Abstract
Regenerative medicine holds the promise of restoring cells and tissues that are destroyed in human disease, including degenerative eye disorders. However, development of this approach in the eye has been limited by a lack of animal models that show robust regeneration of ocular tissue. Here, we test whether MRL/MpJ mice, which exhibit enhanced wound healing, can efficiently regenerate the retinal pigment epithelium (RPE) after an injury that mimics the loss of this tissue in age-related macular degeneration. The RPE of MRL/MpJ and control AKR/J mice was injured by retro-orbital injection of sodium iodate at 20 mg/kg body weight, which titration studies indicated was optimal for highlighting strain differences in the response to injury. Five days after sodium iodate injection at this dose, electroretinography of both strains revealed equivalent retinal responses that were significantly reduced compared to untreated mice. At one and two months post-injection, retinal responses were restored in MRL/MpJ but not AKR/J mice. Brightfield and fluorescence microscopy of eyecup cryosections indicated an initial central loss of RPE cells and RPE65 immunostaining in MRL/MpJ and AKR/J mice, with preservation of peripheral RPE. Phalloidin staining of posterior eye wholemounts confirmed this pattern of RPE loss, and revealed a transition region characterized by RPE cell shedding and restructuring in both strains, suggesting a similar initial response to injury. At one month post-injection, central RPE cells, RPE65 immunostaining and phalloidin staining were restored in MRL/MpJ but not AKR/J mice. BrdU incorporation was observed throughout the RPE of MRL/MpJ but not AKR/J mice after one month of administration following sodium iodate treatment, consistent with RPE proliferation. These findings provide evidence for a dramatic regeneration of the RPE after injury in MRL/MpJ mice that supports full recovery of retinal function, which has not been observed previously in mammalian eyes. This model should prove useful for understanding molecular mechanisms that underlie regeneration, and for identifying factors that promote RPE regeneration in age-related macular degeneration and related diseases.
Keywords: healer mice, regenerative medicine, sodium iodate, macular degeneration, MRL/MpJ, Regeneration, Retinal pigment epithelium
1. Introduction
The motivating concept of regenerative medicine is that physically or functionally damaged cells, tissues, and organs might be restored in patients with severe injuries or chronic diseases (Gurtner et al., 2007). One important disease target is age-related macular degeneration (AMD), a leading cause of blindness worldwide affecting more than 1.75 million people in the U.S. alone (Swaroop et al., 2009). A critical step in AMD pathology is the dysfunction and eventual loss of the retinal pigment epithelium (RPE) (Swaroop et al., 2009). This tissue consists of a cuboidal epithelial monolayer at the interface between choroidal capillaries and the neurosensory retina that is essential for photoreceptor function and overall visual health (Strauss, 2005). Although RPE regeneration is desirable for treating AMD, mammalian RPE is essentially non-renewable (Al-Hussaini et al., 2008; Seagle et al., 2006). Thus, development of a mammalian model exhibiting robust RPE regeneration would be a powerful tool to define pathways capable of repairing RPE loss and in turn may improve AMD treatment.
Several regenerative approaches may be considered for replacing lost RPE. One is to introduce cultured RPE cells derived from embryonic or induced pluripotent stem cells into the subretinal space. This approach has shown some success in animal models, but is limited by the challenge of halting disease progression, immune rejection, a limited source of donor cells, and surgical complications (Boulton et al., 2004; Lee and Maclaren, 2010). A second strategy is to inject bone marrow-derived stem cells into the vitreous or the systemic circulation. We and others have provided evidence in rodent models that injected bone marrow-derived cells reach the choroidal-retinal interface and transdifferentiate into RPE cells, but the efficiency is currently too low to be considered as a robust therapeutic approach (Carr et al., 2009; Harris et al., 2006; Harris et al., 2009; Idelson et al., 2009; Uygun et al., 2009; Vossmerbaeumer et al., 2008). A third, untested, strategy is to induce regeneration chemically by local introduction of factors that reprogram resident stem cells or undamaged RPE cells to repopulate the damaged tissue, as proposed for other organs (Xu et al., 2008). All of these strategies would benefit greatly from the identification of cellular or molecular factors that enhance RPE regeneration.
The characterization of organisms with high regenerative capacity is a promising approach for identifying such factors (Gurley and Alvarado, 2008; Kawakami, 2010). MRL/MpJ mice are recognized as a novel mammalian model for enhanced regeneration (Clark et al., 1998). These mice completely close a 2 mm ear punch without fibrotic scarring. Recent studies have identified molecular attributes that underlie the MRL/MpJ phenotype (Alfaro et al., 2008; Bedelbaeva et al., 2010; Caldwell et al., 2008; Li et al., 2001b; Naviaux et al., 2009; Tucker et al., 2008; Ueno et al., 2005), which may permit targeted improvement of tissue regeneration. However, the regeneration ability under different situations appears to vary considerably in these mice. There are reports of efficient regeneration of the heart, digit tip, digit and articular cartilage and enhanced wound healing for skin transplant (Chadwick et al., 2007; Fitzgerald et al., 2008; Gourevitch et al., 2009; Leferovich et al., 2001; Naseem et al., 2007; Tolba et al., 2010). On the other hand, there are negative results with regard to the regeneration of skin, spinal cord and heart (Abdullah et al., 2005; Cimini et al., 2008; Davis et al., 2007; Grisel et al., 2008; Oh et al., 2004; Robey and Murry, 2008). The different outcomes may be attributed to differences in tissue type and the extent of injury produced in these experiments. In the eye, only the corneal epithelium has been shown to regenerate (Ueno et al., 2005). Thus, further studies are needed to determine whether the RPE regenerates efficiently in MRL/MpJ mice.
Regeneration studies require specific surgical, chemical or genetic ablation of the target tissue. Sodium iodate causes selective toxicity of the RPE (Redfern et al., 2011). High dose (40-50 mg/kg body weight, intravenous injection; 100 mg/kg body weight, intraperitoneal injection) administration of sodium iodate will cause RPE damage over the entire eye (Kiuchi et al., 2002; Redfern et al., 2011). By contrast, low dose sodium iodate (20 mg/kg body weight, intravenous injection) will primarily cause only central damage with less collateral cell damage/loss and sparing of the peripheral RPE (Machalinska et al., 2011). Under normal conditions, RPE cells are considered to be non-regenerative (Seagle et al., 2006), although cell division was observable in peripheral regions (Al-Hussaini et al., 2008) and only minor partial recovery of visual function was observed under lower dose of sodium iodate with sensitive the optokinetic response (OKR) (Franco et al., 2009) and electroretinography (ERG) measurements (Kiuchi et al., 2002; Mizota and Adachi-Usami, 1997). The observed minor recovery was attributed to endogenous regeneration (Machalinska et al., 2011). This potential regenerative capacity has yet to be fully explored experimentally. We believe that it will be clinically attractive if those low-level endogenous repair mechanisms can be defined and manipulated to promote regeneration of RPE in pathological conditions including AMD.
Here, as a step towards improving RPE regenerative strategies, we investigated RPE recovery in MRL/MpJ mice after sodium iodate treatment. Electroretinography and BrdU labeling and histology was performed to assess RPE damage and restoration in MRL/MpJ and control strains. We found that MRL/MpJ mice show more robust structural and functional regeneration than control mice after injury with a full recovery of ERG responsiveness. Our result provides the first demonstration of enhanced RPE regeneration in rodents and provides a significant new tool for future studies of RPE regenerative therapy.
2. Material and methods
2.1. Animals
MRL/MpJ mice were obtained commercially (Jackson Laboratory, Bar Harbor, ME). Two ancestral strains of MRL/MpJ, AKR/J (Jackson Laboratory) and C57BL/6 (Charles River Laboratories, Wilmington, MA) were chosen as albino and pigmented control strains, respectively. For studies of pigmented animals, MRLBL/GFP, a laboratory derivative of MRL/MpJ mice was used. This strain was constructed by crossing MRL/MpJ and Tg (CAG-EGFP)B5Nagy/J mice (Jackson Laboratory) to obtain black mice expressing green fluorescent protein (GFP), which were then backcrossed against MRL/MpJ for >5 generations with retention of these phenotypes. Ear punch closure analysis confirmed the regeneration phenotype. Mice were raised on cage racks with unrestricted access to food and water under fluorescent lighting with a 12-hour light/12-hour dark cycle. All animal procedures were reviewed and approved by the University of Florida Animal Care and Use Committee and performed in an Association for Assessment of Laboratory Animal Care approved facility according to the regulations in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Ablation of RPE with Sodium Iodate
Mice of 8-10 weeks of age were anesthetized and injected with sodium iodate (Sigma-Aldrich, St. Louis, MO) in phosphate buffered saline (PBS), into the retro-orbital sinus, to produce a dose of 20, 30 or 40 mg/kg body weight. Untreated animals were injected with a comparable volume of PBS. Mice (n≥3 for each group) were monitored for RPE regeneration by ERG and by histological analysis at various time points following injection.
2.3. Electroretinography (ERG)
Mice were dark-adapted overnight before analysis. Pupils were dilated with 0.5% proparacaine hydrochloride (Akorn, IL) and 0.5% phenylephrine hydrochloride (Bausch and Lomb, FL). Mice were anesthetized with avertin (2, 2, 2-Tribromoethanol, Sigma-Aldrich, St. Louis, MO) at 0.5 mg/g body weight and placed on a temperature-controlled working platform at 37°C. Gold-rimmed contact electrodes were placed on the corneal surface and visual responses were recorded with a UBA 4204 visual electrodiagnostic ERG system (LKC Technologies, Gaithersburg, MD) using white light stimuli at intensities of −45, −35, −25, −15, −5 and 5 dB with LED light.
2.4. Histology
Eyes were enucleated from euthanized animals and the cornea, iris and lens removed. For cryosections, right eyes were fixed overnight at 4°C in 4% paraformaldehyde in PBS, transferred to 20% sucrose overnight, oriented and embedded in optimal cutting temperature compound (Sakura Tissue Tek; IMEB, San Marcos, CA). Cryosections (14 μm thick) were collected on Superfrost Plus positively-charged slides (Fisher Scientific, Pittsburgh, PA) and air-dried overnight at room temperature. For immunohistochemistry, sections were treated with target retrieval solution (DAKO, Carpinteria, CA), protein blocking buffer (DAKO), and biotin and avidin blocking kit (Vector Laboratories, Burlingame, CA). Sections were stained with mice anti-RPE65 antibody (401.8B11.3D9) at 1:150 dilution (NOVUS, Littleton, CO) as primary and donkey anti-mice IgG AlexaFluor 488 (Invitrogen, Carlsbad, CA) at 1:500 dilution as secondary by using ARK kit (DAKO). After extensive washes in Tris-buffered saline, the slides were counterstained and mounted in antifade medium (Vectashield; Vector Laboratories) with 4′-6-diamidino-2-phenylindole (DAPI). Microscopy was performed with a spinning disk confocal microscope (BX61WI-DSU; Olympus, Center Valley, PA). Green and blue channels were acquired with a 20X objective and merged with Volocity (Perkin Elmer) and tiled with the MosaicJ plugin (Thevenaz and Unser, 2007) in ImageJ (Wayne S. Rasband, U. S. National Institutes of Health, Bethesda, Maryland, USA; available at http://rsb.info.nih.gov/ij/download.html). For whole mounts, left posterior eyecups were stained at room temperature with DAPI and rhodamine-phalloidin (Invitrogen, Carlsbad, CA) in PBS containing 1% Triton X-100 for ≥24 hours. Eyecups were cut radially, mounted with Vectashield and imaged with a spinning disk confocal microscope (IX81-DSU or BX61WI-DSU; Olympus, Center Valley, PA) with acquisition software (Slidebook, Olympus; or Volocity, respectively). To observe cell proliferation after injury, BrdU (50 mg/kg body weight) was administered daily by intraperitoneal injection of sodium iodate treated MRL/MpJ and AKR/J mice. Eyes were harvested 30 days post sodium iodate injection and stained with the BRDU STAINING KIT (Invitrogen, Carlsbad, CA). To check for morphological changes after injury, harvested eyes were fixed overnight in 2% glutaraldehyde and 4% paraformaldehyde in PBS and embedded in epoxy resin. Sections were cut at 1μm thickness and stained with toluidine blue (Li et al., 2001a).
2.5. Statistical methods
SAS software (SAS Institute Inc., Cary, NC) was used for statistical analysis of ERG signals. A proc mixed model process was used to compare the strain, day difference with wave type as random block. Significance was set at P< 0.05, and data are reported as the mean ± SEM, where applicable. All analyses were two-tailed.
3. Results
3.1. Experimental Rationale
To develop a model for RPE regeneration, we sought to induce damage and compare functional and morphological recovery of the RPE in MRL/MpJ with other strains. An immediate issue was the choice of control strains. MRL/MpJ is an inbred albino strain initially generated from a series of crosses among several laboratory strains, making congenic comparison impossible. Albino strains have also been reported to be more sensitive to sodium iodate induced RPE damage (Redfern et al., 2011). We chose control strains with normal wound-healing capability, including C57BL/6 and AKR/J, which were used as stock in the generation of MRL/MpJ mice. Furthermore, AKR/J serves as an albino control for the pigmented C57BL/6 strain. We also have crossed the MRL healer phenotype onto a pigmented GFP-positive background to generate MRLBL-GFP as a pigmented counterpart of albino MRL/MpJ for our study.
3.2. Optimizing sodium iodate dose
To avoid possible secondary effects that might hinder regeneration (Harris et al., 2006), our first objective was to identify conditions in which recovery of injured RPE in MRL/MpJ mice could be easily distinguished from that of control mice. In many mouse studies of RPE injury (Harris et al., 2006; Harris et al., 2009), sodium iodate was used at a dose of 40 mg/kg body weight or higher. This dose resulted in substantial RPE damage with minimal recovery in preliminary experiments. We hypothesized that high-dose sodium iodate may cause excessive damage to the tissue microenvironment, leading to the loss or alteration of repair signals and environmental guidance cues that are required for regeneration. Massive damage would most likely lead to scar formation regardless of strain background.
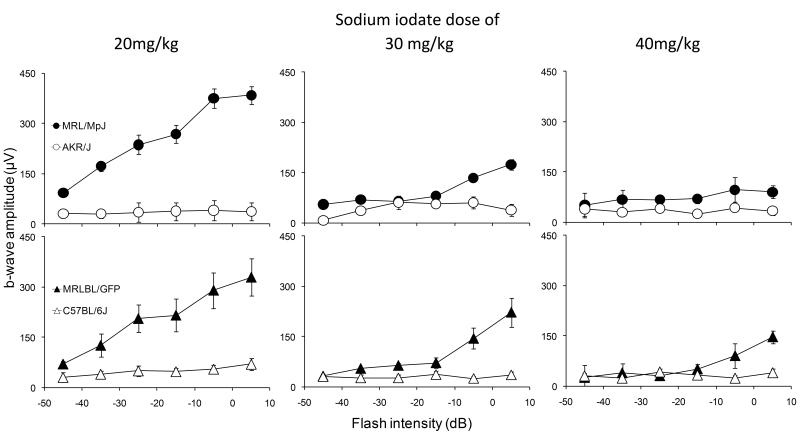
To optimize dosing, strains were injected retro-orbitally with sodium iodate at various doses and examined one month later by ERG. One month is a time point when a plateau stage has been reached in C57BL/6 mice injected with low dose of sodium iodate (Franco et al., 2009). Significantly greater b-wave amplitudes were observed in MRL/MpJ and MRLBL/GFP mice compared with all other strains tested at all sodium iodate doses (Fig. 1). The differences in ERG amplitudes between MRL/MpJ, MRLBL/GFP and control cohorts were greatest at a dose of 20 mg/kg body weight. The 20 mg/kg dose also resulted in a near flat line ERG response in the control strain that was not significantly different from the damage observed with higher doses of sodium iodate. Moreover, regardless of pigment phenotype, MRL strains showed a more robust recovery of the ERG signal than control strains (Fig. 1), suggesting that the previously reported influence of pigment on sodium iodate susceptibility (Redfern et al., 2011) does not apply to recovery. Based on these results, we chose a sodium iodate dose of 20 mg/kg for further studies.
Figure 1.
Comparison of b-wave amplitudes of MRL/MpJ and AKR/J, MRLBL/GFP and C57BL/6, at a dosage of 20, 30 and 40 mg sodium iodate/kg body weight. Significant (P<0.05) differences were observed at 30 days post-injection between the two strains at all doses with n>5. However, among the three doses, 20mg/kg cohorts yield the biggest difference.
3.3. Time course of ERG response in MRL/MpJ mice after chemical ablation of RPE
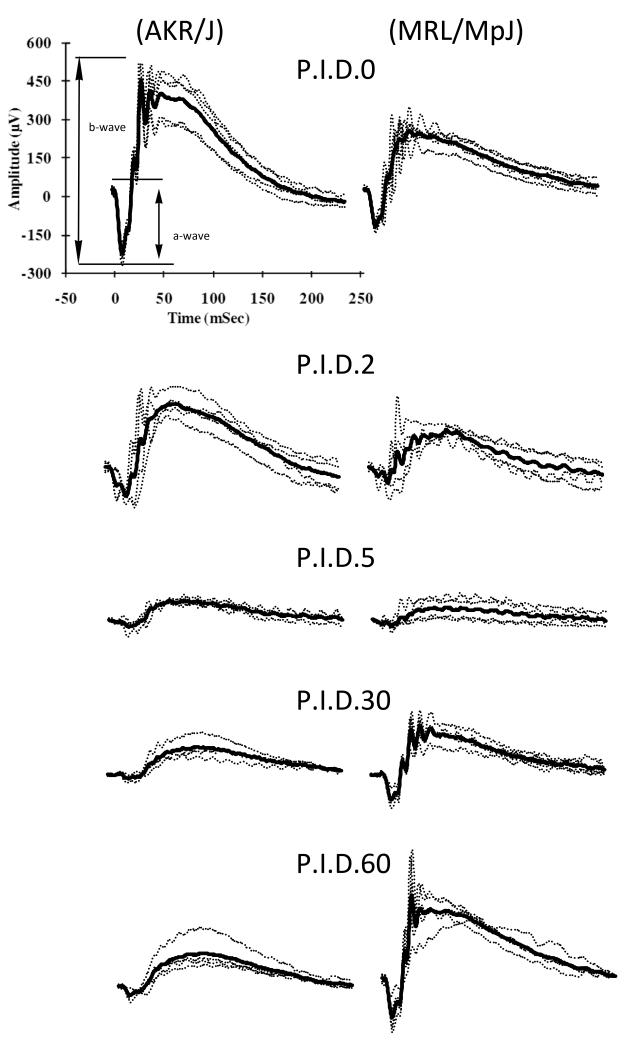
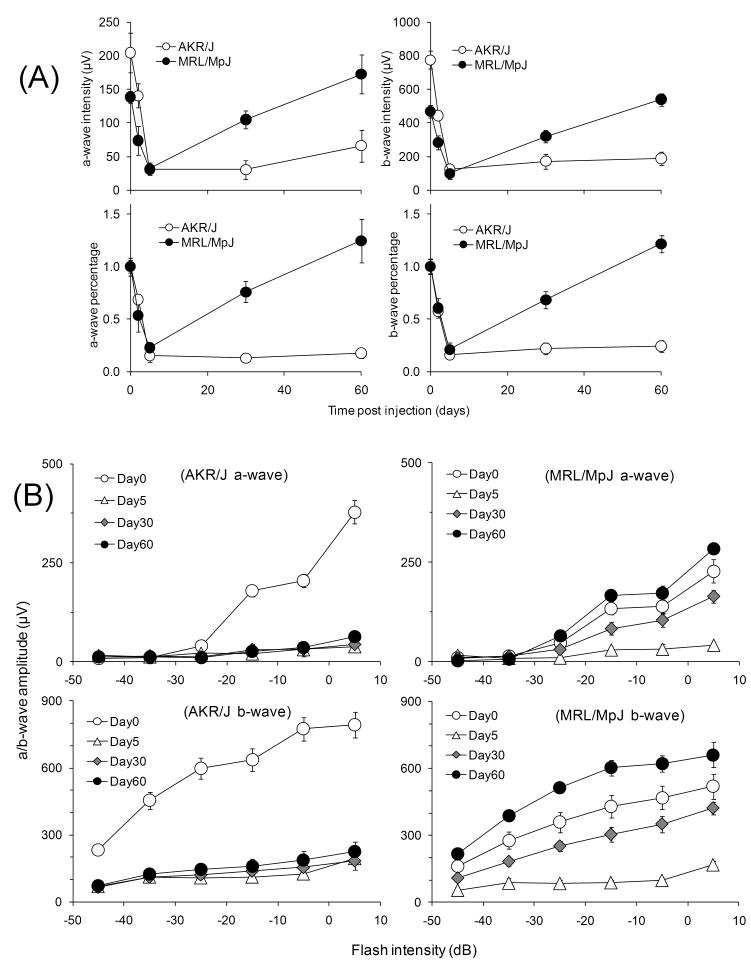
As established in the previous section, MRL/MpJ showed higher ERG responses than control strains at post-injection day 30. To follow the kinetics of the recovery process and to exclude the possibility that MRL/MpJ mice are more resistant to the initial injury, we monitored the ERG response in MRL/MpJ and AKR/J mice as a function of time after sodium iodate injection. Prior to treatment, both strains exhibited typical scotopic ERG responses characterized by a- and b-waves with superimposed oscillatory potentials (Fig. 2). At early times after injection, both strains showed attenuated responses. At later times, the ERG response of MRL/MpJ mice was substantially restored, while that of AKR/J remained low (Fig. 2). To account for strain differences in the pretreatment ERG response, we performed the analysis with ERG amplitudes normalized to the pretreatment values for the b-wave at −5 dB flash intensity. This analysis revealed nearly identical initial damage in both strains followed by recovery in MRL/MpJ but not AKR/J mice (Fig 3. (A)). Similar damage and recovery trends were observed with intensity-response data collected at flash intensities from -45 dB to +5 dB (Fig. 3 (B)). Statistical analysis of the −15 dB, −5 dB and +5 dB b-wave amplitudes at 5, 30 and 60 days post-injection with a mixed model statistical analysis indicated no significant difference in initial damage between the two strains (P=0.1214). Analysis at these stimulus intensities also indicated significant recovery in MRL/MpJ mice at 30 and 60 days compared to 5 days post-injection (P<0.0001 and <0.0001, respectively but not in AKR/J mice (P=0.4910 and 0.6258, respectively). These results suggest that MRL/MpJ and AKR/J mice are equally susceptible to sodium iodate injury, but only MRL/MpJ mice recover significantly. Thus, the increased ERG response in MRL/MpJ mice after sodium iodate treatment is not due to a greater resistance to the initial injury but rather to enhanced regeneration.
Figure 2.
Single and average ERG traces at −5dB intensity. ERG traces in MRL/MpJ and AKR/J mice at an intensity of −5 dB were recorded at 0, 2, 5, 30 and 60 days after sodium iodate injection. Thick line: mean ERG response. Both strains show typical ERG responses before and decreased responses at 2 and 5 days after sodium iodate treatment. A dramatic increase in response was observed at later time points in MRL/MpJ mice.
Figure 3.
Analysis of ERG signal. Respective time based a- and b-wave amplitude and percentage analysis at −5 db (A) and flash dose dependent response of AKR/J and MRL/MpJ at different time points after sodium iodate treatment (B) indicating a significant regeneration difference between the two strains. Both actual amplitude values and the percentage normalized to untreated mice showed a damage-recovery process in the MRL/MpJ mice but only damage trend in AKR/J mice. Flash intensity dependent responses illustrate the true damage and regeneration profile. n≥3.
3.4. Loss and restoration of RPE65 expression in MRL mice posterior cup after injury
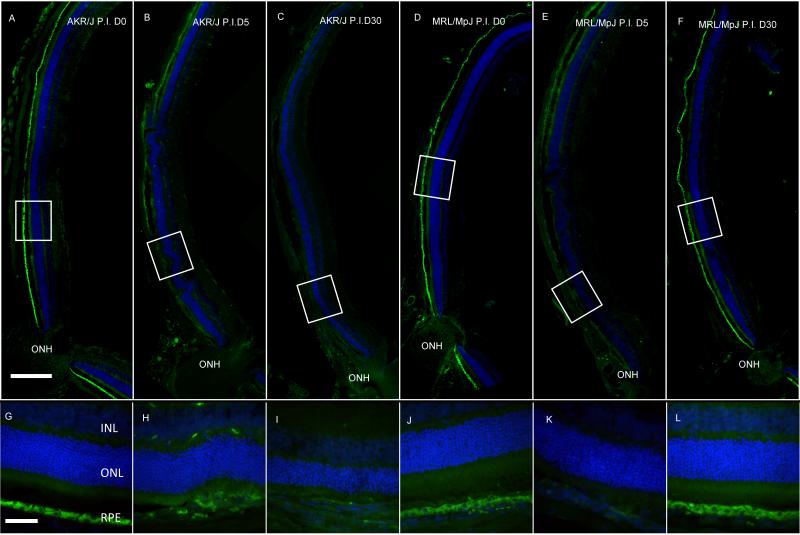
We conducted anti-RPE65 immunostaining to correlate the observed functional recovery with structural changes to the RPE in cryosections of posterior eyecups before and after sodium iodate injury. Prior to injury, immunohistochemical analysis with antibody against RPE65, an RPE-specific protein (Hamel et al., 1993), revealed a thin, positively-stained band at the expected location between the neurosensory retina and the choroid (Fig. 4 (A) and (D)). At higher magnification, polygonal RPE65-positive cells with large central nuclei characteristic of the RPE were clearly identifiable (Fig. 4 (G-L)). RPE65 staining was dramatically reduced in both strains at 5 days post-injection (Fig. 4 (B) and (E)). Strong central RPE65 staining reappeared at 30 days post-injection in MRL/MpJ but not AKR/J mice (Fig. 4 (C) and (F)), consistent with the recovery of ERG responses (Fig. 2 and 3). The decrease in RPE65 staining following injury may be due to a reduction in the cellular expression of RPE65, a loss of RPE cells or both. These results suggest that RPE cellular integrity was similarly disrupted by sodium iodate in both strains, but recovered more effectively in MRL/MpJ mice.
Figure 4.
Immunostaining of RPE65. Fluorescence micrographs of posterior cup cyosections of AKR/J (A-C) and MRL/MpJ (D-F) stained with anti-RPE65 antibody. Higher magnification views of the corresponding boxed areas of AKR/J (G-I) and MRL/MpJ (J-L). A positively-stained band at the expected location between the neurosensory retina and choroid was observed in untreated samples (A and G, D and J). RPE65 staining was dramatically reduced at 5 days after sodium iodate injection (B and H, E and K). Staining was restored in MRL/MpJ but not AKR/J at 30 days post-injection. Scale bar in upper panel: 200 μm; lower panel 50 μm. ONH: optic nerve head. Green: AlexaFluor 488; Blue: 4′-6-diamidino-2-phenylindole (DAPI).
3.5. RPE restructuring and loss within days of sodium iodate injury
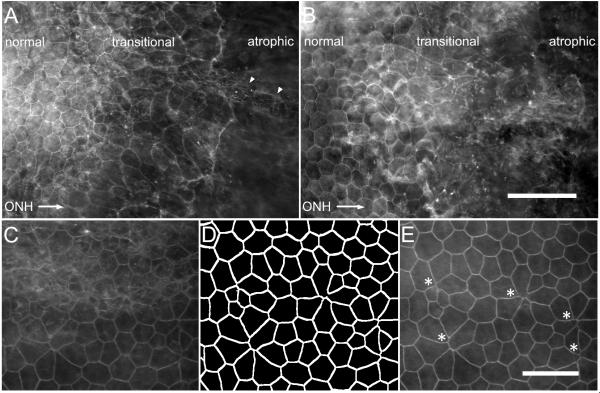
To further confirm the loss of RPE cellular integrity, we analyzed posterior eyecup preparations stained with rhodamine phalloidin, which detects filamentous actin (F-actin) on the apical border of RPE cells. Since conventional RPE/choroid/sclera flat mounts are subject to potential artifacts that arise when the adherent retina is removed, we examined eyecup whole mounts, in which the retina is retained. At two days following sodium iodate injection, both strains showed a similar extent of damage (Fig. 5 (A) and (B)). Polygonal RPE cells were detected in the eyecup periphery, but were absent from the center, consistent with RPE cell atrophy and loss. The transition zone between healthy and atrophic tissue was characterized in both strains by irregularly-shaped RPE cells whose border curvature and area increased towards the eyecup center (Fig. 5 (A) and (B)).
Figure 5.
Initial RPE restructuring and loss two days after sodium iodate injury. Mosaic image of posterior eyecup whole mounts were obtained from AKR/J (A) and MRL/MpJ (B) mice stained with rhodamine phalloidin. Normal polygonal RPE cells were present in the periphery but undetectable in an atrophic region of the eyecup toward the optic nerve head (ONH, direction indicated by arrow). A transitional zone between these regions contained irregularly-shaped cells, with increasing border curvature and cell area towards the ONH, and fibroblast-like cells (arrowhead). The transitional zone was examined at higher magnification (C-E). Merged confocal stacks were difficult to interpret due to the unevenness of the whole mount preparation (C). An ImageJ “flattening” macro written to extract the RPE cell borders (D) revealed the RPE apical surface and possible shedding events within the transitional zone of an MRL/MpJ mouse two days after sodium iodate injury (E, asterisks). Scale bars: A and B, 100 μm; C-E, 50 μm. ONH: optic nerve head.
Visualization of the RPE in whole mount image stacks was challenging because of the abundance of F-actin and unevenness of tissue. Maximum merge z-projection of image stacks encompassing the RPE layer was typically uninterpretable (Fig. 5 (C)). We therefore developed a “flattening” macro in ImageJ to extract the phalloidin-stained RPE cell borders (Fig. 5 (D)) and generate an image containing the RPE apical surface in a single plane (Fig. 5 (E)). These images clearly revealed structures reminiscent of RPE cellular shedding events within the transition zone of sodium iodate-injured mice in both AKR/J and MRL/MpJ (Fig. 5 (E), asterisks). Taken together, these results establish that sodium iodate treatment at 20 mg/kg body weight produces a gradient of RPE loss and cellular tissue restructuring that is similar in both strains.
3.6. Enhanced restoration of RPE morphology at one month
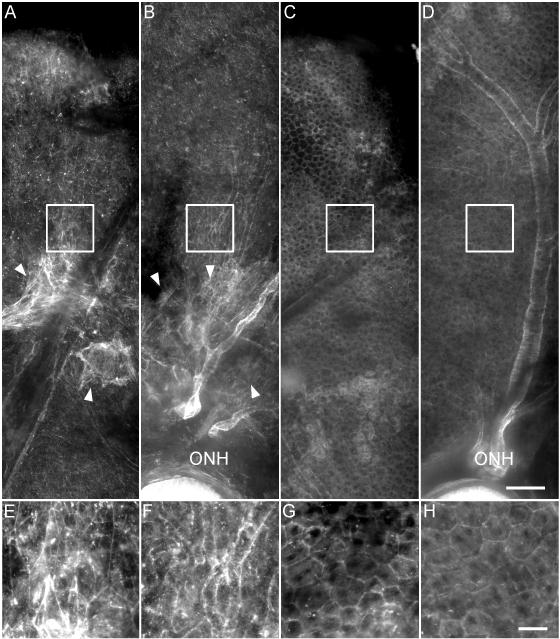
In order to better characterize the loss and regeneration of RPE in the MRL and control animals we examined phalloidin-stained posterior eyecup whole mounts at one month post-injection (Fig. 6). Mosaic images of AKR/J and MRL/MpJ mice were generated starting at the superior, periphery and ending at the optic nerve head. AKR/J mice at one month post-injection retained extensive areas toward the optic nerve head that were completely devoid of polygonal RPE cells as detected by phalloidin staining (Fig. 6 (A) and (B)). By comparison, and in contrast to the appearance at two days post-injection, phalloidin staining of MRL/MpJ eyecups at one month post-injection showed the polygonal RPE cells throughout the posterior eye (Fig. 6 (C) and (D)), a striking improvement to the disrupted morphology observed at two days post-injection. The large, irregularly-shaped cells observed at early times after injury (Fig. 5 (A)) were mostly absent. Polygonal cells were observed mainly in the periphery of AKR/J eyes, which might contribute to the small amount of ERG recovery in the AKR/J strain (Figs. 1-3) Phalloidin-stained F-actin structures reminiscent of those in fibroblasts were observed at the transition between the normal and atrophic RPE, suggestive of scar formation. These results demonstrate that the RPE region damaged by sodium iodate can be efficiently regenerated in MRL/MpJ but not AKR/J mice.
Figure 6.
Regeneration of RPE at 4 weeks after sodium iodate injury. Fluorescence micrographs of posterior eye whole mounts of AKR/J (A, superior; B, central) and MRL/MpJ (C, superior; D, central) were stained with rhodamine-phalloidin. (E-H) Higher magnification views of the corresponding boxed areas. RPE cells appear only in the peripheral region of AKR/J eyes and are irregularly-shaped, and F-actin staining typical of fibroblasts (arrowheads) is evident at the lesion boundaries. Normal polygonal RPE cells appear throughout MRL/MpJ eyes and fibroblasts are absent. ONH: optic nerve head. Scale bars: A-D, 100 μm; E-H, 25 μm.
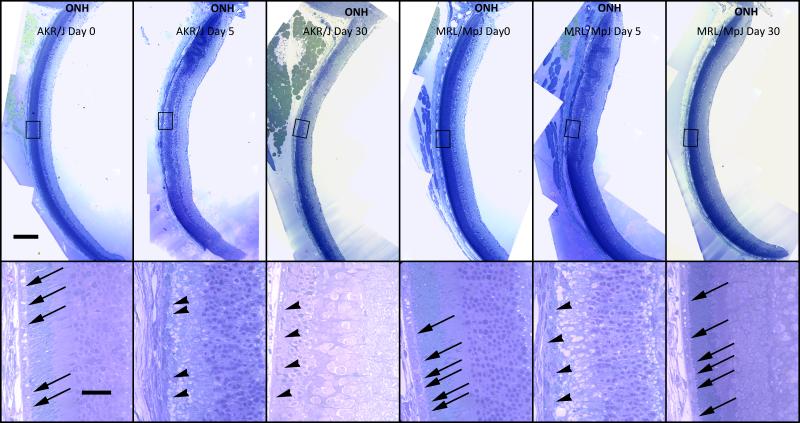
3.7. Confirmation of Morphological changes in plastic embedded sections
To confirm the post injury morphological changes, ultra-thin plastic embedded sections were prepared at day 0, 5 and 30 post-injection. The images revealed severe central RPE damage at 5 days post injection in both MRL/MpJ and AKR/J mice (Fig. 7, arrowheads). At 30 days post injection, the typical structure of the RPE layer was restored in MRL/MpJ mice but not in AKR/J mice (Fig. 7, arrows). At 30 days, the outer nuclear layer and photoreceptor inner and outer segment are largely preserved in MRL/MpJ mice consistent with a restoration of RPE function. These results further confirm RPE65 and phalloidin staining result suggesting that the MRL/MpJ mice are able to regenerate RPE efficiently after sodium iodate injury.
Figure 7.
Structural restoration of RPE cell layer. Overall (upper panel) and magnified (lower panel) bright field images of plastic-embedded eyecups show morphological changes in the posterior eyecup after sodium iodate injury. Normal RPE cells at 0 days post-injection (arrows) were replaced with irregularly-shaped and detached cells (arrowheads) at 5 days in both MRL/MpJ and AKR/J mice. In MRL/MpJ mice, the damaged RPE layer was restored and normal RPE cells were observed in the subretinal space at 30 days post-injection. By contrast, in AKR/J mice at 30 days, the morphology of the RPE and photoreceptor layers deteriorated further than at 5 days post-injection and no normal RPE cells were detectable. Scale bars: upper panel 200 μm; lower panel 50 μm. ONH: optic nerve head.
3.8. Cell proliferation
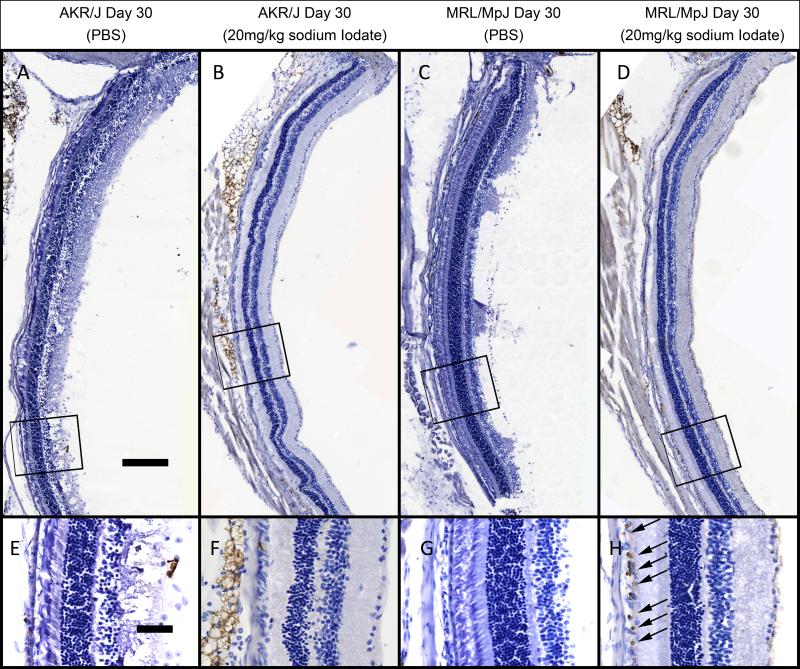
To test if cell proliferation contributes to the observed RPE regeneration, BrdU was injected daily following sodium iodate treatment. At 30 days post injection, many BrdU positive cells were observed in the subretinal space of sodium iodate treated MRL/MpJ mice (Fig. 8 (D) and (H)) but only sporadically in AKR/J mice (Fig. 8 (B) and (F)). Some of these cells have large nuclei and located in a monolayer above Bruch’s membrane, suggesting they are RPE cells. BrdU incorporation was not observed in uninjured mice (Fig. 8 (A), (B), (E) and (F)) indicating that incorporation reflects a post injury proliferation. These results suggest that after injury, there are proliferating cells in the subretinal space in MRL/MpJ mice but not AKR/J mice that may contribute to the regeneration of the RPE.
Figure 8.
Observation of cell proliferation by BrdU staining. Bright field pictures were captured for the eye sections from AKR/J treated with 20mg/kg sodium iodate (B and F) and PBS (A and E), MRL/MpJ treated with 20mg/kg sodium iodate (D and H) and PBS (C and G). Higher magnification views of corresponding boxed areas were displayed in the lower panel. ONH: optic nerve head. BrdU positive cells (arrows) in the subretinal space were only observed in the MRL/MpJ mice 30 days post sodium iodate treatment. Scale bars: A-D, 200 μm; E-H, 50 μm.
4. Discussion
The goal of the current work was to identify a mouse model with robust regeneration of RPE damage for use in future studies to elucidate the underlying mechanisms of regeneration. Our analysis indicates that RPE regeneration after sodium iodate injury is significantly enhanced in MRL/MpJ mice compared to AKR/J mice. We identified a sodium iodate dose of 20 mg/kg under which a clear difference in recovery was observed between these strains. At this dose, the ERG response at 60 days after damage in MRL/MpJ recovered beyond pretreatment amplitudes, consistent with a more than sufficient restoration of RPE function, which is essential for photoreceptor viability and activity (Strauss, 2005). By monitoring tissue histology and morphology as a function of time after sodium iodate injury, we demonstrated that the RPE of both strains undergoes similar cellular damage after injury, but only MRL/MpJ recovers significantly. BrdU incorporation studies indicate that MRL/MpJ recovery correlates with an increased labeling of subretinal nuclei, consistent with a contribution of cell division to regeneration in this MRL/MpJ tissue. The loss and subsequent recovery of RPE function and structure in MRL/MpJ mice satisfies the criteria of a regenerative process (Gurtner et al., 2007).
A modest level of sodium iodate-induced injury appears to be critical for detecting RPE regeneration in rodents. Although published studies are difficult to compare because of differences in the sodium iodate dose, mode of administration, age of animals and time of analysis after injection, major trends can be identified. Complete RPE ablation in mice is often achieved with sodium iodate at 40 – 100 mg/kg body weight injected intravenously or intraperitoneally. Under these conditions, little (Mizota and Adachi-Usami, 1997) to no (Enzmann et al., 2006; Machalinska et al., 2011; Redfern et al., 2011) regeneration has been found. We observed a similarly low level of regeneration in MRL/MpJ and control mice, including pigmented strains, with retro-orbital sodium iodate at 40 mg/kg body weight, in agreement with our previous work (Harris et al., 2006; Harris et al., 2009). By contrast, significant regeneration has been suggested to occur at lower doses. For example, intravenous sodium iodate in C57BL/6 mice at 15 and 25 mg/kg body weight caused a dip and subsequent partial recovery of visual function as measured by a sensitive optomotor kinetic reflex assay, possibly indicating RPE regeneration (Franco et al., 2009). Consistent with these studies, we found robust regeneration of MRL/MpJ RPE with retro-orbital sodium iodate at 20 mg/kg body weight. The importance of a lower dose may reflect a need to preserve adult RPE cells or tissue-resident stem cells that repopulate the damaged tissue. Alternatively, high doses may damage circulating stem cells required for regeneration, as suggested previously (Harris et al., 2006); alter the tissue microenvironment so that it no longer supports RPE regeneration; or induce secondary damage due to inflammatory responses, as suggested as an explanation for RPE cell loss in a genetic ablation model (Longbottom et al., 2009). The observed dependence of regeneration on the severity of sodium iodate injury fits with the variable outcomes of tissue regeneration in MRL/MpJ mice (Abdullah et al., 2005; Chadwick et al., 2007; Cimini et al., 2008; Davis et al., 2007; Fitzgerald et al., 2008; Gourevitch et al., 2009; Grisel et al., 2008; Leferovich et al., 2001; Naseem et al., 2007; Oh et al., 2004; Robey and Murry, 2008; Tolba et al., 2010).
Unlike reports of patchy RPE loss in response to a low sodium iodate dose (Enzmann et al., 2006; Franco et al., 2009), our results indicate contiguous damage of the central posterior RPE with preservation of the periphery. We did not observe patches of RPE cell loss in the strains we examined, possibly because we avoided damage to the RPE by examining whole eyecup preparations in which the retina remained intact. In earlier attempts where the retina was removed, we found that adherence of the RPE and retina introduced artifacts that made it difficult to interpret changes in RPE morphology, especially in sodium iodate-injured eyes. The loss of central RPE with peripheral preservation with low sodium iodate doses has also been reported in C57BL/6 mice (Machalinska et al., 2011) and rabbits (Korte et al., 1994). Greater central damage has also been noted at higher sodium iodate doses in mice (Mizota and Adachi-Usami, 1997; Redfern et al., 2011) and rats (Mizota and Adachi-Usami, 1997), and is supported by fluorescein angiography in rabbits and monkeys, which reveal a sodium iodate-induced breakdown of RPE barrier function in the central eye early after injury (Ringvold et al., 1981). Since major blood vessels of the choroidal and retinal circulation enter and exit the central eye at the optic nerve and have their largest diameter there, we speculate that delivery of and damage from sodium iodate is greatest in this region. However, it is also possible that damage is uniform throughout the eye, but peripheral regions are repaired more rapidly due to the presence of progenitor or stem cells in these regions (von Leithner et al., 2010).
Features of the transition zone between normal and atrophic RPE in MRL/MpJ and AKR/J mice reveal a similar initial response to sodium iodate injury in both strains. Flower-like cell shedding structures in the transition zone have previously been observed in chick embryonic RPE (Nagai and Kalnins, 1996), in transgenic mice following genetic ablation of the RPE (Longbottom et al., 2009), and in cell culture models of epithelial shedding (Florian et al., 2002; Rosenblatt et al., 2001). RPE shedding structures are associated with apical ejection of a central dead or dying RPE cell (Nagai and Kalnins, 1996). Enlarged cells with irregular borders at the central edge of the transition zone may be similar to those reported at the boundary of RPE atrophy in retinal cross sections and in RPE/choroid/sclera flat mounts of sodium iodate-injured mouse and rabbit eyes (Kiuchi et al., 2002; Korte et al., 1995). These observations lead us to propose an initial injury response in which dead or dying RPE cells are shed by a “purse string” closure process characteristic of epithelial monolayers (Garcia-Fernandez et al., 2009). Neighboring cells rearrange to cover the area occupied by the shed cells, as suggested in a genetic RPE ablation model (Longbottom et al., 2009), resulting in enlarged cells with irregular boundaries. In the face of continued atrophy, RPE cells at the edge of the transition zone dedifferentiate, losing their characteristic polygonal shape and RPE65 expression. Further studies may reveal whether the RPE is repopulated in sodium iodate-injured MRL/MpJ mice by proliferation of cells at the central edge of the transition zone.
The establishment of enhanced RPE regeneration in MRL/MpJ mice is an important first step towards identifying factors that may improve regenerative approaches for age-related macular degeneration and related diseases. MRL/MpJ mice are not universal “healer” mice as they were originally nicknamed (Abdullah et al., 2005; Cimini et al., 2008; Davis et al., 2007; Grisel et al., 2008; Oh et al., 2004; Robey and Murry, 2008), and even the original ear-punch regeneration phenotype has been observed at much lower levels in other strains, including C57BL/6 (Costa et al., 2009; Reines et al., 2009). Nevertheless, there is general agreement that tissue regeneration is most robust in MRL/MpJ strain, making it the most experimentally tractable for studying this process. In many ways the fact that MRL/MpJ mice are nearly normal makes them a potentially more relevant model for regenerative studies, as long as the tissue of interest is repaired in this background. Our results clearly show full restoration of ERG function in MRL/MpJ mice following acute sodium iodate injury of the RPE.
Recent studies of this strain suggest several mechanisms for how enhanced RPE regeneration can occur. Circulating stem cells may be superior in MRL/MpJ mice, as suggested in a study of a mouse myocardial injury and recovery model (Alfaro et al., 2008). Alternatively, the peripheral region of the posterior eye, which has been suggested to contain stem or progenitor cells (von Leithner et al., 2010), may be more proliferative in MRL/MpJ mice than in AKR/J mice. Finally, a less destructive inflammatory response in MRL/MpJ mice (Heber-Katz and Gourevitch, 2009; Li et al., 2001b) may promote the engraftment and regeneration of RPE cells or the proliferation of other tissue-derived cells. Studies in MRL/MpJ mice and other animal models have indicated that the status of systemic inflammatory factors and the local balance of pro- and anti-inflammatory cytokines profile are critical in determining whether a wound heals with or without a scar (Anam et al., 2009; Eming et al., 2009; Li et al., 2001b; Zins et al., 2010). Whichever hypothesis proves correct, if the mechanism of enhanced RPE regeneration in the MRL/MpJ mice can be clarified, it will be useful for developing therapies for clinical recovery from RPE damage and loss. The simple fact that RPE regeneration occurs robustly in MRL/MpJ mice may help efforts to identify the cell sources that effect RPE repair in a mammalian system. Studies from our own and other laboratories have shown that HSC derived donor cells can form cells within the RPE layer that are morphologically indistinguishable from native RPE (Carr et al., 2009; Harris et al., 2006; Harris et al., 2009; Idelson et al., 2009; Uygun et al., 2009; Vossmerbaeumer et al., 2008). However, only low levels of donor cell incorporation were observed and it was challenging to confirm that these cells were fully functional. The extensive RPE regeneration in our MRL/MpJ mouse model may permit more robust incorporation of donor cells and facilitate functional testing of these populations within the RPE layer.
Highlights.
Stem cells can replace RPE at a low rate.
We show MRL mice fully regenerate RPE after damage.
A much needed model system.
Acknowledgment
The authors wish to sincerely thank Dr. Liya Pi for project and manuscript discussion, Marda Jorgensen for her suggestions on immunostaining and Hong Li for her help on plastic embedding sectioning.
Grant support: This study is supported by National Institutes of Health grant EY018158 awarded to EWS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdullah I, Lepore JJ, Epstein JA, Parmacek MS, Gruber PJ. MRL mice fail to heal the heart in response to ischemia-reperfusion injury. Wound Repair Regen. 2005;13:205–208. doi: 10.1111/j.1067-1927.2005.130212.x. [DOI] [PubMed] [Google Scholar]
- Al-Hussaini H, Kam JH, Vugler A, Semo M, Jeffery G. Mature retinal pigment epithelium cells are retained in the cell cycle and proliferate in vivo. Molecular vision. 2008;14:1784–1791. [PMC free article] [PubMed] [Google Scholar]
- Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, Davidson JM, Rottman J, Lee E, Young PP. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:18366–18371. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anam K, Amare M, Naik S, Szabo KA, Davis TA. Severe tissue trauma triggers the autoimmune state systemic lupus erythematosus in the MRL/++ lupus-prone mouse. Lupus. 2009;18:318–331. doi: 10.1177/0961203308097479. [DOI] [PubMed] [Google Scholar]
- Bedelbaeva K, Snyder A, Gourevitch D, Clark L, Zhang XM, Leferovich J, Cheverud JM, Lieberman P, Heber-Katz E. Lack of p21 expression links cell cycle control and appendage regeneration in mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:5845–5850. doi: 10.1073/pnas.1000830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, Roanowska M, Wess T. Ageing of the retinal pigment epithelium: implications for transplantation. Graefes Arch Clin Exp Ophthalmol. 2004;242:76–84. doi: 10.1007/s00417-003-0812-8. [DOI] [PubMed] [Google Scholar]
- Caldwell RL, Opalenik SR, Davidson JM, Caprioli RM, Nanney LB. Tissue profiling MALDI mass spectrometry reveals prominent calcium-binding proteins in the proteome of regenerative MRL mouse wounds. Wound Repair Regen. 2008;16:442–449. doi: 10.1111/j.1524-475X.2007.00351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr AJ, Vugler AA, Hikita ST, Lawrence JM, Gias C, Chen LL, Buchholz DE, Ahmado A, Semo M, Smart MJ, Hasan S, da Cruz L, Johnson LV, Clegg DO, Coffey PJ. Protective effects of human iPS-derived retinal pigment epithelium cell transplantation in the retinal dystrophic rat. PloS one. 2009;4:e8152. doi: 10.1371/journal.pone.0008152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick RB, Bu L, Yu H, Hu Y, Wergedal JE, Mohan S, Baylink DJ. Digit tip regrowth and differential gene expression in MRL/Mpj, DBA/2, and C57BL/6 mice. Wound Repair Regen. 2007;15:275–284. doi: 10.1111/j.1524-475X.2007.00216.x. [DOI] [PubMed] [Google Scholar]
- Cimini M, Fazel S, Fujii H, Zhou S, Tang G, Weisel RD, Li RK. The MRL mouse heart does not recover ventricular function after a myocardial infarction. Cardiovasc Pathol. 2008;17:32–39. doi: 10.1016/j.carpath.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Clark LD, Clark RK, Heber-Katz E. A new murine model for mammalian wound repair and regeneration. Clin Immunol Immunopathol. 1998;88:35–45. doi: 10.1006/clin.1998.4519. [DOI] [PubMed] [Google Scholar]
- Costa RA, Ruiz-de-Souza V, Azevedo GM, Jr, Vaz NM, Carvalho CR. Effects of strain and age on ear wound healing and regeneration in mice. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas / Sociedade Brasileira de Biofisica … [et al. 2009;42:1143–1149. doi: 10.1590/s0100-879x2009005000042. [DOI] [PubMed] [Google Scholar]
- Davis TA, Amare M, Naik S, Kovalchuk AL, Tadaki D. Differential cutaneous wound healing in thermally injured MRL/MPJ mice. Wound Repair Regen. 2007;15:577–588. doi: 10.1111/j.1524-475X.2007.00266.x. [DOI] [PubMed] [Google Scholar]
- Eming SA, Hammerschmidt M, Krieg T, Roers A. Interrelation of immunity and tissue repair or regeneration. Seminars in cell & developmental biology. 2009;20:517–527. doi: 10.1016/j.semcdb.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Enzmann V, Row BW, Yamauchi Y, Kheirandish L, Gozal D, Kaplan HJ, McCall MA. Behavioral and anatomical abnormalities in a sodium iodate-induced model of retinal pigment epithelium degeneration. Exp Eye Res. 2006;82:441–448. doi: 10.1016/j.exer.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthritis Cartilage. 2008;16:1319–1326. doi: 10.1016/j.joca.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Florian P, Schoneberg T, Schulzke JD, Fromm M, Gitter AH. Single-cell epithelial defects close rapidly by an actinomyosin purse string mechanism with functional tight junctions. The Journal of physiology. 2002;545:485–499. doi: 10.1113/jphysiol.2002.031161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco LM, Zulliger R, Wolf-Schnurrbusch UE, Katagiri Y, Kaplan HJ, Wolf S, Enzmann V. Decreased visual function after patchy loss of retinal pigment epithelium induced by low-dose sodium iodate. Invest Ophthalmol Vis Sci. 2009;50:4004–4010. doi: 10.1167/iovs.08-2898. [DOI] [PubMed] [Google Scholar]
- Garcia-Fernandez B, Campos I, Geiger J, Santos AC, Jacinto A. Epithelial resealing. The International journal of developmental biology. 2009;53:1549–1556. doi: 10.1387/ijdb.072308bg. [DOI] [PubMed] [Google Scholar]
- Gourevitch DL, Clark L, Bedelbaeva K, Leferovich J, Heber-Katz E. Dynamic changes after murine digit amputation: the MRL mouse digit shows waves of tissue remodeling, growth, and apoptosis. Wound Repair Regen. 2009;17:447–455. doi: 10.1111/j.1524-475X.2009.00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel P, Meinhardt A, Lehr HA, Kappenberger L, Barrandon Y, Vassalli G. The MRL mouse repairs both cryogenic and ischemic myocardial infarcts with scar. Cardiovasc Pathol. 2008;17:14–22. doi: 10.1016/j.carpath.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Gurley KA, Alvarado AS. Stem cells in animal models of regeneration. 2008. [PubMed]
- Gurtner GC, Callaghan MJ, Longaker MT. Progress and potential for regenerative medicine. Annual review of medicine. 2007;58:299–312. doi: 10.1146/annurev.med.58.082405.095329. [DOI] [PubMed] [Google Scholar]
- Hamel CP, Tsilou E, Pfeffer BA, Hooks JJ, Detrick B, Redmond TM. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-transcriptionally regulated in vitro. The Journal of biological chemistry. 1993;268:15751–15757. [PubMed] [Google Scholar]
- Harris JR, Brown GA, Jorgensen M, Kaushal S, Ellis EA, Grant MB, Scott EW. Bone marrow-derived cells home to and regenerate retinal pigment epithelium after injury. Investigative ophthalmology & visual science. 2006;47:2108–2113. doi: 10.1167/iovs.05-0928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR, Fisher R, Jorgensen M, Kaushal S, Scott EW. CD133 progenitor cells from the bone marrow contribute to retinal pigment epithelium repair. Stem cells (Dayton, Ohio) 2009;27:457–466. doi: 10.1634/stemcells.2008-0836. [DOI] [PubMed] [Google Scholar]
- Heber-Katz E, Gourevitch D. The relationship between inflammation and regeneration in the MRL mouse: potential relevance for putative human regenerative(scarless wound healing) capacities? Ann N Y Acad Sci. 2009;1172:110–114. doi: 10.1111/j.1749-6632.2009.04499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idelson M, Alper R, Obolensky A, Ben-Shushan E, Hemo I, Yachimovich-Cohen N, Khaner H, Smith Y, Wiser O, Gropp M, Cohen MA, Even-Ram S, Berman-Zaken Y, Matzrafi L, Rechavi G, Banin E, Reubinoff B. Directed differentiation of human embryonic stem cells into functional retinal pigment epithelium cells. Cell stem cell. 2009;5:396–408. doi: 10.1016/j.stem.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Kawakami A. Stem cell system in tissue regeneration in fish. Development, growth & differentiation. 2010;52:77–87. doi: 10.1111/j.1440-169X.2009.01138.x. [DOI] [PubMed] [Google Scholar]
- Kiuchi K, Yoshizawa K, Shikata N, Moriguchi K, Tsubura A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Current eye research. 2002;25:373–379. doi: 10.1076/ceyr.25.6.373.14227. [DOI] [PubMed] [Google Scholar]
- Korte GE, Mrowiec E, Landzberg KS, Youssri A. Reorganization of actin microfilaments and microtubules in regenerating retinal pigment epithelium. Experimental eye research. 1995;61:189–203. doi: 10.1016/s0014-4835(05)80039-9. [DOI] [PubMed] [Google Scholar]
- Korte GE, Perlman JI, Pollack A. Regeneration of mammalian retinal pigment epithelium. International review of cytology. 1994;152:223–263. doi: 10.1016/s0074-7696(08)62558-9. [DOI] [PubMed] [Google Scholar]
- Lee E, Maclaren RE. Sources of RPE for replacement therapy. Br J Ophthalmol. 2010 doi: 10.1136/bjo.2009.171918. [DOI] [PubMed] [Google Scholar]
- Leferovich JM, Bedelbaeva K, Samulewicz S, Zhang XM, Zwas D, Lankford EB, Heber-Katz E. Heart regeneration in adult MRL mice. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:9830–9835. doi: 10.1073/pnas.181329398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Timmers AM, Hunter K, Gonzalez-Pola C, Lewin AS, Reitze DH, Hauswirth WW. Noninvasive imaging by optical coherence tomography to monitor retinal degeneration in the mouse. Investigative ophthalmology & visual science. 2001a;42:2981–2989. [PubMed] [Google Scholar]
- Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mamm Genome. 2001b;12:52–59. doi: 10.1007/s003350010230. [DOI] [PubMed] [Google Scholar]
- Longbottom R, Fruttiger M, Douglas RH, Martinez-Barbera JP, Greenwood J, Moss SE. Genetic ablation of retinal pigment epithelial cells reveals the adaptive response of the epithelium and impact on photoreceptors. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18728–18733. doi: 10.1073/pnas.0902593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machalinska A, Lubinski W, Klos P, Kawa M, Baumert B, Penkala K, Grzegrzolka R, Karczewicz D, Wiszniewska B, Machalinski B. Sodium iodate selectively injuries the posterior pole of the retina in a dose-dependent manner: morphological and electrophysiological study. Neurochemical research. 2011;35:1819–1827. doi: 10.1007/s11064-010-0248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizota A, Adachi-Usami E. Functional recovery of retina after sodium iodate injection in mice. Vision research. 1997;37:1859–1865. doi: 10.1016/s0042-6989(97)00015-1. [DOI] [PubMed] [Google Scholar]
- Nagai H, Kalnins VI. Normally occurring loss of single cells and repair of resulting defects in retinal pigment epithelium in situ. Experimental eye research. 1996;62:55–61. doi: 10.1006/exer.1996.0007. [DOI] [PubMed] [Google Scholar]
- Naseem RH, Meeson AP, Dimaio J. Michael, White MD, Kallhoff J, Humphries C, Goetsch SC, De Windt LJ, Williams MA, Garry MG, Garry DJ. Reparative myocardial mechanisms in adult C57BL/6 and MRL mice following injury. Physiological genomics. 2007;30:44–52. doi: 10.1152/physiolgenomics.00070.2006. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, Le TP, Bedelbaeva K, Leferovich J, Gourevitch D, Sachadyn P, Zhang XM, Clark L, Heber-Katz E. Retained features of embryonic metabolism in the adult MRL mouse. Mol Genet Metab. 2009;96:133–144. doi: 10.1016/j.ymgme.2008.11.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh YS, Thomson LE, Fishbein MC, Berman DS, Sharifi B, Chen PS. Scar formation after ischemic myocardial injury in MRL mice. Cardiovasc Pathol. 2004;13:203–206. doi: 10.1016/j.carpath.2004.03.610. [DOI] [PubMed] [Google Scholar]
- Redfern WS, Storey S, Tse K, Hussain Q, Maung KP, Valentin JP, Ahmed G, Bigley A, Heathcote D, McKay JS. Evaluation of a convenient method of assessing rodent visual function in safety pharmacology studies: effects of sodium iodate on visual acuity and retinal morphology in albino and pigmented rats and mice. Journal of pharmacological and toxicological methods. 2011;63:102–114. doi: 10.1016/j.vascn.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Reines B, Cheng LI, Matzinger P. Unexpected regeneration in middle-aged mice. Rejuvenation research. 2009;12:45–52. doi: 10.1089/rej.2008.0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringvold A, Olsen EG, Flage T. Transient breakdown of the retinal pigment epithelium diffusion barrier after sodium iodate: a fluorescein angiographic and morphological study in the rabbit. Experimental Eye Research. 1981;33:361–369. doi: 10.1016/s0014-4835(81)80088-7. [DOI] [PubMed] [Google Scholar]
- Robey TE, Murry CE. Absence of regeneration in the MRL/MpJ mouse heart following infarction or cryoinjury. Cardiovasc Pathol. 2008;17:6–13. doi: 10.1016/j.carpath.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Curr Biol. 2001;11:1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Seagle BL, Gasyna EM, Mieler WF, Norris JR., Jr. Photoprotection of human retinal pigment epithelium cells against blue light-induced apoptosis by melanin free radicals from Sepia officinalis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:16644–16648. doi: 10.1073/pnas.0605986103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiological reviews. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Swaroop A, Chew EY, Rickman CB, Abecasis GR. Unraveling a multifactorial late-onset disease: from genetic susceptibility to disease mechanisms for age-related macular degeneration. Annual review of genomics and human genetics. 2009;10:19–43. doi: 10.1146/annurev.genom.9.081307.164350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevenaz P, Unser M. User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microscopy research and technique. 2007;70:135–146. doi: 10.1002/jemt.20393. [DOI] [PubMed] [Google Scholar]
- Tolba RH, Schildberg FA, Decker D, Abdullah Z, Buttner R, Minor T, von Ruecker A. Mechanisms of improved wound healing in Murphy Roths Large (MRL) mice after skin transplantation. Wound Repair Regen. 2010;18:662–670. doi: 10.1111/j.1524-475X.2010.00631.x. [DOI] [PubMed] [Google Scholar]
- Tucker B, Klassen H, Yang L, Chen DF, Young MJ. Elevated MMP Expression in the MRL Mouse Retina Creates a Permissive Environment for Retinal Regeneration. Invest Ophthalmol Vis Sci. 2008;49:1686–1695. doi: 10.1167/iovs.07-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno M, Lyons BL, Burzenski LM, Gott B, Shaffer DJ, Roopenian DC, Shultz LD. Accelerated wound healing of alkali-burned corneas in MRL mice is associated with a reduced inflammatory signature. Invest Ophthalmol Vis Sci. 2005;46:4097–4106. doi: 10.1167/iovs.05-0548. [DOI] [PubMed] [Google Scholar]
- Uygun BE, Sharma N, Yarmush M. Retinal pigment epithelium differentiation of stem cells: current status and challenges. Critical reviews in biomedical engineering. 2009;37:355–375. doi: 10.1615/critrevbiomedeng.v37.i4-5.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Leithner PL, Ciurtin C, Jeffery G. Microscopic mammalian retinal pigment epithelium lesions induce widespread proliferation with differences in magnitude between center and periphery. Molecular vision. 2010;16:570–581. [PMC free article] [PubMed] [Google Scholar]
- Vossmerbaeumer U, Kuehl S, Kern S, Kluter H, Jonas JB, Bieback K. Induction of retinal pigment epithelium properties in ciliary margin progenitor cells. Clinical & experimental ophthalmology. 2008;36:358–366. doi: 10.1111/j.1442-9071.2008.01770.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Shi Y, Ding S. A chemical approach to stem-cell biology and regenerative medicine. Nature. 2008;453:338–344. doi: 10.1038/nature07042. [DOI] [PubMed] [Google Scholar]
- Zins SR, Amare MF, Anam K, Elster EA, Davis TA. Wound trauma mediated inflammatory signaling attenuates a tissue regenerative response in MRL/MpJ mice. Journal of inflammation (London, England) 2010;7:25. doi: 10.1186/1476-9255-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]