Abstract
As additional states of CD4 T cell differentiation are uncovered, their flexibility is also beginning to be recognized. Components that control the plasticity of CD4 T cell populations include their cellular conditions, clonality, transcriptional circuitry and chromatin modifications. Appearance of cellular flexibility may arise from truly flexible genetic programs or alternately heterogeneous populations. New tools will be needed to define the rules that allow or prohibit cellular transitions.
The differentiation of the CD4+ T cell lineage into effector cells underlies successful adaptive immune responses aimed at distinct categories of pathogens. Their functional specialization is coordinated by genetic programs that use different transcription factors to direct expression of distinct soluble mediators and surface molecules that support interactions with other immune cells (Fig. 1). The first paradigm for this functional diversification was the description of TH1 and TH2 CD4+ effector subsets by Mosmann and Coffman in 1986 (ref 1). TH1 cells were thought to be responsible for delayed type hypersensitivity (DTH), activating macrophages via release of interferon (IFN)γ and enabling them to kill intracellular pathogens. TH2 cells were considered the classical helper T cells providing help to B cells to generate class switched antibodies.
Figure 1.
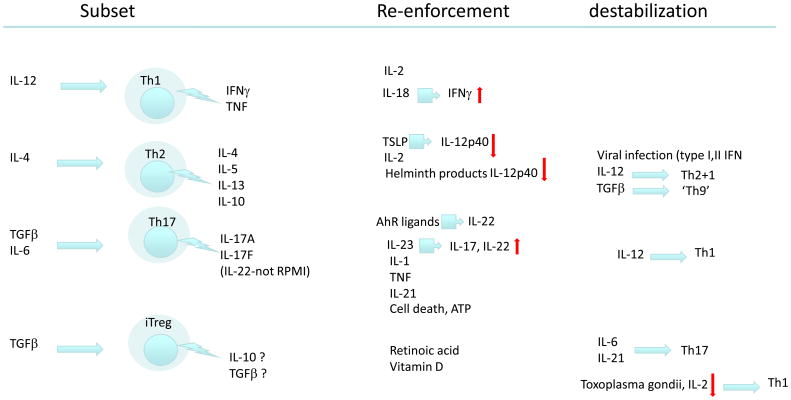
Re-enforcement and destabilization of CD4+ T cell subsets. These four CD4+ subsets are induced in distinct conditions, but also can be reinforced or destabilized by other conditions, as discussed throughout the text.
Several attempts to add new subsets to this dichotomy were thwarted by the inability to identify consistent robust inducing conditions or transcriptional “signature”. As such, the formal status of the subsets previously known as TH3 or TR1 appears uncertain. But in 2003, the requirement for interleukin-23 (IL-23) in IL-17-producing CD4+ T cells was recognized and a role for such cells, rather than TH1 cells, was established in the experimental autoimmune encephalomyelitis (EAE) model2. This data established a role for CD4+ T cells secreting IL-17 rather than IFNγ in diseases such as EAE or collagen-induced arthritis. Initially presumed to diverge from a common TH1 precursor3 the IL-17 producing cells, named TH17, were classified as a new subset on the basis of being independent of the transcription factors GATA-3 and T-bet4, 5. The robust inducing conditions of IL-6 and TGFβ6 and the identification of RORγt and RORα as lineage defining transcription factors7, 8 finalized support of TH17 as a separate subset.
The fourth major subset of CD4+ T cells are Treg cells9 characterized by expression of the transcription factor Foxp3. Tregs derived from the thymus are thought to be a stable subset. However, Tregs can be induced in the periphery from naïve CD4+ T cells by exposure to TGFβ. Similar to the stable thymus-derived Treg, inducible Treg (iTreg) express Foxp3, but may be less stable and share circuitry with TH17 cells which also require TGFβ for their differentiation (reviewed in 10).
Subsets ‘in the making’
Follicular helper T cells (TFH) residing in B cell follicles are essential for the generation of high-affinity isotype switched antibodies and B cell memory11-14, a characteristic that originally defined CD4+ T cells as ‘helpers’. Although all CD4+ T cells migrate to follicular regions, TFH cells are preferentially resident there by virtue of their continuous expression of the chemokine receptor CXCR5. CD4+ T cells expressing CXCR5 have the potential to secrete TH1, TH2 or TH17 cytokines. Therefore, it is unclear whether TFH are a distinct subset or rather a ‘chameleon’ state of other subsets that are imprinted by follicular location. TFH produce high levels of IL-21, which acts in an autocrine manner together with IL-6 on their differentiation and expansion, a process that also depends on the Bcl-6 transcription factor11, 15-19.
A population of IL-9 producing cells, derived from TH2 cells by treatment with TGFβ, has also been described20. IL-9 was once considered a TH2 cytokine, but is now recognized as not being co-expressed with IL-4, IL-5 or IL-13. Although suggested to be produced by TH17 cells or iTreg21-23, IL-9 is not co-expressed with IL-17 or with IL-22, and is not expressed by nTregs or iTreg20. Having been only examined in vitro, it is unclear whether IL-9 producers should be considered as a new subset, to be called TH9, or whether expression of this cytokine reflects adaptation of TH2 cells to a change in the microenvironment in the course of a response triggered by a pathogen or allergen.
More recently, human, but not mouse, TH22 T cells (expressing IL-22 but not IL-17 or RORγt) were described24, 25, and may represent a skin-homing subset responsible for skin inflammation such as psoriasis. These cells preferentially develop when cultured with plasmacytoid DC (pDC) which infiltrate psoriatic skin, but are independent of (and even inhibited by) IFNα,24, making their link to skin inflammation still uncertain.
Flexibility of T cell subsets- plasticity or chaos?
The TH1-TH2 paradigm has been useful but also overused. It provided the needed framework to identify the cytokines, signaling molecules and transcription factors controlling important effector pathways26. Overuse as a simplistic dichotomy was evident as well27, 28, but within this framework came suggestions of flexibility in polarization both in mouse and human cells29-33. The relative flexibility of early TH1 cells compared to rapidly stabilized TH2 cells is partly explained on the basis of early IL-12 receptor expression 34, and by the fact that irreversible commitment of CD4+ T cells might be the end result of repeated stimulation with antigen in vitro or chronic disease in vivo35.
The finding that irreversible fixation of T cell fate appears to require a certain number of divisions36 established an early precedent for later considerations of chromatin modifications in gene regulation37. This question seems particularly relevant to the origin of memory T cells from either committed effector cells or a branch-point before terminal commitment38, 39. Mouse memory T cell populations are considered flexible with regard to cytokine production rather than irreversibly committed31, and this was later confirmed in human memory populations as well40.
Several modes of plasticity of T cell subsets have recently been described (Fig. 1). First, inducible Treg (iTreg) and TH17 cells (reviewed in 41) show effector differentiation plasticity. Thymus-derived Treg have stable Foxp3 expression, but iTreg generated in vitro are unstable (reviewed in 9). Runx transcription factors control the expression of Foxp3, allowing induction of iTreg and maintaining the Treg program in thymus derived Treg42. TGFβ induces both Foxp3 and RORγt in naïve T cells, but Foxp3 is dominant and antagonizes RORγt function unless IL-6 is present43. Thus, an inflammatory environment tilts the balance between iTreg and TH17 differentiation. The issue could be particularly important in the gut, where high production of TGFβ and retinoic acid by CD103-expressing dendritic cells supports favours transition from naïve CD4 cells to iTreg44-46. Second, in vitro TH17 differentiation shows a STAT4- and T-bet-dependent plasticity towards a TH1 profile in mouse47-49 and humans50 (Fig. 1). Beyond this, even stably committed TH2 cell can re-express IL-12Rβ2 and produce both IL-4 and IFNγ following a viral infection in vivo51. Both these transitions appear to depend on the IL-12 receptor as a central point of control. But certain transitions between subsets apparently do not take place; transition from TH2 to TH-17 or Treg, or TH1 to Treg or naive T cell.
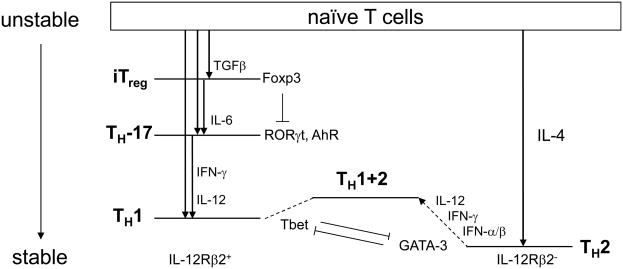
In trying to make sense of these observed or excluded transitions, the general notion of a potential function may be of value. For example, the relationships between CD4 subsets can be displayed in a manner analogous to the energy levels of an electron in the atom (Fig. 2). Stable energy states do not decay; they are not plastic (as if at an energy minimum). For the most part, transitions proceed from a higher energy (i.e., less stable) to lower energy (more stable) state, much like excited atomic states decay into less energetic modes. TH1 cells can arise by differentiation of naïve T cells via IFNγ and IL-12 signaling, or by differentiation of TH17 cells. The plasticity allowing TH17 cells to convert to a TH1 phenotype can thus be described as an allowed transition from ‘unstable’ to ‘stable’, or by higher to lower ‘energy’. Transitions are triggered by TCR stimulation provided in specific conditions. The primary antigen triggered state is the least stable and decays into one of the known subsets following T cell activation. Thus, what might initially appear as chaotic switching between various T cell phenotypes may actually reflect a predictable order of allowed transitions, based on some aspect of this relative stability.
Figure 2.
Transitions of CD4+ T cell subsets. Naïve T cells, when triggered, behave as an ‘unstable state’ that can transition into various other subsets, depending on the conditions as indicated. The diagram suggests a possible hierarchy of stability. Experimentally observed transitions are indicated, along with the required conditions and factors that appear to mediate each step. On the left, subsets that maintain IL-12 receptor remain responsive to IL-12, while on the right, TH2 cells loose IL-12R expression. A hybrid state of TH1+2 has been observed to be induced from TH2 cells, in response to type I interferons, and IFN-γ and IL-12. Known interactions between transcription factors are indicated. Subsets whose status is still uncertain are not presented.
Cytokines, costimulation, circuitry, clonality and chromatin
What could determine the relative stability between CD4+ subsets? Components that regulate T cell stability and plasticity can be divided into four categories; conditions, circuitry, clonality and chromatin. Conditions, both cytokines and costimulation, are the prime factors in differentiation, but also impact stability. Circuitry, meaning the network of interactions between transcription factors, impacts differentiation, stability and plasticity. The issue of T cell clonality has been largely ignored in recent discussions of plasticity, but as we will see, must be considered when examining T cell differentiation. Finally, chromatin modifications associated with active or repressed genes control the maintenance of the phenotype, and, as recognized more recently, its plasticity.
Antigen presenting cells drive CD4+ T cell differentiation through cytokines and costimulation52. Dendritic cells are important in the initial activation and development of CD4+ T cells53, but additional types of innate cells influence CD4+ T cell subsets. For example, neutrophils promote the differentiation of the adaptive TH17 response. Phagocytosis of apoptotic infected neutrophils by dendritic cells is a highly efficient stimulus for TH17 differentiation54. Because IL-17 itself plays an important role in promoting recruitment and differentiation of neutrophils 55 it is clear that γδ TCR T cells that provide the first rapid IL-17 response not only precede differentiation of the adaptive TH17 response, but are most likely instrumental in promoting their differentiation56-58.
TH1 responses on the other hand are helped on the way by natural killer (NK) cells providing the first source of IFNγ59, 60. The crucial issue for TH2 responses had always been the initial source of IL-4, which is important, albeit not indispensable, for their differentiation. Another innate cell type, the basophils, produces substantial amounts of IL-4 have been implicated in the induction of TH2 responses61-63. Other mediators, such as IL-25 and TSLP, coupled with suppression of IL-12 production by dendritic cells, can promote TH2 differentiation even in the absence of IL-4 (reviewed in 64). Beyond these major effects of cytokines derived from innate cells on T cell differentiation, it has been suggested recently that asymmetric cell divisions may contribute to heterogeneity in the expanding T cell population65. How this process might be regulated or come into play with respect to flexibility of CD4+ T cells has not been addressed.
Circuit rules
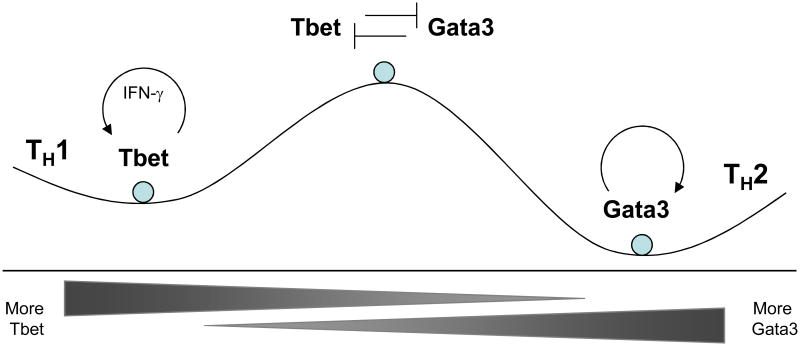
Transcriptional circuitry can promote either stability or plasticity. First, circuits can stabilize phenotypes by being self-reinforcing. For example, the transcriptional circuitry in TH1 and TH2 cells underlies their relative stability compared to TH17 cells and iTregs (Fig. 3). In TH2 cells, the transcriptional autoactivation of GATA-3 provides a self-reinforcing feedback circuit66. Likewise, T-bet induces its own expression, either directly67 or indirectly68. Second, transcriptional circuits can promote developmental divergence of subsets, and this divergent impulse acts to stabilize one phenotype over another. For example, the repression of IL-12 receptors during TH2 differentiation makes these cells less responsive to IL-12, further promoting TH2 development34 Likewise, transcriptional interference between T-bet and GATA-3 proteins, where one factor neutralizes the transcriptional activities of the other, magnifies developmental imbalances and promotes divergence69.
Figure 3.
Transcriptional circuits can stabilize or destabilize CD4+ T cell subsets. The relative stability of states is shown by a hypothetical energy function, with more stable states residing within low energy ‘wells’. The mutual antagonism between T-bet and GATA-3 activity makes the states that co-express the two factors unstable. The positive feedback loops known to reinforce the expression of these factors stabilize the states with high expression of one of the two factors.
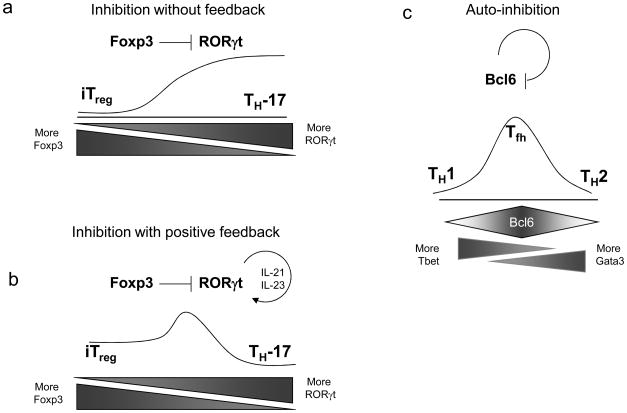
By contrast, the transcriptional circuitry of iTregs and TH-17 cells may dictate a hierarchy of instability relative to TH1 and TH2 cells. First, no functional evidence for transcriptional autoactivation for RORγt or Foxp3 has been established, although Foxp3 may play a role in maintenance of its expression in iTreg cells70. Foxp3 can inhibit RORγt's transcriptional activity43, but no reciprocal effect has been reported. At first, such unilateral inhibition of RORγt by Foxp3 would imply that iTreg should be more stable than TH17 cells (Fig. 4a); in this scenario TH17 cells would require continuous IL-6 input to maintain RORγt expression, at least without additional re-enforcement. However, RORγt induces IL-21, which acts by an autocrine pathway to maintain RORγt expression via STAT3 activation71-73 (Fig. 4b); in this scenario, the inhibition of RORγt's transcriptional activity by Foxp3 produces only a transient stability for iTreg that is overcome by the self-reinforcement of RORγt through IL-21. It is not yet clear how the aryl hydrocarbon receptor (AhR) integrates into the transcriptional network of TH-17 cells. Transduction of AhR deficient T cells with RORγt, AhR or both indicated that there does not seem to be cross talk between the two transcription factors; AhR drives the expression of IL-22, but only does so if both IL-6 and TGFβ are present74 IL-23 appears responsible for amplification of IL-22 (ref 75), but cannot restore IL-22 induction in AhR deficient TH17 cells.
Figure 4.
Possible transcription factor interactions regulating intermediate CD4+ T cell transitions. (a) Hypothetical circuit in which Foxp3 inhibits the activity of RORγt, without a reciprocal inhibition or feedback from RORγt. This circuit might favor iTreg development over TH17 development, making iTreg more stable. Without continuous stimulus by IL-6 to induce RORγt, Foxp3 might tend to repress TH17 development. (b) This hypothetical TH17 state is stabilized despite inhibition of RORγt by Foxp3 due to a known feedback loop, in which RORγt induces expression of IL-21, which acts in an autocrine manner to further induce STAT3 and stimulate RORγt expression. This feedback stabilizes the TH17 state relative to iTreg cells. (c) The transcriptional inhibitor Bcl-6 inhibits its own transcription. Such a mechanism could destabilize the TFH phenotype, consistent with the fact that in vitro cultures of TFH phenotype cells have not been reported as stable, in contrast to the robust stability of TH1 and TH2 cells.
While the lineage status of TFH cells is debated, their dependence on Bcl6 is clear16, 17, 76, and this transcription factor is considered a master regulator for TFH cells. It should be noted that after 10 years77, TFH cells have still not been stably derived in vitro, as it was readily achieved for the self-reinforcing TH1 and TH2 cells. Again, circuitry may be at work. It turns out that Bcl6 protein actually inhibits Bcl6 transcription78, 79, the opposite pattern from the GATA-3 autoactivation seen in TH2 cells (Fig. 4c). Such autoinactivation by Bcl6 could promote TFH instability, much like the transcriptional interference between T-bet and GATA-3, or Foxp3 and RORγt43, promotes developmental divergence.
Two other transitions can occur (Fig. 1). TH17 can transit to TH1 cells (at least in vitro), and TH2 cells can acquire a stable, hybrid state of TH1+2 in vivo in response to the combined actions of type I interferon, IFN-γ and IL-12 during viral infection80. No known circuit explains the instability of TH17 cells and their transition to TH1 cells, but this transition requires the same factors already known to induce TH1 differentiation. Conceivably, T-bet, like Foxp3, could directly bind and neutralize RORγt, or simply might inhibit RORγt transcription. Evidence that T-bet inhibits the level RORγt protein was recently provided, although a transcriptional basis for this was not revealed81. In the development of TH1+2 cells, a key step is the re-expression of the IL-12 receptor through the actions of type I interferons and IFN-γ, although the transcription factors involved were not identified.
Clonality, plasticity and heterogeneity
Consideration of the plasticity or stability of CD4+ T cells is complicated by issues of clonality or heterogeneity. For example, early TH2 cultures rapidly become refractory to reversal to a TH1 phenotype34; this correlated with the early loss of IL-12 receptor in TH2 conditions. In contrast, early TH1 populations remain reversible for longer34. Importantly, this could either be due to maintenance of functional IL-4 signaling by cell in TH1 conditions or simply from uncommitted cells present in a heterogeneous TH1 population34. The alternative explanations of flexibility versus heterogeneity need consideration whenever a population is described as showing ‘plasticity’. Indeed, in vitro polarization never yields 100% pure cells. Early TH17 polarizations were in the range of 5 to 20%. Attributing a cytokine profile to such mixed populations on the basis of ELISA or RT-PCR data generates a considerable amount of ‘noise’.
Some of the variability in TH17 polarization has been attributed to the presence or absence of natural AhR ligands in some culture media74, 82. Iscove's modified Dulbecco medium (IMDM) promotes far better TH17 polarization and IL-22 induction than RPMI and the addition of an AhR inhibitor to IMDM medium reduces polarization and abrogates IL-22 production to the level of that seen in cultures of AhR-deficient CD4+ T cells. Thus, the role of AhR in the induction of IL-22 highlights the great potential for variability in TH17 cell generation in vitro. This fact directly impacts the studies of epigenetic modifications in TH17 cells, as these have been so far carried out with in vitro generated TH17 cells that lacked the full arsenal of signals83.
The role of AhR in TH17 development and effector function revealed an environmental effect on TH17 generation 84-86. Activation of AhR enhances the pathology of EAE by increasing TH17 polarization and cytokine secretion and is indispensable for the induction of IL-22 in TH17 cells as well as IL-17 producing γδ T cells57, 84 Because this transcription factor is activated by a wide range of ligands, including environmental pollutants as well as endogenous physiological ligands87, there is substantial scope for external influences on the TH17 program. Of particular interest, because of their wide availability, are ligands derived from tryptophan metabolism such as 6-formylindolo[3,2-b]carbazole (FICZ)88. Remarkably, L-tryptophan is associated with the development of clinical disorders such as eosinophilia-myalgia-syndrome89, but it remains to be determined if TH17 cells and AhR ligands play any role in this syndrome.
Because studies of T cell subsets involve multiple rounds of cell division, distinguishing individual cell flexibility from heterogeneity should involve interrogation of responses of single cells, such as lineage tracing studies, or alternately, cloning studies. At least one study carried out clonal analysis to demonstrate the instructive mechanisms of GATA-3 in TH2 commitment90. However, no study describing plasticity of TH17 or iTreg cells so far has taken the step to examine clonal populations. Lineage tracing using Cre-lox systems have not yet been applied to iTreg, TH17 or the TH1+2 patterns of flexibility. However, this approach will bring new difficulties, such as whether levels of Cre recombinase expressed by a heterologous locus accurately reflects the level of the endogenous gene in the committed state.
In vivo versus in vitro
In vitro plasticity or stability may not always reflect in vivo behaviour. In vitro differentiation conditions may differ from those in vivo, which may influence the degree of polarization and the nature of the cells generated. Indeed, some studies report that in vivo generated TH17 cells are more stable than those derived in vitro91, while others suggest considerable plasticity in Tregs isolated ex vivo47. Treg cells were shown to acquire the capacity to express either IL-17 or IFNγ (reviewed in 47). More intriguingly, Treg seem to be able to adapt their suppressive mechanisms to particular effector programs. Thus, expression of T-bet in some Treg cells leads to upregulation of CXCR3 and correlates with their capacity to control TH1 responses, while a TH1 inducing environment promotes T-bet expression in Treg cells92 without inducing differentiation into effector cells. On the other hand, under highly inflammatory conditions that lead to host death, Treg cells not only express T-bet, but lose of their regulatory capacity and adopt an effector profile associated with production of IFNγ93.
Similarly, IRF4, which is essential for TH2 effector differentiation, seems to be required by Treg cells to suppress TH2 responses. Conditional ablation of Irf4 in Treg causes excessive TH2 dominated responses94. Furthermore, Treg cells depend on expression of STAT3, a transcription factor that is also essential for the differentiation of TH17 cells, to suppress TH17 responses95. Thus, it appears that environmental factors that drive effector T cell subset differentiation also trigger the expression of transcription factors that can modulate Treg suppressive function, indicating a surprising adaptability in this T cell subset.
Chromatin and the maintenance or plasticity of phenotypes
Epigenetic modifications have recently been put forth as major determinants of the stability or plasticity of CD4+ T cell phenotypes41, 96. This notion had its roots in the initial demonstration that cell division is required for CD4+ T cells to acquire differentiated cytokine expression patterns97, followed by the demonstration that other epigenetic modifications, rather than a cell cycle requirement, were involved in active transcription of cytokine genes98. Active or repressed states of transcription are now extensively correlated with specific modifications of histones, or chromatin marks. Such modifications have been suggested as responsible for the heritability of the differentiated states96, in the case of active chromatin marks, and for the plasticity of a subset83, in the case of “bivalent” marks, which posses features of both active and repressed chromatin. Formally, the causality of chromatin marks in phenotype stability or plasticity remains untested, since no study has directly manipulated chromatin states independently of the conditions or circuitry required to establish them99, 100.
A current controversy is whether chromatin modifications influence heritability of gene expression in dividing cells, as compared with their role in non-dividing cells101. Assays that examine CD4+ T cell plasticity involve multiple rounds of cell division. During DNA replication, histones are thought to be removed from the nucleosome, and new histones inserted into nucleosomes on replicated DNA, presumably altering the locations of active or repressive histone marks99, 101. DNA replication occurs from many sites simultaneously throughout the genome. Thus, for histone modifications to provide epigenetic control of phenotype would require a mechanism to restore the original pattern and prevent randomization of histone marks after cell division. However, whether histone modifications are in fact replicated during cell division is unknown99, 102. A recent study showed that a fraction of H3-H4 histone tetramers could be divided and then associate with new H3-H4 histones after cell division102. Such a mechanism potentially could allow for a process to copy the histone marks from the older generation of H3-H4 tetramers onto the next generation of chimeric H3-H4 tetramers. However, no functional evidence for such a copy mechanism nor a mechanism to maintain nucleosomal positioning was provided.
A second concern discussed above is whether chromatin marks described for various subsets are representative of a uniform or heterogeneous population83. This concern also applies to the claim that bivalent chromatin marks can explain the plasticity of a phenotype when measured in cell populations. Much of the recent support for epigenetic control of stability and plasticity arise from genome-wide application of the Chromatin immunoprecipitation (ChIP) technique that is used to interrogate population of cells for association of biochemical marks with regions of DNA. Thus, bivalent marks identified by ChIP may result from mixes of cells with active and repressive marks. Even beyond this, bivalent marks may represent one active locus and one inactive locus within a single cell. Indeed, such mono-allelic expression of cytokines clearly occurs during T cell differentiation103 particularly during early or incomplete polarization104. Thus, bivalent chromatin marks might indicate flexibility of individual gene loci within one cell, but might also result from heterogeneity or even from monoallelic expression of the locus within a single cell.
Besides histone modifications, DNA modifications may provide a means to control the stability of differentiated phenotypes96. Cytosine methylation at CpG is regulated by DNA (cytosine-5-)-methyltransferase 1 (DNMT1); patterns of methylation can persist throughout multiple rounds of cell divisions by the action of this maintenance methylase on hemimethylated CpG sites96. As such, the methylation status of gene regulatory elements could provide epigenetic control that is stable in the setting of cellular division. However, current technologies have not allowed genome-wide description of DNA methylation patterns, making large scale correlations unavailable.
Where the marks are
Another issue complicates the simple interpretation that ChIP studies identify the chromatin marks associated with regions of DNA. Typically, data from ChIP experiments are interpreted as indicating direct interactions of an identified chromatin regions with the modified histone or sequence-specific DNA binding factors, a presumption based on a linear model chromosomal DNA. However, the initial experimental steps in ChIP protocols105 are the same as for the Chromatin Conformation Capture (3C) technique106, 107. In both techniques, the nucleus is chemically fixed to induce cross-linking105, 108, 109, followed by sonic-degradation of the DNA into small fragments that remain attached to the proteins that are about to be immunoprecipitated. In ChIP and 3C, both protein-protein and protein-DNA interactions are captured by chemical fixation, but 3C further ‘captures’ the proximity of elements caused by DNA looping by introducing a DNA ligation step later on. In discussing results based on ChIP, typically only the protein-DNA cross-linking is emphasized. But importantly, even without this DNA ligation step, indirectly associated DNA has already been immunoprecipitated by ChIP, and so will be identified as a ‘binding site’ by ChIP as it is in 3C. In short, ChIP identifies both direct and indirect DNA regions associated with a histone mark or transcription factor. For example, our interpretation that BATF binds to both the IL-17 promoter and an intergenic enhancer might simply be due to an interaction with one of these regions, if the promoter and enhancer are brought into close proximity by DNA looping in vivo110. This caveat applies also when two transcription factors, such as STAT3 and IRF4, are examined by ChIP, with data suggesting that factors must bind at non-canonical sites111. It is possible in such instances that looping is again at work, with each factor binding to distant canonical sites that are brought into proximity by looping mediated by protein-protein interactions. Such complexities have largely been overlooked as global surveys of factor binding sites have supplanted older techniques such as EMSA, DNA foot printing and site-directed mutagenesis83. Clearly, it is important that speculative interpretations derived from ChIP studies be validated with such additional techniques.
Concluding remarks
Flexibility or stability in T helper subsets can be represented as a series of transitions from less stable to more stable states. Transcriptional circuitry that reinforces or destabilizes expression of specific factors may contribute to the relative stability and to the plasticity of CD4+ T cell subsets. Reinforcement can be direct and cell autonomous, or act in a paracrine manner through the actions of cytokines. Transcription factors that reinforce their own expression, or interfere with the expression or activity of alternative factors, produce stable states that behave as ‘attractors’.
To what extent recent findings of plasticity truly reflect individual CD4+ T cell flexibility or simple population-based heterogeneity is still unresolved. Heterogeneity can occur at the level of individual cells within a population, and at the level of individual gene alleles within a single cell. Furthermore, plasticity and stability are evaluated in the context of cells undergoing many rounds of cell division. The actions of the transcription factors, which persist through cell division, should not be ignored as a mechanism to maintain cellular phenotype, since mechanisms that would re-establish the pattern of specific histone modifications to their original nucleosomal location after cell division have not been identified. In contrast, patterns of cytosine methylation can be replicated through the maintenance methylase DNMT1. For these reasons, the emerging paradigm that histone modifications are a major determinant of heritability of differentiated CD4 subsets remains an intriguing, but untested hypothesis. As additional CD4+ ‘subsets’ emerge, it will be important to elucidate their transcriptional regulation to understand whether they represent novel ‘lineages’ or alternative ‘pathways’ of cellular activation.
Acknowledgments
The authors would like to acknowledge T. Murphy for helpful discussion and careful reading of the manuscript.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Murphy CA, et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005;201:169–171. doi: 10.1084/jem.20042279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harrington LE, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 5.Park H, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov II, et al. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Yang XO, et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;30:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30:626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 12.Breitfeld D, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–1552. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim CH, et al. Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med. 2001;193:1373–1381. doi: 10.1084/jem.193.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaerli P, et al. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogelzang A, et al. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Nurieva RI, et al. Bcl6 mediates the development of T follicular helper cells. Science. 2009;325:1001–1005. doi: 10.1126/science.1176676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu D, et al. The Transcriptional Repressor Bcl-6 Directs T Follicular Helper Cell Lineage Commitment. Immunity. 2009 doi: 10.1016/j.immuni.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Nurieva RI, et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–335. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 21.Lu LF, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- 22.Nowak EC, et al. IL-9 as a mediator of Th17-driven inflammatory disease. J Exp Med. 2009;206:1653–1660. doi: 10.1084/jem.20090246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elyaman W, et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A. 2009;106:12885–12890. doi: 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duhen T, Geiger R, Jarrossay D, Lanzavecchia A, Sallusto F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 25.Trifari S, Kaplan CD, Tran EH, Crellin NK, Spits H. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from T(H)-17, T(H)1 and T(H)2 cells. Nat Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 26.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2:933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 27.Gor DO, Rose NR, Greenspan NS. TH1-TH2: a procrustean paradigm. Nat Immunol. 2003;4:503–505. doi: 10.1038/ni0603-503. [DOI] [PubMed] [Google Scholar]
- 28.Murphy KM. In search of the CTD. Nat Immunol. 2003;4:645. doi: 10.1038/ni0703-645. [DOI] [PubMed] [Google Scholar]
- 29.Sornasse T, Larenas PV, Davis KA, de Vries JE, Yssel H. Differentiation and stability of T helper 1 and 2 cells derived from naive human neonatal CD4+ T cells, analyzed at the single-cell level. J Exp Med. 1996;184:473–483. doi: 10.1084/jem.184.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manetti R, et al. Interleukin 12 induces stable priming for interferon gamma (IFN-gamma) production during differentiation of human T helper (Th) cells and transient IFN-gamma production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmadzadeh M, Farber DL. Functional plasticity of an antigen-specific memory CD4 T cell population. Proc Natl Acad Sci U S A. 2002;99:11802–11807. doi: 10.1073/pnas.192263099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paliard X, et al. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988;141:849–855. [PubMed] [Google Scholar]
- 33.Sundrud MS, et al. Genetic reprogramming of primary human T cells reveals functional plasticity in Th cell differentiation. J Immunol. 2003;171:3542–3549. doi: 10.4049/jimmunol.171.7.3542. [DOI] [PubMed] [Google Scholar]
- 34.Szabo SJ, Jacobson NG, Dighe AS, Gubler U, Murphy KM. Developmental commitment to the Th2 lineage by extinction of IL-12 signaling. Immunity. 1995;2:665–675. doi: 10.1016/1074-7613(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 35.O'Garra A. Cytokines induce the development of functionally heterogeneous T helper cell subsets. Immunity. 1998;8:275–283. doi: 10.1016/s1074-7613(00)80533-6. [DOI] [PubMed] [Google Scholar]
- 36.Murphy E, et al. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996;183:901–913. doi: 10.1084/jem.183.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grogan JL, et al. Early transcription and silencing of cytokine genes underlie polarization of T helper cell subsets. Immunity. 2001;14:205–215. doi: 10.1016/s1074-7613(01)00103-0. [DOI] [PubMed] [Google Scholar]
- 38.Wu CY, et al. Distinct lineages of T(H)1 cells have differential capacities for memory cell generation in vivo. Nat Immunol. 2002;3:852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 39.Lohning M, et al. Long-lived virus-reactive memory T cells generated from purified cytokine-secreting T helper type 1 and type 2 effectors. J Exp Med. 2008;205:53–61. doi: 10.1084/jem.20071855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Messi M, et al. Memory and flexibility of cytokine gene expression as separable properties of human T(H)1 and T(H)2 lymphocytes. Nat Immunol. 2003;4:78–86. doi: 10.1038/ni872. [DOI] [PubMed] [Google Scholar]
- 41.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Bruno L, et al. Runx proteins regulate Foxp3 expression. J Exp Med. 2009;206:2329–2337. doi: 10.1084/jem.20090226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee YK, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bending D, et al. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009 doi: 10.1172/JCI37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol. 2009;39:216–224. doi: 10.1002/eji.200838475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Annunziato F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hegazy AN, et al. Interferons Direct Th2 Cell Reprogramming to Generate a Stable GATA-3(+)T-bet(+) Cell Subset with Combined Th2 and Th1 Cell Functions. Immunity. 2010 doi: 10.1016/j.immuni.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 52.Murphy KM, et al. Signaling and transcription in T helper development. Annu Rev Immunol. 2000;18:451–494. doi: 10.1146/annurev.immunol.18.1.451. [DOI] [PubMed] [Google Scholar]
- 53.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1:199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 54.Torchinsky MB, Garaude J, Martin AP, Blander JM. Innate immune recognition of infected apoptotic cells directs T(H)17 cell differentiation. Nature. 2009;458:78–82. doi: 10.1038/nature07781. [DOI] [PubMed] [Google Scholar]
- 55.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 56.Roark CL, Simonian PL, Fontenot AP, Born WK, O'Brien RL. gammadelta T cells: an important source of IL-17. Curr Opin Immunol. 2008;20:353–357. doi: 10.1016/j.coi.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 58.Sutton CE, et al. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 59.Scharton TM, Scott P. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J Exp Med. 1993;178:567–577. doi: 10.1084/jem.178.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martin-Fontecha A, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 61.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 64.Finkelman FD. Basophils as T(H)2-inducing APCs: the dog can sing but is it a diva. Immunol Cell Biol. 2009 [Google Scholar]
- 65.Chang JT, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 66.Ouyang W, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity. 2000;12:27–37. doi: 10.1016/s1074-7613(00)80156-9. [DOI] [PubMed] [Google Scholar]
- 67.Mullen AC, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 68.Afkarian M, et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 69.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 70.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei L, Laurence A, Elias KM, O'Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nurieva R, et al. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 73.Korn T, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Veldhoen M, Hirota K, Christensen J, O'Garra A, Stockinger B. Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J Exp Med. 2009;206:43–49. doi: 10.1084/jem.20081438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng Y, et al. Interleukin-22, a T(H)17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature. 2007;445:648–651. doi: 10.1038/nature05505. [DOI] [PubMed] [Google Scholar]
- 76.Johnston RJ, et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science. 2009;325:1006–1010. doi: 10.1126/science.1175870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–1134. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kikuchi M, et al. Identification of negative regulatory regions within the first exon and intron of the BCL6 gene. Oncogene. 2000;19:4941–4945. doi: 10.1038/sj.onc.1203864. [DOI] [PubMed] [Google Scholar]
- 79.Arguni E, et al. JunD/AP-1 and STAT3 are the major enhancer molecules for high Bcl6 expression in germinal center B cells. Int Immunol. 2006;18:1079–1089. doi: 10.1093/intimm/dxl041. [DOI] [PubMed] [Google Scholar]
- 80.Tokoyoda K, et al. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Mukasa R, et al. Epigenetic Instability of Cytokine and Transcription Factor Gene Loci Underlies Plasticity of the T Helper 17 Cell Lineage. Immunity. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oberg M, Bergander L, Hakansson H, Rannug U, Rannug A. Identification of the tryptophan photoproduct 6-formylindolo[3,2-b]carbazole, in cell culture medium, as a factor that controls the background aryl hydrocarbon receptor activity. Toxicol Sci. 2005;85:935–943. doi: 10.1093/toxsci/kfi154. [DOI] [PubMed] [Google Scholar]
- 83.Wei G, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veldhoen M, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 85.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 86.Kimura A, Naka T, Nohara K, Fujii-Kuriyama Y, Kishimoto T. Aryl hydrocarbon receptor regulates Stat1 activation and participates in the development of Th17 cells. Proc Natl Acad Sci U S A. 2008;105:9721–9726. doi: 10.1073/pnas.0804231105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 88.Rannug A, Fritsche E. The aryl hydrocarbon receptor and light. Biol Chem. 2006;387:1149–1157. doi: 10.1515/BC.2006.143. [DOI] [PubMed] [Google Scholar]
- 89.Roubenoff R, Cote T, Watson R, Levin ML, Hochberg MC. Eosinophilia-myalgia syndrome due to L-tryptophan ingestion. Report of four cases and review of the Maryland experience. Arthritis Rheum. 1990;33:930–938. doi: 10.1002/art.1780330703. [DOI] [PubMed] [Google Scholar]
- 90.Farrar JD, et al. An instructive component in T helper cell type 2 (Th2) development mediated by GATA-3. J Exp Med. 2001;193:643–650. doi: 10.1084/jem.193.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lexberg MH, et al. Th memory for interleukin-17 expression is stable in vivo. Eur J Immunol. 2008;38:2654–2664. doi: 10.1002/eji.200838541. [DOI] [PubMed] [Google Scholar]
- 92.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oldenhove G, et al. Decrease of Foxp3(+) Treg Cell Number and Acquisition of Effector Cell Phenotype during Lethal Infection. Immunity. 2009 doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaudhry A, et al. CD4+ Regulatory T Cells Control TH17 Responses in a Stat3-Dependent Manner. Science. 2009 doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 97.Bird JJ, et al. Helper T cell differentiation is controlled by the cell cycle. Immunity. 1998;9:229–237. doi: 10.1016/s1074-7613(00)80605-6. [DOI] [PubMed] [Google Scholar]
- 98.Richter A, Lohning M, Radbruch A. Instruction for cytokine expression in T helper lymphocytes in relation to proliferation and cell cycle progression. J Exp Med. 1999;190:1439–1450. doi: 10.1084/jem.190.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet. 2009;43:559–599. doi: 10.1146/annurev.genet.032608.103928. [DOI] [PubMed] [Google Scholar]
- 100.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 101.Ptashne M. On the use of the word ‘epigenetic’. Curr Biol. 2007;17:R233–236. doi: 10.1016/j.cub.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 102.Xu M, et al. Partitioning of histone H3-H4 tetramers during DNA replication-dependent chromatin assembly. Science. 328:94–98. doi: 10.1126/science.1178994. [DOI] [PubMed] [Google Scholar]
- 103.Bix M, Locksley RM. Independent and epigenetic regulation of the interleukin-4 alleles in CD4+ T cells. Science. 1998;281:1352–1354. doi: 10.1126/science.281.5381.1352. [DOI] [PubMed] [Google Scholar]
- 104.Riviere I, Sunshine MJ, Littman DR. Regulation of IL-4 expression by activation of individual alleles. Immunity. 1998;9:217–228. doi: 10.1016/s1074-7613(00)80604-4. [DOI] [PubMed] [Google Scholar]
- 105.Barski A, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 106.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 107.Spilianakis CG, Flavell RA. Long-range intrachromosomal interactions in the T helper type 2 cytokine locus. Nat Immunol. 2004;5:1017–1027. doi: 10.1038/ni1115. [DOI] [PubMed] [Google Scholar]
- 108.Barski A, Zhao K. Genomic location analysis by ChIP-Seq. J Cell Biochem. 2009;107:11–18. doi: 10.1002/jcb.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Z, et al. Genome-wide mapping of HATs and HDACs reveals distinct functions in active and inactive genes. Cell. 2009;138:1019–1031. doi: 10.1016/j.cell.2009.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schraml BU, et al. The AP-1 transcription factor Batf controls T(H)17 differentiation. Nature. 2009;460:405–409. doi: 10.1038/nature08114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kwon H, et al. Analysis of interleukin-21-induced Prdm1 gene regulation reveals functional cooperation of STAT3 and IRF4 transcription factors. Immunity. 2009;31:941–952. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]