–54. doi: 10.1210/jcem-72-6-1249. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. The inhibitory effect of testosterone on hypothalamic-pituitary-adrenal responses to stress is mediated by the medial preoptic area. J Neurosci. 1996;16:1866–76. doi: 10.1523/JNEUROSCI.16-05-01866.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viau V, Soriano L, Dallman MF. Androgens alter corticotropin releasing hormone and arginine vasopressin mRNA within forebrain sites known to regulate activity in the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol. 2001;13:442–52. doi: 10.1046/j.1365-2826.2001.00653.x. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and -adrenal axes. J Neuroendocrinol. 2002;14:506–13. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Walker ML, Gordon TP, Wilson ME. Reproductive performance in capture-acclimated female rhesus monkeys (Macaca mulatta) J Med Primatol. 1982;11:291–302. [PubMed] [Google Scholar]
- Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta activation prevents glucocorticoid receptor-dependent effects of the central nucleus of the amygdala on behavior and neuroendocrine function. Brain Res. 1336:78–88. doi: 10.1016/j.brainres.2010.03.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser MJ, Handa RJ. Estrogen impairs glucocorticoid dependent negative feedback on the hypothalamic-pituitary-adrenal axis via estrogen receptor alpha within the hypothalamus. Neuroscience. 2009;159:883–95. doi: 10.1016/j.neuroscience.2008.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Viau V. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1239–48. doi: 10.1016/j.pnpbp.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Williamson M, Viau V. Selective contributions of the medial preoptic nucleus to testosterone-dependant regulation of the paraventricular nucleus of the hypothalamus and the HPA axis. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1020–30. doi: 10.1152/ajpregu.90389.2008. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Chikazawa K, Yoda R, Legendre A, Mook D, Gould KG. Leptin administration increases nocturnal concentrations of luteinizing hormone and growth hormone in juvenile female rhesus monkeys. J Clin Endocrinol Metab. 2003;88:4874–83. doi: 10.1210/jc.2003-030782. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Legendre A, Pazol K, Fisher J, Chikazawa K. Gonadal steroid modulation of the limbic-hypothalamic- pituitary-adrenal (LHPA) axis is influenced by social status in female rhesus monkeys. Endocrine. 2005;26:89–97. doi: 10.1385/ENDO:26:2:089. [DOI] [PubMed] [Google Scholar]