Abstract
Rationale
Thioredoxin 1 (Trx1) inhibits pathological cardiac hypertrophy. MicroRNAs (miRNAs) are small non-coding RNAs that downregulate posttranscriptional expression of target molecules.
Objectives
We investigated the role of miRNAs in mediating the anti-hypertrophic effect of Trx1 upon angiotensin II (Ang II)-induced cardiac hypertrophy.
Methods and Results
Microarray analyses of mature rodent microRNAs and qRT-PCR/Northern blot analyses showed that Trx1 upregulates members of the let-7 family, including miR-98, in the heart and the cardiomyocytes (CMs) therein. Adenovirus-mediated expression of miR-98 in CMs reduced cell size both at baseline and in response to Ang II. Knock-down of miR-98, and of other members of the let-7 family, augmented Ang II-induced cardiac hypertrophy and attenuated Trx1-mediated inhibition of Ang II-induced cardiac hypertrophy, suggesting that endogenous miR-98/let-7 mediates the anti-hypertrophic effect of Trx1. Cyclin D2 is one of the predicted targets of miR-98. Ang II significantly upregulated cyclin D2, which in turn plays an essential role in mediating Ang II-induced cardiac hypertrophy, whereas overexpression of Trx1 inhibited Ang II-induced upregulation of cyclin D2. miR-98 decreased both expression of cyclin D2 and the activity of a cyclin D2 3’UTR luciferase reporter, suggesting that both Trx1 and miR-98 negatively regulate cyclin D2. Overexpression of cyclin D2 attenuated the suppression of Ang II-induced cardiac hypertrophy by miR-98, suggesting that the anti-hypertrophic actions of miR-98 are mediated in part by downregulation of cyclin D2.
Conclusions
These results suggest that Trx1 upregulates expression of the let-7 family, including miR-98, which in turn inhibits cardiac hypertrophy, in part through downregulation of cyclin D2.
Keywords: microRNA, thioredoxin, cardiac hypertrophy, cyclin D2
Thioredoxin 1 (Trx1) is a ubiquitously expressed anti-oxidant which has two cysteine residues in its catalytic center 1. When the cysteine residues in Trx1 are reduced in the presence of NADPH and Trx reductase, they are highly reactive with oxidized proteins with a disulfide bond and reduce them via thiol disulfide exchange reactions. Trx1 reacts with a plethora of proteins, thereby controlling a wide variety of cellular functions, including growth, death and inflammation, through transcription, protein-protein interaction and posttranslational modifications 2. One of the most prominent actions of Trx1 in the heart is suppression of cardiac hypertrophy. Overexpression of wild-type Trx1 in mice reduces cardiac hypertrophy induced by pressure-overload, whereas a mutated Trx1 (which inhibits endogenous Trx1 activity) increases hypertrophy 3. Together with its cell protective actions in the heart, Trx1 could be a promising modality to treat pathological hypertrophy and inhibit the progression of heart failure.
Previous investigations by us and other investigators have suggested that Trx1 inhibits pathological hypertrophy through multiple mechanisms. For example, Trx1 inhibits Ras 3 and ASK-1 4, thereby negatively regulating protein kinase cascades known to stimulate hypertrophy. In the nucleus, Trx1 inhibits NF-κB, thereby inhibiting hypertrophy, but stimulates CREB and Nrf1, thereby stimulating cell survival in the heart 5. We have shown recently that Trx1 induces nuclear localization of class II HDACs, through reduction of evolutionarily conserved cysteine residues 6, thereby inhibiting pathological hypertrophy. Judging from the fact that Trx1 is universally protective and affects a wide variety of signaling molecules and transcription factors, we speculated that Trx1 may inhibit cardiac hypertrophy through regulation of microRNA (miRNA) as well.
miRNAs are naturally existing small noncoding RNA molecules ~22nt in length. Mature miRNAs negatively regulate gene expression by either translational repression or mRNA degradation 7. To date, several miRNAs have been identified that affect cardiac hypertrophy and heart failure 8. For example, miR-1 9 and miR-133 10 negatively regulate cardiac hypertrophy, whereas miR-195 11 induces pathological hypertrophy and heart failure. miR-23a and miR-208a also positively regulate cardiac hypertrophy 12, 13, and miR-21 regulates growth and survival of cardiomyocytes and fibroblasts, thereby positively mediating cardiac hypertrophy 14, 15.
In order to evaluate the involvement of miRNAs in mediating the anti-hypertrophic actions of Trx1, we conducted microarray analyses of miRNA. We found that members of the let-7 family, including miR-98, are upregulated in hearts of transgenic mice with cardiac specific overexpression of Trx1 (Tg-Trx1). Since members of the let-7 family inhibit cell growth responses and are recognized as tumor suppressors 16, we hypothesized that the anti-hypertrophic actions of Trx1 in the heart and cardiomyocytes therein are mediated in part by upregulation of miR-98. Let-7 was one of the first miRNAs identified and cloned in C. elegans, and its presence is evolutionarily conserved16. Furthermore, let-7 is downregulated in proliferating cell types, whereas it is relatively abundant in mature tissues 16. However, the function of let-7 is not well understood in the heart.
Thus, the goals of this study were 1) to examine the function of miR-98 in the heart, 2) to test the hypothesis that the anti-hypertrophic actions of Trx1 are mediated in part by miR-98, and 3) to identify the downstream target of miR-98 regulating cardiac hypertrophy.
Methods
Adenoviral vectors
Adenoviruses harboring genes of interest were made using the AdMax system (Microbix). Short hairpin RNA knockdown adenovirus for cyclin D2 or miR-98 was made using the adenoviral shuttle vector pDC311 (Microbix), into which the U6 RNA polymerase III promoter and the polylinker region of pSilencer 1.0U6 expression vector (Ambion) are subcloned.
Sequence of sh-cyclin D2: 5’—3’ AATCGAGGCTGTGCTGCTTAATTCAAGAGATTAAGCAGCACAGCCTCGATTTTTTTT; anti-miR-98: 5’—3’ CGCGTGGGCCCAACAATACAACTTACTACCTCAGCAACAATACAACTTACTACCTCAA
miRNA microarray
Total RNA was isolated from the left ventricles of Tg-Trx1 and NTg mice at 2–3 months old. Ten micrograms of RNA were sent to L.C. Sciences for miRNA microarray 9.
Cell fractionation
Cells were fractionated into cytosol and nucleus using the NE-PER® kit (Pierce).
Northern blot
Total RNA, extracted using TRIzol reagent (Invitrogen), was separated on a 1% agarose gel, transferred to an uncharged nylon membrane, Hybond-NX (Amersham Biosciences), and UV cross-linked. The membrane was pre-hybridized/hybridized with MiracleHyb Hybridization solution according to the instruction manual (Stratagene). DNA oligonucleotides, anti-sense sequences of mature miRNAs, were obtained from Integrated DNA Technologies. The probes were 5’-end labeled with ET adenosine 5’-triphosphate[γ-32P] (PerkinElmer) using a T4 Polynucleotide Kinase kit (NEB) and used for hybridization (1X106cpm/ml).
Transfection and luciferase assay
Cells were transfected using FuGENE 6 transfection reagent (Roche) according to the manufacturer’s instructions. For the 3’UTR luciferase assay, the cyclin D2 3’UTR miR-98 target site (sequence with miR-98 binding sites) or a control site (sequence with mutated miR-98 binding sites) was cloned into the pMIR-REPORT vector (Ambion).
Statistics
All values are expressed as mean±SEM. Statistical analyses were performed using ANOVA or t-test with a P<0.05 considered significant.
Results
Trx1 negatively regulates Angiotensin II (Ang II)-induced cardiac hypertrophy
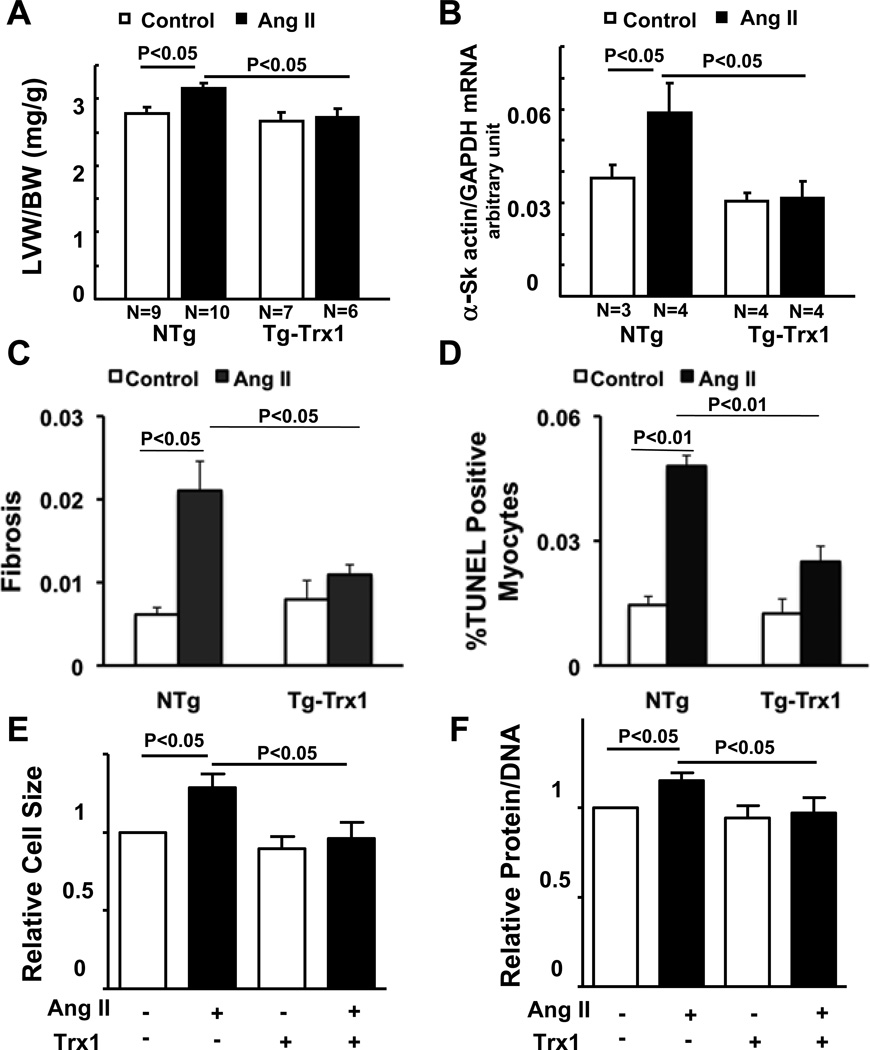
In order to examine whether Trx1 inhibits Ang II-induced cardiac hypertrophy, a subpressor dose of Ang II was continuously infused (200 ng/kg/min) into transgenic mice with cardiac-specific overexpression of Trx1 (Tg-Trx1) 3 and non-transgenic (NTg) mice for 2 weeks. Ang II-induced increases in left ventricular weight/body weight (LVW/BW) and α-skeletal actin expression were abolished in Tg-Trx1 (Fig. 1AB). These results suggest that Trx1 inhibits Ang II-induced cardiac hypertrophy. AngII infusion significantly increased the level of cardiac fibrosis and the number of TUNEL positive myocytes, whereas Trx1 significantly suppressed these pathological changes in the heart (Fig. 1CD, supplemental Fig. S1AB). Treatment of neonatal rat cardiomyocytes with Ang II (100 nM) for 48 hours significantly increased both cell size and protein/DNA content, indicators of cardiac hypertrophy. Transduction of cardiomyocytes with adenovirus harboring Trx1 significantly attenuated Ang II-induced cardiac hypertrophy (Fig. 1EF, supplemental Fig. S1C), suggesting that the anti-hypertrophic effect of Trx1 is cell-autonomous.
Figure 1. Trx1 attenuates Angiotensin II (Ang II)-induced cardiac hypertrophy, fibrosis and apoptosis.
(A–D) Tg-Trx1 and NTg mice were subjected to continuous infusion of either PBS or a subpressor dose (200 ng/kg/min) of Ang II by osmotic pumps for two weeks. (A) Postmortem measurements of left ventricular weight/body weight (LVW/BW, mg/g) after two weeks infusion. (B) qRT-PCR analysis of heart homogenates from Tg-Trx1 and NTg mice with or without Ang II infusion. (C) Fibrosis was assessed by periodic acid-Schiff (PASR) staining and the fibrotic area was measured. (D) Cardiomyocyte apoptosis in the mouse hearts was observed using TUNEL staining, and the TUNEL positive cells were counted. (E, F) Twenty-four hours after transduction of Ad-LacZ or Ad-Trx1, neonatal rat cardiomyocytes (NRCMs) were treated with or without 100 nM Ang II for 48 hours. Cells were stained with anti-α-actinin antibody and DAPI, and relative cell surface area (cell size) was measured (E), or were harvested for protein and DNA content measurement (F). N=3. Values are mean±SEM. P<0.05.
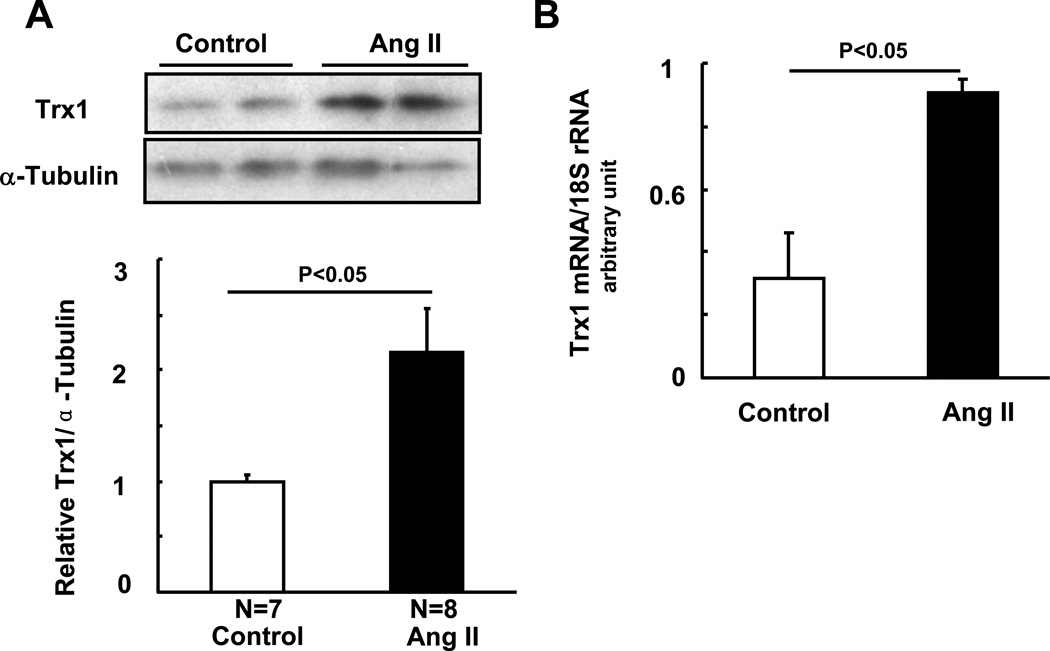
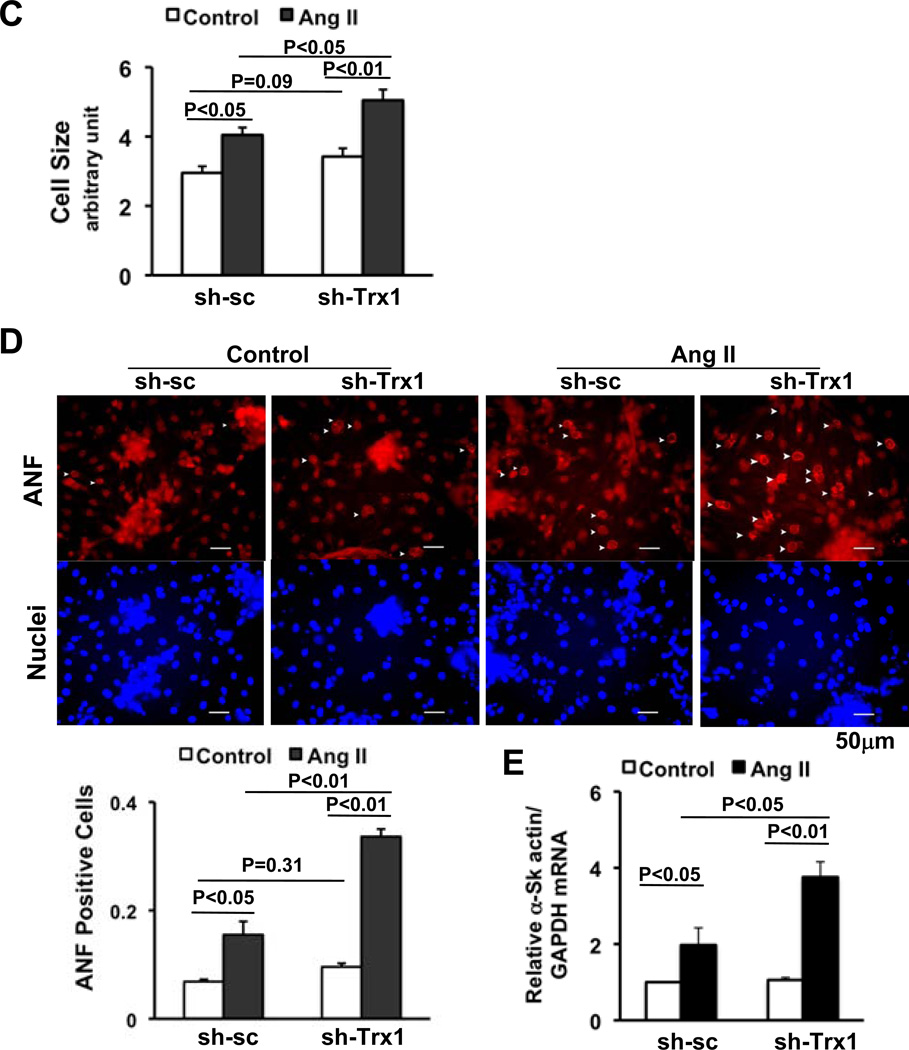
Ang II significantly upregulated protein and mRNA expression of Trx1 both in vivo and in vitro (Fig. 2AB and not shown), suggesting that Trx1 acts as a negative feedback regulator of Ang II-induced cardiac hypertrophy. In order to evaluate the role of endogenous Trx1, we knocked down Trx1 in cultured cardiomyocytes, using adenovirus harboring shRNA-Trx1. Downregulation of Trx1 by shRNA-Trx1 did not significantly enhance these parameters of hypertrophy at baseline in this experimental condition. However, Ang II-induced increases in cell size, ANF expression and α-skeletal actin were enhanced in the presence of shRNA-Trx1 compared to shRNA-scramble (Fig. 2CDE). These results suggest that Trx1 is a negative feedback regulator of Ang II-induced cardiac hypertrophy.
Figure 2. Ang II upregulates Trx1 as a negative feedback mechanism.
(A) FVB mice were subjected to continuous infusion of either PBS or a subpressor dose (200 ng/kg/min) of Ang II by osmotic pump for two weeks. Heart homogenates were subjected to immunoblot analyses with anti-Trx1 and anti-α-tubulin antibodies. Values are mean±SEM. P<0.05. (B) NRCMs were treated with or without 100 nM Ang II. After 24 hours, cells were harvested for qRT-PCR analyses with Trx1 and 18S rRNA primers. Values are mean±SEM. P<0.05. (C–E) Twelve to 24 hours after transduction of either Ad-short hairpin scrambled (sh-sc), or Ad-sh-Trx1, NRCMs were treated with or without 100 nM Ang II for 48 hours. Cells were measured for relative cell surface area (cell size) (C), stained with anti-ANF antibody (red) and DAPI (blue) (D), or harvested for qRT-PCR analysis of α-skeletal actin mRNA (E). Representative ANF perinuclear staining is indicated by arrows (D top), and the percentage of ANF positive cells was quantified (D bottom). N>3. Values are mean±SEM. P<0.05.
Trx1 upregulates miR-98 in cardiomyocytes
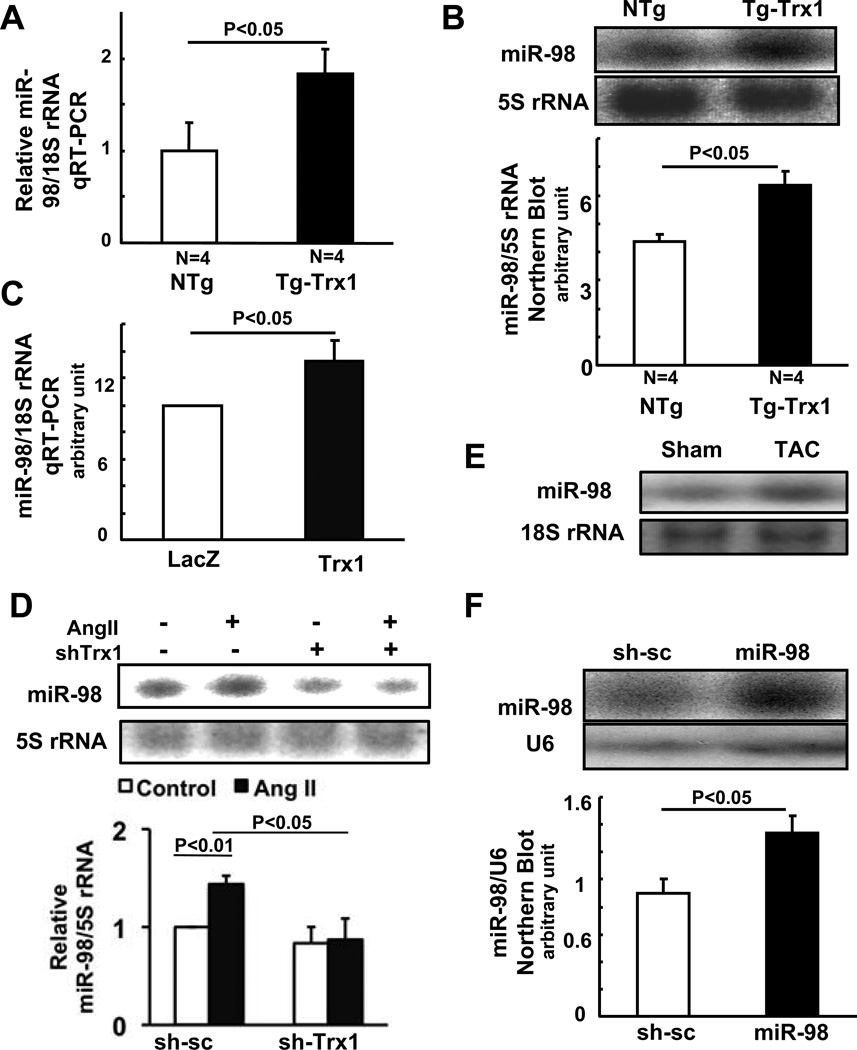
In order to examine the involvement of miRNA in the anti-hypertrophic effect of Trx1, total RNA was extracted from Tg-Trx1 hearts, enriched for small RNA, and analyzed with microarrays containing mature rodent miRNAs. Six out of eleven members of the let-7 family (let-7a, b, c, e, and f, and miR-98) 16 were upregulated in Tg-Trx1 hearts (Table S1). Among them, miR-98 exhibited the highest level of upregulation by Trx1 in the mouse heart. We therefore investigated the role of miR-98 in mediating the anti-hypertrophic effect of Trx1.
Both qRT-PCR and Northern blot analyses showed that miR-98 is significantly upregulated in Tg-Trx1 hearts in vivo (Fig. 3AB), confirming the result of the microarray analysis. Transduction of Ad-Trx1 significantly increased expression of miR-98 compared to transduction of Ad-LacZ in cardiomyocytes in vitro, suggesting that the effect of Trx1 upon miR-98 expression is cell-autonomous (Fig. 3C).
Figure 3. Trx1 and TAC upregulate miR-98.
Heart homogenates from Tg-Trx1 and NTg mice were used for (A) qRT-PCR analyses or (B) Northern blot with a miR-98 probe (N=4.). (C) NRCMs were transduced with Ad-LacZ or Ad-Trx1. After 48 hours, cells were harvested for qRT-PCR analyses with miR-98 and 18S rRNA primers. (D) Twelve to 24 hours after transduction of either Ad-short hairpin scrambled (sh-sc) or Ad-sh-Trx1, NRCMs were treated with or without 100 nM Ang II for 48 hours. Cells were harvested for Northern blot using a miR-98 probe and the results were quantified by densitometry. (E) FVB mice were subjected to TAC operation to generate pressure overload for two weeks, and heart homogenates were used for Northern blot with a miR-98 probe. (F) NRCMs were transduced with Ad-short hairpin scrambled (sh-sc) or Ad-miR-98. After 36 hours, cells were harvested for Northern blot using miR-98 and U6 probes, and the results were quantified by densitometry (N=3). Values are mean±SEM. P<0.05.
Ang II caused a significant increase in expression of miR-98 in cultured cardiomyocytes, which was abolished when Trx1 was downregulated with shRNA-Trx1 (Fig. 3D), suggesting that Trx1 mediates upregulation of miR-98 in response to Ang II. miR-98 was also upregulated by pressure overload in the mouse heart (Fig. 3E).
In order to examine the effect of miR-98 expression upon hypertrophy of cardiomyocytes, we constructed an adenovirus vector harboring pre-miR-98 (Ad-miR-98). Transduction of cultured cardiomyocytes with Ad-miR-98 induced upregulation of mature miR-98 (1.5 fold), as evaluated by Northern blot analyses (Fig. 3F), a level of upregulation similar to that induced by Trx1 overexpression in vivo and in vitro.
miR-98 inhibits myocyte hypertrophy
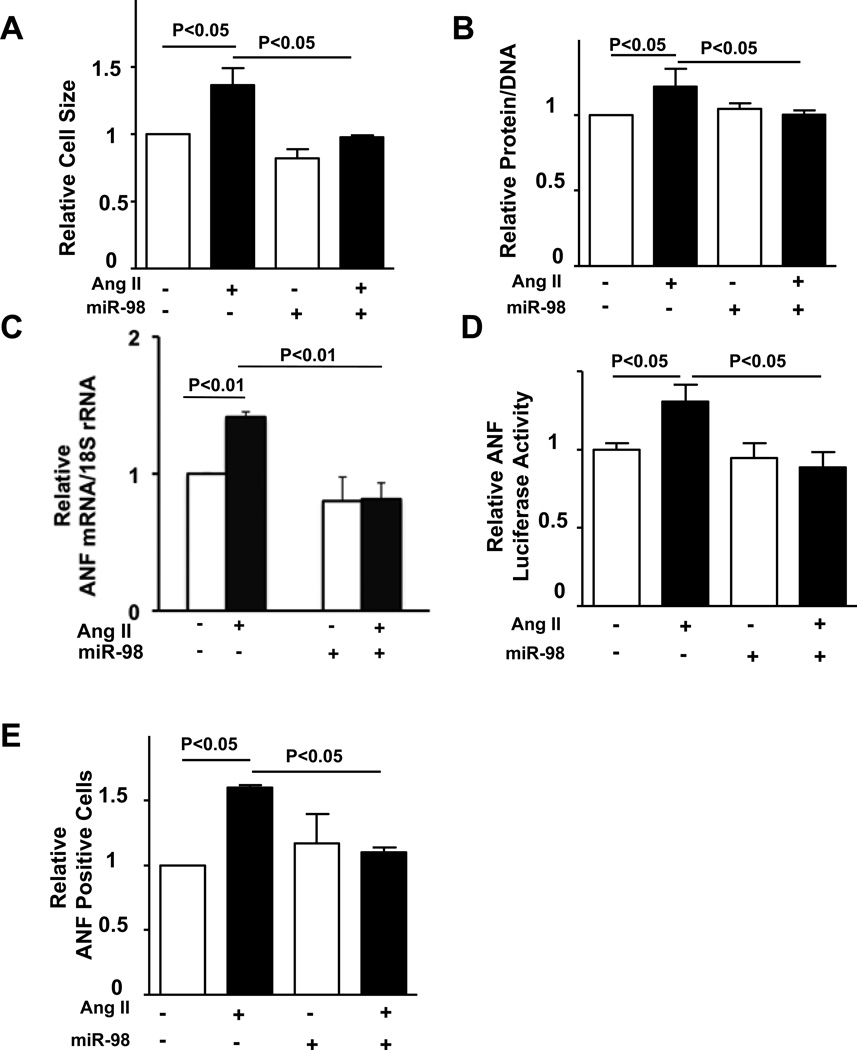
Adenovirus-mediated upregulation of miR-98 significantly reduced Ang II-induced increases in cell size and protein/DNA content in cultured cardiomyocytes, suggesting that miR-98 inhibits Ang II-induced cardiac hypertrophy (Fig. 4AB). Similarly, miR-98 significantly attenuated Ang II-induced increases in ANF mRNA, the activity of an ANF-luciferase reporter gene, and perinuclear staining of ANF in cardiomyocytes (Fig. 4CDE, supplemental Fig. S2). These results suggest that upregulation of miR-98 is sufficient to inhibit Ang II-induced cardiac hypertrophy.
Figure 4. In vitro effect of miR-98 on cardiac myocyte hypertrophy.
Twelve to 24 hours after transduction of Ad-sh-sc, Ad-miR-98 or Ad-anti-miR-98 (anti-98), NRCMs were treated with or without 100 nM Ang II for 48 hours. Relative cell surface area (cell size) was measured (A, F). Cells were harvested for protein/DNA content measurement (B) and qRT-PCR analysis of ANF mRNA (C, G). Cells were harvested for ANF luciferase activity measurement (where ANF promoter luciferase, sh-sc and miR-98 plasmids were used instead of adenovirus) (D), or were stained with anti-ANF antibody (red) and DAPI (blue) (E, H). Representative ANF perinuclear staining is shown (H top), and the percentage of ANF positive cells was quantitated (E, H bottom). N>3. Values are mean±SEM. P<0.05.
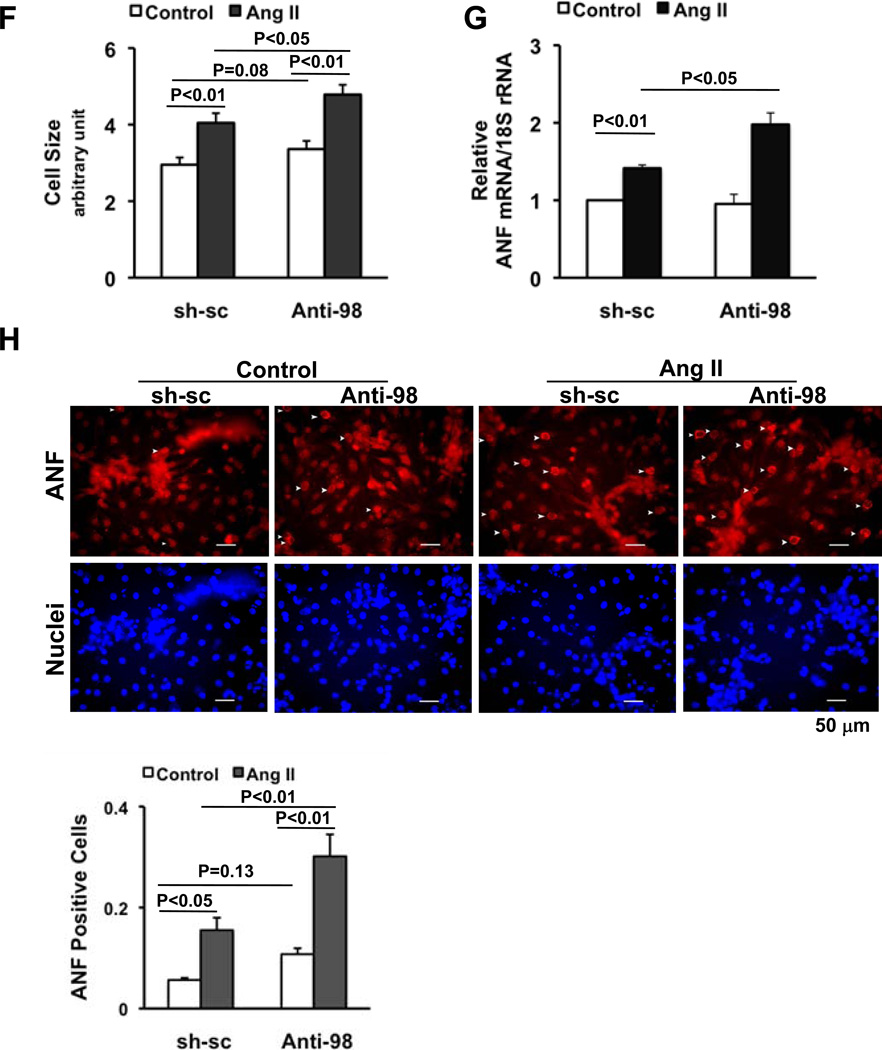
In order to examine the function of endogenous miR-98, adenovirus harboring anti-miR-98 (Ad-anti-miR-98), comprising two repeats of sequence complementary to miR-98 under the control of a U6 promoter, was generated. Adenovirus harboring a scramble sequence (Ad-scramble) was used as control. Transduction of cardiomyocytes with Ad-anti-miR-98 significantly reduced expression of miR-98 (Supplemental Fig. S3A) and reversed miR-98-induced suppression of Ang II-induced ANF expression (Supplemental Fig. S3B) and protein/DNA content (Supplemental Fig. S3C). Downregulation of miR-98 enhanced Ang II-induced increases in cell size and mRNA expression and perinuclear staining of ANF (Fig. 4FGH), suggesting that endogenous miR-98 negatively regulates Ang II-induced hypertrophy in vitro. Although downregulation of miR-98 slightly increased cell size and perinuclear staining of ANF at baseline, these did not reach statistical significance.
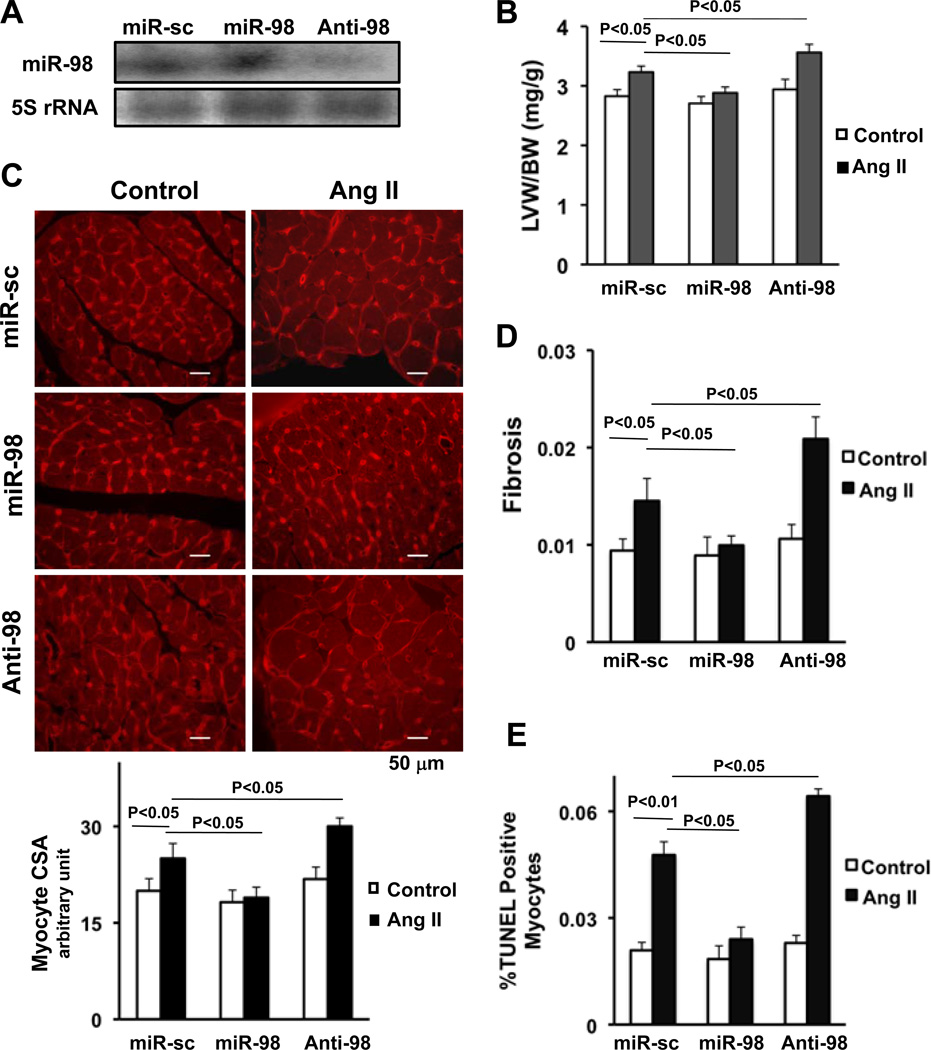
In order to evaluate the function of miR-98 in the heart in vivo, Ad-miR-sc, Ad-miR-98 or Ad-anti-miR-98 was injected into mouse hearts, following which mice were treated with or without continuous infusion of Ang II. Injection of Ad-miR-98 increased, whereas that of Ad-anti-miR-98 decreased expression of miR-98 in heart homogenates (Fig. 5A, supplemental Fig. S4A). Although neither Ad-miR-98 nor Ad-anti-miR-98 significantly affected LVW/BW or LV myocyte cross sectional area without Ang II infusion, Ang II-induced increases in these parameters in the presence of control virus were significantly attenuated by Ad-miR-98 and significantly enhanced by Ad-anti-miR-98 (Fig. 5BC). Similarly, Ang II-induced increases in fibrosis and the number of TUNEL positive myocytes were significantly attenuated by Ad-miR-98 and significantly enhanced by Ad-anti-miR-98 (Fig. 5DE, supplemental Fig. S4BC). These results suggest that miR-98 negatively regulates Ang II-induced cardiac hypertrophy and the accompanying histopathological changes in vivo.
Figure 5. In vivo effect of miR-98 on cardiac hypertrophy, fibrosis and apoptosis.
Ad-miRNA-scrambled (miR-sc), Ad-miR-98 or Ad-anti-miR-98 (anti-98) was injected into C57 mouse hearts with or without continuous infusion of Ang II (200 ng/kg/min) for two weeks. Hearts were harvested for Northern blot with a miR-98 probe (A), postmortem measurements of left ventricular weight/body weight (LVW/BW, mg/g) (B) and myocyte cross sectional area measurement (C). Representative pictures of wheat germ agglutinin (WGA) staining (C top) and quantitative analysis of myocyte cross sectional area (C bottom) are shown. Myocardial fibrosis was determined by PASR staining (D), and myocytes apoptosis was determined by TUNEL staining of the mouse left ventricles (E). N>3. Values are mean±SEM. P<0.05.
miR-98 plays an essential role in mediating the anti-hypertrophic actions of Trx1
We examined the role of miR-98 in mediating the anti-hypertrophic effect of Trx1. To this end, cardiomyocytes were transduced with Ad-Trx1, together with either Ad-anti-miR-98 or Ad-scramble. After 24 hours, cardiomyocytes were treated with or without 100 nM Ang II for an additional 48 hours. Trx1-induced suppression of Ang II-induced increases in protein/DNA was reversed in the presence of Ad-anti-miR-98 (Fig. 6A). Knockdown of miR-98 also significantly attenuated Trx1-mediated suppression of Ang II-induced increases in ANF staining (Fig. 6B). These results suggest that endogenous miR-98 plays an essential role in mediating Trx1-induced suppression of Ang II-induced cardiac hypertrophy.
Figure 6. miR-98 mediates the anti-hypertrophic actions of Trx1.
Twelve to 24 hours after transduction of Ad-sh-sc or Ad-anti-miR-98 (anti-98), and Ad-LacZ or Ad-Trx1, NRCMs were treated with or without 100 nM Ang II for 48 hours. Cells were harvested for protein and DNA content measurement (A) or stained with anti-ANF antibody (red) and DAPI (blue) (B). Representative ANF perinuclear staining is shown and the percentage of ANF positive cells was quantified. N>3. Values are mean±SEM. P<0.05.
Cyclin D2 plays an essential role in mediating Ang II-induced cardiac hypertrophy
Cyclin D2, a member of the G1-phase cyclin family, is one of the predicted targets of miR-98. Increasing lines of evidence suggest that cyclin D2 plays an essential role in mediating cardiac hypertrophy 17, 18. Treatment of cardiomyocytes with Ang II (100 nM) for 24 hours significantly upregulated cyclin D2 in whole cell lysates 1.8-fold (Fig. 7A). Subcellular fractionation experiments showed that Ang II upregulates cyclin D2 in the nuclear fraction 2.1-fold (Supplemental Fig. S5A). The purity of each fraction was confirmed by immunoblots with anti-tubulin and anti-histone H3 antibodies (Supplemental Fig. S5A). Continuous infusion of Ang II (200 ng/kg/min) significantly upregulated cyclin D2 protein 1.9-fold in the mouse heart in vivo (Fig. 7B). These results suggest that Ang II upregulates cyclin D2 in the heart and the cardiomyocytes therein.
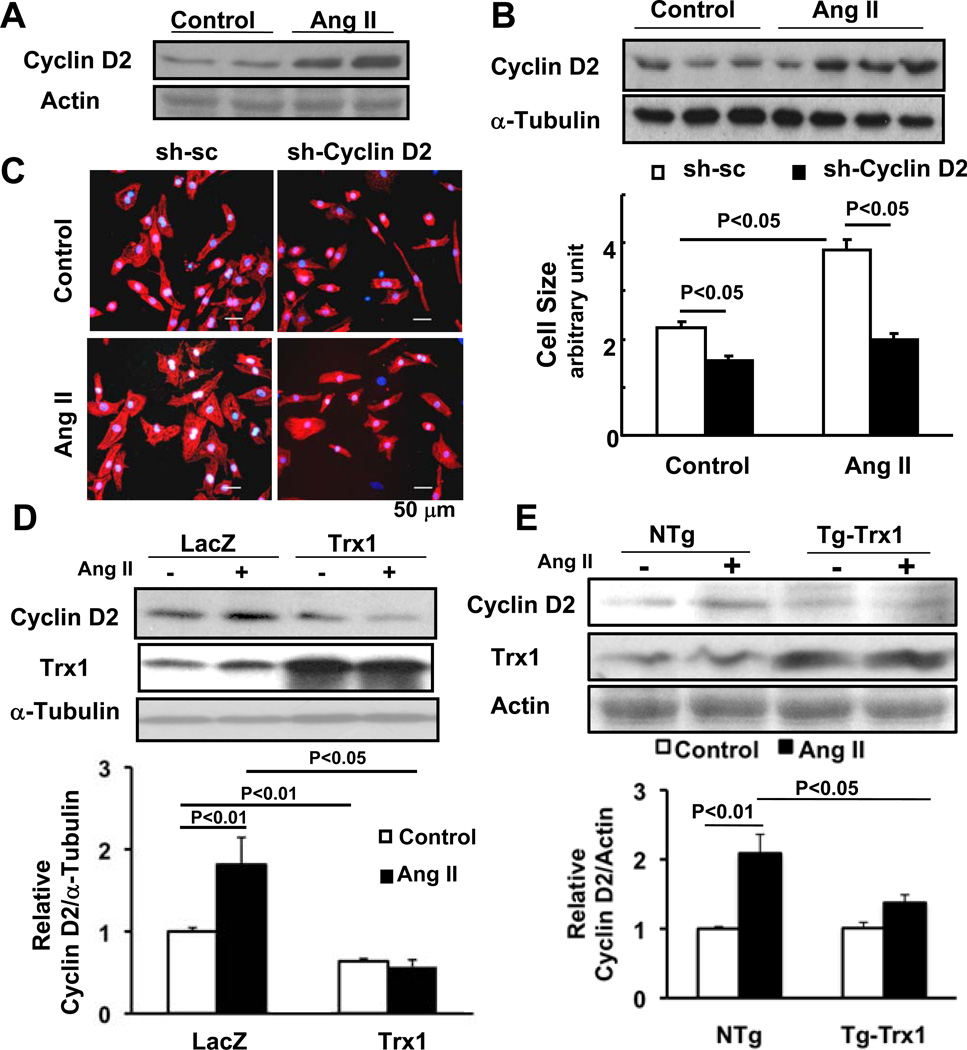
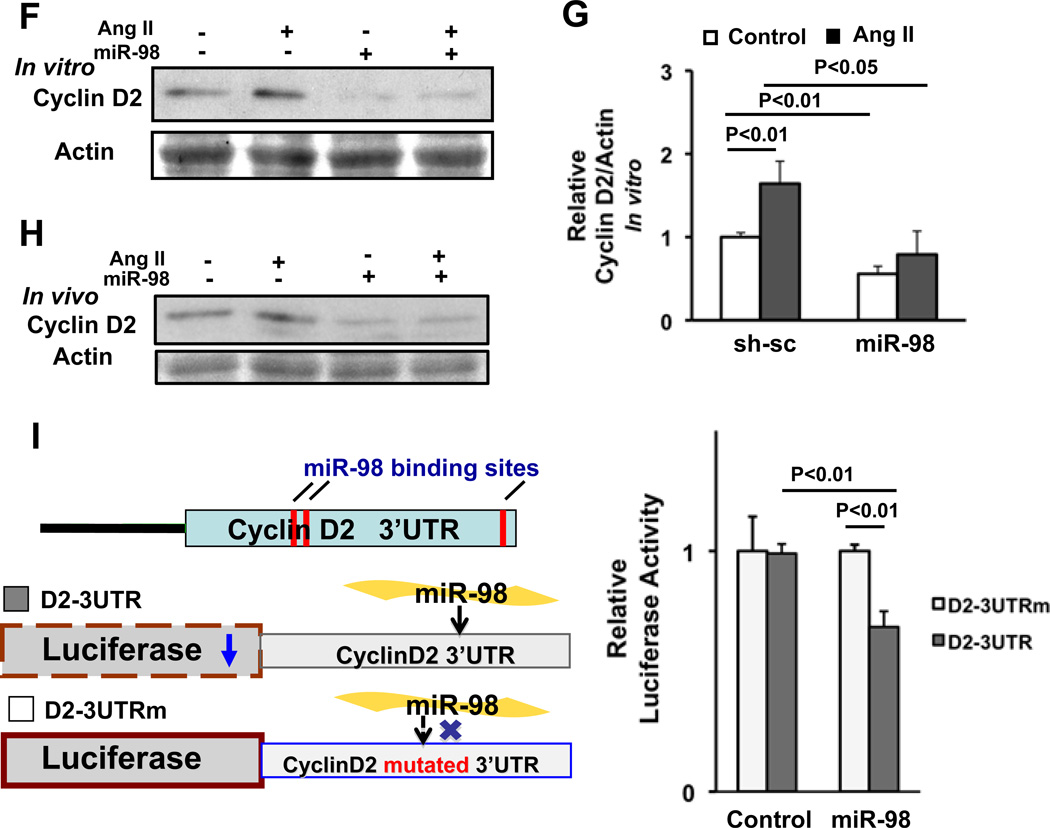
Figure 7. Cyclin D2 is a target of miR-98 and mediates Ang II-induced cardiac hypertrophy in cardiac myocytes.
(A) After 24 hours 100 nM Ang II treatment, NRCMs were harvested for immunoblot analyses of total lysates with anti-cyclin D2 antibody. (B) Mice were subjected to continuous infusion of either PBS (control) or a subpressor dose (200 ng/kg/min) of Ang II by osmotic pump for two weeks. Heart homogenates were subjected to immunoblot analyses with anti-cyclin D2 and anti-α-tubulin antibodies. (C) Twenty-four hours after transduction of Ad-sh-sc or Ad-short hairpin-cyclin D2, NRCMs were treated with or without 100 nM Ang II for 48 hours. Cells were stained with anti-α-actinin antibody (red) and DAPI (blue), and relative cell surface area (cell size) was measured. N>3. Values are mean±SEM. P<0.05. (D) Twelve to 24 hours after transduction of Ad-lacZ or Ad-Trx1, NRCMs were treated with or without 100 nM Ang II for 24 hours. Cells were harvested for immunoblot analyses using anti-cyclin D2, anti-Trx1 and anti-α-tubulin antibodies and the results were quantified by densitometry. N>3. Values are mean±SEM. P<0.05. (E) Tg-Trx1 and NTg mice were subjected to continuous infusion of either PBS or a subpressor dose (200 ng/kg/min) of Ang II by osmotic pumps for two weeks. Heart homogenates were subjected to immunoblot analyses with anti-cyclin D2 and anti-Trx1 antibodies and the results were quantified by densitometry. (F, G) Twenty-four hours after transduction of Ad-sh-sc or Ad-miR-98, NRCMs were treated with or without 100 nM Ang II for 24–48 hours. Cells were harvested for immunoblot analyses using anti-cyclin D2 antibody and the results were quantified by densitometry. (H) Ad-miR-sc or Ad-miR-98 was injected into C57 mice hearts with or without continuous infusion of Ang II (200 ng/kg/min) for two weeks. Heart homogenates were subjected to immunoblot analyses with anti-cyclin D2 antibody. (I) Cartoon shows the cyclin D2 3’UTR with miR-98 binding sites and luciferase constructs harboring either wild type or mutated cyclin D2 3’UTR. NRCMs were co-transfected with one of these two luciferase constructs with or without miR-98 overexpression for 72 hours. Cells were harvested for luciferase assay. N>3. Values are mean±SEM. P<0.05.
In order to examine the role of cyclin D2 in cardiac hypertrophy, cultured cardiomyocytes were transduced with adenovirus harboring shRNA targeting cyclin D2 (Ad-shRNA-cyclin D2) or adenovirus harboring scramble shRNA (Ad-shRNA-scramble) for 72 hours. We confirmed that cyclin D2 is significantly downregulated in cardiomyocytes transduced with Ad-shRNA-cyclin D2 but not with Ad-shRNA-scramble (Supplemental Fig. S5B). Twenty-four hours after transduction, cardiomyocytes were treated with or without Ang II (100 nM) for an additional 48 hours. Downregulation of cyclin D2 significantly reduced the size of cardiomyocytes at baseline and in response to Ang II (Fig. 7C). Downregulation of cyclin D2 expression also reduced expression of ANF (not shown). These results suggest that cyclin D2 plays an essential role in mediating Ang II-induced cardiac hypertrophy. Adenovirus-mediated upregulation of Trx1 in cultured cardiomyocytes downregulated cyclin D2 at baseline and in response to Ang II in vitro (Fig. 7D). Ang II-induced upregulation of cyclin D2 was significantly attenuated in Tg-Trx1 compared to NTg mice (Fig. 7E). These results suggest that Trx1 attenuates Ang II-induced hypertrophy, possibly through downregulation of cyclin D2.
Cyclin D2 is an important target of miR-98 in cardiomyocytes
Cyclin D2 has three predicted miR-98 binding sites in its 3’UTR. We next examined whether miR-98 downregulates cyclin D2 in cardiomyocytes. Upregulation of miR-98 significantly downregulated cyclin D2 in cultured cardiomyocytes at baseline and in response to Ang II stimulation (Fig. 7FG). Ang II-induced upregulation of cyclin D2 was inhibited by miR-98 and enhanced by anti-miR-98 introduced by adenovirus-mediated gene transfer in the mouse heart (Fig. 7H, supplemental Figure S6AB). In order to evaluate whether cyclin D2 is a direct target of miR-98, we constructed a reporter gene harboring the 3’UTR of the cyclin D2 gene (D2-3UTR). Another reporter gene harboring the 3’UTR of the cyclin D2 gene with mutations in the putative miR-98 binding sites (D2-3UTRm) served as a negative control. miR-98 significantly reduced the activity of D2-3UTR compared to that of D2-3UTRm in cardiomyocytes, whereas the control vector did not (Fig. 7I). These results suggest that cyclin D2 is a direct target of miR-98 in cardiomyocytes.
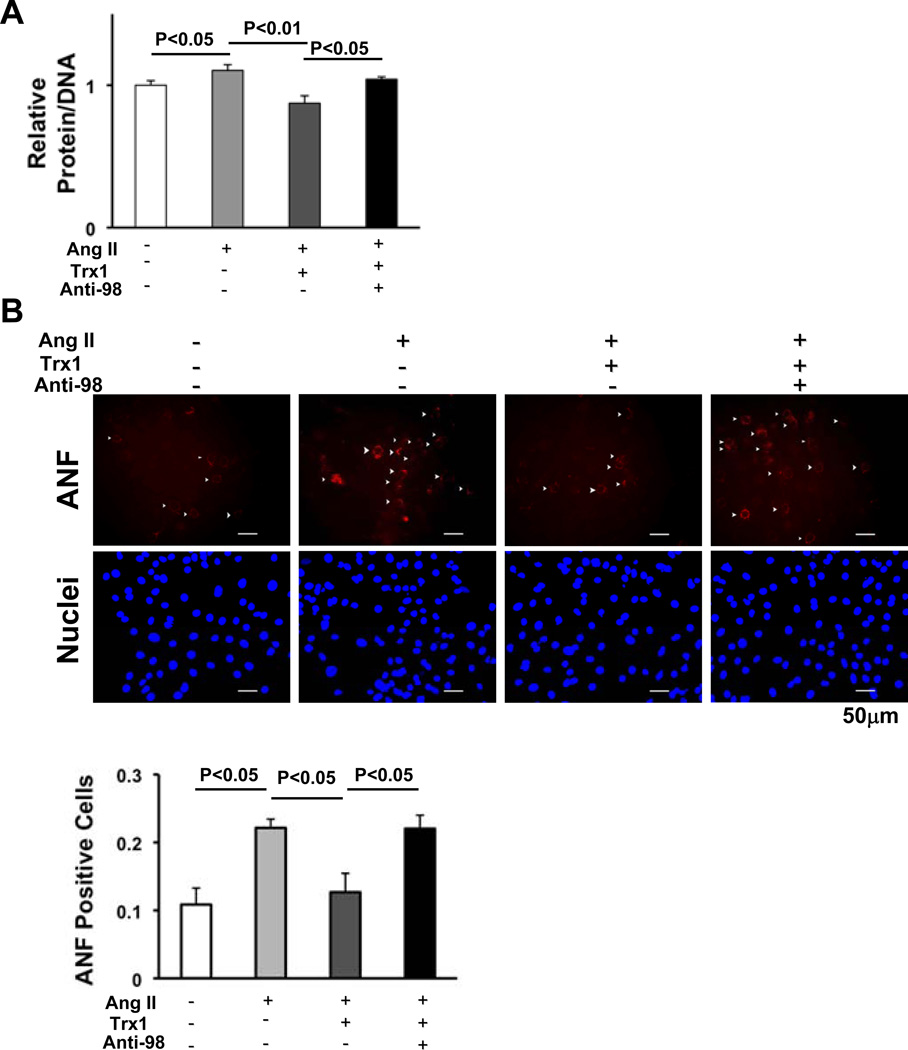
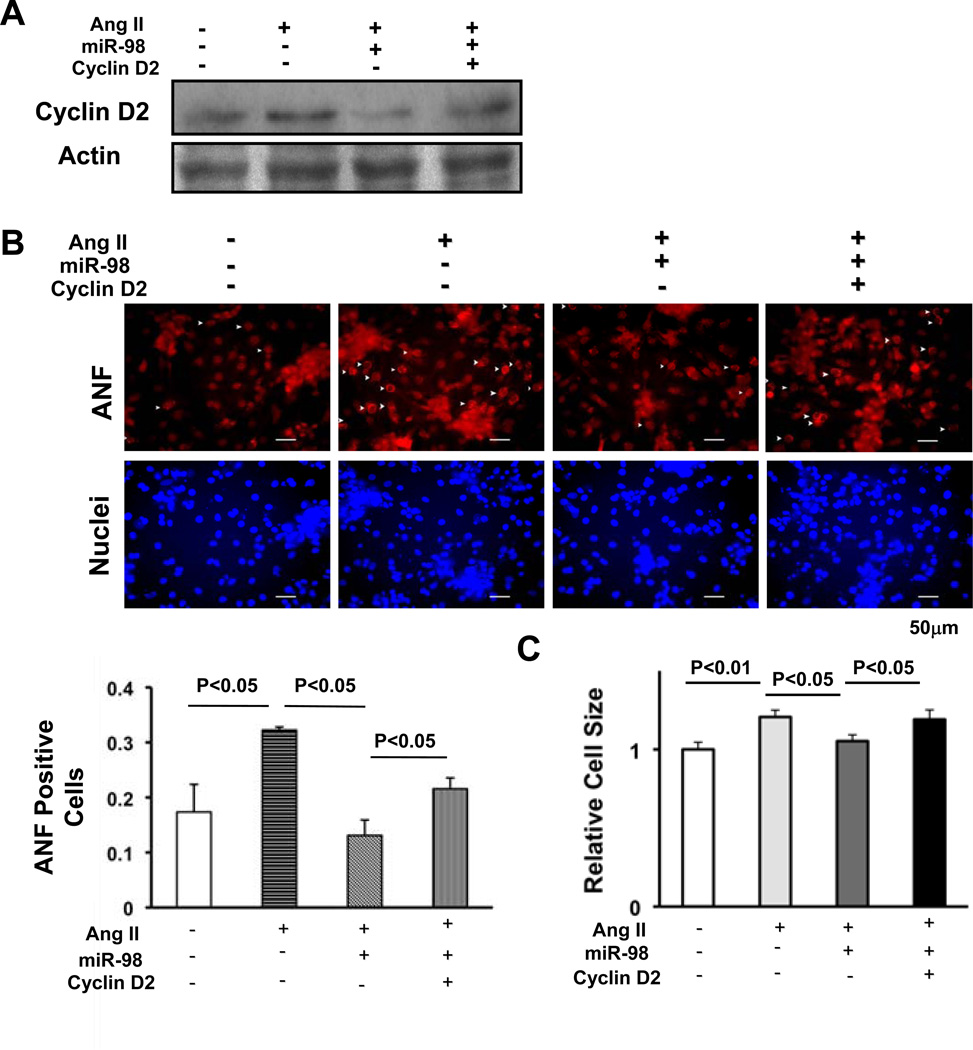
In order to evaluate the role of the cyclin D2 downregulation in mediating miR-98-induced suppression of Ang II-induced cardiac hypertrophy, miR-98-induced downregulation of cyclin D2 was reversed by adenovirus-mediated expression of cyclin D2 (Fig. 8A). Under these experimental conditions, suppression of Ang II-induced ANF expression (Fig. 8B) and increases in cardiomyocyte cell size (Fig. 8C) by miR-98 was significantly attenuated when expression of cyclin D2 was restored, suggesting that downregulation of cyclin D2 plays an important role in mediating the anti-hypertrophic effects of miR-98.
Figure 8. Downregulation of cyclin D2 mediates the anti-hypertrophic effects of miR-98.
Twelve to 24 hours after transduction of Ad-sh-sc or Ad-miR-98, NRCMs were transduced with Ad-LacZ or Ad-cyclin D2 and treated with or without Ang II (100 nM) for 48 hours. Cells were harvested for immunoblot analyses using anti-cyclin D2 antibody (A), or stained with anti-ANF antibody (red) and DAPI (blue) (B top). The percentage of ANF positive cells was quantified (B bottom), and relative cell surface area (cell size) was measured (C). Values are mean±SEM. P<0.05.
Discussion
In this study, we show that Trx1 upregulates expression of the let-7 family miRNA, including miR-98, which in turn inhibits Ang II-induced cardiac hypertrophy. In addition, cyclin D2 is an important target of miR-98/let-7. Upregulation of miR-98/let-7 and inhibition of cyclin D2 may play an important role in mediating the suppression of cardiac hypertrophy by Trx1.
Trx1 acts as a negative feedback regulator of Ang II-induced cardiac hypertrophy. Trx1 is upregulated in response to stress, such as infection, pressure overload, ischemia and heart failure 5. mRNA expression of Trx1 is regulated by binding of transcription factors to the SP-1 site, the antioxidant responsive element (ARE) and the cyclic AMP-responsive element (CRE) in the Trx1 promoter 19. The mechanism by which Ang II upregulates Trx1 remains to be elucidated.
Trx1 suppresses cardiac hypertrophy through multiple mechanisms. Trx1 scavenges reactive oxygen species, primarily through reduction of peroxiredoxins and/or glutathione peroxidases 5. Trx1 also directly reduces signaling molecules and transcription factors, including Ras 3 and class II HDACs 6, 20, through the thiol disulfide exchange reaction, thereby inhibiting cardiac hypertrophy. In addition, Trx1 suppresses apoptosis signal-regulating kinase-1 (ASK-1) through direct protein-protein interaction and degradation of ASK-1, which inhibits cardiac hypertrophy and apoptosis (reviewed in 20). Here we propose that upregulation of miR-98/let-7 also contributes to the suppression of hypertrophy by Trx1. We speculate that Trx1 inhibits cardiac hypertrophy through multiple parallel and serial mechanisms, including miR-98-dependent downregulation of cyclin D2 and posttranslational modifications of other signaling molecules. Since miR-98 inhibits NFAT signaling in cardiac myocytes (Supplemental Fig. S7), it is likely that the effect of miR-98 may converge with other mechanisms mediated by Trx1 upstream of the calcineurin-NFAT pathway to inhibit pathological hypertrophy.
Trx1 upregulates many members of the let-7 family in the mouse heart, among which miR-98 exhibited the highest degree of upregulation. Since Ang II-induced upregulation of miR-98 was inhibited in the absence of Trx1, Trx1 must play an important role in mediating Ang II-induced upregulation of miR-98. Expression of miR-98/let-7 is upregulated in differentiated cells, whereas it is downregulated in cancer cells 16. Expression of miR-98/let-7 is also increased by hypoxia 21, and is downregulated by lipopolysaccharide 22 and c-myc 23. Since Trx1 inhibits cell growth 24 and is also upregulated by hypoxia 25, it will be interesting to test the general involvement of Trx1, as well as the downstream transcription factors regulated by Trx1, in mediating upregulation of miR-98. The fact that miR-98 and let-7f, which form a cluster, are the top two miRNAs upregulated by Trx1 suggests that a Trx1-sensitive transcriptional mechanism may exist. In addition, many members of the let-7 family are posttranscriptionally regulated by Lin-28 26. Whether or not Trx1 is involved in the posttranscriptional regulation of the let-7 family is currently unknown.
Our results suggest that miR-98 negatively regulates cardiac hypertrophy. Since anti-miR-98 significantly enhanced Ang II-induced cardiac hypertrophy, miR-98/let-7 acts as a negative feedback regulator of Ang II-induced cardiac hypertrophy. Importantly, downregulation of miR-98 significantly attenuated the anti-hypertrophic effect of Trx1 against Ang II-induced hypertrophy. This is striking considering the fact that Trx1 mediates its anti-hypertrophic actions by multiple mechanisms 20, and raises the possibility that the downstream mediators of Trx1 may also require miR-98/let-7 for their actions. However, the results of a TargetScan analysis, which predicts the targets of miRNA, suggest that many proteins involved in Trx1-mediated suppression of hypertrophy regulation, except Ras, are not directly regulated by miR-98. It should be noted that miR-98 and some other members of the let-7 family have a very similar, if not identical, seed sequence and share target genes 16 (Supplemental Fig. S8A). Since the anti-miR-98 used in this study partially downregulates expression of other members of the let-7 family (Supplemental Fig. S8B), Trx1-induced upregulation of multiple members of the let-7 family may, in concert, mediate the anti-hypertrophic actions of Trx1. In fact, the members of the let-7 family repress both common and distinct sets of target genes 27.
Let-7 family members have been implicated as tumor suppressors 16. Expression of let-7 is increased in mature tissue, whereas it is downregulated during cancer development. Overexpression of let-7 in human primary fibroblasts reduces the cell number and induces a fraction of cells in the G2/M phase due to cell cycle arrest 28. Hypoxia-induced upregulation of miR-98 inhibits oncofetal genes, such as high mobility group A2 (HMGA2), in head and neck squamous cell carcinoma 21. Thus, the anti-hypertrophic action of miR-98/let-7 in the heart is consistent with the growth suppressive effects of miR-98/let-7 in other tissues.
Let-7 downregulates many genes involved in cell proliferation and tumor growth, including Ras 29, Cdc34 28, HMGA-2 30, c-Myc 31 and insulin-like growth factor II mRNA-binding protein-1 (IMP-1) 32. We here propose that cyclin D2, a component of the cell cycle machinery, is an important target of miR-98 in the heart. Although hypertrophy of the postnatal heart occurs primarily through increases in cell size without cell division, cyclins are also involved in cardiac hypertrophy. In postmitotic cardiomyocytes, hypertrophic stimuli, including Ang II, activate cyclin Ds and cdk4, which in turn causes phosphorylation of retinoblastoma gene product (pRb) 33. Among G1 cyclins, cyclin D2 is particularly important, and both necessary and sufficient for inducing cardiac hypertrophy. Expression of cyclin D2 in the heart reduces the size of myocardial infarction, possibly through proliferation of cardiomyocytes 34. Cardiac hypertrophy induced by overexpression of Myc was blocked in cyclin D2-null mice 35. We here demonstrate that cyclin D2 plays an essential role in mediating Ang II-induced cardiac hypertrophy, and that cyclin D2 is significantly downregulated by miR-98. The fact that the suppressive effect of miR-98 against Ang II-induced hypertrophy is partially reversed in the presence of cyclin D2 overexpression suggests that cyclin D2 is an important target of miR-98/let-7 and that downregulation of cyclin D2 plays an essential role in mediating the anti-hypertrophic action of miR-98/let-7.
The reversal of the anti-hypertrophic actions of miR-98 by overexpression of cyclin D2 was only partial. Thus, the anti-hypertrophic actions of miR-98 may be mediated by cyclin D2-independent mechanisms as well. It will be interesting to evaluate the role of other possible targets of the miR-98/let-7 family in mediating the anti-hypertrophic actions of miR-98/let-7. For example, disrupting the pairing between HMGA2 and let-7 (miR-98 family) promoted anchorage-independent growth indicative of tumorigenesis in NIH3T3 cells 30. We have shown previously that Trx1 inhibits Ras, another known target of let-7, through reduction of cysteine residues 3. Testing whether or not Trx1 also inhibits Ras through miR-98-induced downregulation is of great interest.
In summary, we here report that miR-98/let-7 mediates the anti-hypertrophic action of Trx1 against Ang II-induced hypertrophy, in part through downregulation of cyclin D2 (Supplemental Fig. S9). Since miR-98 is also upregulated by pressure overload, miR-98 may act as a negative feedback regulator against other forms of cardiac hypertrophy as well. The fact that Trx1 upregulates miR-98/let-7, a powerful mechanism of growth suppression, suggests that exploring the signaling mechanisms by which Trx1 upregulates miR-98/let-7 and miR-98/let-7 inhibits cardiac hypertrophy would provide us with important clues regarding controlling pathological hypertrophy and heart failure.
Supplementary Material
Acknowledgments
The authors thank Daniela Zablocki for critical reading of the manuscript.
Sources of Funding
This work was supported in part by U.S. Public Health Service Grants HL59139, HL67724, HL69020, HL91469, HL102738, and AG27211, and Foundation of Leducq Transatlantic Network of Excellence.
List of Abbreviations
- Ang II
Angiotensin II
- ANF
Atrial natriuretic factor
- Anti-98
Anti-microRNA-98
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- miRNA
MicroRNA
- miR-98
MicroRNA-98
- NRCMs /CMs
Neonatal rat cardiomyocytes
- sh-sc
Scramble short hairpin RNA adenovirus
- Trx1
Thioredoxin 1
- 3’UTR
3 prime untranslated region
Footnotes
Disclosures
None
References
- 1.Nakamura H, Nakamura K, Yodoi J. Redox regulation of cellular activation. Annu Rev Immunol. 1997;15:351–369. doi: 10.1146/annurev.immunol.15.1.351. [DOI] [PubMed] [Google Scholar]
- 2.Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Biophys Biomol Struct. 2001;30:421–455. doi: 10.1146/annurev.biophys.30.1.421. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto M, Yang G, Hong C, Liu J, Holle E, Yu X, Wagner T, Vatner SF, Sadoshima J. Inhibition of endogenous thioredoxin in the heart increases oxidative stress and cardiac hypertrophy. J Clin Invest. 2003;112:1395–1406. doi: 10.1172/JCI17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ago T, Sadoshima J. Thioredoxin and ventricular remodeling. J Mol Cell Cardiol. 2006;41:762–773. doi: 10.1016/j.yjmcc.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ago T, Liu T, Zhai P, Chen W, Li H, Molkentin JD, Vatner SF, Sadoshima J. A redox-dependent pathway for regulating class II HDACs and cardiac hypertrophy. Cell. 2008;133:978–993. doi: 10.1016/j.cell.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 7.Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn GW., 2nd Therapeutic potential of microRNAs in heart failure. Curr Cardiol Rep. 12:209–215. doi: 10.1007/s11886-010-0096-7. [DOI] [PubMed] [Google Scholar]
- 9.Sayed D, Hong C, Chen IY, Lypowy J, Abdellatif M. MicroRNAs play an essential role in the development of cardiac hypertrophy. Circ Res. 2007;100:416–424. doi: 10.1161/01.RES.0000257913.42552.23. [DOI] [PubMed] [Google Scholar]
- 10.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Hoydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 11.van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. 2009;119:2772–2786. doi: 10.1172/JCI36154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Z, Murtaza I, Wang K, Jiao J, Gao J, Li PF. miR-23a functions downstream of NFATc3 to regulate cardiac hypertrophy. Proc Natl Acad Sci U S A. 2009;106:12103–12108. doi: 10.1073/pnas.0811371106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem. 2010;285:20281–20290. doi: 10.1074/jbc.M110.109207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
- 16.Roush S, Slack FJ. The let-7 family of microRNAs. Trends Cell Biol. 2008;18:505–516. doi: 10.1016/j.tcb.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 17.Angelis E, Garcia A, Chan SS, Schenke-Layland K, Ren S, Goodfellow SJ, Jordan MC, Roos KP, White RJ, MacLellan WR. A cyclin D2-Rb pathway regulates cardiac myocyte size and RNA polymerase III after biomechanical stress in adult myocardium. Circ Res. 2008;102:1222–1229. doi: 10.1161/CIRCRESAHA.107.163550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Busk PK, Hinrichsen R, Bartkova J, Hansen AH, Christoffersen TE, Bartek J, Haunso S. Cyclin D2 induces proliferation of cardiac myocytes and represses hypertrophy. Exp Cell Res. 2005;304:149–161. doi: 10.1016/j.yexcr.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 19.Hoshino Y, Shioji K, Nakamura H, Masutani H, Yodoi J. From oxygen sensing to heart failure: role of thioredoxin. Antioxid Redox Signal. 2007;9:689–699. doi: 10.1089/ars.2007.1575. [DOI] [PubMed] [Google Scholar]
- 20.Ago T, Sadoshima J. Thioredoxin1 as a negative regulator of cardiac hypertrophy. Antioxid Redox Signal. 2007;9:679–687. doi: 10.1089/ars.2007.1529. [DOI] [PubMed] [Google Scholar]
- 21.Hebert C, Norris K, Scheper MA, Nikitakis N, Sauk JJ. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer. 2007;6:5. doi: 10.1186/1476-4598-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu G, Zhou R, Liu J, Gong AY, Eischeid AN, Dittman JW, Chen XM. MicroRNA-98 and let-7 confer cholangiocyte expression of cytokine-inducible Src homology 2-containing protein in response to microbial challenge. J Immunol. 2009;183:1617–1624. doi: 10.4049/jimmunol.0804362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Deiss LP, Kimchi A. A genetic tool used to identify thioredoxin as a mediator of a growth inhibitory signal. Science. 1991;252:117–120. doi: 10.1126/science.1901424. [DOI] [PubMed] [Google Scholar]
- 25.Hattori I, Takagi Y, Nozaki K, Kondo N, Bai J, Nakamura H, Hashimoto N, Yodoi J. Hypoxia-ischemia induces thioredoxin expression and nitrotyrosine formation in new-born rat brain. Redox Rep. 2002;7:256–259. doi: 10.1179/135100002125000749. [DOI] [PubMed] [Google Scholar]
- 26.Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbott AL, Alvarez-Saavedra E, Miska EA, Lau NC, Bartel DP, Horvitz HR, Ambros V. The let-7 MicroRNA family members mir-48, mir-84, and mir-241 function together to regulate developmental timing in Caenorhabditis elegans. Dev Cell. 2005;9:403–414. doi: 10.1016/j.devcel.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Legesse-Miller A, Elemento O, Pfau SJ, Forman JJ, Tavazoie S, Coller HA. let-7 overexpression leads to an increased fraction of cells in G2/M, direct down-regulation of Cdc34 and stabilization of Wee1 kinase in primary fibroblasts. J Biol Chem. 2009;284:6605–6609. doi: 10.1074/jbc.C900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 30.Mayr C, Hemann MT, Bartel DP. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science. 2007;315:1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampson VB, Rong NH, Han J, Yang Q, Aris V, Soteropoulos P, Petrelli NJ, Dunn SP, Krueger LJ. MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res. 2007;67:9762–9770. doi: 10.1158/0008-5472.CAN-07-2462. [DOI] [PubMed] [Google Scholar]
- 32.Boyerinas B, Park SM, Shomron N, Hedegaard MM, Vinther J, Andersen JS, Feig C, Xu J, Burge CB, Peter ME. Identification of let-7-regulated oncofetal genes. Cancer Res. 2008;68:2587–2591. doi: 10.1158/0008-5472.CAN-08-0264. [DOI] [PubMed] [Google Scholar]
- 33.Sadoshima J, Aoki H, Izumo S. Angiotensin II and serum differentially regulate expression of cyclins, activity of cyclin-dependent kinases, and phosphorylation of retinoblastoma gene product in neonatal cardiac myocytes. Circ Res. 1997;80:228–241. doi: 10.1161/01.res.80.2.228. [DOI] [PubMed] [Google Scholar]
- 34.Pasumarthi KB, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted expression of cyclin D2 results in cardiomyocyte DNA synthesis and infarct regression in transgenic mice. Circ Res. 2005;96:110–118. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- 35.Zhong W, Mao S, Tobis S, Angelis E, Jordan MC, Roos KP, Fishbein MC, de Alboran IM, MacLellan WR. Hypertrophic growth in cardiac myocytes is mediated by Myc through a Cyclin D2-dependent pathway. Embo J. 2006;25:3869–3879. doi: 10.1038/sj.emboj.7601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.