Abstract
Diabetes mellitus causes cerebral microvasculature deterioration and cognitive decline. The specialized endothelial cells of cerebral microvasculature comprise the blood-brain barrier, and the pericytes (PC) that are in immediate contact with these endothelial cells are vital for blood-brain barrier integrity. In diabetes, increased mitochondrial oxidative stress is implicated as a mechanism for hyperglycemia-induced PC loss as a prerequisite leading to blood-brain barrier disruption. Mitochondrial carbonic anhydrases (CA) regulate the oxidative metabolism of glucose and thus play an important role in the generation of reactive oxygen species and oxidative stress. We hypothesize that the inhibition of mitochondrial CA would reduce mitochondrial oxidative stress, rescue cerebral PC loss caused by diabetes-induced oxidative stress, and preserve blood-brain barrier integrity. We studied the effects of pharmacological inhibition of mitochondrial CA activity on streptozotocin-diabetes-induced oxidative stress and PC loss in the mouse brain. At 3 wk of diabetes, there was significant oxidative stress; the levels of reduced glutathione were lower and those of 3-nitrotyrosine, 4-hydroxy-2-trans-nonenal, and superoxide dismutase were higher. Treatment of diabetic mice with topiramate, a potent mitochondrial CA inhibitor, prevented the oxidative stress caused by 3 wk of diabetes. A significant decline in cerebral PC numbers, at 12 wk of diabetes, was also rescued by topiramate treatment. These results provide the first evidence that inhibition of mitochondrial CA activity reduces diabetes-induced oxidative stress in the mouse brain and rescues cerebral PC dropout. Thus, mitochondrial CA may provide a new therapeutic target for oxidative stress related illnesses of the central nervous system.
Diabetes mellitus leads to brain microvasculature dysfunction, disruption of the blood-brain barrier (1), and decline in cognitive function (2, 3). Although the blood-brain barrier is made up of specialized endothelial cells (EC), the pericytes (PC) in immediate contact with the EC regulate the viability and function of the barrier (4–6). In the retina, an extension of the central nervous system, a decline in the PC to EC ratio is a sign of the microvascular degeneration of the blood-retinal barrier that leads to diabetic retinopathy (7). We propose that a similar decline in the number of cerebral PC is responsible for the diabetes-induced pathological changes in the brain.
The mechanisms by which hyperglycemia leads to PC loss are imperfectly understood. However, mitochondrial oxidative stress is a common mediator of all of the hyperglycemia-induced pathways (increased polyol pathway, production of advanced glycation end-products formation, activation of protein kinase C, increased hexosamine pathway flux, and enhanced glucose metabolism via oxidative metabolism) involved in the pathology of diabetes (8, 9). As one of the most metabolically active tissues in the body, the brain is particularly vulnerable to oxidative stress; therefore, reducing oxidative stress may protect the brain from the damage caused by hyperglycemia.
A mechanism for hyperglycemia-induced oxidative stress is overproduction of reactive oxygen species (ROS) (10). ROS are a normal byproduct (11, 12) of electron transport chain (ETC) reactions in the reduction of glucose to H2O and CO2 in the production of ATP. Overproduction of ROS causes oxidative stress. In diabetes, hyperglycemia shuttles more glucose to the Krebs cycle, in insulin-independent tissues such as brain (12), thus increasing the rate of production of electron donors (reduced flavin adenine dinucleotide and reduced nicotinamide adenine dinucleotide). These electron donors generate a proton gradient across the inner mitochondrial membrane during ETC reactions. When the electrochemical potential difference generated by the proton gradient is high, the lifetime of superoxide-generating electron-transport intermediates is prolonged (8). There seems to be a threshold value above which superoxide production is markedly increased (13). In cultured aortic EC, hyperglycemia increases the proton gradient above this threshold value as a result of overproduction of electron donors (13). This, in turn, causes a marked increase in the production of superoxide by these cells and oxidative stress.
We hypothesize that similar to EC, cerebral PC suffer higher oxidative stress in the hyperglycemia of diabetes. We proposed to reduce the oxidative stress by limiting the production of superoxide with the inhibition of carbonic anhydrases (CA) found in the mitochondria. CA are Zn metalloenzymes that catalyze reversible hydration of CO2. Of the 16 known isozymes of CA, only two, CA VA and VB, are found in the mitochondria (14).
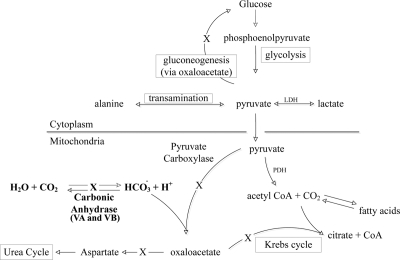
Mitochondrial CA regulate oxidative metabolism of glucose, production of ROS, and oxidative stress. As illustrated in Fig. 1, glucose is metabolized to pyruvate in the cytosol by glycolysis. Being permeable to mitochondrial membranes, pyruvate freely enters mitochondria, where it is carboxylated to oxaloacetate, a key intermediate in the Krebs cycle/ETC pathways (Fig. 1). The conversion of pyruvate to oxaloacetate requires bicarbonate (HCO3−). The mitochondrial membranes are impermeable to HCO3− (15–19); therefore, the latter must be produced inside the mitochondria. Mitochondrial CA produce HCO3− by the reaction CO2 + H2O ⇆ HCO3− + H+ inside the mitochondria. Inhibition of mitochondrial CA blocks the production of HCO3− and thus limits superoxide production and oxidative stress. Pyruvate is instead shuttled through anaerobic metabolism (aerobic glycolysis) to produce ATP without producing superoxide. Aerobic glycolysis is not harmful and indeed is preferred by fast growing cells (20).
Fig. 1.
Role of mitochondrial CA in oxidative metabolism of glucose. Glucose is metabolized to pyruvate by glycolysis, in the cytosol. Being permeable to the mitochondrial membranes, pyruvate freely enters the mitochondrial matrix, where it is converted to oxaloacetate. Oxaloacetate enters Krebs cycle/ETC reactions to produce ATP and superoxide. Conversion of pyruvate to oxaloacetate requires HCO3−. Mitochondrial membranes are impermeable to HCO3−; therefore, HCO3− cannot be imported from the cytosol and must be generated within the mitochondria. Two CA isozymes VA and VB (mitochondrial CA) that exist in the mitochondria generate HCO3− by reversible hydration of carbon dioxide by the reaction: CO2 + H2O⇆H+ + HCO3−. Absence/inhibition of mitochondrial CA slows down the production of HCO3− and thus the synthesis of oxaloacetate. This impairs the Krebs cycle, ETC, and ROS production. X, Impairment of various pathways upon mitochondrial CA blockage. LDH, Lactate dehydrogenase; CoA, coenzyme A.
We now report that streptozotocin (STZ)-diabetes led to oxidative stress and caused a decreased PC to EC ratio in the mouse brain. Inhibition of mitochondrial CA with topiramate prevented the oxidative stress in the brain and rescued the cerebral PC to EC ratio as well. These results provide the first evidence that inhibition of mitochondrial CA reduces oxidative stress in the mouse brain and rescues PC loss in STZ-diabetic mice.
Materials and Methods
Antibodies and reagents
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. The antihuman α-smooth muscle actin (α-SMA) antibody (MAB1420) was from R&D Systems (Minneapolis, MN). The antihuman FVIII antibody (MAB3440) and goat antimouse IgG conjugated with fluorescein isothiocyanate or Rhodamine Red were from Millipore (Billerica, MA). The anti-4-hydroxy-2-trans-nonenal (HNE) (HNE11-S) and antinitrotyrosine antibodies [3-nitrotyrosine (3-NT)] (AB5411) were from Alpha Diagnostics (San Antonio, TX) and Chemicon-Millipore, respectively. Antibodies against mouse mitochondrial CA VA and VB C-tail peptides were the same as previously described (21).
Animals and experimental protocols
Male CD-1 mice were from Charles River (Raleigh, NC) and C57BL/6 mitochondrial CA VA and VB double knockout (DKO) mice (Shah, G. N., T. S. Rubbelke, A. Waheed, W. S. Sly, manuscript in preparation) were from the in-house colonies of William S. Sly at Saint Louis University. Mice were maintained on ad libitum rodent chow diet and citrate water (1.68 mm sodium citrate and 1.57 mm potassium citrate, C7254 and C8385, respectively). Citrate water helps survival of STZ-diabetic mice. Mice were conducted under protocols approved by the Institutional Animal Care and Usage Committee of Saint Louis University.
Experiment 1
Eight-week-old male CD-1 mice were rendered diabetic by one-time iv tail-vein injection of freshly prepared 150 mg/kg STZ in vehicle [0.2 ml of 0.9% NaCl with 2 mg/ml citric acid (pH 4.5)] according to the standard method (22). Control mice received injections of vehicle. Diabetes onset was confirmed by blood glucose levels 48 h after STZ injections as were determined with a Glucometer (ReliOn, Arkray, MN) in the blood collected from the tail vein. Only animals with glucose levels more than or equal to 250 mg/dl were included in the study. Mice were divided in three groups of n = 10 in each group: nondiabetic, diabetic, and diabetic mice injected with topiramate (T0575). Topiramate was dissolved in dimethylsulfoxide at 1:4 (wt/vol) and diluted with saline before injection. Control mice were sham injected with dimethylsulfoxide diluted in saline. Two days after induction of diabetes, topiramate was administered by daily sc injections at 50 mg/kg. For analyses of parameters of oxidative stress, topiramate treatment was carried out for 3 wk. After treatment, mice were anesthetized with ip injections of urethane (4.0 g/kg), and blood was collected for assaying the clinical chemistry. The vascular spaces of the brains were washed free of their contents, and the brains were removed and stored in an antioxidant buffer [8.6 mm Na2HPO4, 26.6 mm NaH2PO4, 50 μm butylhydroxy-toluene, 10 mm aminotriazole, and 0.1 mm diethyltriaminepentaacetic acid] at −80 C until further analysis.
Experiment 2
Eight-week-old nondiabetic mitochondrial CA DKO mice and their littermate controls were anesthetized, the vascular spaces of the brains were washed free of their contents, and the brains were removed in antioxidant buffer for measurements of oxidative stress.
Experiment 3
For PC dropout studies, diabetic male CD-1 mice were treated with topiramate for 4, 8, and 12 wk and were separated into the same three groups as in experiment 1. At the end of each time point, mice were anesthetized, and the vascular spaces of the brains were washed free of their contents. The brains were removed and frozen immediately in liquid nitrogen. The brains were stored at −80 C until further use.
General parameters
At the completion of topiramate treatment, mice were weighed, and arterial blood samples (abdominal aorta) were freshly collected into heparin capillary tubes (Radiometer America, Inc., Louisville, KY). Serum creatinine, and electrolyte chemistries (Na, K, hemoglobin, hematocrit, total CO2 ((TCO2), blood urea nitrogen (BUN), and anion gap) were determined by an i-STAT Clinical Analyzer (Abbott Laboratories, Abbott Park, IL). Serum insulin levels were determined by rat/mouse insulin Elisa kit (EZRMI-13K; Millipore).
Throughout the study, blood glucose levels were monitored in the tail vein blood as described earlier.
Cells
Mouse cerebral PC were isolated according to the method of Hayashi et al. (23). Pure cultures of PC were obtained by prolonged culture of isolated brain microvessel fragments under selective culture conditions. The cerebral cortices from 8-wk-old CD-1 mice were cleaned of meninges and minced. The homogenate was digested with collagenase CLS2 (1 mg/ml; Worthington, Lakewood, NJ) and deoxyribonuclease I (37.5 μg/ml) in growth media (DMEM containing 100 U/ml penicillin, 100 μg/ml streptomycin, 50 mg/ml gentamicin, and 2 mm glutamine) at 37 C for 1.5 h. Neurons and glial cells were removed by centrifugation in 20% BSA-DMEM (1000 × g for 20 min). The microvessel pellets were further digested with collagenase/dispase (1 mg/ml; Roche, Mannheim, Germany) and deoxyribonuclease I (16.7 μg/ml) in DMEM at 37 C for 1 h. The microvessel fragments were washed twice in DMEM (first 1000 × g for 8 min, then 700 × g for 5 min) and placed in uncoated culture flasks in growth media at 37 C in a humidified atmosphere of 5% CO2/95% air. After 14 d in culture, PC reached 80–90% confluence. Positive staining for α-SMA identified the cultured cells as PC. Absence of EC was ascertained by negative staining for antihuman FVIII antibody. These cells were used to ascertain the presence of mitochondrial CA VA and VB by RT-PCR and immunoblot analysis.
Immunoblot analysis
Immunoblotting was performed by standard procedures. Briefly, whole brain and cultured PC were homogenized in lysis buffer [25 mm Tris (pH 7.5), 0.15 m NaCl, and 1 mm phenylmethylsulfonylfluoride], sonicated, and cleared by centrifugation. Protein concentration in the final supernatants was determined by BCA Protein Assay (Pierce, Rockford, IL). The proteins (50 μg) were separated on 4–12% Bis-Tris reducing gels (NuPAGE Novex; Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membranes. Polypeptides were identified by probing with mitochondrial CA VA and VB primary antibodies and horseradish peroxidase-conjugated secondary antibodies and visualized by chemiluminescent substrate (Pierce).
RT-PCR analysis
Total RNA was extracted from the whole brain and the primary cultured PC with RNeasy kit (QIAGEN, Valencia, CA). RNA (1 μg) was reverse transcribed with SuperScript III first Strand Synthesis System for RT-PCR (Invitrogen). The double-stranded DNA thus produced was used as template for PCR amplification of mitochondrial CA with specific primers for either CA VA or VB (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). The PCR conditions for mitochondrial CA VA were 10 cycles at 92 C for 15 sec, 63 C for 30 sec, 68 C for 1 min, and 20 cycles with 10 sec autoextension. The PCR conditions were the same for mitochondrial CA VB except that the annealing was at 60 C for 30 sec. The RT reactions without reverse transcriptase were used as negative controls.
Measurement of oxidative stress in the brain
Glutathione (GSH) determination
GSH concentrations in the brain were determined by reverse phase HPLC by the method of Winters et al. (24). The HPLC system (Thermo Electron Corp., Marietta, OH) consisted of a Finnigan Spectra System vacuum membrane degasser (model SCM1000), a gradient pump (model P2000), autosampler (model AS3000), and a fluorescence detector (model FL3000) with λex = 330 nm and λem = 376 nm. The HPLC column used was a Reliasil ODS-1 C18 column (5-μm packing material) with 250 mm × 4.6 mm internal diameter (Column Engineering, Ontario, CA). The mobile phase (70% acetonitrile and 30% water) was adjusted to a pH of 2.0 through the addition of 1 ml/liter of both acetic and o-phosphoric acids (Fisher Scientific, Fair Lawn, NJ). Brains were homogenized in a serine-borate buffer [100 mm Tris-HCl, 10 mm boric acid, 5 mm l-serine, and 1 mm diethyltriaminepentaacetic acid (pH 7.4)] on ice. Homogenates were derivatized with 1.0 mm N-(1-prenyl)-maleimide (NPM) in acetonitrile. HPLC grade water was added to each sample to make a volume of 250 μl, and 750 μl of NPM (1 mm in acetonitrile) were added. This mixture was incubated for 5 min at room temperature, and the reaction was stopped by adding 10 μl of 2 n HCl. The samples were then filtered through a 0.45-μm acrodisc filter (Advantec MFS, Inc., Dublin, CA) and injected into the HPLC system. The NPM derivatives were eluted from the column isocratically at a flow rate of 1 ml/min (24).
Superoxide dismutase (SOD) activity determination
The brain homogenates were centrifuged (10,000 × g, 10 min, 4 C), and cell-free supernatants were subjected to enzyme assays immediately. The SOD activity in extracts was determined by measuring the inhibition of cytochrome c reduction using the xanthine/xanthine oxidase O2•− generating system at 550 nm (25). One unit of SOD activity was defined as the amount of enzyme that inhibits the rate of cytochrome c reduction by 50% at 25 C. Specific activity was defined as the amount of the enzyme causing a half maximum inhibition of cytochrome c reduction and expressed as U/mg−1 protein.
Determination of lipid peroxidation and protein oxidation
Mice brains were homogenized in Triton X-100 lysis buffer [0.5% Triton X-100, 20 mm Tris (pH 7.4), 0.15 m NaCl, 2 mm EDTA, 1 mm EGTA, and a protease inhibitor cocktail (P2714)], centrifuged at 1000 × g for 10 min, and supernatants were agitated for 30 min. After centrifugation at 20,000 × g for 40 min, final supernatants were used for detection of HNE (byproduct of lipid peroxidation) and 3-NT (a marker of protein oxidation) by immunoblotting. The proteins (25 μg) were electrophoresed on 4–12% Bis-Tris reducing gels. After transfer, the nitrocellulose membranes (0.45 μm pore size) were incubated with anti-HNE or anti-3-NT antibodies (1:2000) overnight at 4 C and horseradish peroxidase-conjugated secondary antibodies (1:10,000) for 1 h at room temperature. The polypeptides were visualized by chemiluminescent substrate. The membranes were reprobed with anti-β-actin antibody to validate the loading efficiency, and the bands were quantified by ImageJ analysis software. Optical density values of individual HNE or 3-NT bands within each lane were added to obtain a total value.
In vivo PC loss
Mouse brains were harvested from diabetic mice at 4, 8, and 12 wk of topiramate treatment. The microvessels were isolated by a modified method of Gerhart et al. (26). Briefly, brains were collected and homogenized on ice in 1 ml of cold stock buffer [low glucose DMEM plus 25 mm HEPES and 1% dextran (pH 7.4)] in a glass tissue grinder with a Teflon pestle. The homogenate was filtered through a series of nylon mesh membranes (300 μm, then twice through 100 μm; Spectrum, Houston, TX), mixed with an equal volume of 40% dextran in stock buffer, and centrifuged at 3500 × g for 30 min at 4 C. The supernatant with the lipid layer was removed and the pellet resuspended in stock buffer. The suspension was passed through a 25-μm nylon mesh membrane (Bio-Design, Carmel, NY). The microvessels on the surface of the membrane were washed with the stock buffer four times, collected from the membrane, and then centrifuged at 3000 × g for 15 min at 4 C. The supernatant was discarded, and the microvessel pellet was resuspended in the incubation buffer. The purity and quantity of each preparation was routinely checked by light microscopy.
Periodic acid-Schiff (PAS)-hematoxylin staining was used to analyze PC dropout. Equal volumes of the isolated microvessels were put on two-well chamber slides and air dried. The microvessels were rinsed with PBS and fixed with 3% paraformaldehyde in PBS for 45 min at room temperature. The cells were washed twice with PBS and permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2 min on ice. The slides were washed twice with distilled water stained with PAS and hematoxylin and analyzed by light microscopy. Captured PAS-stained images of the microvessels were examined by a blinded observer to count the EC and PC nuclei in the same microscope field. Intraluminal cell nuclei with large oval shape were ascribed as EC. Typically, cell nuclei placed laterally on the capillary wall (between the two blades of microvascular basal lamina) with a small round shape are PC. Segmented nuclei or nuclei without contact with the basal lamina were excluded. Both PC and EC were counted in at least five random microscopic fields of 100 cells each. Results are presented as the percent of total cells that are PC.
Statistical analysis
All means are reported with their n and sem. Two means were compared by the unpaired two-tailed Student's t test. For more than two means, ANOVA, followed by Newman-Keuls multiple comparison test, was used. When there were two independent variables, a two-way ANOVA, followed by a multiple group comparisons test (Bonferroni), was used to assess statistical significance. P < 0.05 was considered significant. Statistical analyses were made using GraphPad Prism 5.0 package program (GraphPad Software, Inc., San Diego, CA).
Results
Mitochondrial CA in primary cultured PC
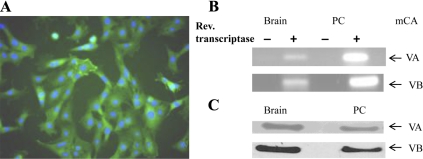
Our hypothesis states that oxidative stress caused by hyperglycemia leads to cerebral PC death, and this oxidative stress can be reduced by inhibition of mitochondrial CA. Because there are no published reports that cerebral PC contain mitochondrial CA, we ascertained their presence in the primary cultured PC by RT-PCR and immunoblotting. Our results confirm the presence of both CA VA and CA VB in the PC (Fig. 2). Using the same methods, we analyzed PC for CA II, a cytosolic; CA IV, a glycosylphopatidylinositol-anchored; and CA XII and CA XIV, two transmembrane CA. None of these CA were expressed in the PC (data not shown).
Fig. 2.
Mitochondrial CA (mCA) in the primary cultured PC derived from mouse brain microvessels. A, Cerebral PC stained for α-SMA are green, nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI) are blue. PC (1 × 104) were fixed on chamber slides with 4% paraformaldehyde in PBS, permeabilized, blocked with 0.3% Triton, 10% normal donkey serum (NDS) in PBS, and incubated with mouse antihuman α-SMA antibody at 10 μg/ml in 1% NDS in PBS. Secondary antibody was goat antimouse fluorescein isothiocyanate-conjugated IgG (1:200). After incubation and washing, slides were counterstained with DAPI (10 μg/ml) in mounting media. B, Transcripts of CA VA and VB in the PC and the brain. RNA isolated from PC was reversed transcribed and PCR amplified by mitochondrial CA VA-specific (top) and CA VB-specific (bottom) primers. Mouse brain RNA was used as a positive control. C, Mitochondrial CA VA and VB polypeptides in the PC and the brain. Proteins (50 μg) isolated from the PC were separated on polyacrylamide gels and probed with antimouse CA VA or VB antibody at 1:3000 dilution. Secondary antibody was goat antirabbit horseradish peroxidase (1:5000)-conjugated IgG. Proteins from the mouse brain were used as a positive control.
Effect of pharmacological inhibition of mitochondrial CA activity on oxidative stress in the mouse brain
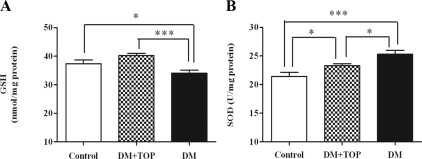
Mitochondrial CA inhibition was accomplished by sc injections of topiramate, a potent mitochondrial CA inhibitor. GSH is a sensitive indicator of oxidative stress, and GSH levels in the diabetic mice were significantly less (Fig. 3A) than the controls (34.1 ± 1.0 vs. 37.4 ± 1.3, P < 0.05), indicating oxidative stress. Topiramate treatment of diabetic mice significantly increased the levels of GSH in these mice (topiramate-treated diabetic, 40.2 ± 0.8 vs. diabetic, 34.1 ± 1.0; P < 0.001) compared with untreated diabetic mice.
Fig. 3.
Effect of topiramate treatment on diabetes-induced alterations in GSH levels and SOD activity in the mouse brain. Two days after induction of diabetes, mice (n = 10/group) were given daily sc injections of topiramate for 3 wk, and brains were analyzed for oxidative stress. GSH levels were determined by HPLC and SOD activity by measuring the inhibition of cytochrome c reduction using the xanthine/xanthine oxidase O2•− generating system. A, The significantly lower GSH levels in the diabetic animals (DM) were restored to control levels by topiramate treatment (DM + TOP). B, The SOD activity was significantly lower in the mice treated with topiramate compared with untreated diabetic mice. The values are expressed as mean ± sem (*, P < 0.05; ***, P < 0.001).
SOD plays an important role in detoxification of oxidative stress by scavenging superoxide radicals. The SOD activity (Fig. 3B) in the diabetic mice was significantly higher than that of the control mice (25.30 ± 0.72 vs. 21.45 ± 0.68, P < 0.001). In topiramate-treated diabetic mice, SOD activity was significantly less than that of the untreated diabetic mice (topiramate-treated diabetic, 23.29 ± 0.37 vs. diabetic, 25.30 ± 0.72; P < 0.05).
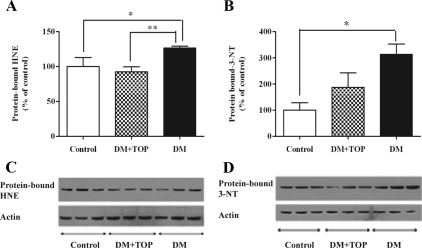
Lipid peroxidation, another measure of oxidative stress, can be assessed by the levels of protein-bound HNE, a stable byproduct of lipid peroxidation. Higher levels of HNE signify oxidative stress. A significant elevation in HNE-bound proteins was observed in the diabetic mice (126.2 ± 2.7 vs. 100 ± 12.72, P < 0.05), compared with control mice (Fig. 4A). Topiramate treatment significantly reduced the protein bound HNE (topiramate-treated diabetic, 92.15 ± 7.3 vs. diabetic, 126.2 ± 2.7; P < 0.01) in diabetic mice.
Fig. 4.
Effect of topiramate treatment on diabetes-induced lipid peroxidation and protein oxidation in the mouse brain. Two days after induction of diabetes, mice (n = 10/group) were given daily sc injections of topiramate for 3 wk, and brains were analyzed for HNE and 3-NT by immunoblot analysis. The significantly high levels of HNE (A) and 3-NT (B) in the diabetic (DM) mice were reversed in topiramate-treated (DM + TOP) mice. C and D, Representative immunoblots of protein-bound HNE and 3-NT, respectively. The values are expressed as mean ± sem (*, P < 0.05; **, P < 0.01).
Elevated levels of 3-NT, a marker of protein oxidation, are another measure of oxidative stress. The levels of 3-NT were significantly higher (Fig. 4B) in diabetic mice (274.4 ± 12.7 vs. 100.0 ± 28.1, P < 0.05) compared with controls. In topiramate-treated diabetic mice, there was reduction in 3-NT levels compared with untreated diabetic mice (topiramate-treated diabetic, 186.5 ± 55.6 vs. diabetic, 274.4 ± 12.7; ns).
Serum chemistry and phenotype of diabetic and topiramate-treated diabetic mice.
To assess the possible side effects of topiramate, we determined the serum chemistry of the mice. Both diabetic and topiramate-treated diabetic mice had increased serum glucose, decreased serum sodium, and chloride. Diabetic mice also had lower serum potassium compared with controls. The control, diabetic, and topiramate-treated groups did not differ in BUN and creatinine nor in serum values for anion gap, hemoglobin, hematocrit, TCO2, or calcium (Table 1). Both diabetic and topiramate-diabetic mice had insulin levels below the lower detection limit of the assay. At 12 wk of diabetes, the untreated diabetic mice were cachectic, whereas the topiramate-treated diabetic mice were as healthy as the control mice (Fig. 5).
Table 1.
Body weight, blood glucose concentration, and serum chemistry in experimental mice (duration of diabetes is 3 wk)
| Control | DM | DM + TOP | |
|---|---|---|---|
| Body weight (g) | 39.0 ± 3.3 | 31.6 ± 2.2b | 29.4 ± 3.3b |
| Blood glucose (mg/dl) | 165 ± 53.3 | >250a | >250a |
| Na (mmol/liter) | 149 ± 2.1 | 142 ± 4.1a | 143 ± 4.4a |
| K (mmol/liter) | 8.1 ± 0.9 | 6.1 ± 2.6a | 7.1 ± 1.1 |
| Cl (mmol/liter) | 121 ± 2.3 | 111 ± 3.0a | 112 ± 4.4a |
| Ca+2 (mmol/liter) | 1.24 ± 0.1 | 1.23 ± 0.1 | 1.26 ± 0.1 |
| TCO2 (mmol/liter) | 15 ± 2.6 | 18 ± 3.4 | 16 ± 5.0 |
| BUN (mg/dl) | 21 ± 2.8 | 22 ± 7.6 | 24 ± 4.5 |
| Creatinine (mg/dl) | 0.21 ± 0.03 | 0.20 ± 0.00 | 0.20 ± 0.00 |
| Hematocrit (% PCV) | 43 ± 3.0 | 46 ± 3.3 | 46 ± 2.2 |
| Hemoglobin (g/dl) | 14.7 ± 1.0 | 15.7 ± 1.1 | 15.5 ± 0.8 |
| AnGap (mmol/liter) | 20.6 ± 2.5 | 19.3 ± 4.4 | 22.9 ± 4.5 |
Values are mean ± sem, n = 10.
P < 0.05 for difference from controls.
P < 0.0001 for difference from controls. PCV, Packed cell volume; AnGap, anion gap.
Fig. 5.

Comparison of phenotype of mice. The diabetic mice (B) were cachetic compared with control mice (A), whereas the topiramate-treated diabetic mice (C) were as healthy as the controls.
Effect of genetic knockout of mitochondrial CA genes on oxidative stress in the mouse brain
We hypothesized that inhibition of mitochondrial CA reduces oxidative stress in the mouse brain. We measured oxidative stress in the brain of nondiabetic mice in which both mitochondrial CA VA and VB genes were knocked out (mitochondrial CA DKO). Consistent with our hypothesis, oxidative stress in these animals was significantly less than in their littermate controls. As shown in Table 2, GSH levels in DKO mice were significantly higher, and SOD activity was lower compared with the littermate controls. The protein-bound HNE and 3-NT levels were also significantly less in DKO mice (Table 2).
Table 2.
Oxidative stress in the brain of nondiabetic mitochondrial CA DKO and littermate controls
| Group | GSH (nmol/mg protein) | HNE (% of control) | 3-NT (% of control) | SOD (U/mg protein) |
|---|---|---|---|---|
| Control | 16.0 ± 0.2 | 100 ± 4.6 | 100 ± 18.46 | 23.6 ± 0.6 |
| DKO | 20.9 ± 2.0a | 83.9 ± 3.6a | 94.1 ± 2.25a | 19.5 ± 0.6b |
Values are mean ± sem, n = 3–4.
P < 0.05 for difference from controls.
P < 0.009 for difference from controls.
Rescue of STZ-diabetic induced cerebral PC loss by topiramate treatment
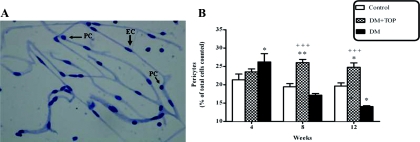
The numbers of PC and EC were determined in PAS-stained, isolated brain microvessels (Fig. 6A). At 4 wk of diabetes (Fig. 6B), there was a significant increase in PC numbers in the diabetic mice (26.23 ± 2.25 vs. 21.35 ± 1.56, P < 0.05), compared with the controls. This was followed by a decrease in PC numbers at 8 wk of diabetes (diabetic, 17.17 ± 0.44 vs. control, 19.44 ± 0.87; ns) that reached statistical significance at 12 wk of diabetes (diabetic, 14.06 ± 0.24 vs. control, 19.66 ± 0.84; P < 0.05). Topiramate-treated diabetic mice also showed an increase in PC numbers (Fig. 6B) at 4 wk of diabetes (topiramate treated, 23.51 ± 0.81 vs. control, 21.35 ± 1.56; ns), which did not reach statistical significance. Most importantly, the PC numbers in topiramate-treated diabetic mice remained statistically higher at 8 (topiramate-treated diabetic, 26.03 ± 0.88 vs. controls, 19.44 ± 0.87; P < 0.01) and 12 wk (topiramate-treated diabetic, 24.76 ± 1.19 vs. controls, 19.66 ± 0.84; P < 0.001) compared with controls.
Fig. 6.
Topiramate treatment rescued diabetes-induced PC loss in the cerebral microvasculature. Diabetic mice (n = 10) were treated with daily sc injections of topiramate for up to 12 wk. Brains were harvested at 4, 8, and 12 wk of topiramate treatment, cerebral microvessels were isolated and stained with PAS-hematoxylin on chamber slides. A, Captured PAS-hematoxylin-stained microscope image of isolated mouse brain microvessels. Thinner arrows point to prominent round nuclei of PC, and thick arrows points to elongated cigar-shaped nucleus of EC. The PC and EC, in the isolated microvessels, were counted, and percent of PC/total cells was calculated. B, Percent of PC at 4, 8, and 12 wk of topiramate treatment. After an initial significant increase in PC numbers in diabetic mice at 4 wk of diabetes, the numbers declined below normal at 8 wk and became significantly less at 12 wk. The PC numbers remain high in topiramate-treated mice at 8 and 12 wk of diabetes. Values are mean ± sem [*, P < 0.05; **, P < 0.01 compared with control; +++, P < 0.001 comparison between treatments (DM vs. DM+TOP)]. DM, Diabetic mice; DM + TOP, diabetic mice treated with topiramate.
Discussion
The main findings of this study were that STZ-diabetes caused oxidative stress and PC dropout in the mouse brain and pharmacological inhibition of mitochondrial CA reduced the oxidative stress and rescued cerebral PC dropout.
The oxidative stress in the brain was measured after 3 wk of mitochondrial CA inhibition by topiramate treatment of diabetic mice. Topiramate is a potent inhibitor of mitochondrial CA VA and VB (inhibition constant, Ki, 63 nM and 30 nM, respectively) (27). The absence of CA II, a cytosolic; CA IV, a glycosylphophatidylinositol anchored; and CA XII and XIV, two transmembrane CA in cultured cerebral PC adds to chemical specificity of topiramate. Topiramate is U.S. Food and Drug Administration approved for treatment of migraine, seizures, and Lennox-Gastaut syndrome and is being studied for use in alcohol dependence, bipolar disorder, bulimia nervosa, essential tremor, obesity, and West syndrome. The low toxicity of topiramate is further underscored by our results that topiramate-treated diabetic mice were as healthy as the control mice (Fig. 5) compared with cachectic diabetic mice not treated with topiramate. These mice did not differ in serum chemistry from the diabetic mice, including their serum glucose levels (Table 1).
In addition to being a potent CA inhibitor, topiramate also has effects on Na+-independent Cl−/HCO3− channels, sodium channels, and calcium channels (28) and on N-methyl-D-aspartic acid receptors in hippocampus (29). Our current study suggests an additional role for topiramate, the inhibition of mitochondrial CA leading to reduced mitochondrial oxidative stress in the mouse brain.
Topiramate is also reported to preserve β-cell function and to be antidiabetic (30, 31). In the current study, the insulin levels in both STZ-diabetic and topiramate-treated diabetic mice were below the lower detection limit of the assay. This along with hyperglycemia in both these groups eliminates the possibility that topiramate effect was mediated by modulation of β-cell function and the PC effect was secondary to milder diabetes.
In our study, the parameters of oxidative stress were GSH, HNE, and 3-NT. GSH, an indicator of the cellular redox state and a direct scavenger of ROS, is important in brain's defense against oxidative stress (32). In the cerebral microvessels, GSH is a critical factor in maintaining blood-brain barrier integrity (33). Decreased concentration of GSH in the brain is associated with both types of diabetes (34, 35). HNE, another frequently used indicator of oxidative stress (36), is one of the most toxic products of lipid peroxidation of polyunsaturated fatty acids. HNE has been implicated in the pathogenesis of many degenerative disorders (37), including natural (38) and chemically induced diabetes (39). The brain is very sensitive to ROS-mediated HNE damage because of its high oxygen consumption and high polyunsaturated lipid content (40). 3-NT, another indicator of oxidative stress, is formed from the reaction of nitric oxide with superoxide anion (O2−) and is a marker of protein oxidation. The presence of 3-NT has been reported in the brain tissue of STZ-diabetic rats (41).
Significant oxidative stress in the diabetic mouse brain was demonstrated by the low levels of GSH (Fig. 3A) and high levels of both the protein-bound HNE (Fig. 4A) and 3-NT (Fig. 4B). As expected, topiramate treatment of these mice significantly decreased oxidative stress (Figs. 3A and 4A). These data provide the first evidence that topiramate treatment reduces diabetes-induced oxidative stress in the mouse brain possibly by inhibition of mitochondrial CA. In addition to being a potent CA inhibitor, topiramate also has effects on Na+-independent Cl−/HCO3− channels, sodium channels, and calcium channels (28), and it modulates N-methyl-D-aspartic acid receptors in hippocampus (29). To provide further evidence that it is mitochondrial CA inhibitory effect of topiramate that reduces oxidative stress, we measured GSH, HNE, and 3-NT levels in the brains of nondiabetic mitochondrial CA DKO mice. The oxidative stress in these mice, even at the basal level, was significantly less than their littermate controls (Table 2). These results clearly show that inhibition of mitochondrial CA function pharmacologically as well as removal of mitochondrial CA proteins by genetic engineering reduces oxidative stress in the mouse brain.
We also measured the enzymatic activity of SOD, an antioxidant enzyme that plays an important role in detoxification of oxidative stress by converting superoxide anions radicals (O2•−) to H2O2 (42). The activity of SOD in topiramate-treated diabetic mice was significantly lower than the untreated diabetic mice (Fig. 3B). These results are not surprising and actually are consistent with our hypothesis that mitochondrial CA inhibition reduces oxidative stress by limiting the production of ROS, rather than up-regulation of SOD activity, which is a protective response to pathologic levels of superoxide radicals already produced. We propose that reduction in ROS production is accomplished by reducing the supply of mitochondrial HCO3− (Fig. 1). Mitochondrial HCO3− is required for the production of oxaloacetate, a key intermediate in Krebs cycle/ETC (major producer of ROS) from pyruvate. HCO3− cannot be imported from the cytosol, because mitochondrial membranes are impermeable to the former (15–19). Therefore, HCO3− is provided by the CA (mitochondrial CA) inside the mitochondria, by reversible hydration of CO2 in the reaction: CO2 + H2O⇆HCO3− + H+. The low SOD activity in the mitochondrial CA DKO (Table 2) mice provide further evidence that the reduced oxidative stress in these mice is a result of the reduction in the rate of ROS production caused by low levels of mitochondrial HCO3−.
Significant reduction in oxidative stress due to mitochondrial CA gene knockout in C57BL/6 (DKO) and pharmacological inhibition of mitochondrial CA function in CD-1 (STZ-diabetic) mice shows that the effect is not strain specific. These findings point to a wider implication for mitochondrial CA as new therapeutic target for reducing oxidative stress in the brain.
To see the effect of hyperglycemia induced oxidative stress on cerebral PC, the number of PC in isolated brain microvessels was determined after 4, 8, and 12 wk of topiramate treatment of the diabetic mice. In the brain capillaries, PC are located next to the EC, surrounded by the basal lamina (43–47). The PC have a prominent round nucleus that clearly differs in shape from the elongated cigar-shaped nucleus of the EC (Fig. 6A). It is becoming increasingly clear that PC are integral to blood-brain barrier function (48, 49). In experimental models of diabetic retinopathy, hyperglycemia has been found to lead to PC loss (50). We postulated that hyperglycemia-induced intracellular oxidative stress will lead to cerebral PC loss in diabetic animals, and mitochondrial CA inhibition will rescue the PC by decreasing the oxidative stress. In the absence of published reports of mitochondrial CA in mouse cerebral PC, we first ascertained the presence of mitochondrial CA in the primary cultured cerebral PC by RT-PCR and immunoblotting (Fig. 2). We then determined the PC to EC ratio in the isolated brain capillaries (Fig. 6A), by established procedure (49). Our results showed a significant decrease in the PC numbers in diabetic animals at 12 wk of diabetes. The most exciting finding was the rescue of PC by pharmacologic inhibition of mitochondrial CA (Fig. 6B). PC loss or a reduced PC to EC ratio under pathological conditions may be achieved through migration of PC from their microvascular location (51, 52) or PC apoptosis (53–55). The exact mechanism for reduction in PC numbers, in our study, remains to be elucidated. An initial increase in PC number, observed at 4 wk of diabetes, may be a transient adaptation to diabetes. Cerebral PC are involved in the control of brain glucose homeostasis and have been shown to undergo numerous adaptive changes in response to glucose levels (56).
Loss of PC with reduced PC to EC ratios results in a focal increase in permeability (49). A close correlation between PC density and blood-brain barrier permeability has been reported in PC-deficient adult mice (57). STZ-diabetes has been shown to progressively increase blood-brain barrier permeability in specific brain regions in rats (1). Recent clinical evidence suggests that diabetes-induced changes in the blood-brain barrier may be a predisposing factor for Alzheimer's disease (58), and people suffering from both types of diabetes have an increased risk of cognitive impairment (59–63). Thus, determining the pathophysiological role of diabetes on blood-brain barrier function and structure is an important step toward understanding the altered neuronal function and increased susceptibility to cerebrovascular diseases. Cerebral PC may provide a new therapeutic target toward achieving this goal.
Studies are in progress, in our lab, to investigate the effect of STZ-diabetes-induced PC loss on blood-brain barrier integrity and cognitive function and their protection by mitochondrial CA inhibition. Such a correlation may provide a mechanism for cognitive decline in diabetes, and mitochondrial CA may turn out be a valuable new therapeutic target for oxidative stress-related illnesses of the central nervous system.
Supplementary Material
Acknowledgments
We thank Prof. William Sly for providing mitochondrial CA DKO mice; Sandra Cornell (Saint Louis University), Ping Patrick (Saint Louis University), and Xinsheng Zhang (Missouri University of Science and Technology), for their technical assistance; and Barbara Harris (Missouri University of Science and Technology) for editorial assistance.
This work was supported by the National Institutes of Health Grant RO1DK083485 and by the Veterans Affairs merit review.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BUN
- Blood urea nitrogen
- CA
- carbonic anhydrase
- DKO
- double knockout
- EC
- endothelial cell
- ETC
- electron transport chain
- GSH
- glutathione
- HCO3−
- bicarbonate
- HNE
- 4-hydroxy-2-trans-nonenal
- NPM
- N-(1-prenyl)-maleimide
- 3-NT
- 3-nitrotyrosine
- ns
- not significant
- PAS
- periodic acid-Schiff
- PC
- pericyte
- ROS
- reactive oxygen species
- α-SMA
- α-smooth muscle actin
- SOD
- superoxide dismutase
- STZ
- streptozotocin
- TCO2
- total CO2.
References
- 1. Huber JD, VanGilder RL, Houser KA. 2006. Streptozotocin-induced diabetes progressively increases blood-brain barrier permeability in specific brain regions in rats. Am J Physiol Heart Circ Physiol 291:H2660–H2668 [DOI] [PubMed] [Google Scholar]
- 2. Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. 2003. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatr 74:70–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wessels AM, Rombouts SA, Simsek S, Kuijer JP, Kostense PJ, Barkhof F, Scheltens P, Snoek FJ, Heine RJ. 2006. Microvascular disease in type 1 diabetes alters brain activation: a functional magnetic resonance imaging study. Diabetes 55:334–340 [DOI] [PubMed] [Google Scholar]
- 4. Dore-Duffy P, Katychev A, Wang X, Van Buren E. 2006. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab 26:613–624 [DOI] [PubMed] [Google Scholar]
- 5. Dore-Duffy P. 2008. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des 14:1581–1593 [DOI] [PubMed] [Google Scholar]
- 6. Daneman R, Zhou L, Kebede AA, Barres BA. 2010. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468:562–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Katychev A, Wang X, Duffy A, Dore-Duffy P. 2003. Glucocorticoid-induced apoptosis in CNS microvascular pericytes. Dev Neurosci 25:436–446 [DOI] [PubMed] [Google Scholar]
- 8. Brownlee M. 2001. Biochemistry and molecular cell biology of diabetic complications. Nature 414:813–820 [DOI] [PubMed] [Google Scholar]
- 9. Brownlee M. 2005. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 10. Hawkins BT, Lundeen TF, Norwood KM, Brooks HL, Egleton RD. 2007. Increased blood-brain barrier permeability and altered tight junctions in experimental diabetes in the rat: contribution of hyperglycaemia and matrix metalloproteinases. Diabetologia 50:202–211 [DOI] [PubMed] [Google Scholar]
- 11. Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. 2003. Production of reactive oxygen species by mitochondria: central role of complex III. J Biol Chem 278:36027–36031 [DOI] [PubMed] [Google Scholar]
- 12. Liu Y, Fiskum G, Schubert D. 2002. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem 80:780–787 [DOI] [PubMed] [Google Scholar]
- 13. Korshunov SS, Skulachev VP, Starkov AA. 1997. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18 [DOI] [PubMed] [Google Scholar]
- 14. Shah GN, Hewett-Emmett D, Grubb JH, Migas MC, Fleming RE, Waheed A, Sly WS. 2000. Mitochondrial carbonic anhydrase CA VB: differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc Natl Acad Sci USA 97:1677–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dodgson SJ, Forster RE., 2nd 1986. Inhibition of CA V decreases glucose synthesis from pyruvate. Arch Biochem Biophys 251:198–204 [DOI] [PubMed] [Google Scholar]
- 16. Dodgson SJ, Forster RE., 2nd 1986. Carbonic anhydrase: inhibition results in decreased urea production by hepatocytes. J Appl Physiol 60:646–652 [DOI] [PubMed] [Google Scholar]
- 17. Hazen SA, Waheed A, Sly WS, LaNoue KF, Lynch CJ. 1996. Differentiation-dependent expression of CA V and the role of carbonic anhydrase isozymes in pyruvate carboxylation in adipocytes. FASEB J 10:481–490 [DOI] [PubMed] [Google Scholar]
- 18. Hazen SA, Waheed A, Sly WS, LaNoue KF, Lynch CJ. 1997. Effect of carbonic anhydrase inhibition and acetoacetate on anaplerotic pyruvate carboxylase activity in cultured rat astrocytes. Dev Neurosci 19:162–171 [DOI] [PubMed] [Google Scholar]
- 19. Parkkila AK, Scarim AL, Parkkila S, Waheed A, Corbett JA, Sly WS. 1998. Expression of carbonic anhydrase V in pancreatic β cells suggests role for mitochondrial carbonic anhydrase in insulin secretion. J Biol Chem 273:24620–24623 [DOI] [PubMed] [Google Scholar]
- 20. Vander Heiden MG, Cantley LC, Thompson CB. 2009. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nagao Y, Srinivasan M, Platero JS, Svendrowski M, Waheed A, Sly WS. 1994. Mitochondrial carbonic anhydrase (isozyme V) in mouse and rat: cDNA cloning, expression, subcellular localization, processing, and tissue distribution. Proc Natl Acad Sci USA 91:10330–10334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banks WA, Jaspan JB, Kastin AJ. 1997. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides 18:1577–1584 [DOI] [PubMed] [Google Scholar]
- 23. Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, Niwa M. 2004. Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. Regul Pept 123:77–83 [DOI] [PubMed] [Google Scholar]
- 24. Winters RA, Zukowski J, Ercal N, Matthews RH, Spitz DR. 1995. Analysis of glutathione, glutathione disulfide, cysteine, homocysteine, and other biological thiols by high-performance liquid chromatography following derivatization by n-(1-pyrenyl)maleimide. Anal Biochem 227:14–21 [DOI] [PubMed] [Google Scholar]
- 25. McCord JM, Fridovich I. 1969. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055 [PubMed] [Google Scholar]
- 26. Gerhart DZ, Broderius MA, Drewes LR. 1988. Cultured human and canine endothelial cells from brain microvessels. Brain Res Bull 21:785–793 [DOI] [PubMed] [Google Scholar]
- 27. Nishimori I, Vullo D, Innocenti A, Scozzafava A, Mastrolorenzo A, Supuran CT. 2005. Carbonic anhydrase inhibitors. The mitochondrial isozyme VB as a new target for sulfonamide and sulfamate inhibitors. J Med Chem 48:7860–7866 [DOI] [PubMed] [Google Scholar]
- 28. Leniger T, Thöne J, Wiemann M. 2004. Topiramate modulates pH of hippocampal CA3 neurons by combined effects on carbonic anhydrase and Cl−/HCO3− exchange. Br J Pharmacol 142:831–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Deutsch SI, Schwartz BL, Rosse RB, Mastropaolo J, Marvel CL, Drapalski AL. 2003. Adjuvant topiramate administration: a pharmacologic strategy for addressing NMDA receptor hypofunction in schizophrenia. Clin Neuropharmacol 26:199–206 [DOI] [PubMed] [Google Scholar]
- 30. Liang Y, Chen X, Osborne M, DeCarlo SO, Jetton TL, Demarest K. 2005. Topiramate ameliorates hyperglycaemia and improves glucose-stimulated insulin release in ZDF rats and db/db mice. Diabetes Obes Metab 7:360–369 [DOI] [PubMed] [Google Scholar]
- 31. Roy Chengappa KN, Levine J, Rathore D, Parepally H, Atzert R. 2001. Long-term effects of topiramate on bipolar mood instability, weight change and glycemic control: a case-series. Eur Psychiatry 16:186–190 [DOI] [PubMed] [Google Scholar]
- 32. Schafer FQ, Buettner GR. 2001. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30:1191–1212 [DOI] [PubMed] [Google Scholar]
- 33. Agarwal R, Shukla GS. 1999. Potential role of cerebral glutathione in the maintenance of blood-brain barrier integrity in rat. Neurochem Res 24:1507–1514 [DOI] [PubMed] [Google Scholar]
- 34. Mastrocola R, Restivo F, Vercellinatto I, Danni O, Brignardello E, Aragno M, Boccuzzi G. 2005. Oxidative and nitrosative stress in brain mitochondria of diabetic rats. J Endocrinol 187:37–44 [DOI] [PubMed] [Google Scholar]
- 35. Pastore A, Federici G, Bertini E, Piemonte F. 2003. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta 333:19–39 [DOI] [PubMed] [Google Scholar]
- 36. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. 2007. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39:44–84 [DOI] [PubMed] [Google Scholar]
- 37. Armstrong D, Abdella N, Salman A, Miller N, Rahman EA, Bojancyzk M. 1992. Relationship of lipid peroxides to diabetic complications. Comparison with conventional laboratory tests. J Diabetes Complicat 6:116–122 [DOI] [PubMed] [Google Scholar]
- 38. Nishigaki I, Hagihara M, Tsunekawa H, Maseki M, Yagi K. 1981. Lipid peroxide levels of serum lipoprotein fractions of diabetic patients. Biochem Med 25:373–378 [DOI] [PubMed] [Google Scholar]
- 39. Higuchi Y. 1982. Lipid peroxides and α-tocopherol in rat streptozotocin-induced diabetes mellitus. Acta Med Okayama 36:165–175 [DOI] [PubMed] [Google Scholar]
- 40. Ozkaya YG, Agar A, Yargiçoglu P, Hacioglu G, Bilmen-Sarikçioglu S, Ozen I, Alicigüzel Y. 2002. The effect of exercise on brain antioxidant status of diabetic rats. Diabetes Metab 28:377–384 [PubMed] [Google Scholar]
- 41. Dalle-Donne I, Scaloni A, Giustarini D, Cavarra E, Tell G, Lungarella G, Colombo R, Rossi R, Milzani A. 2005. Proteins as biomarkers of oxidative/nitrosative stress in diseases: the contribution of redox proteomics. Mass Spectrom Rev 24:55–99 [DOI] [PubMed] [Google Scholar]
- 42. Halliwell B. 1992. Reactive oxygen species and the central nervous system. J Neurochem 59:1609–1623 [DOI] [PubMed] [Google Scholar]
- 43. Balabanov R, Dore-Duffy P. 1998. Role of the CNS microvascular pericyte in the blood-brain barrier. J Neurosci Res 53:637–644 [DOI] [PubMed] [Google Scholar]
- 44. Fisher M. 2009. Pericyte signaling in the neurovascular unit. Stroke 40:S13–S15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shepro D, Morel NM. 1993. Pericyte physiology. FASEB J 7:1031–1038 [DOI] [PubMed] [Google Scholar]
- 46. Sims DE. 1991. Recent advances in pericyte biology—implications for health and disease. Can J Cardiol 7:431–443 [PubMed] [Google Scholar]
- 47. Tilton RG. 1991. Capillary pericytes: perspectives and future trends. J Electron Microsc Tech 19:327–344 [DOI] [PubMed] [Google Scholar]
- 48. Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, Zlokovic BV. 2010. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 68:409–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P. 2011. The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, Clermont A, Leitges M, Marette A, Aiello LP, Kern TS, King GL. 2009. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med 15:1298–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA. 2000. Pericyte migration from the vascular wall in response to traumatic brain injury. Microvasc Res 60:55–69 [DOI] [PubMed] [Google Scholar]
- 52. Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. 2008. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes 57:2495–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li W, Liu X, Yanoff M, Cohen S, Ye X. 1996. Cultured retinal capillary pericytes die by apoptosis after an abrupt fluctuation from high to low glucose levels: a comparative study with retinal capillary endothelial cells. Diabetologia 39:537–547 [DOI] [PubMed] [Google Scholar]
- 54. Shojaee N, Patton WF, Hechtman HB, Shepro D. 1999. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem 75:118–129 [PubMed] [Google Scholar]
- 55. Yamagishi S, Imaizumi T. 2005. Pericyte biology and diseases. Int J Tissue React 27:125–135 [PubMed] [Google Scholar]
- 56. Virgintino D, Robertson D, Monaghan P, Errede M, Bertossi M, Ambrosi G, Roncali L. 1997. Glucose transporter GLUT1 in human brain microvessels revealed by ultrastructural immunocytochemistry. J Submicrosc Cytol Pathol 29:365–370 [PubMed] [Google Scholar]
- 57. Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, Johansson BR, Betsholtz C. 2010. Pericytes regulate the blood-brain barrier. Nature 468:557–561 [DOI] [PubMed] [Google Scholar]
- 58. Ristow M. 2004. Neurodegenerative disorders associated with diabetes mellitus. J Mol Med 82:510–529 [DOI] [PubMed] [Google Scholar]
- 59. Bruce DG, Davis WA, Casey GP, Starkstein SE, Clarnette RM, Almeida OP, Davis TM. 2008. Predictors of cognitive decline in older individuals with diabetes. Diabetes Care 31:2103–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. 2007. Relation of diabetes to mild cognitive impairment. Arch Neurol 64:570–575 [DOI] [PubMed] [Google Scholar]
- 61. Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman AP, Kappelle LJ, Mali WP. 2008. Diabetes increases atrophy and vascular lesions on brain MRI in patients with symptomatic arterial disease. Stroke 39:1600–1603 [DOI] [PubMed] [Google Scholar]
- 62. van Harten B, Oosterman J, Muslimovic D, van Loon BJ, Scheltens P, Weinstein HC. 2007. Cognitive impairment and MRI correlates in the elderly patients with type 2 diabetes mellitus. Age Ageing 36:164–170 [DOI] [PubMed] [Google Scholar]
- 63. Whitmer RA. 2007. Type 2 diabetes and risk of cognitive impairment and dementia. Curr Neurol Neurosci Rep 7:373–380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.