Allogeneic hematopoietic cell transplantation (HCT), which is a treatment option for leukemia and lymphoma (reviewed in[1]), represents a form of adoptive T cell therapy (the graft-versus-tumor [GVT] effect). The majority of allogeneic transplants are performed using un-manipulated donor T cells, although as we will describe below, ex vivo manipulated donor T cell therapy represents a promising new area of clinical research. And, in the autologous setting, ex vivo expanded T cells, in particular T cells that are gene-modified to enhance tumor targeting or avoid viral infection, have been evaluated in a diversity of diseases including melanoma and sarcoma[2], leukemia [3], and HIV disease[4] [5]. Successful adoptive T cell therapy in part requires that the transferred T cells: (1) differentiate into a favorable T cell subset, which may minimally include Th1, Th2, TREG, or Th17 populations depending on the clinical context [6]; and (2) persist in vivo [7]. In this review, we will focus on the ex vivo use of rapamycin to generate functionally defined T cell subsets that possess a favorable pattern of in vivo persistence after adoptive transfer. Current results indicate that rapamycin induces autophagy during ex vivo T cell differentiation, with resultant T cell acquisition of an anti-apoptotic phenotype that confers in vivo persistence.
Autophagy is triggered during cellular stresses such as starvation, growth factor withdrawal, or through rapamycin pharmacologic blockade of mTOR [8], which is a central gatekeeper of cell signaling pathways and cellular energetics. Three types of autophagy exist: (1) macroautophagy, which involves the enclosure of cytoplasm, cytoplasmic protein aggregates, or whole organelles in a double membrane bound structure that is degraded by lysosomes; (2) microautophagy, which is the capture of cytoplasmic contents in small vesicles that bud directly into lysosomes; and (3) chaperone-mediated autophagy, which involves translocation of proteins containing a target sequence into the lysosomal membrane. Rapamycin primarily results in macroautophagy (hereafter referred to as autophagy) that occurs in three stages: (1) initiation, which involves the formation of an isolation membrane around the cytoplasm; (2) maturation, where membrane expansion results in the formation of an autophagosomal double membrane; and (3) degradation in the lysosomes. These three events are successfully orchestrated by various molecules such as the ATG series of proteins and beclin-1, with mTOR serving as a distal autophagy gatekeeper (mTOR on: autophagy inhibition; mTOR off: autophagy promotion) (reviewed in [9]).
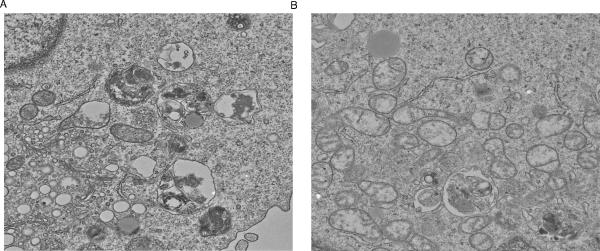
Given the importance of autophagy in determining T cell survival (reviewed in [10]), we evaluated whether rapamycin exposure during ex vivo T cell culture induced autophagy and modulated T cell survival. For these studies, we evaluated human CD4+ T cells that were polarized to a Th1 cytokine phenotype. Indeed, as shown in the electron microscopy images (Fig 1 (A and B), human T cells manufactured ex vivo in rapamycin were replete with autophagosomes; consistent with an autophagy mechanism, T cells knocked-down for Beclin-1 had reduced autophagosome formation during mTOR blockade. Initially, rapamycin was characterized as an agent that induces T cell anergy even in the presence of co-stimulation [11]; in this light, it was somewhat paradoxical that the post-autophagy, rapamycin-resistant T cells that we evaluated had increased in vivo function upon adoptive transfer into a human-into-mouse model of xenogeneic graft-versus-host disease (x-GVHD) [12]. In these experiments, we also found that rapamycin-resistant T cells underwent mitochondrial autophagy (mitophagy), thereby resulting in reduced T cell mitochondrial mass but increased mitochondrial function (more stable mitochondrial membrane potential). These results extended previous findings in human cell lines, where it was observed that autophagy altered mitochondrial biology in T cells, including a reduction in mitochondrial mass [13].
Fig 1. Rapamycin induces autophagy in human Th1 cell.
CD4+ T cells were co-stimulated with anti-CD3, anti-CD28 and cultured for 6 days in media containing rhlL2 (20 IU/ml), INF-α2b (1 M IU/ml), and rapamycin. (A) Resultant CD4+ Th1 cells expressed autophagosomes by electron microscopy. (B) Culture initiation with CD4+ T cells knocked-down for Beclin-1 expression by siRNA methodology had greatly reduced autophagy.
In these experiments, we also observed that ex vivo manufacturing in rapamycin decreased T cell free oxygen radical formation, was associated with reduced T cell expression of pro-apoptotic bcl-2 family gene members (BAX and BAK), and yielded an anti-apoptotic phenotype in rapamycin-resistant T cells[12]. We found that the anti-apoptotic phenotype of rapamycin-resistant T cells was relatively optimized: that is, such T cells had similar engraftment and persistence characteristics as transgenic T cells over-expressing anti-apoptotic bcl-2. Importantly, we observed a nearly all-or-nothing phenomenon with respect to human T cell engraftment and persistence in the x-GVHD model: that is, rapamycin-resistant T cells (but not control, co-stimulated T cells) engrafted and persisted for months after adoptive transfer. In addition, such long-term engrafting, rapamycin-resistant human T cells vigorously engrafted upon transfer to secondary immune-deficient murine hosts (S. Amarnath, unpublished observation); this latter observation is consistent with a conclusion that ex vivo rapamycin promoted long-term memory T cell characteristics. These results run parallel to findings in models of malignant glioma[14] and tuberous sclerosis complex[15], where autophagy promotes tumor cell clonogenicity.
We have found that post-autophagy, rapamycin-resistant T cells can be manufactured in both Th1- and Th2-polarizing conditions [16], thereby offering diverse opportunities for clinical translation. In this study, we found that ex vivo rapamycin promoted a T central memory (TCM) phenotype; this result is consistent with the known linkage of mTOR and T cell homing through modulation of KLF2 transcription factor levels[17] and with findings of the superior in vivo persistence of TCM cells [18]. An ability to polarize towards both Th1 and Th2 phenotypes in the presence of high-dose rapamycin contrasts somewhat with results obtained using T cells genetically-deficient in TORC1/TORC2, where mTOR status was shown to influence T cell polarization [19]; and, our findings differ somewhat from studies using T cell culture without polarizing cytokine addition, where rapamycin was shown to promote TREG cell differentiation [20]. Because of the association of Th1-type cells with GVHD[21], rapamycin-resistant T cells may have application primarily in the autologous transplantation setting; along these lines, we have sponsored an Investigational New Drug Application (IND) with the U.S. Food and Drug Administration for evaluation of rapamycin-resistant Th1 cells for immune reconstitution after autologous HCT therapy of multiple myeloma (clinical trial registration, NCT 01239368).
On the other hand, rapamycin-resistant Th2-type cells may have clinical translational application in the setting of allogeneic transplantation. We found that allogeneic murine Th2 cells manufactured ex vivo in rapamycin prevented GVHD in an IL-4 dependent manner, and when combined with T cells that induced a Th1-type response, could yield a favorable balance between GVHD protection and mediation of GVT effects[22]. Furthermore, we found that anti-apoptotic, rapamycin-resistant Th2-type cells prevented murine hematopoietic cell graft rejection [23]; in other studies, we demonstrated that host-based therapy with rapamycin-generated Th2 cells prevented graft rejection in a rat model of cardiac transplantation [24]. Based on these results, we have initiated a pilot clinical trial of rapamycin-resistant human Th2 cells in the setting of low-intensity allogeneic HCT for therapy of refractory hematologic malignancy (NCT 00074490). Initial results, presented in abstract form [25, 26], indicate that this approach can promote alloengraftment and preserve anti-tumor effects with a low rate of GVHD.
In conclusion, through use of ex vivo methods of T cell expansion that incorporate rapamycin and polarizing cytokines, autophagy can be harnessed for the generation of Th1- or Th2-type T cells that mediate increased in vivo effects upon adoptive transfer. This flexibility in generation of cross-regulatory Th1/Th2 subsets indicates that this approach may have application for both autologous and allogeneic transplantation. The beneficial effects of autophagy on T cell function are certainly complex, and are likely mediated at least in part through alterations in mitochondrial biology, apoptotic tendency, and effector maturation. Further studies will be required to more fully characterize the role of autophagy for enhancement of T cell function and to evaluate whether alternative pro-autophagy agents may work in a different or synergistic manner with rapamycin.
Acknowledgements
The authors would like to thank Dr. Francis A Flomerfelt, Experimental Transplantation Immunology Branch, National Cancer Institute, National Institute of Health for his expertise in Electron Microscopy.
Footnotes
Financial Disclosures The authors have no relevant financial disclosures with respect to the subject matter or materials discussed in this manuscript.
BIBLIOGRAPHY
- 1.Chakraverty R, Mackinnon S. Allogeneic transplantation for lymphoma. J Clin Oncol. 2011;29(14):1855–1863. doi: 10.1200/JCO.2010.32.8419. [DOI] [PubMed] [Google Scholar]
- 2.Robbins Pf, Morgan Ra, Feldman Sa, et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol. 2011;29(7):917–924. doi: 10.1200/JCO.2010.32.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Porter Dl, Levine Bl, Kalos M, Bagg A, June Ch. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine Bl, Bernstein Wb, Aronson Ne, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nat Med. 2002;8(1):47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 5.Heslop He, Slobod Ks, Pule Ma, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'shea Jj, Paul We. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. doi: 10.1126/science.1178334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinrichs Cs, Borman Za, Gattinoni L, et al. Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood. 2011;117(3):808–814. doi: 10.1182/blood-2010-05-286286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kundu M, Thompson Cb. Autophagy: basic principles and relevance to disease. Annu Rev Pathol. 2008;3:427–455. doi: 10.1146/annurev.pathmechdis.2.010506.091842. [DOI] [PubMed] [Google Scholar]
- 9.Walsh Cm, Edinger Al. The complex interplay between autophagy, apoptosis, and necrotic signals promotes T-cell homeostasis. Immunol Rev. 2010;236:95–109. doi: 10.1111/j.1600-065X.2010.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lunemann Jd, Munz C. Autophagy in CD4+ T-cell immunity and tolerance. Cell Death Differ. 2009;16(1):79–86. doi: 10.1038/cdd.2008.113. [DOI] [PubMed] [Google Scholar]
- 11.Powell Jd, Lerner Cg, Schwartz Rh. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162(5):2775–2784. [PubMed] [Google Scholar]
- 12.Amarnath S, Flomerfelt Fa, Costanzo Cm, et al. Rapamycin generates anti-apoptotic human Th1/Tc1 cells via autophagy for induction of xenogeneic GVHD. Autophagy. 2010;6(4) doi: 10.4161/auto.6.4.11811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colell A, Ricci Je, Tait S, et al. GAPDH and autophagy preserve survival after apoptotic cytochrome c release in the absence of caspase activation. Cell. 2007;129(5):983–997. doi: 10.1016/j.cell.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 14.Fan Qw, Cheng C, Hackett C, et al. Akt and autophagy cooperate to promote survival of drug-resistant glioma. Sci Signal. 2010;3(147):ra81. doi: 10.1126/scisignal.2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parkhitko A, Myachina F, Morrison Ta, et al. Tumorigenesis in tuberous sclerosis complex is autophagy and p62/sequestosome 1 (SQSTM1)-dependent. Proc Natl Acad Sci U S A. 2011;108(30):12455–12460. doi: 10.1073/pnas.1104361108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung U, Foley Je, Erdmann Aa, et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 Effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol Blood Marrow Transplant. 2006;12(9):905–918. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 17.Finlay D, Cantrell D. Phosphoinositide 3-kinase and the mammalian target of rapamycin pathways control T cell migration. Ann N Y Acad Sci. 2010;1183:149–157. doi: 10.1111/j.1749-6632.2009.05134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger C, Jensen Mc, Lansdorp Pm, Gough M, Elliott C, Riddell Sr. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118(1):294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell Jd, Delgoffe Gm. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33(3):301–311. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Battaglia M, Stabilini A, Roncarolo Mg. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105(12):4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 21.Fowler Dh, Breglio J, Nagel G, Hirose C, Gress Re. Allospecific CD4+, Th1/Th2 and CD8+, Tc1/Tc2 populations in murine GVL: type I cells generate GVL and type II cells abrogate GVL. Biol Blood Marrow Transplant. 1996;2(3):118–125. [PubMed] [Google Scholar]
- 22.Foley Je, Jung U, Miera A, et al. Ex vivo rapamycin generates donor Th2 cells that potently inhibit graft-versus-host disease and graft-versus-tumor effects via an IL-4-dependent mechanism. J Immunol. 2005;175(9):5732–5743. doi: 10.4049/jimmunol.175.9.5732. [DOI] [PubMed] [Google Scholar]
- 23.Mariotti J, Foley J, Jung U, et al. Ex vivo rapamycin generates apoptosis-resistant donor Th2 cells that persist in vivo and prevent hemopoietic stem cell graft rejection. J Immunol. 2008;180(1):89–105. doi: 10.4049/jimmunol.180.1.89. [DOI] [PubMed] [Google Scholar]
- 24.Amarnath S, Chen H, Foley Je, et al. Host-based Th2 cell therapy for prolongation of cardiac allograft viability. PLoS One. 2011;6(4):e18885. doi: 10.1371/journal.pone.0018885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossoba Me, Mariotti J, Yan Xy, et al. T Rapa Cell Clinical Products Contain a Balance of Minimally Differentiated Th2/Th1 Effector Cells Depleted of Treg Cells. Blood. 2010;116(21):158–159. [Google Scholar]
- 26.Fowler Dh, Mossoba Me, Schuver Bb, et al. Adoptive Transfer of Treg Depleted Donor Th1 and Th2 Cells Safely Accelerates Alloengraftment After Low Intensity Chemotherapy. Blood. 2010;116(21):230–231. [Google Scholar]