Abstract
The clinical use of synthetic glucocorticoids in preterm infants to promote lung development has received considerable attention due to the potential for increased risk of developing metabolic disease in adulthood after such treatment. In this study, we examined the hypothesis that exposure to the synthetic glucocorticoid, dexamethasone (DEX), during late gestation in the rat results in the development of nonalcoholic fatty liver disease in adult offspring. Pregnant Sprague Dawley dams were treated with 0.4 mg/kg DEX beginning on gestational d 18 until parturition (gestational d 23). At postnatal d 21, offspring were weaned onto either a standard chow or high-fat (60% fat-derived calories) diet. In adulthood (postnatal d 60–65), hepatic tissue was harvested and examined for pathology. Liver steatosis, or fat accumulation, was found to be more severe in the DEX-exposed female offspring that were weaned onto the high-fat diet. This finding corresponded with decreased plasma IGF-I concentrations, as well as decreased hypothalamic expression of GHRH mRNA. Morphological measurements on body and long bone length further implicate a GH signaling deficit after fetal DEX exposure. Collectively, these data indicate suppression of GH axis function in the female DEX/high-fat cohort but not in the male offspring. Because deficits in the GH signaling can be linked to the development of nonalcoholic fatty liver disease, our results suggest that the prominent liver injury noted in female offspring exposed to DEX during late gestation may stem from abnormal development of the GH axis at the hypothalamic level.
The fetal origins of adult disease hypothesis proposes that adverse environments during fetal development may predispose individuals to disease later in life (1). Studies supporting this hypothesis report an elevated frequency of metabolic disorders, such as heart disease, obesity, glucose intolerance, and diabetes, in individuals who were malnourished during fetal development (1–3). Recently, concern has also arisen regarding the widespread clinical use of synthetic glucocorticoid (GC) treatment, a practice that began in the mid 1980s, to promote lung development in preterm infants (4). Indeed, morphometric studies document a correlation between fetal GC administration and reductions in size at birth (5), which predicts metabolic disease in adulthood (6). Given the prevalence of clinical GC use in preterm infants, further understanding of the potential risk for developing disease later in life is warranted.
Abnormal metabolic programming in rodents after excessive fetal GC levels results in reduced birth weight and abnormal distribution of fat pads and muscle mass (7). Similar fetal GC exposure paradigms have also revealed the development of other facets of the metabolic syndrome, including glucose intolerance (8) and susceptibility to hypertension in the adult offspring (9). An additional report employing a random stress paradigm in pregnant rats documented that the offspring from the stressed dams were more susceptible to obesity when weaned on a high-fat diet and proposed that this susceptibility was due to excessive exposure of the developing fetus to maternal GC (10). In contrast, administration of dexamethasone (DEX) to pregnant rats during late gestation does not predispose offspring to excessive adipose accumulation (11) but does increase hepatic triglyceride accumulation in the male offspring that had been weaned onto a high-fat diet (11). Such observations indicate the possibility of increased risk for nonalcoholic fatty liver disease (NAFLD) in offspring after GC exposure during fetal life.
Hepatic lipid accumulation, or steatosis, constitutes the first stage of NAFLD and is the hepatic manifestation of the metabolic syndrome. Although steatosis is typically a fully reversible phenomenon, prolonged occurrence of this condition is associated with progression of liver injury to hepatitis and the irreversible deposition of fibrotic tissue. Ultimately, sustained liver disease may lead to cirrhosis of the liver, an irreversible and often fatal condition (12). Although molecular mechanisms explaining susceptibility to NAFLD remain unclear, recent evidence has emerged, suggesting that deficiencies in the GH axis may potentiate the development of hepatic steatosis (13–15). In our current studies, we have employed a fetal DEX exposure paradigm in rats to test the hypothesis that prenatal exposure to GC causes a predisposition to hepatic injury. Our findings support this hypothesis and show that there is also a profound sex difference in susceptibility. In addition, the results of these studies demonstrate that a dysregulation of the GH axis involving the arcuate nucleus (ArcN) and paraventricular nucleus (PVN) of the hypothalamus may underlie the observed pathology.
Materials and Methods
Animals
Timed-pregnant Sprague Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) to arrive at the Arizona State University Department of Animal Care and Technologies (Tempe, Arizona) at gestational day (GD)7. This arrival time is before the birthdate of neurons destined to populate the hypothalamus and was chosen to limit any transport-induced stress effects on the developing brain. Beginning on GD14, dams were handled daily for several minutes to acclimate them to manipulation, thus limiting any confounding effect of stress responses to the treatment procedure. Beginning on GD18, rats were injected sc with 0.4 mg/kg DEX or vehicle (2% ethanol in safflower oil) daily until parturition (GD23). This dosing regimen was designed to fall within the range of clinical human exposure (0.1–0.5 mg/kg) but also remain consistent with existing reports using Sprague Dawley rats in which dosing between 0.2 and 0.8 mg/kg resulted in abnormal neurodevelopmental (16, 17). On the day of birth, all offspring were weighed and randomly assigned to a litter. This ensured that each litter was composed of five male and five female offspring and was also designed to minimize any litter effect that may influence the study. Although DEX or vehicle-exposed offspring were always assigned to a dam that had received that respective injection, concern over any confounding effects due to our DEX regimen on maternal care/nutrition was minimized based on an existing cross-fostering study, which concluded that the effects of fetal DEX exposure are not due to an effect on the dam (18). At postnatal day (PND)21, litters were weaned onto either a high-fat diet containing 60% fat-derived calories (Research Diets, New Brunswick, NJ) or a standard chow diet. At termination of the study (animals were 60–65 d of age), rats were killed by rapid decapitation, trunk blood was collected, a portion of the liver was postfixed in 4% neutral-buffered paraformaldehyde, and the remaining liver, as well as the brain tissue, was harvested, snap frozen, and stored at −80 C until processing. Each treatment group included at least six animals, each derived from separate litters. All procedures were approved by the Arizona State University Institutional Animals Care and Use Committee, under subcontract from the University of Arizona College of Medicine-Phoenix, and were in keeping with National Institutes of Health guidelines. Offspring were weighed weekly to track weight gain over time, and nose-to-anus distance was measured using Vernier calipers.
Histological analysis
Formaldehyde-fixed adult liver tissue was paraffin embedded, and 5- to 7-μm-thick tissue sections were taken with a microtome. Macrosteatosis was identified by the appearance of a large fat droplet that displaced the nucleus of the hepatocyte. Centrilobular and periportal macrosteatosis was measured using an approach similar to that reported by Iimuro et al. (19), in which scoring was performed after hematoxylin and eosin staining using regions of interest (ROI) consisting of the first five cell layers surrounding the central vein or portal triad to emphasize regional pathology. Because fat accumulation may lead to hepatocyte swelling, the total number of hepatocytes within each ROI was counted, and macrosteatotic cells are reported as a percentage of total cells within the ROI. No less than four lobules, never adjacent to each other, were scored per section, and the mean of this value was used to represent the findings from that particular animal. This approach was taken ensure that results reflected the status of the entire organ.
Western blot analysis
At the termination of the study, approximately 5 ml of trunk blood per rat were collected in polystyrene culture tubes containing 100 μl of 0.5 m EDTA and 200 μl of 4 μg/ml aprotinin to prevent coagulation and proteolytic degradation, respectively. Plasma was saved after centrifugation and stored at −20 C. For assay, plasma was diluted 1:25 in standard radioimmunoprecipitation assay buffer supplemented with a protease inhibitor cocktail (Sigma-Aldrich Chemical Co., St. Louis, MO) and separated by SDS-PAGE. Protein was transferred to nitrocellulose and probed for IGF-I using monocolonal antibodies generated against IGF-I (catalog MS-1508; Thermo Scientific, Freemont, CA) diluted 1:1000 in Tris-buffered saline with Tween 20 and incubated overnight at 4 C. IGF-I immunoreactivity was detected using a fluorescent secondary antibody (LiCOR Biosciences, Lincoln, NE) diluted 1:14,000 in 3% nonfat dry milk in Tris-buffered saline with Tween 20 and visualized and quantified using an Odyssey imaging system (LiCOR Biosciences). Immunofluorescence intensity is reported in arbitrary units based on all six samples per treatment group. Protein identity with this particular antibody was determined based on mass information for rat IGF-I described elsewhere (20). The coefficient of variance within groups was typically between 4 and 10%, indicating adequate consistency throughout the Western blotting, as demonstrated by Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org.
RT quantitative PCR (qPCR)
Frozen rat brains were cryosectioned at −20 C through the hypothalamic regions containing the PVN and ArcN; 200-μm-thick tissue sections were punched bilaterally using a 1 mm diameter stainless steel cannula with the third ventricle, lateral ventricles, and hippocampus as landmarks, according to the atlas of Paxinos and Watson (21). Tissue containing the PVN was collected immediately adjacent to the dorsal portion of the third ventricle between Bregma −1.80 and −2.00, whereas tissue containing the ArcN was collected immediately adjacent to ventral portion of the third ventricle between Bregma −2.50 and −4.30. Tissue punches were never allowed to thaw and were stored on dry ice until RNA extraction, which was always performed on the same day. Isolated hypothalamic tissue was homogenized in guanidium isothiocyanate supplemented with β-mercaptoethanol, and RNA was purified by phenol/chloroform/isoamyl alcohol extraction according to procedures described elsewhere (22). RNA quantity and purity were confirmed spectrophotometrically, and 1 μg of total RNA per sample was reverse transcribed using iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). Total cDNA per RT reaction was quantified using the Quant-iT Oligreen ssDNA Reagent (Molecular Probes, Eugene, OR), and cDNA concentrations were normalized before qPCR. Gene expression was measured with a LightCycler 480 (Roche Diagnostics, Indianapolis, IN) using primers designed to amplify specific regions within the coding sequence of GHRH (Table 1). Absolute target mRNA (femtograms) was calculated based on comparison of the crossing point of each individual sample with a standard curve constructed from purified PCR product.
Table 1.
Primer sequences for RT-qPCR
| Accession no. | Gene | Description | Primer sequence (5′-3′) | |
|---|---|---|---|---|
| NM_001109218 | CD36 | CD36/fatty acid translocase | Forward | TAAACCTCCTGGACCTGGTAGAGA |
| Reverse | GCGCACACCACCATTTCTTCAACT | |||
| NM_017332 | Fasn | Fatty acid synthase | Forward | ATTCTGAATGCCGGGACGAACACA |
| Reverse | AGCCAATTAACAAAGGGCTCTGGC | |||
| NM_031577 | Ghrh | GHRH | Forward | GGGCCAATTATATGCCCGCAAACT |
| Reverse | CACTCTGTCCAAATGGCGGTTGAA | |||
| NM_032075 | Ghsr | GH secretagogue receptor | Forward | TCTTCTGCCTCACTGTGCTCTACA |
| Reverse | AGCCAGGCTCGAAGGACTTGGAAA | |||
| NM_178866 | Igf1 | IGF-I | Forward | TGGCACTCTGCTTGCTCACCTTTA |
| Reverse | AGTACATCTCCAGCCTCCTCAGAT | |||
| NM_031347 | Pgc1α | PPAR-γ coactivator 1-α | Forward | ATGTGAATGACCTGGACACAGACAGC |
| Reverse | AATGAGGGCAATCCGTCTTCATCC | |||
| NM_013196 | Ppar-α | Peroxisome proliferator-activated receptor-α | Forward | AACTGCAGACCTCAAATCTCTGGC |
| Reverse | AATCGGACCTCTGCCTCCTTGTTT | |||
| NM_013124 | Ppar-γ | Peroxisome proliferator-activated receptor-γ | Forward | GACAAGGATTCATGACCAGGGAGT |
| Reverse | TCTGCCTGAGGTCTGTCATCTTCT | |||
| NM_012659 | Sst | Somatostatin | Forward | GCTCTGCATCGTCCTGGCTTT |
| Reverse | ATCGTTCTCTGTCTGGTTGGG | |||
X-ray imaging and bone length measurements
The effect of fetal DEX exposure on bone length was measured at weaning of the litters (PND21). Offspring were euthanized by CO2 inhalation and then decapitated. Muscle and connective tissue around the femur was dissected away to allow greater freedom of movement, and both legs were secured flat to the sample tray with tape before x-ray imaging. Imaging was performed using a Kodak In-Vitro Multispectral Imaging System (Kodak, Rochester, NY). Images were captured and femur and tibia length measured using the Carestream Molecular Imaging Software version 5.0.2.26 (Carestream Health, Inc., Rochester, NY). Bone measurements (in millimeters) were recorded between the head and medial condyle of the femur and the medial condyle and fibular notch of the tibia (see figure 6 below). Both the right and left sides were measured per animal and respective bone lengths averaged.
Statistical analysis
Statistical analyses were performed using GraphPad Prism version 5.0b (GraphPad Software, Inc., La Jolla, CA). Body length and initial weight gain data were analyzed by two-way ANOVA across time and fetal DEX exposure factors, with time being a repeated measure. Weight at PND60 was also analyzed by two-way ANOVA with fetal DEX exposure and diet (e.g. chow or high fat) as the factors. Two-way ANOVA was also used to determine interactions between sex and fetal DEX exposure in the remaining experiments. For all experiments, post hoc comparison of means was performed using the method of Bonferroni. Results of the two-way ANOVA are presented in respective results section, and post hoc significance is denoted by asterisk within the figures. Differences were deemed significant if P < 0.05.
Results
Weight gain after fetal DEX exposure
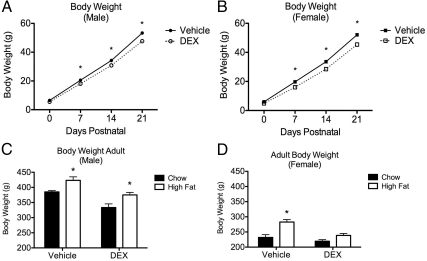
The fetal DEX exposure paradigm employed in the present studies resulted in decreased body weight in both male and female offspring early in life (PND0–PND21) (Fig. 1, A and B). For body weight in the male offspring, two-way ANOVA (DEX treatment × time), with repeated measures across time, revealed significant effects of time [F(3,93) = 3290, P < 0.0001] and treatment [F(1,93) = 30.27, P < 0.0001] and a significant interaction between treatment and time [F(3,93) = 8.863, P = 0.0007]. Similar results were observed in the female offspring regarding the effect of time [F(3,96) = 2403, P < 0.0003] and treatment [F(1,96) = 85.39, P <0.0001], as well as an interaction between time and DEX exposure [F(3,96) = 10.08, P < 0.0001] on the body weight. Post hoc comparison of data from animals at PND0, PND7, PND14, and PND21 revealed a significant difference in body weight between vehicle and DEX-treated offspring at PND7–PND21 in both male and female offspring (Fig. 1, A and B).
Fig. 1.
Fetal DEX exposure during late gestation reduces body weight. Offspring exposed during the final 5 d of gestation to DEX weighed significantly less than controls throughout early life (A and B) and into adulthood (C and D). In female offspring, fetal DEX exposure limited the increase in body weight from chronic consumption of a high-fat diet (D). Early weight gain reported as mean weight (g) ± sem of 13 animals, with significant differences between vehicle and DEX-exposed offspring indicated by asterisk (A and B). Weight at PND60 is reported as mean weight (g) ± sem of six animals, with significance between chow and high-fat diet indicated by asterisk (C and D).
Two-way ANOVA also revealed main effects of fetal DEX treatment [F(1,20) = 14.23, P = 0.0012] and diet [F(1,20) = 40.75, P < 0.0001] on terminal (PND60–PND65) body weight in the male offspring, although no significant interaction between these variables was found (Fig. 1C). Post hoc comparison of body weight in the male offspring at termination of the study indicated a significant difference resulting from the diet, regardless of fetal DEX exposure (Fig. 1C). Significant effects of fetal DEX exposure [F(1,20) = 108.17, P < 0.0001] and diet [F(1,20) = 87.62, P < 0.0001] on body weight were likewise observed in the female offspring at termination of the study (Fig. 1D). However, in contrast to the males, a significant interaction between DEX exposure and diet was observed [F(1,20) = 22.77, P = 0.0001] in this cohort. Furthermore, post hoc comparison of female body weight did not reveal a significant difference between body weight in DEX-exposed females weaned onto either the chow or high-fat diet (Fig. 1D). These data suggest that the fetal DEX exposure limited elevated weight gain in response to the high-fat diet in females (Fig. 1D).
Linear growth measurements
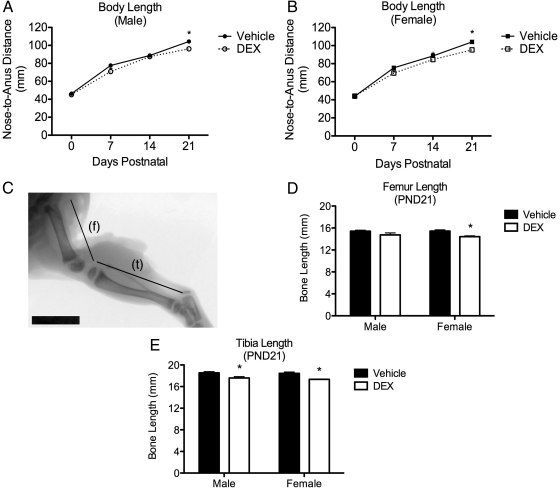
Two-way ANOVA for each sex (DEX treatment × time) with repeated measures across time was used to measure the effects of fetal DEX exposure and time on body length (Fig. 2, A and B). These analysis revealed an effect of DEX exposure [F(1,93) = 60.39, P < 0.0001] and time [F(3,93) = 1001, P < 0.0001] on nose-to-anus distance in the male offspring, along with a significant interaction [F(3,93) = 6.233, P = 0.0007] between these variables (Fig. 2A). Similar findings were also noted in the female cohort regarding effects of DEX exposure [F(1,93) = 29.15, P < 0.0001] and time [F(3,93) = 1409, P < 0.0001], as well as a significant interaction between these two variables female [F(3,93) = 8.470, P < 0.0001] on body length (Fig. 2B). Post hoc analysis of the nose-to-anus distance indicated significantly shorter body length in both male and female offspring after fetal exposure to DEX, with the exception of PND0 (both sexes) and PND14 in the male offspring, although the possibility of high measurement variance may underlie the lack of difference in the treated and control male offspring at PND14 (Fig. 2, A and B).
Fig. 2.
Fetal DEX exposure decreases body and posterior limb length during early development. Nose-to-anus distance (A and B) and leg bone length (C–E) were measured as markers of GH axis activity during early life. Fetal DEX exposure decreases body length through PND21 in both male and female offspring (A and B). Furthermore, x-ray images taken postmortem at PND21 (f, Femur; t, tibia) (Scale bar, 1 cm) (C) show that femur length was significantly decreased in female, but not male, offspring in response to the fetal DEX exposure (D). Tibia length was significantly decreased in male and female offspring in response to fetal DEX exposure (E). Early longitudinal growth reported as mean nose-to-anus distance (mm) ± sem of 13 animals, with significant differences between vehicle and DEX-exposed offspring indicated by asterisk (A and B). Femur and tibia lengths at PND21 are reported as mean ± sem of three to five animals, with significant differences in bone length between vehicle and DEX exposed offspring indicated by asterisk (D and E).
Both the femur and tibia were imaged by x-ray on PND21 and bone length measured (Fig. 2C). Two-way ANOVA (DEX exposure × sex) of the posterior limb bone lengths revealed an effect of fetal DEX exposure on femur [F(1,12) = 13.36, P = 0.0033] and tibia [F(1,12) = 18.00, P = 0.0011] bone length (Fig. 2, D and E). Sex did not affect the length of either bone, nor was any interaction between fetal DEX exposure and sex observed (Fig. 2, D and E). Post hoc analysis of bone length revealed that fetal DEX exposure resulted in significantly shorter femur length in the female, but not male, offspring, although tibia length was significantly reduced in both male and female offspring that were exposed during gestation to DEX (Fig. 2, D and E).
Hepatic steatosis after chronic high-fat feeding
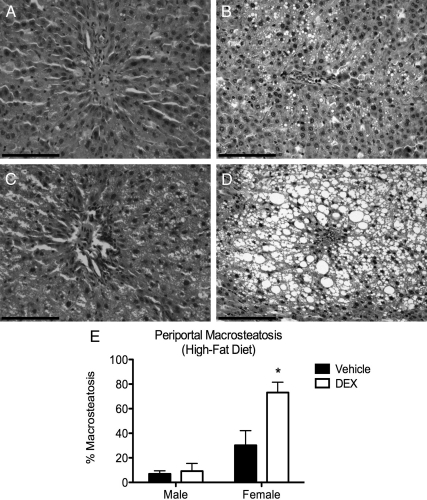
The effect of fetal DEX exposure on hepatic macrosteatosis was evaluated microscopically in paraffin-embedded liver tissue samples stained with hematoxylin and eosin (Fig. 3, A–D). After fetal DEX exposure and weaning onto the high-fat diet, macrosteatotic pathology surrounding the periportal region of the liver lobules was observed to be present in female (Fig. 3D) and to a much lesser extent in the male offspring (Fig. 3B). Two-way ANOVA of the number of periportal steatotic cells within the female periportal regions revealed an effect of fetal DEX exposure [F(1,19) = 8.67, P = 0.0083] and sex [F(1,19) = 32.30, P < 0.0001], as well as an interaction between these variables [F(1,19) = 7.06, P = 0.0156], whereas similar effects were not observed in the male cohort (Fig. 3F). Furthermore, post hoc comparison between vehicle or DEX-exposed offspring revealed a significant difference in the number of steatotic cells in the female cohort, whereas this effects was not observed in the males (Fig. 3F).
Fig. 3.
Fetal DEX exposure causes elevated periportal steatosis in DEX-exposed females (magnification, ×20). Scale bars, 100 μm. A significant increase in macrosteatotic vesicles was found in livers from female offspring that had been exposed prenatally to DEX (D), but not vehicle (C). This was not observed in the male offspring (A and B). Macrosteatosis in the female liver also resulted in substantial disruption of hepatic architecture (D). Cell counts are reported as mean ± sem of six animals, with significant differences between vehicle and DEX exposed offspring indicated by asterisk (E).
Similar analysis of the centrilobular region of the liver lobules did not reveal any significant elevation in pathology, and the presence of inflammatory cell infiltration and hepatocyte necrosis was not observed in any treatment group. Furthermore, histological evaluation of tissue from the chow-fed groups did not indicate the presence of any hepatic injury regardless of sex or fetal DEX exposure (data not shown).
IGF-I suppression
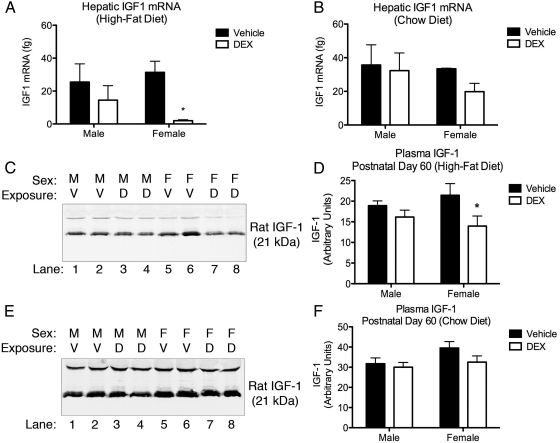
Expression of hepatic Igf-1 mRNA was measured in adult (PND60–PND65) tissue by RT-qPCR (Fig. 4, A and B). Two-way ANOVA of data (DEX exposure × sex) from the offspring weaned onto the high-fat cohort revealed a significant effect of fetal DEX exposure [F(1,13) = 8.367, P = 0.0125] on the expression of hepatic Igf-1 mRNA (Fig. 4A). Post hoc analysis of these data also indicated a significant decrease in expression of Igf-1 mRNA in female offspring exposed during gestation to DEX when compared with their vehicle-exposed counterparts, whereas this effect was not observed in the male offspring. Similar comparison of Igf-1 mRNA expression in hepatic tissue isolated from the chow-fed cohort did not reveal any significant effects (Fig. 4B).
Fig. 4.
Fetal DEX exposure decreases hepatic Igf-1 expression. Fetal exposure to DEX results in decreased expression of Igf-1 transcript in female, but not male, offspring weaned onto the high-fat diet (A). This effect was not observed in animals weaned onto the chow diet (B). These observations are consistent with relative plasma IGF-I immunoreactivity demonstrating a similar trend in fetal DEX-exposed female offspring weaned onto the high-fat diet (C and D), whereas no effect was observed in animals weaned onto the chow diet (E and F). qPCR and densitometry are reported as mean ± sem of six animals, with significance between vehicle and DEX-exposed offspring indicated by asterisk (B and D). D, DEX; M, male; V, vehicle; F, female.
Relative comparison of plasma IGF-I was performed by Western blotting followed by densitometric analysis and was employed as a marker of GH axis activity. Two-way ANOVA (DEX exposure × sex) of the relative IGF-I immunoreactivity revealed an effect of DEX treatment [F(1,20) = 5.753, P = 0.0263] on circulating levels of IGF-I in blood samples taken from the high-fat diet cohort (Fig. 4, C and D). Furthermore, post hoc analysis of IGF-I immunoreactivity from blood collected from the high-fat cohort revealed a decrease in circulating IGF-I in the female but not male offspring in response to the fetal DEX exposure (Fig. 4D). Similar analysis of plasma harvested from offspring weaned onto a standard chow diet revealed no differences in the amount of circulating IGF-I (Fig. 4, E and F).
Hypothalamic gene expression
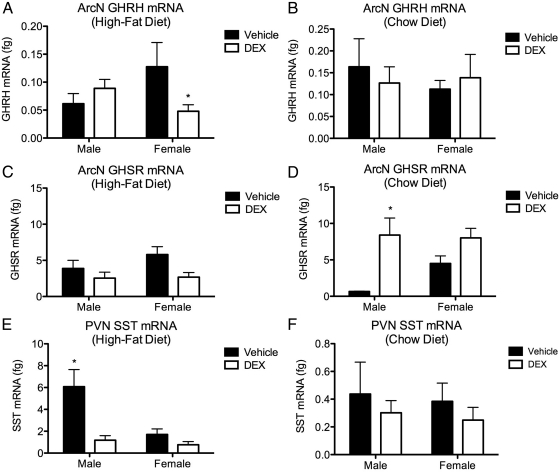
Real-time qPCR was used to measure gene expression of GH-regulating neuropeptides in the PVN and ArcN of the hypothalamus harvested from adult offspring (PND60–PND65). Two-way ANOVA (DEX exposure × sex) of Ghrh mRNA in ArcN harvested from offspring weaned onto the high-fat diet did not reveal a main effect from either fetal DEX exposure or sex. However, a significant interaction between these variables on Ghrh expression was observed [F(1,6) = 6.040, P = 0.0493]. Post hoc analysis of these same data revealed a significant decrease in the expression of Ghrh in the female, but not male, ArcN (Fig. 5A). Similar analysis of Ghrh expression in ArcN harvested from the chow-fed offspring did not reveal any significant differences (Fig. 5B).
Fig. 5.
Sex differences in hypothalamic gene expression in response to fetal DEX exposure. Exposure to DEX during late gestation decreases the expression of GHRH mRNA in the ArcN harvested from female, but not male, offspring after weaning onto a high-fat diet (A). This effect was not observed in any offspring weaned onto the chow diet (B). Fetal DEX exposure did not affect GHSR expression in rats weaned onto the high fat diet (C), however, expression of this gene was increased by DEX exposure in male offspring weaned onto the chow diet (D). Fetal DEX exposure decreases expression of SST transcript in adult male, but not female, offspring that had been weaned onto a high-fat diet (E). No effect on SST was observed in offspring weaned onto the chow diet (F). Gene expression is reported as mean ± sem of six brains, with significance between vehicle and DEX-exposed offspring indicated by asterisk.
Expression of GH secretagogue receptor (Ghsr) in the ArcN harvested from offspring weaned onto the high-fat diet was also compared by two-way ANOVA (DEX exposure × sex), which revealed a main effect of DEX exposure on the expression of this gene [F(1,6) = 6.312, P = 0.0458], although post hoc comparison of these data did not reveal any significant decreases in expression of Ghsr in either sex (Fig. 5C). Similarly, two-way ANOVA (DEX exposure × sex) of ArcN Ghsr harvested from the chow-fed cohort also revealed a main effect from fetal DEX exposure [F(1,8) = 9.473, P = 0.0152]. However, in this cohort, post hoc analysis did indicate an increase in Ghsr expression in DEX-exposed males, whereas a similar increase was not observed in the females (Fig. 5D).
The expression of somatostatin (Sst) mRNA in the PVN was also compared among samples harvested from the high-fat cohort using two-way ANOVA (DEX exposure × sex), revealing an effect of fetal DEX exposure [F(1,5) = 13.96, P = 0.0135] and the sex of the offspring [F(1,5) = 9.40, P = 0.0279] on the expression of this gene (Fig. 5E). Post hoc analysis of these data also indicated that expression of Sst was suppressed in the DEX-exposed males, whereas no effect was observed in the female offspring (Fig. 5E). Two-way ANOVA (DEX exposure × sex) of Sst expression in the PVN harvested from offspring weaned onto the chow diet did not reveal any differential expression of this gene (Fig. 5F).
Hepatic gene expression
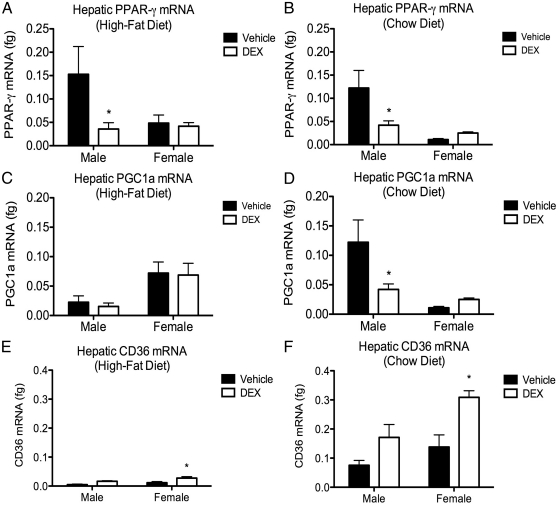
The expression of genes involved in hepatic lipid homeostasis was measured adult (PND60–PND65) tissue using RT-qPCR, and comparisons were made by two-way ANOVA (fetal DEX exposure × sex). Peroxisome proliferator-activated receptor-γ (Ppar-γ) expression in offspring weaned onto the high-fat diet revealed a main effect of fetal DEX exposure [F(1,18) = 5.307, P = 0.0409] on gene expression, and post hoc analysis of these data also indicated a significant decrease in Ppar-γ expression in the DEX-exposed male, but not female, offspring when compared with their respective vehicle-treated controls (Fig. 6A). Analysis of the chow-fed cohort revealed an interaction between fetal DEX exposure and sex on Ppar-γ expression [F(1,18) = 4.852, P = 0.409], as well as a decrease in Ppar-γ expression in the male, but not female, offspring (Fig. 6B).
Fig. 6.
Effects of fetal DEX exposure on hepatic gene expression. Fetal exposure to DEX resulted in a significant decrease in the expression of Ppar-γ in male, but not female, offspring regardless of diet (A and B). Expression of mRNA encoding the PPAR-γ interacting protein PGC1a was similarly decreased in male offspring that were exposed during late gestation to DEX and weaned onto a chow diet. However, no effect was observed in the female offspring (C). The effect of DEX on expression of Pgc1a was not observed in either male or female offspring that were weaned onto the high-fat diet (D). Expression of the fatty acid transporter CD36, which is inversely regulated by GH, was influenced by fetal DEX exposure resulting in significantly elevated transcript in female, but not male, offspring weaned onto either the high-fat (E) or chow (F) diets. Gene expression is reported as mean ± sem of no less than six animals with significance between vehicle and DEX-exposed offspring indicated by asterisk.
Analysis PPAR-γ coactivator protein-α (Pgc1α) expression indicated a main effect of sex [F(1,16) = 8.264, P = 0.0110], but not fetal DEX exposure, on expression of this gene in tissue harvested from rats weaned onto the high-fat diet (Fig. 6C). Conversely, two-way ANOVA of Pgc1α in tissue harvested from the chow-fed rats revealed a main effect of fetal DEX exposure [F(1,20) = 10.77, P = 0.0037], but not sex on this gene, and post hoc comparison indicated a significant decrease in Pgc1α expression in the male, but not female, offspring (Fig. 6D).
The mRNA for the fatty acid transporter CD36 was similarly measured, revealing a significant effect of fetal DEX exposure [F(1,13) = 10.96, P = 0.0056] on the hepatic expression of this gene in animals consuming the high-fat diet, whereas post hoc comparison indicated a significant induction of CD36 in the female, but not male, rats (Fig. 6E). Comparison of CD36 in the chow-fed cohort revealed main effects of both fetal DEX exposure [F(1,17) = 14.34, P = 0.0015] and sex [F(1,13) = 8.095, P = 0.0112] on the expression of this gene, and post hoc analysis showed a significant increase in expression of CD36 in the fetal DEX-exposed female, but not male, offspring (Fig. 6F).
Discussion
Fetal exposure to excess GC has been linked in humans and animals to decreased size at birth. Although the long-term effects of clinical GC administration in infants remain unknown, low birth weight in humans is documented to predict metabolic disease in adulthood (2), and observations from animal models of prenatal GC exposure have likewise revealed the increased potential to develop an array of metabolic disturbances in adulthood (7, 8, 23, 24).
The model for GC exposure used in this study resulted in reduced body weight at birth that persisted into adulthood (Fig. 1) and is consistent with published reports from studies employing similar synthetic GC exposure paradigms (7, 11). Furthermore, similar studies have concluded that this dosing regimen does not result in abnormal nutrition or maternal care that may impact the offspring, suggesting that the reduced birth weight observed after maternal GC administration is due to direct actions of these steroids on the developing fetus (18, 25). In addition to reduced body weight, our model of fetal DEX exposure also resulted in decreased axial growth over the first 21 d of life, as well as decreased femur and tibia bone length (Fig. 2), suggesting that decreased size observed in the DEX-exposed offspring may be due in part to reduced GH signaling (26).
Interestingly, although predisposition to obesity is predicted by decreased size at birth, fetal DEX exposure did not lead to excess abdominal fat pad accumulation in either sex after chronic consumption of a high-fat diet (Supplemental Fig. 2) and actually limited weight gain in the female offspring that were weaned onto a high-fat diet (Fig. 1). Although the failure of fetal DEX exposure to increase susceptibility to diet-induced obesity was unexpected in light of the reduced birth weight that results from this exposure, our findings are in agreement with an existing report by Drake et al. (11), which also documented increased diet-induced hepatic triglyceride accumulation in the prenatal GC-exposed male offspring, although females were not examined.
Histologic evaluation of hepatic tissue from the offspring weaned onto the standard chow diet did not display any overt pathology, regardless of sex or fetal DEX exposure. However, hepatic macrosteatosis was strikingly prevalent in the female offspring that had been exposed to DEX during late gestation and then weaned onto the high-fat diet. Nonalcoholic steatosis in humans and Sprague Dawley rats is typically associated with fat vesicle accumulation surrounding the central vein of the classic hepatic functional lobule (27), in which blood enters through the portal triad, percolates through the hepatocyte-lined sinusoids, and exits the lobule at the central vein. Interestingly, centrilobular pathology in our rats was very limited. However, elevated periportal steatosis, which is associated with oxidative-type injury, such as iron overload (28), was significantly elevated in the female offspring (Fig. 3).
To date, no mechanism explaining the elevation in liver triglycerides after fetal exposure to DEX has been reported. However, the reduced birth weight and reduced axial and long bone length that we have observed suggest an underlying GH signaling deficit resulting from fetal DEX exposure. Given the established role for GH deficits in the development of hepatic steatosis in rodent models (13, 14), the possibility exists that suppressed GH axis signaling may play a role in the elevated liver pathology that we observe after fetal DEX exposure. Unfortunately, the inherent uncertainty in interpreting direct GH measurements due to the pulsatile nature of this hormone precludes acquiring meaningful data after single point measures of blood levels. However, because GH acts through hepatic signal transducer and activator of transcription to induce the expression of Igf-1, we measured the hepatic expression of this gene as a marker of GH signaling; thus, demonstrating a sex-specific decrease in Igf-1 expression in fetal DEX-exposed females that had been weaned onto the high-fat diet (Fig. 4A). Curiously, no effect was observed in the chow-fed offspring, suggesting the possibility of a “two-hit” scenario, in which fetal DEX exposure primes the female offspring for a more severe response to a second challenge later in life. Because blood IGF-I levels are commonly employed as markers of GH signaling, we also measured immunoreactive IGF-I in plasma harvested from these animals, which similarly demonstrated a sex-specific decrease in circulating IGF-I in response to fetal DEX exposure followed by the high-fat diet (Fig. 4, C–F). Interestingly, in a transgenic model of liver-specific GH receptor deletion, circulating IGF-I was decreased. However, reduced tibial growth was not observed (14). Therefore, it was concluded that the modest IGF-I decrease observed was insufficient to cause reductions in bone length, because IGF-I augments direct GH action on the growth of bones (14). These data support our hypothesis of a direct GH deficit, because this would result in both IGF-I suppression and reduction in long bone growth, whereas modest IGF-I reduction alone would not be expected to impact the bone growth.
Fetal exposure to excess GC is recognized to influence development of the hypothalamus (23). We therefore examined hypothalamic transcript levels of the neuropeptides GHRH (stimulatory), GHSR (stimulatory), and SST (inhibitory), which influence GH release. In adult offspring that had been weaned onto the high-fat diet, it was observed that exposure to DEX during gestation significantly reduced the expression Ghrh mRNA in the ArcN in the female, but not male, offspring (Fig. 5A). This effect was not observed in any group that was weaned onto the chow diet. Because GHRH-mediated secretion of GH is augmented by GHSR (29), we also measured expression of the gene encoding this receptor in the ArcN (Fig. 5, C and D). Measurement of Ghsr revealed a significant effect of fetal DEX exposure on the expression of this gene in offspring weaned onto the high-fat diet. However, because no sex differences were observed, it is not clear whether the expression of this gene plays a role in the female-specific potentiation of liver injury that we have observed. Expression of Sst mRNA in the PVN harvested from offspring weaned onto the high-fat diet was significantly reduced in the male animals that had been exposed during gestation to DEX, but this effect was not observed in the females (Fig. 5E). As with Ghrh mRNA, no effects on Sst mRNA expression in either male or female offspring were observed in the PVN isolated from animals weaned onto the chow diet (Fig. 5F). These results represent a significant finding based on a previous report, which demonstrated that destruction of the hypothalamic GHRH neurons with the neurotoxin monosodium glutamate, which left the SST-expressing neurons intact, resulted in a 90% inhibition of GH release in both male and female rats (30). This previous report thus indicates that GHRH expression in the ArcN may play an important role in GH secretion. Interestingly, suppression of Sst in the male offspring (Fig. 5E) would be predicted to result in elevated IGF-I, but this effect was not observed. A possible explanation for this phenomenon may be explained by different sensitivity of male and female pituitary glands to inhibition of GH release by SST. Specifically, it has been documented that increases in pituitary GH mRNA expression do not occur in mice lacking Sst, whereas GH expression is elevated in females lacking this gene (31). The gene expression data presented here suggest deleterious modification of the female hypothalamus in offspring exposed during fetal development to DEX and later challenged with a high-fat diet, whereas male offspring appear to exhibit an adaptive measure in the decreased expression of SST expression, potentially compensating for suboptimal stimulation of GH release.
In an effort to elucidate events occurring at the level the hepatic tissue that may underlie our observations, we measured both Ppar-γ and Pgc1a, because these genes were reported to be differentially regulated in a previous study (11). As with the study by Drake et al. (11), hepatic Ppar-γ expression was decreased in the male offspring after gestational DEX exposure (Fig. 4, A and B). However, no effect on the expression of this gene was observed in the female offspring. We similarly demonstrate a decrease in Pgc1a expression after gestational DEX exposure in male offspring weaned onto the high-fat diet, but this effect was not conserved in the chow-fed offspring. Although our data regarding Ppar-γ and Pgc1a fail to describe a mechanism behind the fetal DEX-induced potentiation of diet-induced liver disease, an additional experiment measuring the expression of CD36 gene expression suggests that GH deficits may result in an increase in this enzyme, leading to elevated hepatic steatosis. Indeed, elevations in hepatic CD36 are documented to accompany observations of insulin resistance, hyperinsulinaemia, and fatty liver disease in humans (32). Supporting the notion that CD36 may play a role in our observations is a report by Fan et al. (14), which demonstrates elevated levels of this enzyme in GH-receptor knockout mice, which occurs concurrently with fatty liver. Elevated CD36 may therefore play a key role in our findings, in that it is negatively regulated by GH, and elevations in this enzyme are linked to the development of hepatic steatosis. Changes in the expression of fatty acid synthase and PPAR-α in response to fetal DEX were also measured, given the role for these enzymes in hepatic lipid homeostasis. However, no differences were observed (data not shown).
In summary, the results presented here support existing studies that document the development of metabolic disease in adulthood after fetal overexposure to the synthetic GC DEX. We report the potentiation of diet-induced hepatic injury, particularly in female offspring, in rats exposed to DEX during the final weeks of gestation and weaned onto a high-fat diet in adulthood. Our studies indicate that this is possibly as a consequence of GH signaling deficits. Furthermore, our data suggest that a GH deficit may stem from hypothalamic dysregulation. However, an alternate explanation may be abnormal estrogen secretion, because elevated estrogen in the females can inhibit GH signaling through disruption of signal transducer and activator of transcription 5-mediated IGF-I expression (33). Collectively, our results suggest that fetal DEX exposure during late gestation in rats may program the physiology of the offspring such that greater susceptibility in females to pathology arising from suboptimal GH signaling occurs.
Supplementary Material
Acknowledgments
We thank Dr. Stuart Tobet and Dr. Jill M. Goldstein for their editorial assistance in the preparation of this manuscript.
Present address for C.D.F.: Department of Anatomy, Physiology and Pharmacology, College of Veterinary Medicine, Auburn University, Auburn, Alabama 36849-5518.
This work was supported by National Institutes of Health Grants NS039951 (to R.J.H.) and MH082679 (to R.J.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ArcN
- Arcuate nucleus
- DEX
- dexamethasone
- GC
- glucocorticoid
- GD
- gestational day
- Ghsr
- GH secretagogue receptor
- NAFLD
- nonalcoholic fatty liver disease
- Pgc1α
- PPAR-γ coactivator protein-α
- PND
- postnatal day
- Ppar-γ
- peroxisome proliferator-activated receptor-γ
- PVN
- paraventricular nucleus
- qPCR
- quantitative PCR
- ROI
- regions of interest
- Sst
- somatostatin.
References
- 1. Hales CN, Barker DJ. 1992. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 35:595–601 [DOI] [PubMed] [Google Scholar]
- 2. Hales CN, Barker DJ, Clark PM, Cox LJ, Fall C, Osmond C, Winter PD. 1991. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 303:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Painter RC, Roseboom TJ, Bleker OP. 2005. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol 20:345–352 [DOI] [PubMed] [Google Scholar]
- 4. Watterberg KL. 2010. Policy statement—postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 126:800–808 [DOI] [PubMed] [Google Scholar]
- 5. Norberg H, Stålnacke J, Heijtz RD, Smedler AC, Nyman M, Forssberg H, Norman M. 2011. Antenatal corticosteroids for preterm birth: dose-dependent reduction in birthweight, length and head circumference. Acta Paediatr 100:364–369 [DOI] [PubMed] [Google Scholar]
- 6. Barker DJ. 2006. Adult consequences of fetal growth restriction. Clin Obstet Gynecol 49:270–283 [DOI] [PubMed] [Google Scholar]
- 7. Cleasby ME, Kelly PA, Walker BR, Seckl JR. 2003. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology 144:999–1007 [DOI] [PubMed] [Google Scholar]
- 8. Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. 1998. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest 101:2174–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. 2008. Prenatal dexamethasone ‘programmes’ hypotension, but stress-induced hypertension in adult offspring. J Endocrinol 196:343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tamashiro KL, Terrillion CE, Hyun J, Koenig JI, Moran TH. 2009. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58:1116–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. 2010. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology 151:1581–1587 [DOI] [PubMed] [Google Scholar]
- 12. Roberts SE, Goldacre MJ, Yeates D. 2005. Trends in mortality after hospital admission for liver cirrhosis in an English population from 1968 to 1999. Gut 54:1615–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qin Y, Tian YP. 2010. Preventive effects of chronic exogenous growth hormone levels on diet-induced hepatic steatosis in rats. Lipids Health Dis 9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fan Y, Menon RK, Cohen P, Hwang D, Clemens T, DiGirolamo DJ, Kopchick JJ, Le Roith D, Trucco M, Sperling MA. 2009. Liver-specific deletion of the growth hormone receptor reveals essential role of growth hormone signaling in hepatic lipid metabolism. J Biol Chem 284:19937–19944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Laron Z, Ginsberg S, Webb M. 2008. Nonalcoholic fatty liver in patients with Laron syndrome and GH gene deletion—preliminary report. Growth Horm IGF Res 18:434–438 [DOI] [PubMed] [Google Scholar]
- 16. Duksal F, Kilic I, Tufan AC, Akdogan I. 2009. Effects of different corticosteroids on the brain weight and hippocampal neuronal loss in rats. Brain Res 1250:75–80 [DOI] [PubMed] [Google Scholar]
- 17. Kreider ML, Tate CA, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. 2006. Lasting effects of developmental dexamethasone treatment on neural cell number and size, synaptic activity, and cell signaling: critical periods of vulnerability, dose-effect relationships, regional targets, and sex selectivity. Neuropsychopharmacology 31:12–35 [DOI] [PubMed] [Google Scholar]
- 18. Nyirenda MJ, Welberg LA, Seckl JR. 2001. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? J Endocrinol 170:653–660 [DOI] [PubMed] [Google Scholar]
- 19. Iimuro Y, Frankenberg MV, Arteel GE, Bradford BU, Wall CA, Thurman RG. 1997. Female rats exhibit greater susceptibility to early alcohol-induced liver injury than males. Am J Physiol 272:G1186–G1194 [DOI] [PubMed] [Google Scholar]
- 20. Stavropoulou A, Halapas A, Sourla A, Philippou A, Papageorgiou E, Papalois A, Koutsilieris M. 2009. IGF-1 expression in infarcted myocardium and MGF E peptide actions in rat cardiomyocytes in vitro. Mol Med 15:127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paxinos G, Watson C. 2007. The rat brain in stereotaxic coordinates. Amsterdam: Elsevier; [DOI] [PubMed] [Google Scholar]
- 22. Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159 [DOI] [PubMed] [Google Scholar]
- 23. de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. 2007. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest 117:1058–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nyirenda MJ, Carter R, Tang JI, de Vries A, Schlumbohm C, Hillier SG, Streit F, Oellerich M, Armstrong VW, Fuchs E, Seckl JR. 2009. Prenatal programming of metabolic syndrome in the common marmoset is associated with increased expression of 11β-hydroxysteroid dehydrogenase type 1. Diabetes 58:2873–2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. LaBorde JB, Hansen DK, Young JF, Sheehan DM, Holson RR. 1992. Prenatal dexamethasone exposure in rats: effects of dose, age at exposure, and drug-induced hypophagia on malformations and fetal organ weights. Fundam Appl Toxicol 19:545–554 [DOI] [PubMed] [Google Scholar]
- 26. Westwood M, Maqsood AR, Solomon M, Whatmore AJ, Davis JR, Baxter RC, Gevers EF, Robinson IC, Clayton PE. 2010. The effect of different patterns of growth hormone administration on the IGF axis and somatic and skeletal growth of the dwarf rat. Am J Physiol Endocrinol Metab 298:E467–E476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kučera O, Garnol T, Lotková H, Staňková P, Mazurová Y, Hroch M, Bolehovská R, Roušar T, Červinková Z. 2011. The effect of rat strain, diet composition and feeding period on the development of a nutritional model of non-alcoholic fatty liver disease in rats. Physiol Res 60:317–328 [DOI] [PubMed] [Google Scholar]
- 28. Turlin B, Mendler MH, Moirand R, Guyader D, Guillygomarc'h A, Deugnier Y. 2001. Histologic features of the liver in insulin resistance-associated iron overload. A study of 139 patients. Am J Clin Pathol 116:263–270 [DOI] [PubMed] [Google Scholar]
- 29. Lall S, Balthasar N, Carmignac D, Magoulas C, Sesay A, Houston P, Mathers K, Robinson I. 2004. Physiological studies of transgenic mice overexpressing growth hormone (GH) secretagogue receptor 1A in GH-releasing hormone neurons. Endocrinology 145:1602–1611 [DOI] [PubMed] [Google Scholar]
- 30. Maiter D, Underwood LE, Martin JB, Koenig JI. 1991. Neonatal treatment with monosodium glutamate: effects of prolonged growth hormone (GH)-releasing hormone deficiency on pulsatile GH secretion and growth in female rats. Endocrinology 128:1100–1106 [DOI] [PubMed] [Google Scholar]
- 31. Luque RM, Kineman RD. 2007. Gender-dependent role of endogenous somatostatin in regulating growth hormone-axis function in mice. Endocrinology 148:5998–6006 [DOI] [PubMed] [Google Scholar]
- 32. Miquilena-Colina ME, Lima-Cabello E, Sánchez-Campos S, García-Mediavilla MV, Fernández-Bermejo M, Lozano-Rodríguez T, Vargas-Castrillón J, Buqué X, Ochoa B, Aspichueta P, González-Gallego J, García-Monzón C. 2011. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 60:1394–1402 [DOI] [PubMed] [Google Scholar]
- 33. Leung KC, Doyle N, Ballesteros M, Sjogren K, Watts CK, Low TH, Leong GM, Ross RJ, Ho KK. 2003. Estrogen inhibits GH signaling by suppressing GH-induced JAK2 phosphorylation, an effect mediated by SOCS-2. Proc Natl Acad Sci USA 100:1016–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.