Abstract
We previously demonstrated that H2 relaxin (RLN2) facilitates castrate-resistant (CR) growth of prostate cancer (CaP) cells through PI3K/Akt/β-catenin-mediated activation of the androgen receptor (AR) pathway. As inhibition of this pathway caused only ~50% reduction in CR growth, the goal of the current study was to identify additional RLN2-activated pathways that contribute to CR growth. Next-generation sequencing-based transcriptome and gene ontology analyses comparing LNCaP stably transfected with RLN2 versus LNCaP-vector identified differential expression of genes associated with cell proliferation (12.7% of differentially expressed genes), including genes associated with the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) and nuclear factor–kappaB (NF–κB) pathways. Subsequent molecular analyses confirmed that the cAMP/PKA and NF–κB pathways play a role in facilitating H2 relaxin-mediated CR growth of CaP cells. Inhibition of PKA-attenuated RLN2-mediated AR activity inhibited proliferation and caused a small but significant increase in apoptosis. Combined inhibition of the PKA and NF–κB signaling pathways via inhibition of PKA and Akt induced significant apoptosis and dramatically reduced clonogenic potential, outperforming docetaxel, the standard of care treatment for CR CaP. Immunohistochemical analysis of tissue microarrays in combination with multispectral quantitative imaging comparing RLN2 levels in patients with benign prostatic hyperplasia (BPH), prostatic intraepithelial neoplasia, and CaP determined that RLN2 is significantly upregulated in CaP vs BPH (p = 0.002). The combined data indicate RLN2 overexpression is frequent in CaP patients and provides a growth advantage to CaP cells. A near-complete inhibition of RLN2-induced CR growth can be achieved by simultaneous blockade of both pathways.
Keywords: H2 relaxin, Castrate-resistant prostate cancer, Androgen receptor, Cyclic AMP, Protein kinase A, NF–kappaB
Introduction
Androgen ablation is the standard therapy for disseminated prostate cancer (CaP); however, patients develop resistance to this treatment regimen within 18–24 months [13]. Patients with castration-resistant (CR) CaP are offered limited options, usually chemotherapy treatment with docetaxel, and more recently, the cancer vaccine “Provenge”, whose effect is to increase patient survival time by a median of 3 or 4 months, respectively [18, 30, 42]. The identification and elucidation of pathways that promote CR CaP is critical for the development of successful new therapies to treat this disease.
H2 relaxin (RLN2) is a peptide hormone that is a member of the insulin-like superfamily. Several groups, including ours, have demonstrated that H2 relaxin plays a role in prostate carcinogenesis. Overexpression of RLN2 can induce tumor growth in a mouse model of CaP [37], and stimulation with RLN2 increases cell proliferation, invasiveness, and adhesion of CaP cells in vitro [7, 8]. Inhibition of RLN2 using an inhibitory analog [38] or suppression of its receptor LGR7 (also called RXFP1) [9] blocks RLN2-mediated CaP growth. Studies in human CaP have demonstrated that RLN2 expression is increased in radical prostatectomy specimens after 6 months of androgen ablation and in CR CaP, and that expression is highest in bone metastases [44]. Our group has demonstrated that RLN2 mediates CR growth of CaP cells by a mechanism that involves PI3K-dependent co-translocation of the androgen receptor (AR) and β-catenin to the nucleus and transactivation of the prostate-specific antigen (PSA) promoter [21, 46]. Based on our studies, others have also shown that RLN2-induced β-catenin stabilization is mediated by ProtocadherinY [43]. While it is clear that β-catenin plays an important role in mediating the effects of RLN2 in CaP cells, inhibition of β-catenin stabilization or of Akt activation only partially inhibits RLN2-mediated growth of LNCaP, while blocking the RLN2 receptor, RXFP1, causes near-complete inhibition. Thus, the goal of the current study was to further elucidate the mechanism(s) by which RLN2 contributes to AR activation and CaP progression.
In the current study, we confirm that H2 relaxin expression is elevated in CaP patient specimens and in addition demonstrates that RLN2 is expressed at low levels in benign prostatic hyperplasia (BPH) relative to CaP specimens (p = 0.002). This finding has not previously been reported. We have used next-generation sequencing (NGS)-based transcriptome and gene ontology (GO) analyses to identify additional downstream effectors of RLN2 in CaP cells. These data, combined with molecular and inhibitor studies, have identified the nuclear factor–kappaB (NF–κB) and protein kinase A (PKA) signaling pathways as being activated by RLN2. Most importantly, when both pathways were simultaneously attenuated by the use of the PKA inhibitor H-89 as well as perifosine, which is an upstream inhibitor of NF–κB, RLN2-induced cell growth and survival were effectively downregulated.
Materials and Methods
Cell Lines and Culture
LNCaP, PC-3, and Rwpe1 cells were purchased from the American Type Culture Collection (ATCC, Manassas, VA). CWR22Rv1 and PC346C (an androgen-dependent cell line [22]) were kindly provided to us by Drs. Hsing-Jien Kung and Van Weerden, respectively. All cell lines were maintained as previously described [46].
Generation of Stable Cell Lines
The RLN2 LNCaP sublines (LNCaP-rlx3 and LNCaP-rlx5) were generated in-house. Briefly, the RLN2 allele was cloned into the pCR3.1 vector (Invitrogen, Carlsbad, CA) downstream of the cytomegalovirus promoter. Plasmids containing the RLN2 allele or empty pCR3.1 plasmid (LNCaP-vector) were stably transfected into LNCaP cells using Effectene (Qiagen, Valencia, CA). After 48 h, cells were grown under G418 (500 μg/ml) selection for 2–3 weeks until isolated colonies appeared. Colonies were selected and expanded in 24-well plates before being transferred to culture flasks.
Reagents
Antibodies used were: total and phospho inhibitor of kappaB (IκB)-alpha, and Bcl-xL (Cell Signaling Technology, Beverly, MA), B-actin (Sigma-Aldrich Corporation, St. Louis, MO), NF–κB (Santa Cruz BioTechnologies, Santa Cruz, CA), H2 relaxin (ALPCO, Salem, NH). The inhibitors were: IκB kinase inhibitor (IKK inhibitor; a 14-amino acid peptide corresponding to the active IκB phosphorylation recognition sequence fused to the hydrophobic region of the fibroblast growth factor signal peptide; Calbiochem, Gibbstown, NJ), and PKA inhibitors, H89 and PKI (Calbiochem, Gibbstown, NJ).
SiRNA
Pre-validated siRNA specific for beta-catenin was purchased from Dharmacon (Lafayette, CO). To achieve knockdown, cells were transfected with either 20 or 50 nM siRNA using Lipofectamine 2000 as previously described [21].
Clonogenic Assay
Single cells (12,000) were seeded into 60-mm culture dishes containing fetal bovine serum (FBS) as media on day 0 and allowed to attach for 24 h at 37°C. After 24 h, FBS media was replaced with charcoal-stripped serum (CSS) media and cultured for 14 days. Colonies were fixed in 1.0% crystal violet and 0.5% glacial acetic acid in ethanol, and visible colonies containing approximately 50 or more cells were counted.
Flow Cytometry
Cell cycle analysis: Analyses were performed as previously described [45]. Apoptosis analysis: The TACS annexin V-FITC kit (R&D Systems) was used to quantitate both early and late apoptosis. The analysis was performed using a Coulter Epics XL flow cytometer (Beckman Coulter); compensation was performed using FlowJo software (FlowJo, Ashland, OR). The assay was performed in accordance with the protocol described by the manufacturer. All samples were run in triplicate; all experiments were performed at least three times.
Immunoblot Analysis
Analyses were performed as previously described [46].
Cell Culture Immunohistochemistry
Cells (50,000 per chamber) were seeded in chamber slides (BD Falcon, Two Oak Park, MA) in FBS media and allowed to adhere for 24 h. After 24 h, FBS media was replaced with CSS media. After 3 days, cells were washed with phosphate buffered saline (pH 7.4) then fixed in ice-cold methanol for 7 min. After further washing, cells were incubated with 0.5% bovine serum albumin for 20 min prior to the NF–κB antibody (see “Reagents” section for details) being added at a dilution of 1:250. After 60 min, cells were washed and then incubated with a fluorescein-isothiocyanate-labeled secondary antibody (Sigma-Aldrich), 1:500. Cells were again washed and then mounted using DAPI-containing mountant (Vector labs, Burlingame, CA). Staining was visualized by fluorescence microscopy using an Olympus BX61 microscope equipped with SlideBook digital imaging software.
cAMP and PKA Kinase Assays
cAMP. The cyclic adenosine monophosphate (cAMP) Biotrak competitive enzyme immunoassay (Amersham-Pharmacia, RPN225) was used to determine cAMP activity levels 10 min after treatment of parental LNCaP with recombinant human (rh) H2 relaxin. Forskolin (Calbiochem) was used as a positive control. LNCaP were serum-starved for 24 h prior to the addition of rh H2 relaxin or forskolin. The assay was performed in accordance with the Protocol 3 described by the manufacturer. PKA. The PKA kinase activity assay (Stressgen, EKS-390A) was used to determine PKA activity levels 5, 15, and 30 min after treatment of parental LNCaP with rh H2 relaxin or rh H2 relaxin and H89. LNCaP were serum-starved for 24 h prior to the addition of rh H2 relaxin or H89. The assay was performed in accordance with the protocol described by the manufacturer. All samples were run in triplicate; all experiments were performed at least three times.
NF–κB DNA Binding Assay
The NF–κB p65 EZ-TFA Transcription Factor assay (Millipore) was used to determine whether H2 relaxin is able to mediate binding of NF–κB to its DNA consensus sequence. This assay was performed using protein extracted from both LNCaP-vector treated with 100 ng/ml rh H2 relaxin for 60 min and from the LNCaP-RLN2/C1 and LNCaP-RLN2/C2 sublines cultured in CSS media. The Whole Cell Extraction kit (Millipore) was used to prepare the protein extracts used in this assay. The assay was performed in accordance with the protocol described by the manufacturer. All samples were run in triplicate; all experiments were performed at least three times.
Tissue Microarray and TMA Immunohistochemistry
The tissue microarrays were constructed using a semi-automated tissue arrayer, TMAarrayer (Pathology Devices, Inc., Westminster, MD). Cores from 49 CaP (with known grade), 15 prostatic intraepithelial neoplasia (PIN), and 24 BPH patients (four core replicates of each) were analyzed. The protocol described by Thompson et al. was used to stain the CaP tissue microarrays (TMAs) for H2 relaxin [44].
Multispectral Imaging and Quantification of Staining
Images were obtained using an Olympus BX51 microscope with an attached Nuance CRI multispectral imaging system version 2.10.0 (Cambridge Research and Instrumentation, Woburn, MA). This imaging system is able to separate signals generated by different chromatogens and thereby improve quantitative accuracy (review; [20]). Isolation of background tissue and hematoxylin wavelengths was achieved by spectral analysis of hematoxylin-only stained cores and slide-mounted pure liquid hematoxylin. The 3,3′-diaminobenzidine (DAB) wavelength was isolated by positive control core samples and slide-mounted pure liquid DAB. Multispectral analysis was then used to assess the ratio of DAB level to TMA core area for each individual TMA core. Images were converted to a false fluorescence format.
RNA Extraction and qRT-PCR
RNA extraction methods have previously been described [46]. RLN2 and GAPDH expression was assessed using pre-designed TaqMan primer/probes sets in combination with the TaqMan Reverse Transcription and Universal PCR Master Mix kits as per manufacturer’s protocol (Applied Biosystems, Foster City, CA). Samples were run on an ABI 7900HT and data analyzed using the corresponding software. Triplicate samples were run for each experimental group. Care was taken to ensure that the resulting Ct values for each group were within 0.5 Ct of each other.
Cell Proliferation Assay
Described by Vinall et al. [46].
PSA ELISA
LNCaP were seeded into 24 well plates (50,000 cells/well) in FBS media and allowed to attach for 24 h. After 24 h the FBS media was replaced with CSS media and the relevant inhibitors added (see reagents section for details. The level of PSA present in culture supernatants was measured using a PSA ELISA kit (MEDICORP, Montreal, Quebec, Canada) according to the manufacturer’s protocol. Absorbance was measured at OD 450 using a Benchmark Plus Microplate Spectrophotometer (Bio-Rad Labs, Hercules, CA).
Sequencing-Based Transcriptome Analysis
NGS was utilized for whole transcriptome analysis of LNCaP-vector and the LNCaP stably transfected with RLN2 (LNCaP-RLN2)and LNCaP-p53/R273H sublines. For this, RNA-Sequencing (RNA-Seq) libraries were prepared from 1 ug total RNA using the mRNA-Seq Sample Prep Kit (Illumina, San Diego, CA) according to the manufacturer’s protocol. The libraries were then loaded on paired-end flow cells for cluster generation (cBot) followed by sequencing (2 × 40-bp) with the Genome Analyzer IIx (Illumina, Inc.) using kitted reagents (Illumina) and according to the manufacturer’s protocols [4]. Data and bioinformatics analysis: Image processing, base calling, and quality scoring (Phred) were executed by SCS 2.6/RTA 1.6 software (Illumina, Inc.). CASAVA 1.7 (Illumina) was used for read alignment to the reference human genome sequence (GRCh37/hg19) using ELANDv2 and allowing for a maximum of two mismatches. Normalized transcript levels were quantified by calculation for RPKM (reads per kilobase of exon model per million mapped reads) [25]. Comparison analysis was performed in order to identify genes differentially expressed in the LNCaP-RLN2 clones as compared to the LNCaP-vector control. For this, genes up- or downregulated in LNCaP-RLN2 were filtered based upon having RPKM values ≥25 (i.e., moderate expression) in either the LNCaP-RLN2 or LNCaP-vector cell lines, respectively, exhibiting the same trend in regulation in both LNCaP-RLN2 clones, and having ≥1.5-fold change in expression (RLN2/Vector). Subsequently, GO analysis [2] was performed in order to classify the genes according to biological process and molecular function.
Statistical Analysis
At least three independent experiments were completed for each analysis described in this article. Data are shown as mean ± SD. Multiple group comparison was performed by one-way ANOVA followed by the Scheffe procedure for comparison of means using STATA software (College Station, TX). p < 0.05 was considered statistically significant (* signifies p < 0.05).
Results
RLN2 Is Elevated in CaP Patient Specimens Relative to BPH
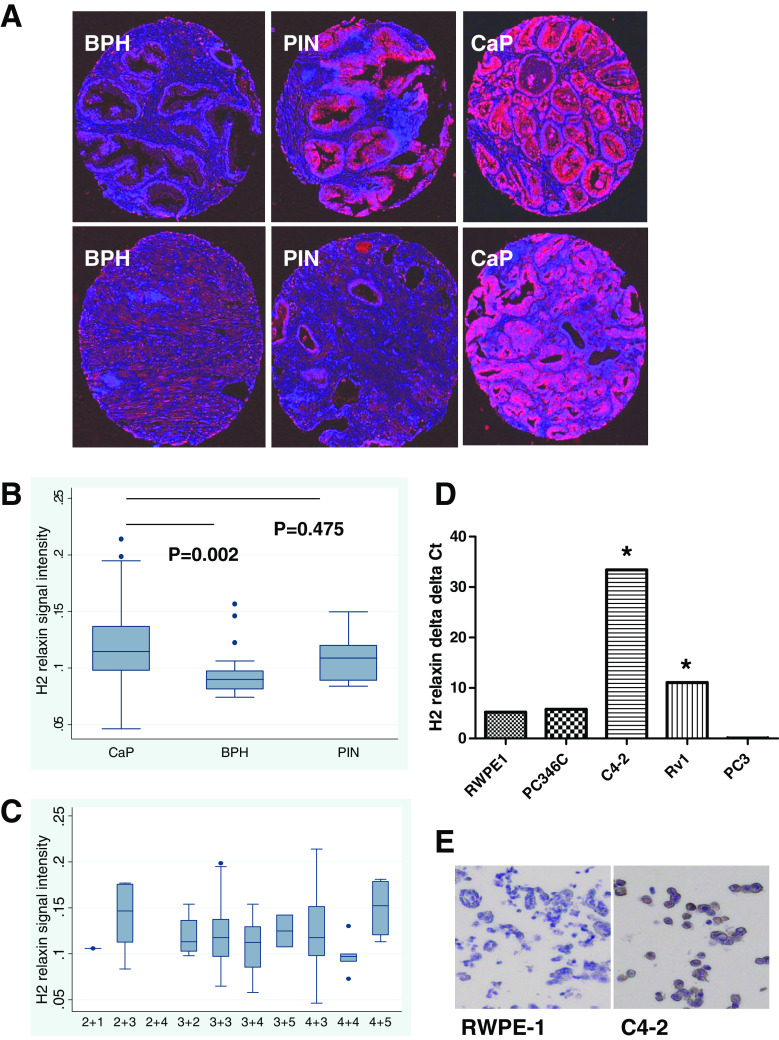
To determine the significance of RLN2 in human prostate cancer (CaP) patients, we conducted immunohistochemical (IHC) analysis of 49 CaP, 15 PIN and 24 BPH specimens taken from patients with primary CaP undergoing prostatectomy (for PIN and CaP specimens) or TURP (for BPH) as initial treatment for the disease (Fig. 1a). Note that RLN2 stained the epithelial cells strongly while some staining could also be seen for the stromal cells. However, the nuclei did not stain for this peptide hormone at all, demonstrating the specificity of the staining. Specifically, the staining level increased from BPH <PIN = CaP indicating the increased accumulation of RLN2 during initiation of CaP. Quantification of staining using the Nuance multispectral imaging system demonstrated that RLN2 expression is significantly higher in CaP specimens relative to the BPH specimens (Fig. 1b, 0.119+/−0.032 versus 0.95+/−0.021, p = 0.002), a finding that has not previously been reported. The increase of RLN2 in CaP compared to BPH is of clinical relevance because in patients the serum levels of PSA increase in both cases. Since RLN2 can be detected in the serum [37], this means that the serum levels of this peptide could potentially be used as an independent marker of CaP as opposed to BPH. Further studies are required to test this hypothesis. A significant difference in RLN2 expression was not observed between the PIN and CaP specimens (p = 0.475) and RLN2 expression did not correlate with Gleason grade (Fig. 1c). Specimens from CaP patients with CR disease were not assessed due to lack of availability, however, other studies have observed very high H2 relaxin expression levels in bone metastases specimens from CR CaP patients [44]. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) analysis and RLN2 IHC was used to assess relative expression of RLN2 in “normal-like” RWPE-1 and multiple CaP cell lines (Fig. 1d, e). Note that triplicate samples were run for each experimental group, and the resulting Ct values for each group were within 0.5 Ct of each other. Excluding PC3, the aggressive tumors C4-2 and CWR22Rv1 expressed significantly higher levels of RLN2 compared with the RWPE-1 cells (p < 0.05) derived from a normal human prostate and compared with the PC-346C cells (p < 0.05) derived from an androgen-dependent CaP, thereby supporting our hypothesis that RLN2 plays a role in progression to CR CaP.
Fig. 1.
RLN2 expression is elevated in CaP patients. a Representative images from cores of BPH, PIN, and CaP were obtained from patients with BPH (who underwent TURP) or CaP (who underwent prostatectomy) with IRB consent and assembled as a TMA. The cores were immunostained with an antibody to RLN2 and counterstained with hematoxylin. Multispectral imaging technologies were utilized to convert the brown staining (representing RLN2 localization) and the blue hematoxylin counterstain to false fluorescent imaging for better visualization and staining quantitation. Here shown are two representative cores from each group. b Box plot comparing median H2 relaxin expression values and interquartile ranges between the patient groups. Statistical analysis of RLN2 expression based on quantitation of multispectral imaging determined that RLN2 expression is higher in CaP patients compared with patients with BPH (p = 0.002), while the difference in staining between BPH and PIN or PIN and CaP was not significant. All patient cores were assessed based on their DAB level to area score. c RLN2 expression did not correlate with Gleason grade. d Quantitative RT-PCR (qRT-PCR) analysis of RWPE-1 cells (derived from a normal prostate) vs several CaP cell lines demonstrated that, excluding PC3 cells, RLN2 expression is elevated in CaP cell lines. Triplicate samples were run for each experimental group, and the resulting Ct values for each group were within 0.5 Ct of each other. e Immunocytochemical analysis in RWPE-1 vs C4-2 with RLN2 antibody (brown staining) cells confirmed this trend. The cells were counterstained with hematoxylin (blue) which allows visualization of unstained cells
Generation of LNCaP Sublines that Stably Overexpress H2 Relaxin
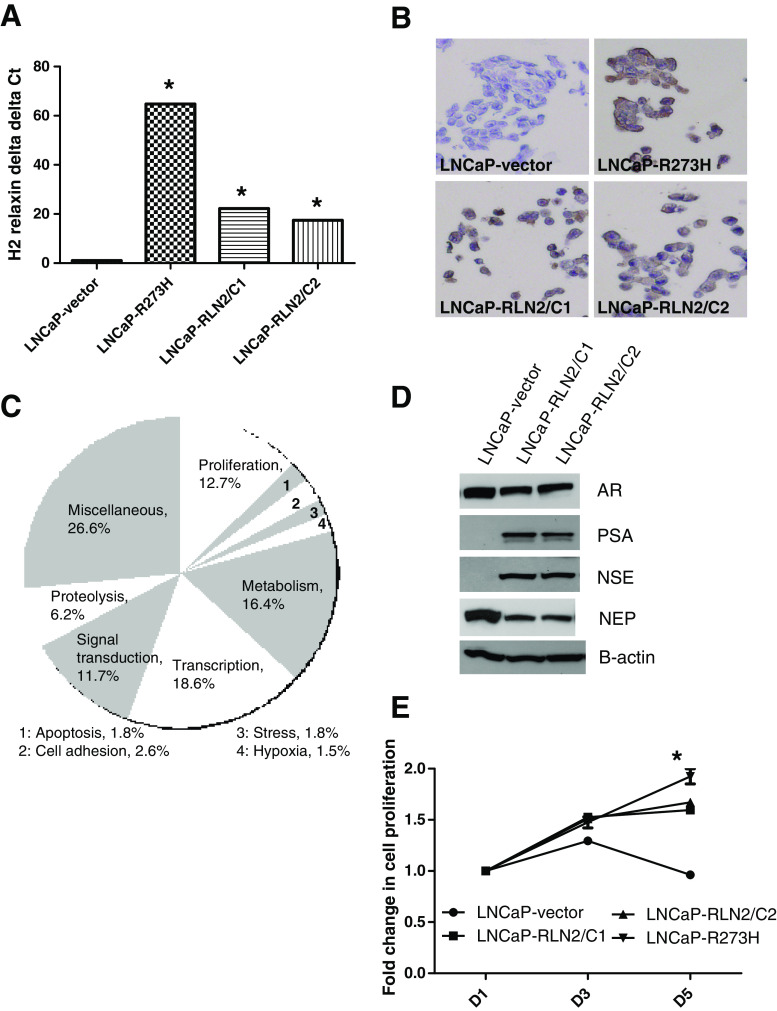
To understand the functional significance of increased RLN2 expression in CaP, we stably transfected LNCaP with plasmid expressing the RLN2 gene. LNCaP are an androgen-dependent CaP cell line and are the cell lines used for all our previous RLN2-related studies [21, 46]. Two clones (LNCaP-RLN2/C1 and LNCaP-RLN2/C2) were chosen for further investigation of the role played by RLN2 in CaP. These sublines expressed ~20-fold higher levels of RLN2 mRNA relative to LNCaP stably transfected with the vector control (LNCaP-vector; Fig. 2a), p < 0.005 for both LNCaP-RLN2/C1 and /C2 versus LNCaP-vector. LNCaP-R273H, an androgen-independent LNCaP subline that stably expresses a p53R273H mutant allele, expressed the highest level of RLN2 mRNA, ~60-fold higher than the androgen-dependent LNCaP-vector subline (p < 0.0005), and was used as a positive control. Note that triplicate samples were run for each experimental group, and the resulting Ct values for each group were within 0.5 Ct of each other. We have previously demonstrated that the p53R273H mutant, which is a hotspot mutation in CaP patients, can bind to the RLN2 promoter and drive RLN2 expression [44]. In the present study, the increase in RLN2 expression was observed at both the mRNA level (as determined by qRT-PCR) and at the protein level (as determined by immunocytochemistry in the same cell lines; Fig. 2b). It is noteworthy that the LNCaP-RLN2 sublines appear to be good models of CR CaP as they behave similarly to the CaP cells found in CR CaP patient tumors; they are able to grow in the absence of androgens and express normal levels of AR, but high levels of PSA even in the absence of androgen, i.e., the AR pathway, can be activated in ligand-independent manner.
Fig. 2.
Effect of increased expression of RLN2 in LNCaP prostate cancer cells. a LNCaP sublines LNCaP-RLN2/C1 and LNCaP-RLN2/C2, which express elevated levels of RLN2, were generated by stable transfection of RLN2 in LNCaP cells. Quantitative RT-PCR (qRT-PCR) analysis for RLN2 mRNA levels in these sublines compared with vector-transfected LNCaP cells and LNCaP-R273H, a subline previously shown to express extremely high levels of this peptide hormone, demonstrated that the two RLN2 LNCaP sublines expressed RLN2 at significantly higher levels compared with LNCaP but not as high as in p53R273H-transfected cells. Triplicate samples were run for each experimental group, and the resulting Ct values for each group were within 0.5 Ct of each other. b Immunocytochemical analysis of the cell lines confirmed this trend. c Next-generation sequencing (NGS) followed by gene ontology (GO) analysis revealed 12.7% of genes that are differentially expressed between the RLN2 LNCaP and LNCaP-vector sublines are linked to proliferation. Other key processes associated with increased RLN2 expression were transcription (18.6%), metabolism (16.4%), signal transduction (11.7%), and proteolysis (6.2%). d The RLN2 LNCaP sublines express high levels of NSE and low levels of NEP, suggesting a neuroendocrine-like phenotype. While lower levels of AR were observed in the RLN2 LNCaP sublines, assessment of PSA levels indicates that the AR pathway is much more active. e MTT proliferation assay determined that the RLN2 LNCaP sublines are able to grow in the absence of androgen, as was the LNCaP-R273H subline, and that the difference in proliferation at the day 5 time point was statistically significant when comparing the LNCaP-vector and all three LNCaP sublines (asterisk signifies p < 0.05)
Identification of Downstream Effectors of RLN2 Using NGS Analysis
To help identify downstream effectors of RLN2 and to further elucidate the role played by RLN2 in CaP, we conducted next-generation DNA sequencing analysis (i.e., RNA-Seq) followed by GO analysis using LNCaP-RLN2/C1 and LNCaP-RLN2/C2 vs LNCaP-vector sublines. Comparison of gene sequence analysis (Fig. 2c) revealed 12.7% of genes with >1.5-fold increased expression in the LNCaP-RLN2 sublines relative to the LNCaP-vector were related to proliferation. Other key processes associated with increased RLN2 expression were transcription (18.6%), metabolism (16.4%), signal transduction (11.7%), and proteolysis (6.2%; Fig. 2c). As expected, NGS analysis determined that the LNCaP-RLN2 sublines express high levels of RLN2 compared with the LNCaP-vector subline (350-fold and 582-fold increased expression in the LNCaP-RLN2/C1 and LNCaP-RLN2/C2 sublines, respectively). Table 1 lists the top ten differentially expressed genes in the RLN2 versus vector control sublines. It is of note that H1 relaxin (RLN1) is also expressed at high levels in the RLN2 sublines. While the RLN1 isoform has been shown to be expressed in the prostate at the mRNA level, only the RLN2 is translated and secreted [12, 16, 17, 31, 35, 47] indicating that upregulation of RLN1 expression in the RLN2 sublines likely has no functional consequence. Expression of PSA (KLK3, eightfold and 22-fold increased expression in LNCaP-RLN2/C1 and LNCaP-RLN2/C2, respectively), a downstream target of the AR, and cyclin D1 (1.44-fold and 1.2-fold increased expression), an important cell cycle regulator, was increased in the RLN2 sublines. Expression of TRAF1 and C-IAP1, which can be driven by NF–κB, were also overexpressed (TRAF1, 2.01-fold and 3.08-fold increased expression; C-IAP1, 1.47-fold and 1.5-fold increased expression; Table 1). Both of these molecules have been shown to promote cell survival.
Table 1.
Genes that are differentially expressed by LNCaP-RLN2 versus LNCaP-vector sublines
| Gene symbol | LNCaP-RLN2/C1 (fold change relative to LNCaP-vector) | LNCaP-RLN2/C2 (fold change relative to LNCaP-vector) | GO function |
|---|---|---|---|
| SALL2 | 932 | 1,827 | Transcription |
| CCL20 | 701 | 166 | Chemotaxis |
| KLHL13 | 693 | 187 | Catabolism |
| TCEA3 | 498 | 705 | Transcription |
| RLN1 | 419 | 241 | Signal transduction |
| TUSC3 | 413 | 264 | Glycosylation |
| PEG3 | 401 | 231 | Transcription |
| RLN2 | 350 | 582 | Female pregnancy |
| S100A10 | 267 | 165 | Signal transduction |
| MEST | 196 | 106 | Mesoderm development |
| KLK3 | 8 | 22 | Catalytic activity |
| MYC | 1.18 | 0.94 | Survival |
| CCND1 | 1.44 | 1.20 | Cell cycle |
| BCL2L1 | 0.83 | 0.93 | Survival |
| TRAF1 | 2.01 | 3.08 | Survival |
| CIAPIN1 | 1.47 | 1.50 | Survival |
| NSE | 9.532 | 18.796 | NED |
| NEP | 2.544 | 1.668 | NED |
NGS was performed on mRNA-Seq libraries prepared from total RNA isolated from LNCaP-RLN2 and vector control cell lines. Data analysis was performed as described in “Materials and Methods.” Normalized transcript expression (RPKM values) was used for calculation of fold expression changes in the RLN2 LNCaP sublines relative to the vector control. The numbers listed in the LNCaP-RLN2/C1 and LNCaP-RLN2/C2 columns of the table are fold change in gene expression relative to LNCaP-vector. This table also lists the relative expression of genes that have been shown to be driven by either the beta-catenin/AR complex or by NF–κB and those involved in neuroendocrine differentiation (in gray). Both PSA (KLK3) and cyclin D1 (CCND1) have increased expression in the LNCaP-RLN2 sublines, as does TRAF1 and C-IAP1 (CIAPIN1). C-Myc and Bcl-xL were not differentially expressed
To verify the observations made with the NGS/GO analysis, we demonstrated that stable expression of RLN2 increased PSA levels, but not AR expression, in LNCaP cells, confirming that RLN2 affects AR transcriptional activity but not expression (Fig. 2d). These results thereby validate the use of these clones as a model for determining RLN2 function in CaP. Increased expression of RLN2 has been observed during neuroendocrine differentiation (NED) of CaP cells [10], a process that is associated with the development of CR CaP in CaP patients [48]. Our data also indicate that H2 relaxin is associated with NED and thereby further support a role for H2 relaxin in progression to CR CaP. The LNCaP-RLN2/C1 and LNCaP-RLN2/C2 sublines express increased levels of neuron-specific enolase (NSE) and decreased levels of neural endopeptidase (NEP) compared with LNCaP-vector (Fig. 2c). NSE is a key marker of NED (review; [33]), and increased expression is associated with CaP progression [19]. NEP is an enzyme that is expressed at high levels by normal CaP cells and is responsible for degrading neuropeptides such as bombesin, ET-1, and neurotensin that promote NED [26, 27]. NGS analysis revealed that mRNA levels of NSE and NEP were also altered in the LNCaP-RLN2 sublines, 9.532-fold and 18.796-fold increase in NSE in LNCaP-RLN2/C1 and LNCaP-RLN2/C2, respectively, and a 2.544-fold and 1.668-fold decrease in NEP expression. Since cyclin D1 and several survival-related genes appeared to be upregulated in H2-relaxin overexpressing cells (Table 1), we determined whether RLN2 overexpression stimulated cell numbers. MTT assay verified that RLN2 overexpressing sublines have a significantly increased rate of cell growth in culture medium containing charcoal-stripped serum (containing castrate levels of androgens) compared with vector-transfected LNCaP cells (Fig. 2e, statistical analysis compared the day 5 data for each subline and revealed a significant difference between the LNCaP-vector compared with all LNCaP-RLN2/C1 and LNCaP-RLN2/C2 as well as LNCaP-R273H, p < 0.005). These observations are supported by our previous observations indicating ligand-independent activation of the AR by RLN2 [46]. It should be noted that increased AR activity and increase cell proliferation are not characteristics that associated with NED. Our data indicate that the LNCaP-RLN2 sublines have some NED-like characteristics they are clearly not NE cells. While this is somewhat unusual, other groups have reported similar findings. For example, Snail induces NSE and chromogranin A expression in LNCaP as well as mediating nuclear translocation of AR and increased PSA expression [23].
RLN2 Promotes Activation of an NF–κB-Dependent Cell Survival Pathway in LNCaP Prostate Cancer Cells
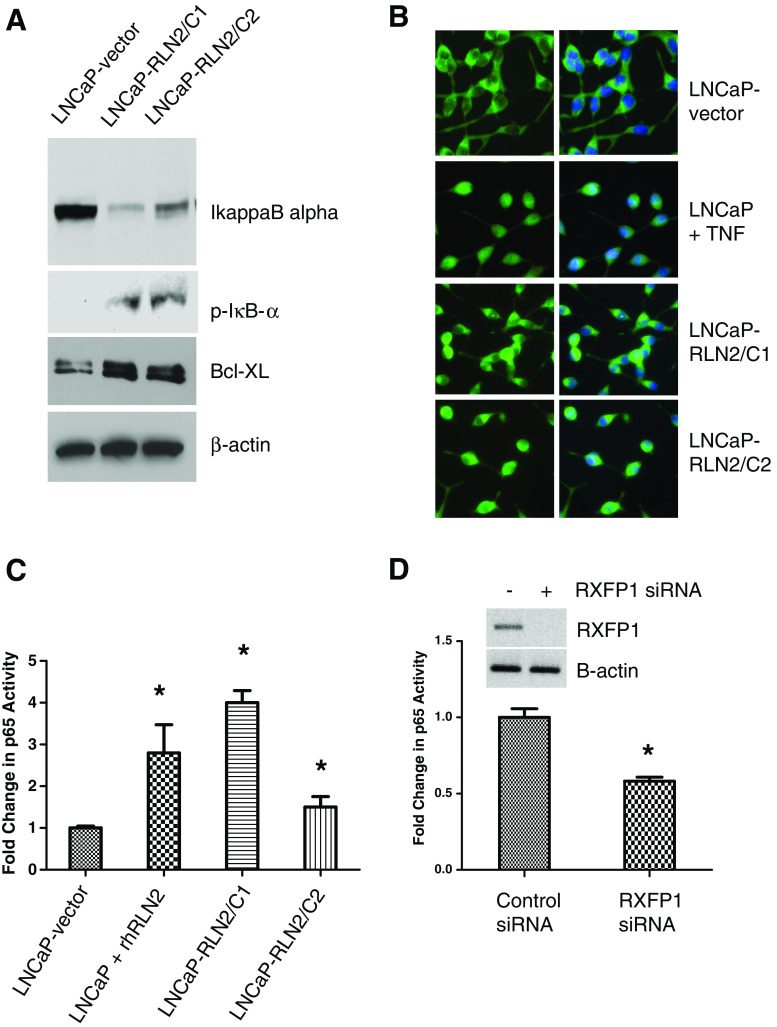
Since the NGS/GO analysis demonstrated an increase in TRAF1 and C-IAP1, which can be driven by NF–κB in LNCaP sublines overexpressing RLN2, we also investigated the activation of NF–κB in these cells. The NF–κB subunits (p65, p50) remain bound to IκB-α in the cytoplasm; upon stimulation, IκB-α is degraded and p65/p50 released, which then translocates to the nucleus and helps transcribe anti-apoptotic genes such as Bcl-xL [49]. To determine whether the NF–κB pathway is active in the RLN2 LNCaP sublines, we assessed IκB-α expression levels and phosphorylation state, NF–κB localization, and binding of NF–κB to its DNA consensus sequence. IκB-α levels were significantly lower in the RLN2 LNCaP sublines, and IκB-α was phosphorylated indicating that active degradation of IκB-α occurs in these cells (Fig. 3a). Increased expression of Bcl-xL, a downstream effector of NF–κB, was also observed in the LNCaP sublines overexpressing RLN2. Immunofluorescence analysis of the LNCaP-rlx sublines revealed a significant increase in levels of nuclear NF–κB compared with LNCaP-vector (Fig. 3b). LNCaP treated with TNF-α were used as a positive control for nuclear staining. We also demonstrate that RLN2 is able to facilitate binding of the NF–κB p65 subunit to its DNA consensus sequence (Fig. 3c). Significantly increased binding was observed in both LNCaP-vector treated with recombinant human (rh) RLN2 and in the RLN2 LNCaP sublines relative to the LNCaP-vector only control (p < 0.05 for all three LNCaP sublines compared with LNCaP-vector). These studies indicate that activation of NF–κB is an important mediator of RLN2-mediated cell survival and point to a mechanism by which RLN2 may induce CR CaP.
Fig. 3.
RLN2 induces activation of the NF–κB pathway. a The RLN2 LNCaP sublines expressed decreased levels of IκB-α and increased levels of P-IκB-α, indicating RLN2 expression causes IκB-α degradation. Increased levels of Bcl-xL, a downstream effector of NF–κB was also observed. b Increased nuclear translocation of NF–κB is observed in RLN2 LNCaP sublines. LNCaP cells or the RLN2 overexpressing sublines were immunostained with anti-p65 antibody (green) or with DAPI to detect the nuclei (blue). Merger of the two stains indicated NF–κB nuclear localization. LNCaP cells treated with TNF-α (10 ng/ml) were used as positive control. Note that, in LNCaP cells, NF–κB remained in the cytoplasm whereas in the RLN2-overexpressing sublines, the complex is localized to the nucleus. c NF–κB transcriptional activity as determined by reporter assay was significantly elevated in RLN2 LNCaP sublines and in parental LNCaP treated with rhRLN2 (human recombinant). Nuclear localization of functional NF–κB was confirmed by assessment of the ability of NF–κB to bind to its DNA binding consensus sequence. d Upper panels Knockdown of RXFP1, the RLN2 receptor, inhibited the ability of NF–κB to bind to its DNA consensus sequence in the RLN2 LNCaP sublines (lower panels; asterisk signifies p < 0.05)
It is known that RLN2 signals via the G protein-coupled receptor (GPCR) RXFP1 in CaP cells. To determine whether the effects of RLN2 on NF–κB are mediated by RXFP1, we investigated the effect of RXFP1 knockdown on NF–κB activity. RXFP1-mediated activation of NF–κB could be inhibited using siRNA specific to RXFP1, the RLN2 receptor (Fig. 3d), indicating that the effects of RLN2 on NF–κB are indeed mediated by RXFP1.
RLN2 Stimulates cAMP Production and PKA Activation Independent of NF–κB
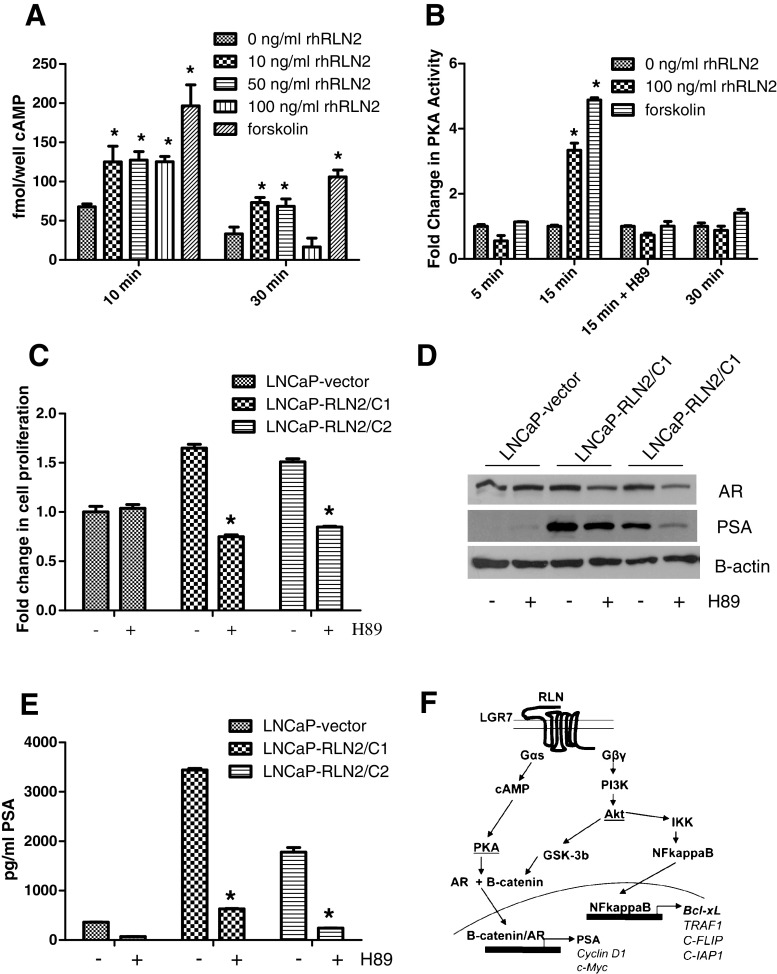
Activation of the H2 relaxin receptor RXFP1 by RLN2 activates the Gs class of G-proteins (Gsα), resulting in cyclic AMP (cAMP)-dependent PKA activation. Hence, we investigated whether RLN2s effects are mediated by the PKA pathway in CaP cells. Previous studies performed in other cell types have demonstrated that RLN2 causes activation of the adenylate cyclase/AMP/PKA pathway [3, 15]. Our results validate these findings in CaP cells. Treatment of parental LNCaP with 10, 50, and 100 ng/ml recombinant human RLN2 (rhRLN2) induced a significant increase (p < 0.005), ~2.2- and 2.1-fold increase in cAMP activity respectively compared with a 3.2-fold forskolin-induced response (positive control, p < 0.005; Fig. 4a). However, this increase was transient as shown by the decrease in cAMP levels after 30 min of treatment. Similarly, treatment of parental LNCaP with 50 ng/ml RLN2 induced a ~3.3-fold increase in PKA activity (p < 0.005) compared with a ~4.9-fold forskolin-induced increase (positive control, p < 0.005; Fig. 4b). On the other hand, co-treatment with the PKA inhibitor H89 was able to completely inhibit this response to RLN2, indicating the assay is PKA-specific (Fig. 4b). Inhibition of PKA using H89 also caused a decrease in growth rate in both LNCaP-RLN2/C1 and LNCaP-RLN2/C2 cultured in CSS media—in LNCaP-RLN2/C1, a ~1.8-fold decrease in growth rate was observed (p < 0.05), and in LNCaP-RLN2/C2 a ~2.1-fold decrease ensued (Fig. 4c, p < 0.05). Inhibition of the PKA pathway did not directly affect the activation of NF–κB (data not shown), indicating that the PKA and the NF–κB pathways represent two different arms of the signaling mechanisms downstream of RLN2 (Fig. 4f). Inhibition of PKA had a more dramatic effect on PSA expression (Fig. 4d, e). Treatment with H89 caused a ~7-fold decrease in PSA levels in LNCaP-RLN2/C1 (p < 0.005) and a ~5.6-fold decrease in LNCaP-RLN2/C2 (p < 0.05). It is of note that the RLN2 LNCaP sublines express very high levels of PSA even when cultured in castrate conditions. These data indicate that H2 relaxin induces the activation of the AR signaling pathway and cell growth in a ligand-independent manner by a mechanism mediated by the activation of the cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) pathway.
Fig. 4.
RLN2 stimulates cAMP production and PKA activation. a LNCaP cells were treated with 0, 10, 50, and 100 ng/ml rhRLN2 (human recombinant) and cAMP levels measured by ELISA. Forskolin 50 μM, which directly stimulates cAMP production, is used as a positive control. b The 100 ng/ml of rhRLN2 induced PKA activation comparable to the positive control Forskolin as measured by the phosphorylation of Kemptide, a phosphate group acceptor synthetic peptide. Activation of PKA was observed 15 min post-treatment upon treatment with 100 ng/ml rhRLN2. This activation could be inhibited using H89, a PKA inhibitor. c, d Inhibition of PKA activity in LNCaP-RLN2/C1 and LNCaP-RLN2/C2 cells resulted in inhibition of c cell growth as measured by MTT assay and d, e PSA levels, as measured by both Western blotting as well as PSA ELISA. f Shows a schematic representation of RLN2 signaling in CaP cells based on data obtained from this and other studies of the RLN2 pathway in CaP cells. RLN2 is able to cause activation of both the cAMP/PKA and NF–κB signaling pathways by two independent mechanisms. Note that LGR7 is an alternate name for RXFP1 (asterisk signifies p < 0.05)
RLN2 Overexpression Confers Resistance to Treatment with Therapeutic Agents
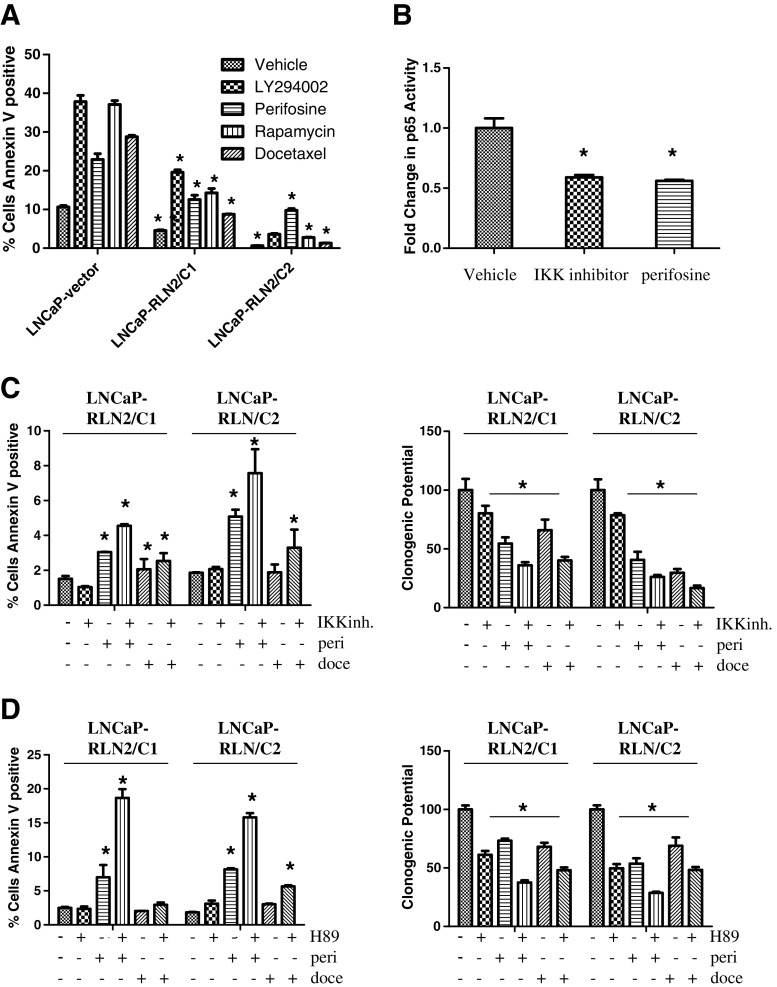
Activation of the NF–κB pathway has been frequently associated with drug resistance. Since RLN2 induces an increase in NF–κB activity, we investigated whether RLN2 expression is also associated with resistance to various therapeutic drugs. Annexin V/propidium iodide (PI) labeling followed by flow cytometry analysis to investigate the effects on apoptosis showed that LNCaP cells transfected with vector only are highly susceptible to induction of apoptosis by various inhibitors including LY294002 (PI3K inhibitor), perifosine (Akt inhibitor), rapamycin (mTOR inhibitor), and docetaxel (anti-mitotic), whereas the LNCaP-RLN2/C1 and LNCaP-RLN2/C2 sublines are more resistant to treatment with the same drugs (Fig. 5a). Perifosine, rapamycin, and docetaxel are all clinical agents. Perifosine has been shown to reduce PSA levels in 20% CaP patients with recurrent disease [5]. Several ongoing clinical trials are testing the efficacy of rapamycin and analogs of rapamycin alone and in combination with other agents (review; [11]). Docetaxel is the standard of care treatment for CaP patients with castrate-resistant CaP (review; [39]). For LNCaP-vector, treatment with vehicle control, LY294002, perifosine, rapamycin, and docetaxel induced ~10%, 38%, 22%, 37%, and 29% apoptosis, respectively (Fig. 5a), but was reduced in LNCaP-RLN2/C1 (4%, 20%, 12%, 13%, and 9%) and LNCaP-RLN2/C2 (1%, 4%, 10%, 2%, and 1.5%). The levels of apoptosis in LNCaP-vector were statistically higher compared with those observed in the LNCaP RLN2 sublines regardless of the type of drug treatment (p < 0.05). These data indicate that there is a link between RLN2 expression and chemoresistance in LNCaP cells and provide rationale for combining targeted inhibition of the RLN2 pathway with conventional chemotherapy.
Fig. 5.
Combined blockade of the PKA and PI3K/Akt signaling pathways that are activated by RLN2 promotes apoptosis. a The RLN2 LNCaP sublines are resistant to apoptosis by several drugs, including LY294002, perifosine, rapamycin, and docetaxel. The levels of apoptosis in LNCaP-vector were statistically higher compared with those observed in the LNCaP RLN2 sublines regardless of the type of drug treatment. b Perifosine, similar to the IKK inhibitor, decreases binding of NF–κB to its DNA consensus sequence. c Inhibition of IKK in the LNCaP-RLN2/C1 and LNCaP-RLN2/C2 sublines did not cause an increase in apoptosis, while perifosine alone caused only a moderate increase in apoptosis (~2-fold increase). Simultaneous blockade of IKK and Akt resulted in only a minimal increase in apoptosis compared with treatment with perifosine alone (left panel). A similar trend was observed by clonogenic assay (right panel). d On the other hand, simultaneous blockade of PKA with H-89 together with perifosine resulted in a significant increase in apoptosis compared with blockade of either individual pathway (~2–3-fold increase in apoptosis compared with treatment with perifosine alone, ~15–18% apoptosis in the combination treatment; left panel), an increase that was far greater than that observed with docetaxel treatment (~2–3% apoptosis). Clonogenic assay showed this same trend (right panel; asterisk signifies p < 0.05)
Combined Treatment with Perifosine and a PKA Inhibitor in CaP Cells Overexpressing RLN2 Promotes Apoptosis
IKK causes phosphorylation of IκB-α and subsequent proteasome-mediated degradation. This degradation allows NF–κB to translocate to the nucleus. Hence, an IKK inhibitor would inhibit the activation of the NF–κB pathway. Perifosine is known to be an Akt inhibitor; however, it inhibits NF–κB activation to the same extent as the IKK inhibitor (Fig. 5b). As perifosine has been Food and Drug administration (FDA)-approved and is currently in clinical trials for the treatment of CaP, we investigated whether its effects on NF–κB would be of significance in the treatment of patients who overexpress RLN2 and may have developed resistance to commonly used drugs as a result. Hence, we compared the effects of perifosine to that of the IKK inhibitor (Fig. 5b, c). Inhibition of IKK in the LNCaP-RLN2/C1 and LNCaP-RLN2/C2 sublines did not cause a significant increase in apoptosis or decrease in clonogenic potential (Fig. 5c), whereas perifosine alone caused only a moderate increase in apoptosis and decrease in clonogenic potential (~2-fold increase and ~40% decrease respectively, Fig. 5c). Similarly, simultaneous blockade of IKK and Akt resulted in only a modest increase in apoptosis and decrease in clonogenic potential compared with treatment with perifosine alone (Fig. 5c). We concluded that the lack of a clinically relevant increase with dual blockade is due to the fact that both the IKK inhibitor and perifosine are acting on the same target, either directly or indirectly (see scheme in Fig. 4f) resulting in decreased binding of NF–κB to its DNA consensus sequence (Fig. 5b).
We hypothesized that simultaneous blockade of pathways leading to PKA and NF–κB would therefore be the only way to completely block signaling downstream of RLN2-induced cell proliferation and survival (based on scheme in Fig. 4f). H-89 alone had little or no effect on the RLN2 overexpressing clones (Fig. 5d); in addition, inhibition of either IKK or PKA caused only a moderate increase in the sensitivity of the RLN2 LNCaP sublines to treatment with docetaxel (Fig. 5c, d). However, dual inhibition of both arms of the RLN2/RXFP1 pathway, with H-89 and perifosine, resulted in a larger and significant increase in apoptosis compared with blockade of either individual pathway (~2–3-fold increase in apoptosis compared with treatment with perifosine alone, ~15–18% apoptosis in the combination treatment, Fig. 5d) and compared with treatment with docetaxel (docetaxel induced only ~2–3% apoptosis in the LNCaP-RLN2 sublines, Fig. 5d). It is of note that this dual inhibition induced a similar level of apoptosis in the LNCaP-RLN2 sublines (15–18%) as docetaxel treatment in the LNCaP-vector subline (~25%). A significant decrease in clonogenic potential was also observed (~20–30% decrease compared with treatment with either the IKK inhibitor or perifosine alone (Fig. 5d). Taken together, these results indicate that overexpression of RLN2, which is commonly seen in tumors from patients with CaP (Fig. 1a), provides a growth advantage to CaP cells by causing activation of both the NF–κB and PKA pathways. A near-complete inhibition of this growth advantage can be achieved only by simultaneous blockade of both pathways.
Discussion
The key finding of this study is that dual blockade of the PKA and NF–κB signaling pathways inhibits H2 relaxin-mediated castrate-resistant growth of prostate cancer cells. This finding is of clinical relevance as our group and others have determined H2 relaxin is expressed at increased levels in CaP patients and increases in CaP patients following androgen ablation [44]. In addition, H2 relaxin has been demonstrated to play an important role in mediating CR CaP growth [7, 8, 21, 37, 46]. Focus was placed on determining the importance of the NF–κB and PKA pathways in facilitating H2 relaxin-mediated CR CaP growth because (1) NGS analyses identified the differential expression of several PKA and NF–κB-related genes in the LNCaP-RLN2 sublines; (2) these pathways are known to be dysregulated in CaP and are linked to CaP progression; (3) both pathways have been demonstrated to be activated by H2 relaxin in other cell types.
Several groups have demonstrated a link between PKA activity and CaP progression (review; [24]). PKA can mediate ligand-independent activation of AR and can therefore play an important role in facilitating both androgen-dependent and androgen-independent CaP. We have previously demonstrated that H2 relaxin mediates PI3K-dependent co-translocation of the AR and β-catenin to the nucleus and causes transactivation of the PSA promoter [21, 46]. Our current data indicate that H2 relaxin can also activate AR via PKA. H2 relaxin has been demonstrated to cause activation of PKA in other cell types [14, 34]. H2 relaxin signals via RXFP1 and 2, both of which are GPCRs that activate the Gs class of G-proteins (Gsα) resulting in cAMP-dependent PKA activation. Only RXFP1 is expressed in CaP cells [44]. Our data demonstrate AR activity is very high in the LNCaP-RLN2 sublines relative to LNCaP-vector and that inhibition of PKA in the LNCaP-RLN2 sublines causes a significant inhibition of this elevated AR activity. These data indicate that PKA-mediated activation of AR is very important in a setting of elevated H2 relaxin expression.
NF–κB is a transcription factor which controls expression of genes associated with both cell proliferation and apoptosis [36]. NF–κB has been demonstrated to be constitutively active in several CaP cell lines and expressed at high levels in both PIN and CaP patient samples [1, 6, 19, 28, 40]. Usually, NF–κB is sequestered in the cytoplasm through interaction of its p65 and p50 subunits with IkBα. Growth and survival stimuli induce phosphorylation of IkBα by IKKα resulting in IkBα degradation, followed by p65 phosphorylation and NF–κB translocation to the nucleus. We demonstrate that H2 relaxin is one of these growth stimuli; forced overexpression of H2 relaxin caused IkBα degradation, nuclear translocation of NF–κB, and binding to the NF–κB DNA binding consensus sequence. Relaxin has previously been shown to activate NF–κB in other organs, but this is the first time it has been shown to activate NF–κB in CaP. While it has been shown that Akt phosphorylation such as that induced by H2 relaxin can phosphorylate the p65 subunit of NF–κB [41], we show that H2 relaxin promotes NF–κB activity by degrading IkBα.
There is sound rationale to simultaneously block both the PKA and NF–κB pathways in CaP cells that express elevated levels of H2 relaxin; both pathways are activated by H2 relaxin yet mediate proliferation by different mechanisms, and both pathways have been shown to be active in CaP patients. While inhibition of either pathway alone resulted in growth inhibition and/or a small increase in apoptosis, it was only when both pathways were inhibited simultaneously that a clinically relevant increase in apoptosis occurred. As inhibition of both IKK and Akt caused similar levels of NF–κB inhibition, we chose to use perifosine, an Akt inhibitor, for the drug combination studies as it has been FDA-approved and tried in CaP clinical trials. In contrast, common IKK inhibitors such as Bay11-7082 have not and are therefore of limited relevance for translational studies. It is possible that our future studies may employ other inhibitors such as bortezomib, a proteasome inhibitor that is currently in clinical use and has been found to inhibit NF–κB [32]. Currently, PKA inhibitors are not in clinical use in CaP patients. For future translational studies, we may employ drugs that lower cAMP levels such as beta-blockers, which are in clinical use, to determine if these have an effect on H2 relaxin signaling. It is of note that beta-blockers have been found to have a small effect on prevention of CaP [29]. It is also of note that the simultaneous blockade of the PKA and NF–κB pathways outperformed docetaxel, the standard of care treatment for advanced CR CaP.
In summary, our data indicate that simultaneous inhibition of PKA and NF–κB would prevent RLN2-mediated cell survival in CaP and that, in a setting of elevated RLN2 expression, this combined inhibition is superior to docetaxel. The number of patients who would potentially benefit from this study is likely to be extensive since a significant portion of CaP patients overexpress RLN2.
Acknowledgments
The authors would like to thank Stephanie Soares for the construction of the TMA.
Financial support
DoD PC074103 (RLV, RGE, DVW)
The UC Davis Cancer Center Genomics Shared Resource is supported by Cancer Center Support Grant P30 CA93373-01 (RW deVere White) from the NCI.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Arlt A, Schafer H. NFkappaB-dependent chemoresistance in solid tumors. Int J Clin Pharmacol Ther. 2002;40(8):336–347. doi: 10.5414/cpp40336. [DOI] [PubMed] [Google Scholar]
- 2.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bathgate RA, Samuel CS, Burazin TC, Gundlach AL, Tregear GW. Relaxin: new peptides, receptors and novel actions. Trends Endocrinol Metab. 2003;14(5):207–213. doi: 10.1016/S1043-2760(03)00081-X. [DOI] [PubMed] [Google Scholar]
- 4.Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, Hall KP, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456(7218):53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chee KG, Longmate J, Quinn DI, Chatta G, Pinski J, Twardowski P, Pan CX, et al. The AKT inhibitor perifosine in biochemically recurrent prostate cancer: a phase II California/Pittsburgh cancer consortium trial. Clin Genitourin Cancer. 2007;5(7):433–437. doi: 10.3816/CGC.2007.n.031. [DOI] [PubMed] [Google Scholar]
- 6.Domingo-Domenech J, Mellado B, Ferrer B, Truan D, Codony-Servat J, Sauleda S, Alcover J, et al. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer. 2005;93(11):1285–1294. doi: 10.1038/sj.bjc.6602851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S, Agoulnik IU, Bogatcheva NV, Kamat AA, Kwabi-Addo B, Li R, Ayala G, Ittmann MM, Agoulnik AI. Relaxin promotes prostate cancer progression. Clin Cancer Res. 2007;13(6):1695–1702. doi: 10.1158/1078-0432.CCR-06-2492. [DOI] [PubMed] [Google Scholar]
- 8.Feng S, Agoulnik IU, Li Z, Han HD, Lopez-Berestein G, Sood A, Ittmann MM, Agoulnik AI. Relaxin/RXFP1 signaling in prostate cancer progression. Ann N Y Acad Sci. 2009;1160:379–380. doi: 10.1111/j.1749-6632.2008.03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng S, Agoulnik IU, Truong A, Li Z, Creighton CJ, Kaftanovskaya EM, Pereira R, et al. Suppression of relaxin receptor RXFP1 decreases prostate cancer growth and metastasis. Endocr Relat Cancer. 2010;17(4):1021–1033. doi: 10.1677/ERC-10-0073. [DOI] [PubMed] [Google Scholar]
- 10.Figueiredo KA, Palmer JB, Mui AL, Nelson CC, Cox ME. Demonstration of upregulated H2 relaxin mRNA expression during neuroendocrine differentiation of LNCaP prostate cancer cells and production of biologically active mammalian recombinant 6 histidine-tagged H2 relaxin. Ann N Y Acad Sci. 2005;1041:320–327. doi: 10.1196/annals.1282.051. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther. 2008;7(6):1347–1354. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garibay-Tupas JL, Bao S, Kim MT, Tashima LS, Bryant-Greenwood GD. Isolation and analysis of the 3′-untranslated regions of the human relaxin H1 and H2 genes. J Mol Endocrinol. 2000;24(2):241–252. doi: 10.1677/jme.0.0240241. [DOI] [PubMed] [Google Scholar]
- 13.Gittes RF. Carcinoma of the prostate. N Engl J Med. 1991;324(4):236–245. doi: 10.1056/NEJM199101243240406. [DOI] [PubMed] [Google Scholar]
- 14.Halls ML, Bathgate RA, Roche PJ, Summers RJ. Signaling pathways of the LGR7 and LGR8 receptors determined by reporter genes. Ann N Y Acad Sci. 2005;1041:292–295. doi: 10.1196/annals.1282.043. [DOI] [PubMed] [Google Scholar]
- 15.Hsu SY, Kudo M, Chen T, Nakabayashi K, Bhalla A, van der Spek PJ, van Duin M, Hsueh AJ. The three subfamilies of leucine-rich repeat-containing G protein-coupled receptors (LGR): identification of LGR6 and LGR7 and the signaling mechanism for LGR7. Mol Endocrinol. 2000;14(8):1257–1271. doi: 10.1210/me.14.8.1257. [DOI] [PubMed] [Google Scholar]
- 16.Ivell R, Einspanier A. Relaxin peptides are new global players. Trends Endocrinol Metab. 2002;13(8):343–348. doi: 10.1016/S1043-2760(02)00664-1. [DOI] [PubMed] [Google Scholar]
- 17.Ivell R, Hunt N, Khan-Dawood F, Dawood MY. Expression of the human relaxin gene in the corpus luteum of the menstrual cycle and in the prostate. Mol Cell Endocrinol. 1989;66(2):251–255. doi: 10.1016/0303-7207(89)90037-3. [DOI] [PubMed] [Google Scholar]
- 18.Kasamon KM, Dawson NA. Update on hormone-refractory prostate cancer. Curr Opin Urol. 2004;14(3):185–193. doi: 10.1097/00042307-200405000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Lessard L, Karakiewicz PI, Bellon-Gagnon P, Alam-Fahmy M, Ismail HA, Mes-Masson AM, Saad F. Nuclear localization of nuclear factor-kappaB p65 in primary prostate tumors is highly predictive of pelvic lymph node metastases. Clin Cancer Res. 2006;12(19):5741–5745. doi: 10.1158/1078-0432.CCR-06-0330. [DOI] [PubMed] [Google Scholar]
- 20.Levenson RM, Mansfield JR. Multispectral imaging in biology and medicine: slices of life. Cytometry A. 2006;69(8):748–758. doi: 10.1002/cyto.a.20319. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Vinall RL, Tepper C, Shi XB, Xue LR, Ma AH, Wang LY, et al. Inappropriate activation of androgen receptor by relaxin via beta-catenin pathway. Oncogene. 2008;27(4):499–505. doi: 10.1038/sj.onc.1210671. [DOI] [PubMed] [Google Scholar]
- 22.Marques RB, Erkens-Schulze S, de Ridder CM, Hermans KG, Waltering K, Visakorpi T, Trapman J, Romijn JC, van Weerden WM, Jenster G. Androgen receptor modifications in prostate cancer cells upon long-termandrogen ablation and antiandrogen treatment. Int J Cancer. 2005;117(2):221–229. doi: 10.1002/ijc.21201. [DOI] [PubMed] [Google Scholar]
- 23.McKeithen D, Graham T, Chung LW, and Odero-Marah V Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells. Prostate 70 (9):982–992 doi:10.1002/pros.21132 [DOI] [PMC free article] [PubMed]
- 24.Merkle D and Hoffmann R Roles of cAMP and cAMP-dependent protein kinase in the progression of prostate cancer: cross-talk with the androgen receptor. Cell Signal 23 (3):507–515. doi:10.1016/j.cellsig.2010.08.017 [DOI] [PubMed]
- 25.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- 26.Osman I, Dai J, Mikhail M, Navarro D, Taneja SS, Lee P, Christos P, Shen R, Nanus DM. Loss of neutral endopeptidase and activation of protein kinase B (Akt) is associated with prostate cancer progression. Cancer. 2006;107(11):2628–2636. doi: 10.1002/cncr.22312. [DOI] [PubMed] [Google Scholar]
- 27.Osman I, Yee H, Taneja SS, Levinson B, Zeleniuch-Jacquotte A, Chang C, Nobert C, Nanus DM. Neutral endopeptidase protein expression and prognosis in localized prostate cancer. Clin Cancer Res. 2004;10(12 Pt 1):4096–4100. doi: 10.1158/1078-0432.CCR-04-0120. [DOI] [PubMed] [Google Scholar]
- 28.Palayoor ST, Youmell MY, Calderwood SK, Coleman CN, Price BD. Constitutive activation of IkappaB kinase alpha and NF-kappaB in prostate cancer cells is inhibited by ibuprofen. Oncogene. 1999;18(51):7389–7394. doi: 10.1038/sj.onc.1203160. [DOI] [PubMed] [Google Scholar]
- 29.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15(6):535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 30.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 31.Samuel CS, Tian H, Zhao L, Amento EP. Relaxin is a key mediator of prostate growth and male reproductive tract development. Lab Invest. 2003;83(7):1055–1067. doi: 10.1097/01.LAB.0000079784.81186.B9. [DOI] [PubMed] [Google Scholar]
- 32.Sartore-Bianchi A, Gasparri F, Galvani A, Nici L, Darnowski JW, Barbone D, Fennell DA, Gaudino G, Porta C, Mutti L. Bortezomib inhibits nuclear factor-kappaB dependent survival and has potent in vivo activity in mesothelioma. Clin Cancer Res. 2007;13(19):5942–5951. doi: 10.1158/1078-0432.CCR-07-0536. [DOI] [PubMed] [Google Scholar]
- 33.Shariff AH, Ather MH. Neuroendocrine differentiation in prostate cancer. Urology. 2006;68(1):2–8. doi: 10.1016/j.urology.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Shaw EE, Wood P, Kulpa J, Yang FH, Summerlee AJ, Pyle WG. Relaxin alters cardiac myofilament function through a PKC-dependent pathway. Am J Physiol Heart Circ Physiol. 2009;297(1):H29–H36. doi: 10.1152/ajpheart.00482.2008. [DOI] [PubMed] [Google Scholar]
- 35.Sherwood OD. Relaxin’s physiological roles and other diverse actions. Endocr Rev. 2004;25(2):205–234. doi: 10.1210/er.2003-0013. [DOI] [PubMed] [Google Scholar]
- 36.Shih VF, Tsui R, Caldwell A and Hoffmann A A single NFkappaB system for both canonical and non-canonical signaling. Cell Res 21 (1):86–102. doi:10.1038/cr.2010.161 [DOI] [PMC free article] [PubMed]
- 37.Silvertown JD, Ng J, Sato T, Summerlee AJ, Medin JA. H2 relaxin overexpression increases in vivo prostate xenograft tumor growth and angiogenesis. Int J Cancer. 2006;118(1):62–73. doi: 10.1002/ijc.21288. [DOI] [PubMed] [Google Scholar]
- 38.Silvertown JD, Symes JC, Neschadim A, Nonaka T, Kao JC, Summerlee AJ, Medin JA. Analog of H2 relaxin exhibits antagonistic properties and impairs prostate tumor growth. FASEB J. 2007;21(3):754–765. doi: 10.1096/fj.06-6847com. [DOI] [PubMed] [Google Scholar]
- 39.Singh P, Yam M, Russell PJ, and Khatri A Molecular and traditional chemotherapy: a united front against prostate cancer. Cancer Lett 293 (1):1–14. doi:10.1016/j.canlet.2009.11.019 [DOI] [PubMed]
- 40.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10(16):5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 41.Takeshima E, Tomimori K, Kawakami H, Ishikawa C, Sawada S, Tomita M, Senba M, et al. NF-kappaB activation by Helicobacter pylori requires Akt-mediated phosphorylation of p65. BMC Microbiol. 2009;9:36. doi: 10.1186/1471-2180-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 43.Thompson VC, Hurtado-Coll A, Turbin D, Fazli L, Lehman ML, Gleave ME, Nelson CC. Relaxin drives Wnt signaling through upregulation of PCDHY in prostate cancer. Prostate. 2010;70(10):1134–1145. doi: 10.1002/pros.21148. [DOI] [PubMed] [Google Scholar]
- 44.Thompson VC, Morris TG, Cochrane DR, Cavanagh J, Wafa LA, Hamilton T, Wang S, Fazli L, Gleave ME, Nelson CC. Relaxin becomes upregulated during prostate cancer progression to androgen independence and is negatively regulated by androgens. Prostate. 2006;66(16):1698–1709. doi: 10.1002/pros.20423. [DOI] [PubMed] [Google Scholar]
- 45.Vinall RL, Hwa K, Ghosh P, Pan CX, Lara PN, Jr, de Vere White RW. Combination treatment of prostate cancer cell lines with bioactive soy isoflavones and perifosine causes increased growth arrest and/or apoptosis. Clin Cancer Res. 2007;13(20):6204–6216. doi: 10.1158/1078-0432.CCR-07-0600. [DOI] [PubMed] [Google Scholar]
- 46.Vinall RL, Tepper CG, Shi XB, Xue LA, Gandour-Edwards R, de Vere White RW. The R273H p53 mutation can facilitate the androgen-independent growth of LNCaP by a mechanism that involves H2 relaxin and its cognate receptor LGR7. Oncogene. 2006;25(14):2082–2093. doi: 10.1038/sj.onc.1209246. [DOI] [PubMed] [Google Scholar]
- 47.Welsh JB, Sapinoso LM, Kern SG, Brown DA, Liu T, Bauskin AR, Ward RL, et al. Large-scale delineation of secreted protein biomarkers overexpressed in cancer tissue and serum. Proc Natl Acad Sci U S A. 2003;100(6):3410–3415. doi: 10.1073/pnas.0530278100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu JT, Astill ME, Liu GH, Stephenson RA. Serum chromogranin A: early detection of hormonal resistance in prostate cancer patients. J Clin Lab Anal. 1998;12(1):20–25. doi: 10.1002/(SICI)1098-2825(1998)12:1<20::AID-JCLA4>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yardy GW, Brewster SF. Wnt signalling and prostate cancer. Prostate Cancer Prostatic Dis. 2005;8(2):119–126. doi: 10.1038/sj.pcan.4500794. [DOI] [PubMed] [Google Scholar]