Abstract
Dissociated human fetal skeletal muscle contains myogenic cells, as well as non-myogenic cells such as adipocytes, fibroblasts, and lymphocytes. It is therefore important to determine an efficient and reliable isolation method to obtain a purer population of myoblasts. Toward this end, fluorescence-activated cell sorting in conjunction with robust myogenic cell surface markers can be utilized to enrich for myoblasts in dissociated muscle. In this chapter, we describe a method to significantly enrich for myoblasts using melanoma cell adhesion molecule (MCAM), which we have determined to be an excellent marker of human fetal myoblasts. The myoblasts resulting from this isolation method can then be expanded in vitro and still retain significant myogenic activity as shown by an in vitro fusion assay. The ability to isolate a highly myogenic population from dissociated muscle facilitates the in vitro study of skeletal muscle development and muscle diseases. Furthermore, robust expansion of these cells will lead to new insights in the development of cell-based therapies for human muscle disorders.
Keywords: Human muscle, Tissue dissociation, Fluorescence-activated cell sorter, Myoblast purification
1. Introduction
Efficient isolation and maintenance of human myoblasts in vitro is an essential technique for the investigation of myogenic progenitor commitment and differentiation, the characterization of muscle development and human muscle disorders, and the development of cell-based therapies for muscle diseases. Initial studies involved the use of tissue explants or unpurified dissociated cells (1–7). However, due to the presence of non-myogenic mononuclear cells in human skeletal muscle, including adipocytes, fibroblasts, and lymphocytes, methods for the isolation of purer populations of myoblasts were developed. In 1974, Stephen Hauschka determined in vitro conditions for the clonal culture and differentiation of human muscle cells (8, 9). These culture conditions were further adapted by Blau and Webster, who introduced the use of preplating to remove fibroblasts followed by the generation of myogenic clones (10).
For more specific and efficient isolation of myoblasts within a dissociated human muscle sample, Webster et al. utilized fluorescence-activated cell sorting (FACS) to positively select for cells expressing human neural cell adhesion molecule (NCAM) (11), a cell surface antigen shown to be expressed on myogenic cells (12). Alternatively, Baroffio et al. used FACS to enrich for human myoblasts on the basis of cell size; after expansion, the cells were tested for the expression of NCAM to confirm their myogenicity (13).
The prospective isolation of pure populations of myogenic progenitors is highly desirable for translational research. Currently, many types of stem/progenitor cells are under study for their potential to repair diverse tissues, including skeletal muscle. As new technologies enable reliable propagation of multipotent or pluripotent cells, such as ES or induced pluripotent (iPS) cells, there is also an emerging need to optimize methods for the selection of lineage-specific progenitors obtained following the differentiation of these pluripotent cells. As a result, the identification of markers that prospectively enrich for cells with a specific potential is currently being researched. To identify such markers, gene expression studies on progenitor cells derived from embryonic or fetal tissues might be useful as these cells are likely still immature and retain long-term expansion potential, yet are developmentally specified toward a particular tissue.
Recently, melanoma cell adhesion molecule (MCAM), also known as CD146, Mel-CAM, MUC18, A32 antigen, and S-Endo-1, was detected in human skeletal muscle as well as in other normal tissues such as smooth muscle, endothelium, and the nervous system (reviewed in refs. (14–16)). Interestingly, studies in chick embryos also showed that MCAM is expressed in somatic cells that specify the myotome during development (17). Following a microarray screening to identify genes regulated during human fetal myoblast fusion, MCAM was found highly expressed in proliferating myoblasts and significantly downregulated during fusion (18). Freshly isolated MCAM-positive cells were shown to undergo fusion in vitro whereas MCAM-negative cells did not (18). Furthermore, inhibition of MCAM expression in myoblasts by RNAi enhanced myoblast differentiation and fusion (18). Therefore, significant enrichment of myoblasts from dissociated muscle can be obtained by using MCAM as a positive selection marker in cell sorting.
The present chapter describes the dissociation of human fetal skeletal muscle and the subsequent purification of myoblasts using cell sorting based upon MCAM expression. In vitro culture of these purified cells is then detailed as well as an assessment of myogenicity using an in vitro fusion assay.
2. Materials
2.1. Dissociation of Primary Human Fetal Skeletal Muscle Tissue (see Note 1)
Protected disposable scalpels with stainless steel blade size #10.
Sterile 10 cm tissue culture-treated plastic dishes.
Assorted sterile 5, 10, and 25 mL pipettes.
Sterile 0.22 μm PES (low protein binding) filters – 250 and 500 mL volumes.
0.22 μm CN filter unit – 500 mL volume.
Sterile 15 and 50-mL conical centrifuge tubes.
BD Falcon sterile nylon cell strainers – 100 and 40-μm pore sizes.
10× Hank’s balanced saline solution (HBSS), free of calcium chloride, magnesium chloride and magnesium sulfate, diluted to 1× with double distilled water and filter sterilized with a 0.22 μm CN filter. This solution can be stored at 4°C or room temperature.
Complete growth medium (500 mL): Mix 395 mL of high glucose Dulbecco’s modified Eagle’s medium (DMEM) with 100 mL of fetal bovine serum (FBS; see Note 2) and 5 mL of 100× penicillin–streptomycin–glutamine (PSG). Sterilize by filtering the solution through a 500 mL 0.22 μm PES filter unit. Store at 4°C and use within 1 month.
Sterile red blood cell lysis solution (Qiagen), stored at room temperature.
Sterile HEPES buffered saline solution, without phenol red.
1 M calcium chloride solution (CaCl2·2H2O, FW 147). Dissolve 1.47 g powder in 10 mL of double distilled water. Store at 4°C.
Dispase stock solution: dissolve 1 g powder dispase II (Roche Applied Science) in 100 mL HEPES buffered saline. Add 316 mL of high glucose DMEM to generate a stock solution of 2.4 U/mL. Filter-sterilize the solution through a PES 500 mL filter; aliquot into 15-mL conical tubes (10 mL/tube) and store aliquots at −20°C.
Collagenase D stock solution: dissolve 2.5 g powder collagenase D (Roche Applied Science) in 250 mL solution of 1× HBSS supplemented with 1.25 mL of 1 M CaCl2. Sterilize by filtering through a PES filter unit. The filtered solution can be dispensed in 15-mL conical tubes (10 mL/tube) and stored at −20°C.
Sterile freezing medium: 90% FBS and 10% dimethyl sulfoxide (DMSO). Prepare freezing medium and immediately store on ice. Unused sterile freezing medium can be stored at 4°C for up to 4 weeks.
Sterile 1.8 mL CryoTube™ vials.
Bench top centrifuge.
Hemocytometer.
Sterile laminar flow hood.
−150°C freezer, liquid nitrogen storage tank.
Humidified 5% CO2 incubator set to 37°C.
2.2. Isolation of Myoblasts from Dissociated Human Fetal Skeletal Muscle
2.2.1. Thawing of Cryopreserved Sample Prior to FACS
CryoTube™ vial containing dissociated human fetal skeletal muscle (Hu Fe SkM).
Sterile 0.22 μm PES filter – 500 mL volume.
Sterile 50-mL conical centrifuge tubes.
Sterile tissue culture-treated plastic dishes – 10 or 15 cm size.
Sterile complete growth medium, as mentioned above.
Sterile laminar flow hood.
Water bath set to 37°C.
CO2 incubator, as above.
2.2.2. Preparation of Sample for FACS
Dissociated Hu Fe SkM sample thawed 1 day prior to FACS.
Sterile 0.22 μm PES filter – 500 mL volume.
Sterile 15 and 50-mL conical centrifuge tubes.
Sterile nylon cell strainers – 40-μm pore size.
Sterile 1× HBSS, as above.
Sterile cell dissociation buffer, enzyme free, PBS-based (Invitrogen) stored at room temperature.
Sterile 0.5% BSA/HBSS solution (500 mL): dissolve 2.5 g bovine serum albumin (BSA) in 1× HBSS. Sterilize by filtering the solution through a 500 mL 0.22 μm PES filter unit. Store at 4°C.
-
Antibodies (all stored at 4°C):
Anti-MCAM antibody, clone P1H12 (Millipore).
Mouse IgG1 monoclonal antibody (BD Pharmingen).
Alexa Fluor® 488 goat anti-mouse IgG (Invitrogen). Protect from light.
10 mL syringe and 0.22 μm Acrodisc® Supor membrane low protein binding syringe filters (Pall Life Sciences).
1 mg/mL Propidium iodide (PI): resuspend 10 mg powder in 10 mL of double distilled water, and then filter sterilize using a 0.22-μm syringe filter. Dispense in 1 mL aliquots and store at 4°C.
Sterile FACS or 5 mL round-bottom tubes.
Sterile laminar flow hood.
Bench top centrifuge.
Inverted microscope.
Hemocytometer.
2.2.3. Fluorescence-Activated Cell Sorting
FACS or 5-mL round-bottom tubes.
Cell sorting machine.
Cell sorting software.
2.3. In Vitro Culture and Analysis of Human Fetal Skeletal Myoblasts
2.3.1. In Vitro Cell Culture
Sterile 50-mL conical centrifuge tubes.
Sterile tissue culture-treated plastic dishes.
BD Falcon black with clear bottom 96-well Microtest™ Optilux™ plates.
Sterile 0.22 μm PES (low protein binding) filter – 150 mL tube top volume.
Sterile 1× HBSS.
Sterile complete growth medium, as above.
Differentiation medium (50 mL): Mix 48.5 mL of low glucose Dulbecco’s Modified Eagle’s Medium (DMEM) with 1 mL of horse serum (HS) and 0.5 mL of 100× PSG. Sterilize by filtering the solution through a 150 mL 0.22 μm PES filter unit. Store at 4°C and use within 1 month.
0.15% Gelatin: add 0.75 g of gelatin to 500 mL of double distilled water. Do not shake. Sterilize the solution by autoclaving for 20 min and store at 4°C.
TrypLE™ Express Dissociation Enzyme with Phenol Red (Invitrogen).
Sterile laminar flow hood.
Water bath set to 37°C.
CO2 incubator.
Bench top centrifuge.
Inverted microscope.
Hemocytometer.
2.3.2. Immunocyto-chemistry for In Vitro Fusion Assay
10× Phosphate buffered saline (PBS), diluted to 1× with double distilled water. Store at room temperature.
4% Paraformaldehyde (4% PFA): Dilute 16% paraformaldehyde with 1× PBS. Use with caution as paraformaldehyde is extremely toxic; it is recommended that paraformaldehyde be used in a fume hood for safety. Aliquot and store at −20°C. Aliquots should not be repeatedly freezed and thawed; discard unused PFA after initial use.
Permeabilization solution: Mix 50 μL of Triton ×-100 with 10 mL of 1× PBS.
Blocking solution: Mix together 1 mL of fetal bovine serum (FBS), 10 μL of Triton ×-100, and 9 mL of 1× PBS.
-
Antibodies (all stored at 4°C):
Anti-Human Desmin, clone D33 (Dako).
Alexa Fluor® 488 goat anti-mouse IgG (Invitrogen). Protect from light.
-
DAPI solution
DAPI stock solution (5 mg/mL): Dissolve 10 mg DAPI in 2 mL of double distilled water. Aliquot and store at −20°C.
DAPI working solution (100 ng/mL): Mix 2 μL of DAPI stock solution with 100 mL of PBS. Store at 4°C wrapped in aluminum foil to protect from light.
Inverted microscope with epi-fluorescence capabilities including ultraviolet/DAPI and FITC/GFP filter sets.
3. Methods
3.1. Dissociation of Primary Human Fetal Skeletal Muscle Tissue
All steps in this protocol should be performed in a sterile laminar flow hood using sterile tissue culture technique.
Preweigh one 10 cm tissue culture plate, and place the tissue sample to be dissociated in a second (non preweighed) 10 cm tissue culture plate.
Using sterile scalpels, remove and discard any remaining skin and bone from the muscle tissue. Tissue should be kept moist in sterile 1× HBSS. Add a few drops of sterile 1× HBSS to tissue as necessary, to prevent it from drying out. After skin is removed, place muscle tissue in the preweighed 10 cm tissue culture plate and weigh the plate again. Subtract from this number the tare of the empty plate to calculate the amount of muscle tissue to be dissociated.
Thaw frozen aliquots of dispase II and collagenase D in a 37°C water bath. Thawed collagenase and dispase stocks will be added at a volume of 3.5 mL each per gram of muscle tissue to be dissociated. Thaw only the amounts of collagenase D and dispase II necessary for dissociation. If an excess of enzymes is thawed, it can be refrozen once and reused.
Using sterile scalpels, mince muscle tissue until it resembles a fine paste. During mincing, add a few drops of sterile 1× HBSS to prevent exposed tissue from drying out. Tissue should always appear moist, but with no excess of liquid.
After tissue is finely minced, add equal amounts of the thawed dispase II and collagenase D solutions. The final concentration will be 5 mg/mL for collagenase D and 1.2 U/mL for dispase II in this solution. Pipette minced tissue and enzyme solution up and down through a sterile 25 mL pipette a few times.
Incubate plate in a tissue culture incubator at 37°C with 5% CO2 for 15 min. Then pipette the digestion solution up and down through a sterile 25 mL pipette a few times and incubate again for 15 min. Repeat this step an additional 1–2 times, until the slurry easily passes though a sterile 5 mL pipette and all tissue chunks are dissolved. The total digestion time will range between 45 min and 1 h 15 min.
Add 2 volumes of complete growth medium to the digested slurry and filter the digestion solution through a 100-μm cell strainer over a 50-mL conical tube. Change cell strainer if it appears clogged.
Pellet cells for 10 min at 329 × g, room temperature.
Resuspend the pellet in 1 volume of complete growth medium (i.e., 3 mL) and add 7 volumes (i.e., 21 mL) of red blood cell lysis solution. Invert the tube a few times and then filter the solution through a 40-μm cell strainer over a 50-mL conical tube.
Count cells using a hemocytometer, and then pellet the cells for 10 min at 329 × g, room temperature.
Freeze cells at a concentration of 107 cells/mL in ice-cold freezing medium. Store cryovials at −80°C for ~2–3 days, then transfer them to −150°C where they can be permanently stored until necessary.
3.2. Isolation of Myoblasts from Dissociated Human Fetal Skeletal Muscle
3.2.1. Thawing of Cryopreserved Sample Prior to FACS
All steps in this protocol except for cell sorting (see Subheading 3.2.3) should be performed in a sterile laminar flow hood using sterile tissue culture technique. Cell sorting should be performed in as clean an environment as possible.
Cryopreserved cells should be carefully thawed and plated 1 day prior to cell sorting. This allows the cells to recover from the freezing process before undergoing FACS.
Prewarm complete growth medium in a water bath set to 37°C. Then, pipette 10 mL prewarmed medium into a sterile 50-mL conical tube.
Carefully and quickly thaw a vial of cryopreserved, dissociated Hu Fe SkM cells in a 37°C water bath and transfer the cells into the 50-mL conical tube with prewarmed proliferation medium using a 1-mL pipette. Rinse the inside of the cryovial with fresh complete growth medium to remove as many cells as possible. This step should be performed very quickly as the DMSO used during the cryopreservation process is toxic to the cells at room temperature.
Plate the cells in the prewarmed medium onto sterile, tissue-culture treated plates at approximately 0.5–1 × 107 cells/10 cm plate or 1.5–3 × 107 cells/15 cm plate (see Note 3). If using a 15 cm plate, add 15 mL prewarmed medium to bring the total medium volume to 25 mL.
Incubate the cells in a CO2 incubator overnight at 37°C.
3.2.2. Preparation of Sample for FACS
Prewarm the following in a 37°C water bath: complete growth medium, 1× HBSS, and cell dissociation buffer. Place the 0.5% BSA/HBSS on ice.
Check your cells under a phase contrast microscope with 10× magnification (see Note 4). Ensure that there is no contamination and that the cells look healthy.
Set-up two 50-mL conical tubes per plate of cells thawed.
Save the old plate medium by carefully removing the medium from the plate and pipetting it into the first 50-mL conical tube.
Wash the cells with 5 mL (10 cm plate) or 10 mL (15 cm plate) 1× HBSS, and then save the wash by pipetting it into the second 50-mL conical tube (see Note 5).
Pipette 3 mL (10 cm plate) or 10 mL (15 cm plate) cell dissociation buffer onto the plate of washed cells and incubate in a humidified CO2 incubator for 10–12 min (see Note 6).
After incubation, remove the cells by gently lifting the cells off the plate. This is done by tilting the plate at an angle (~45°) and carefully pipetting the dissociation buffer currently in the plate onto the surface of the plate. Repeat this process 5–10 times (see Note 7). Pipette the dissociation buffer (containing the cells) into the 50-mL conical tube with the old medium (see Note 8).
Repeat step 7 twice more with 1× HBSS washes (5 mL for 10 cm plate and 10 mL for 15 cm plate). Save the washes in the second 50-mL conical tube.
Check the plate under a phase contrast microscope at 10× magnification for the presence of cells. There should be very few cells on the surface of the plate after this process.
Centrifuge the 50-mL conical tubes containing the cells and washes at 329 × g at 4°C for 10 min.
Resuspend the cells in 0.5–2 mL ice-cold 0.5% BSA/HBSS and combine the cells from both 50 mL conical tubes.
Filter the cells through a sterile 40 μm cell strainer over a 50 mL conical tube. Wash the cell strainer with an additional 0.5–1 mL of ice-cold 0.5% BSA/HBSS.
Determine the cell concentration using a hemocytometer or other cell counting device.
Adjust the cell concentration to 2 × 107 cells/mL in ice-cold 0.5% BSA/HBSS. This may require an extra centrifugation step to spin down and resuspend the cells in a smaller volume. Cells should be centrifuged for 10 min at 329 × g at 4°C.
-
For FACS compensation controls, remove the following number of cells, pipette into a FACS tube (see Note 9), and adjust the volume to 200 μL by adding 175 μL ice-cold 0.5% BSA/HBSS:
“No stain” compensation control → 0.5×106 cells. Store on ice in the dark.
“PI” compensation control → 0.5×106 cells. Add 0.4 μL of 1 mg/mL PI (final conc.: 2 μg/mL). Store on ice in the dark (see Note 10).
-
Primary antibody incubation:
For the primary antibody isotype control (mIgG), remove 0.5×106 cells (in 25 μL) and pipette into a 15 mL conical tube. Add 0.25 μL of unconjugated mIgG1 isotype control antibody (1:100 dilution). Place on ice.
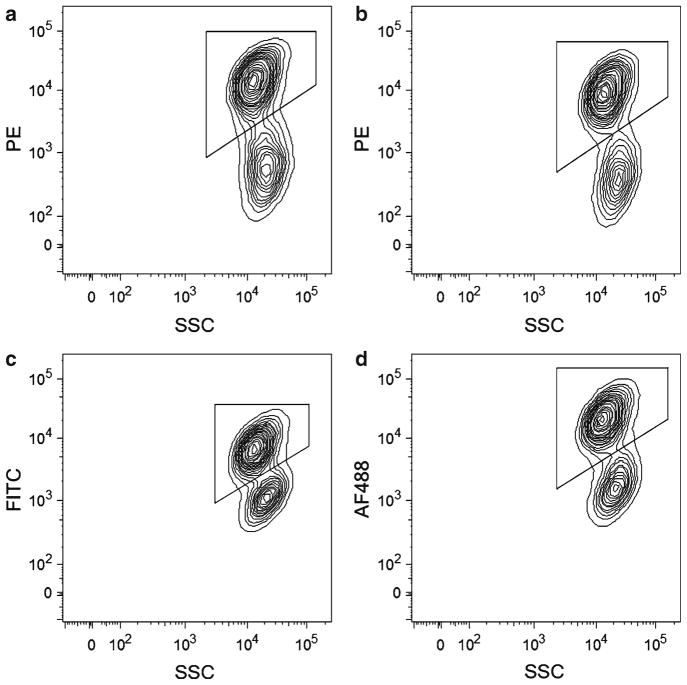
For the remainder of the sample, add the unconjugated MCAM antibody to the sample at a final concentration of 0.5 μg/106 cells (1:100 dilution). Place on ice. Although this antibody is available in several conjugated formats, we have noted that the separation between the positive and negative MCAM populations is decreased by the conjugation. As a result, we recommend the use of the unconjugated antibody with a conjugated secondary antibody for maximum separation and sort purity (see Fig. 1).
Incubate the isotype control and MCAM samples on ice for 30 min.
After this incubation, wash the cells by adding ice-cold 0.5% BSA/HBSS (2–3 mL for isotype control and 10–20 mL for every 2×107 MCAM-labeled cells). Centrifuge the cells for 10 min at 329 × g at 4°C.
Resuspend the cells at 2 × 107 cells/mL in ice-cold 0.5% BSA/HBSS.
-
Secondary antibody incubation (see Note 11):
For the primary antibody isotype control (mIgG), add 0.25 μL of Alexa Fluor® 488-conjugated anti-mouse antibody (1:100 dilution). Place on ice.
For the MCAM antibody-stained sample, add Alexa Fluor® 488-conjugated anti-mouse antibody at a 1:100 dilution. Place on ice.
Incubate the isotype control and MCAM samples with the secondary antibody on ice for 30 min.
After this incubation, wash the cells by adding ice-cold 0.5% BSA/HBSS (2–3 mL for isotype control and 10–20 mL for every 2×107 MCAM-labeled cells). Centrifuge the cells for 10 min at 329 × g at 4°C.
Resuspend the primary antibody isotype control in 200 μL ice-cold 0.5% BSA/HBSS and pipette into a new FACS tube. Add 0.4 μL of 1 mg/mL PI (final conc.: 2 μg/mL). Store on ice in the dark.
Resuspend the MCAM-labeled sample at 2 × 107 cells/mL in ice-cold 0.5% BSA/HBSS.
Transfer 0.5×106 cells (25 μL) of the MCAM-labeled sample to a new FACS tube. Adjust the volume to 200 μL by adding 175 μL ice-cold 0.5% BSA/HBSS. Reserve these cells as the “Alexa Fluor® 488” compensation control. Store on ice in the dark.
Filter the remainder of the MCAM-labeled cells through a sterile 40 μm cell strainer over a 50 mL conical tube. Wash the cell strainer with ice cold 0.5% BSA/HBSS (approximately half the volume that the cells are currently in).
Add 1 mg/mL PI to the MCAM-labeled sample at a final concentration of 2 μg/mL.
Transfer the MCAM-labeled sample to a new FACS tube. Store on ice in the dark.
Prepare collection tube for sorted cells by pipetting 0.5 mL ice-cold 0.5% BSA/HBSS into a new FACS tube. Store on ice.
Fig. 1.
Comparison of unconjugated and conjugated anti-MCAM, clone P1H12, antibodies. FACS plots illustrate a 17-week human fetal sample stained with (a ) unconjugated anti-MCAM primary and PE-conjugated secondary antibodies, or (b) anti-MCAM antibody directly conjugated to PE, (c ) FITC, or (d ) AF488. Note the separation between the MCAM positive and negative populations. Populations positive for MCAM are marked by the gates.
3.2.3. Fluorescence-Activated Cell Sorting
It is beyond the scope of this chapter to review FACS or flow cytometry in detail. Please refer to literature specific to this technique, such as Current Protocols in Cytometry (Wiley and Sons) for further information. Here, gating specifications are briefly indicated.
Determine optimal excitation voltages and compensation values using the “no stain” and single color compensation controls.
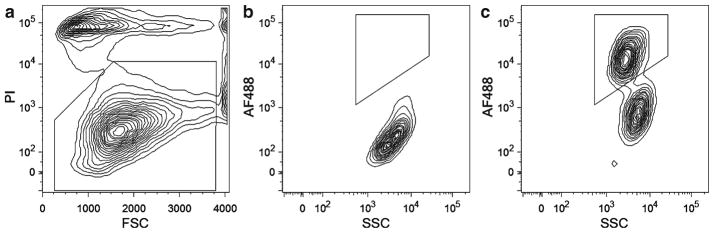
Visualize the “PI” compensation control on a PI vs. forward or side scatter graph. Gate for live cells based upon PI exclusion (i.e., PI-negative cells) (see Fig. 2a).
Determine the positive and negative MCAM populations: visualize the “primary antibody isotype” control and MCAM-labeled sample on an Alexa Fluor® 488 vs. forward or side scatter graph (see Note 12). There should be one MCAM-negative population in the “primary antibody isotype” control, while two populations, one MCAM-positive and one MCAM-negative, should be seen in the MCAM-labeled sample (see Fig. 2b, c). Gate and sort for the MCAM-positive cell population based upon this comparison.
Fig. 2.
Example of FACS gating for human fetal cells immunostained with anti-MCAM antibody and PI: (a ) Live cells selected by gating on the PI-negative population. (b ) Cells labeled with mIgG isotype control. (c ) Cells labeled with anti-MCAM antibody. Comparison of the plots in (b, c) clearly defines a single MCAM-positive population in the anti-MCAM immunostained sample.
3.3. In Vitro Culture and Analysis of Human Fetal Skeletal Myoblasts
3.3.1. In Vitro Cell Culture
All steps in this protocol should be performed in a sterile laminar flow hood using sterile tissue culture technique.
Coat sterile tissue culture-treated plates with 10 mL (10 cm plate) 0.15% gelatin for 1 h at 37°C. After incubation, remove the gelatin solution by aspiration. Then, slightly angle the plates for ~10 min to pool any excess liquid. Remove any excess liquid by aspiration. Coated plates can be stored at 4°C for up to 1 week; prewarm stored, coated plates in a CO2 incubator prior to use.
Prewarm complete growth medium in a water bath set to 37°C.
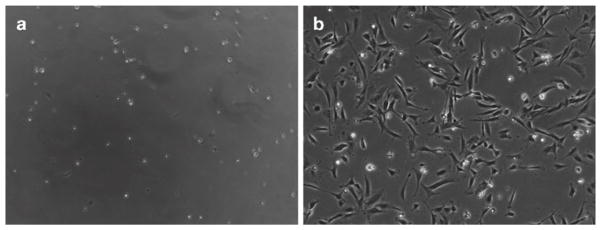
Resuspend sorted MCAM-positive cells at 0.5–1 × 106 cells/10 mL complete growth medium and plate on coated plates from step 1 (10 mL/plate). Gently rock plate(s) to evenly distribute cells, and then incubate in a CO2 incubator. Sorted cells will be small and have a bright, rounded appearance but will attach within 1–2 days postsorting. Figure 3a illustrates sorted MCAM-positive cells 1 day after sorting.
Propagate the cells to 60–75% confluency (see Fig. 3b; Note 13). This should take approximately 2–3 days; however, if necessary, replace the medium with fresh growth medium every 2 days until the plate is at 60–75% confluency.
-
To passage cells:
Coat sterile tissue culture-treated plates as mentioned above.
Prewarm the following in a water bath set to 37°C: 1× HBSS, TrypLE™ Express dissociation enzyme, and complete growth medium.
Remove medium from plate by aspiration and wash the cells with 10 mL (10 cm plate) 1× HBSS. Remove HBSS by aspiration.
Pipette 1 mL TrypLE™ Express onto the plate and incubate in a humidified 37°C CO2 incubator for 2–3 min. Gently remove the cells from the plate using 9 mL complete growth medium and pipette into a sterile 50-mL conical tube. Wash any remaining cells from the surface of the plate with additional complete growth medium.
Centrifuge the cells at 329 × g at room temperature for 10 min.
Resuspend the cells in 10 mL fresh complete growth medium.
Determine the cell concentration using a hemocytometer and plate the cells at 0.5–1 × 106 cells in 10 mL complete growth medium/10 cm plate.
Cells should be passaged every 2–3 days and should not be grown past 75% confluency.
-
To freeze cells:
Trypsinize the cells as in step 5 and then resuspend in ice-cold freezing medium at desired cell concentration.
Store cryovials at −150°C where they can be permanently stored until necessary.
-
To perform an in vitro fusion assay:
Coat BD Falcon 96-well plates with 0.15% gelatin (50–100 μL/well) as above (see Note 14).
Trypsinize the cells and determine the cell concentration as in step 5 and then plate 7,500 cells in 100 μL complete growth medium/96-well.
Incubate the cells in a CO2 incubator overnight.
Carefully remove the growth medium from each well and replace with 100 μL prewarmed differentiation medium (see Note 15). Incubate the cells in a CO2 incubator overnight.
Replace the differentiation medium in each well daily during the course of the fusion assay.
Monitor the differentiation of the cells using a phase contrast microscope at 10 or 20× magnification. Fusion should occur within 1 week of exposure to differentiation medium. When mature myotubes of >10 nuclei/myotube are present, perform the following immunocytochemistry protocol to visualize the cells by fluorescence microscopy.
Fig. 3.
In vitro culture of FACS sorted MCAM-positive human fetal cells. (a ) Sorted MCAM+ cells 1 day after sorting and plating. Most cells appear bright and rounded, while some cells have flattened and firmly adhered to the plate. (b) Sorted MCAM+ cells 7 days after sorting. Most cells are now elongated and firmly adhered to the plate.
3.3.2. Immunocyto-chemistry for In Vitro fusion Assay (see Note 16)
Thaw 4% PFA at room temperature.
Carefully wash the cells with 50 μL of 1× PBS/96-well. Remove PBS using a 1–200 μL pipette (see Note 17).
Fix the cells with 50 μL 4% PFA for 20 min at room temperature; remove fixation liquid using a pipette, then permeabilize the cells with 50 μL permeabilization solution for 3 min at room temperature.
Remove permeabilization solution using a pipette, and then block the cells for 30 min at room temperature with 50 μL blocking solution.
Prepare the primary antibody solution by diluting the anti-human desmin antibody 1:100 in fresh blocking solution. Incubate the cells with primary antibody solution overnight at 4°C.
Wash the cells 3 times with 1× PBS for 5 min at room temperature. The plate may be gently agitated on a rotating shaker.
Prepare the secondary antibody solution by diluting the Alexa Fluor® 488 anti-mouse antibody 1:1,000 in blocking solution. Incubate the cells in the dark with secondary antibody solution for 1 h at room temperature.
Wash the cells 3 times with 1× PBS for 5 min at room temperature in the dark. The plate may be gently agitated on a rotating shaker.
Store the cells in 100–200 μL DAPI working solution at 4°C and protect the cells from light with aluminum foil.
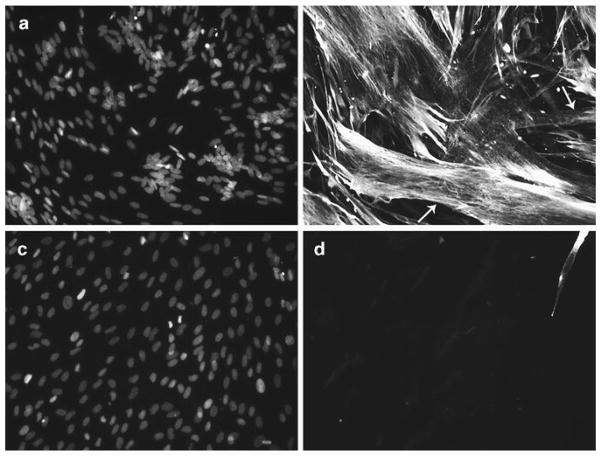
Visualize the cells by fluorescent microscopy using ultraviolet/DAPI and FITC/GFP filter sets for DAPI and desmin, respectively. An example of fused MCAM-positive cells is shown in Fig. 4.
Fig. 4.
In vitro fusion assay of sorted MCAM-positive and MCAM-negative cells. MCAM+ and MCAM− cells were isolated from a human fetal sample and cultured for 2 weeks prior to plating for a fusion assay. Cells were then differentiated for 3 days, fixed and stained for expression of desmin. MCAM+ cells fused as depicted in (a, b), while MCAM− cells were desmin-negative and not fused as shown in (c, d). (a, c ) DAPI; (b, d) desmin of the same microscopic fields. White arrows point to typical myotubes observed in the MCAM+ fraction.
Acknowledgments
This work was supported by grants from NIH/NINDS 2R01NS047727 and 5P50NS040828.
Footnotes
Institutional review and protocol approval are required prior to collection and processing of human fetal tissue. All personnel handling human tissue must receive appropriate safety and human subject education training.
FBS varies considerably between companies and even lot to lot from the same company. Therefore, several different FBS samples should be tested using the in vitro methods described in Subheading 3.3 to determine which lot/company works best for your myogenic cells.
No plate coating is required for this step as the cells are only plated for a day prior to use.
There will be many floating, live cells in your culture, which is normal for dissociated Hu Fe SkM. It is also likely that there will be small clumps of cells in the culture, and the number of clumps will vary. These clumps will be filtered out prior to cell sorting. Additionally, the dissociation process results in a large amount of debris in addition to cells. This will make the culture appear “dirty” (i.e., little black specks, etc.), but again, this is normal and should not be considered contamination. This debris will be removed during the FACS sample preparation process.
Given the large number of floating, live cells, it is important to save the old medium and the wash prior to removing the cells from the plate with cell dissociation buffer. This ensures that you maximize the number of cells available for FACS and subsequent analysis.
Cell dissociation buffer MUST be used to remove the cells from the plate when using the Chemicon anti-MCAM antibody. Trypsin removes/destroys the epitope for this antibody and therefore cannot be used for this protocol.
When the plate is tilted at an angle, the cells can be seen on the surface of the plate as a light opaque coating. Repeatedly rinse the cells off the plate until this coating is no longer visible.
The cell suspension containing cells and cell dissociation buffer must be pipetted into the old medium (or fresh medium) to deactivate the cell dissociation buffer. Prolonged exposure of the cells to the cell dissociation buffer may negatively affect the health of the cells.
Some FACS machines may require FACS tubes that are different in diameter/size from the tube specified in this protocol. Check that your tubes fit in your machine prior to use.
Propidium iodide (PI) is a membrane impermeant DNA dye that is used in this protocol to discriminate live from dead cells during the FACS.
For the best possible separation between antigen-expressing and nonexpressing populations, it is important to use a secondary antibody conjugated to a bright and photostable fluorophore. Invitrogen Alexa Fluor® -conjugated antibodies are brighter and more photostable than conventional conjugates such as FITC and Cy3, and they are recommended for applications requiring fluorescence detection in this protocol.
The Alexa Fluor® 488 dye is equivalent to FITC and GFP for fluorescence excitation and emission. Therefore, standard FITC and GFP filter sets for FACS and fluorescent microscopy can be used to visualize this dye.
Cells should never reach 100% confluency when proliferating as they will begin to differentiate and fuse on contact. The high serum growth medium will lower in serum concentration over time and will not be able to prevent fusion (see Note 15).
Black with optically clear bottom plates are recommended for optimal fluorescence detection. However, the anti-human desmin antibody used in this protocol is a robust antibody that can be detected in clear 96-well tissue culture plates if needed.
This immunocytochemical protocol can also be utilized for the detection of other myogenic markers of proliferating or differentiating cells.
A 1–200 μL pipette is recommended for removal of liquids in this immunocytochemical protocol due to the small surface area of a 96-well. Aspiration or large pipette tips (i.e., 1 mL pipette tip) may remove a significant number of cells from the well surface.
References
- 1.Pogogeff IA, Murray MR. Form and behavior of adult mammalian skeletal muscle in vitro. Anat Rec. 1946;95:321–335. doi: 10.1002/ar.1090950308. [DOI] [PubMed] [Google Scholar]
- 2.Geiger RS, Garvin JS. Pattern of regeneration of muscle from progressive muscular dystrophy patients cultivated in vitro as compared to normal human skeletal muscle. J Neuropathol Exp Neurol. 1957;16:532–543. [PubMed] [Google Scholar]
- 3.Herrmann H, Konigsberg UR, Robinson G. Observations on culture in vitro of normal and dystrophic muscle tissue. Proc Soc Exp Biol Med. 1960;105:217–221. doi: 10.3181/00379727-105-26059. [DOI] [PubMed] [Google Scholar]
- 4.Goyle SS, Kalra SL, Singh B. The growth of normal and dystrophic human skeletal muscle in tissue culture. Neurol India. 1967;15:149–151. [PubMed] [Google Scholar]
- 5.Kakulas BA, Papadimitriou JM, Knight JO, Mastaglia FL. Normal and abnormal human muscle in tissue culture. Proc Aust Assoc Neurol. 1968;5:79–85. [PubMed] [Google Scholar]
- 6.Skeate Y, Bishop A, Dubowitz V. Differentiation of diseased human muscle in culture. Cell Tissue Kinet. 1969;2:307–310. [Google Scholar]
- 7.Bishop A, Gallup B, Skeate Y, Dubowitz V. Morphological studies on normal and diseased human muscle in culture. J Neurol Sci. 1971;13:333–350. doi: 10.1016/0022-510x(71)90037-2. [DOI] [PubMed] [Google Scholar]
- 8.Hauschka SD. Clonal analysis of vertebrate myogenesis: II. Environmental influences upon human muscle differentiation. Dev Biol. 1974;37:329–344. doi: 10.1016/0012-1606(74)90153-5. [DOI] [PubMed] [Google Scholar]
- 9.Hauschka SD. Muscle cell culture: Future goals for facilitating the investigation of human muscle disease. In: Schotland DL, editor. Disorders of the motor unit. Wiley; New York: 1982. [Google Scholar]
- 10.Blau HM, Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci. 1981;78:5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Webster C, Pavlath GK, Parks DR, et al. Isolation of human myoblasts with the fluorescence-activated cell sorter. Exp Cell Res. 1988;174:252–265. doi: 10.1016/0014-4827(88)90159-0. [DOI] [PubMed] [Google Scholar]
- 12.Walsh FS, Ritter MA. Surface antigen differentiation during human myogenesis in culture. Nature. 1981;289:60–64. doi: 10.1038/289060a0. [DOI] [PubMed] [Google Scholar]
- 13.Baroffio A, Aubry JP, Kaelin A, et al. Purification of human muscle satellite cells by flow cytometry. Muscle Nerve. 1993;16:498–505. doi: 10.1002/mus.880160511. [DOI] [PubMed] [Google Scholar]
- 14.Shih IeM. The role of CD146 (Mel-CAM) in biology and pathology. J Pathol. 1999;189:4–11. doi: 10.1002/(SICI)1096-9896(199909)189:1<4::AID-PATH332>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 15.Ouhtit A, Gaur RL, Abd Elmageed ZY, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795:130–136. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Lecourt S, Marolleau JP, Fromigué O, et al. Characterization of distinct mesenchy-mal-like cell populations from human skeletal muscle in situ and in vitro. Exp Cell Res. 2010;316:2513–2526. doi: 10.1016/j.yexcr.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Pujades C, Guez-Guez B, Dunon D. Melanoma cell adhesion molecule (MCAM) expression in the myogenic lineage during early chick embryonic development. Int J Dev Biol. 2002;46:263–266. doi: 10.1387/ijdb.011493. [DOI] [PubMed] [Google Scholar]
- 18.Cerletti M, Molloy MJ, Tomczak KK, et al. Melanoma cell adhesion molecule is a novel marker for human fetal myogenic cells and affects myoblast fusion. J Cell Sci. 2006;119:3117–3127. doi: 10.1242/jcs.03056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simpson SB, Jr, Cox PG. Vertebrate regeneration system: culture in vitro. Science. 1967;157:1330–1332. doi: 10.1126/science.157.3794.1330. [DOI] [PubMed] [Google Scholar]
- 20.Yaffe D, Saxel O. A myogenic cell line with altered serum requirements for differentiation. Differentiation. 1977;7:159–166. doi: 10.1111/j.1432-0436.1977.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 21.Webster C, Filippi G, Rinaldi A, et al. The myoblast defect identified in Duchenne muscular dystrophy is not a primary expression of the DMD mutation. Hum Genet. 1986;74:74–80. doi: 10.1007/BF00278789. [DOI] [PubMed] [Google Scholar]