Abstract
In the bone marrow cavity, adipocyte numbers increase, whereas osteoblast progenitor numbers decrease with aging. Because adipocytes and osteoblasts share a common progenitor, it is possible that this shift is due to an increase in adipocyte-lineage cells at the expense of osteoblast-lineage commitment. Estrogens inhibit adipocyte differentiation, and in both men and women, circulating estrogens correlate with bone loss with aging. In bone cells, estrogens stimulate expression of TGF-β and suppress mesenchymal cell adipogenesis. Using a tripotential mesenchymal cell line, we have examined whether estradiol suppression of adipocyte differentiation is due to stimulation of TGF-β and the mechanism by which TGF-β suppresses adipogenesis. We observed that estradiol-mediated suppression of adipogenic gene expression required at least 48 h treatment. TGF-β expression increased within 24 h of estradiol treatment, and TGF-β inhibition reversed estradiol influences on adipogenesis and adipocyte gene expression. Connective tissue growth factor (CTGF) mediates TGF-β suppression of adipogenesis in mouse 3T3-L1 cells. CTGF expression was induced within 24 h of TGF-β treatment, whereas estradiol-mediated induction required 48 h treatment. Moreover, estradiol-mediated induction of CTGF was abrogated by TGF-β inhibition. These data support that estradiol effects on adipogenesis involves TGF-β induction, which then induces CTGF to suppress adipogenesis.
In both men and women, aging leads to significant loss of both trabecular and cortical bone (1, 2). One contributing factor to age-related bone loss may be alterations in the osteoblast progenitor pool, which has led to consideration of factors that could influence the commitment of mesenchymal progenitors to differentiate toward the osteoblast lineage. Evidence in many model systems has supported the concept that osteoblasts and adipocytes develop from a common progenitor pool (3–9). With aging, the net loss of bone is paralleled by an increase in the adiposity of the marrow in both men and women (3, 10). These observations have generated the hypothesis that the increase in adipocytes observed in the marrow with aging leads to a concomitant decrease in available progenitors to replenish the osteoblast lineage, contributing to the net loss of bone observed with aging.
The search for effective therapies to counter bone loss with aging will likely benefit from understanding what drives this shift in progenitors toward the adipocyte lineage. Although it is well known that loss of estrogens accelerates bone loss in women, it has been found that reduced estradiol levels leads to bone loss in men as well (11). Several studies have supported that estrogens influence marrow adiposity. Astudillo et al. (12) observed that the bone marrow samples of osteoporotic women contain more adipocytes than aged matched controls, indicating a correlation between circulating levels of estrogens and bone marrow adipocyte numbers. Syed et al. (13) observed that a 1-yr treatment of osteoporotic postmenopausal women with estradiol led to a significant decrease in bone marrow adipocyte volume and prevented an increase in adipocyte number compared with placebo-treated controls. In ovariectomized rats, there was a significant increase in bone marrow adipocytes that accompanied bone loss (14). These in vivo studies, which indicate a role for estrogens in modulating adipogenesis, have been supported by in vitro studies. Okazaki et al. (15) generated cell lines overexpressing either estrogen receptor α or estrogen receptor β in the mouse tripotential bone marrow stromal cell line ST2. They observed that bone morphogenetic protein 2 (BMP2) induced both osteogenic and adipogenic differentiation in these cell lines and that estradiol suppressed basal and BMP2-induced adipogenesis in both cell lines. This effect was blocked by the estrogen receptor antagonist ICI182780. Similarly, in the clonal mouse cell line KS483, estradiol stimulated osteoblastic cell differentiation while inhibiting adipocyte differentiation when the cells were treated with isobutylmethylxanthine, dexamethasone, and insulin (16). As above, this response was estrogen receptor dependent.
Heim et al. (17) found that the phytoestrogen genistein suppressed expression of peroxisome proliferator-activated receptor isoform γ (PPARγ) gene, the master regulator of adipocyte differentiation. They also observed that genistein induced TGF-β1 gene expression in human bone marrow stromal cells and blocking TGF-β partially blocked genistein-mediated suppression of TGF-β gene expression. Phytoestrogens bind weakly to the estrogen receptor and have physiological influences through multiple other pathways including inhibiting enzymes involved in steroid biosynthesis, direct binding to and activating peroxisome proliferator regulators, effects on natural killer cells, inhibition of tyrosine kinases, and inhibiting metastasis (reviewed in Ref. 18). Thus, influences of phytoestrogens and estrogens are not identical and conclusions about the mechanisms by which estrogens impact cells cannot be determined without direct studies of influences of estrogens.
We and others have observed that estradiol induces TGF-β in osteoblasts and osteoclasts (19–22). TGF-β has been shown to inhibit adipocyte formation in bone marrow mesenchymal progenitor cells and NIH3T3 cells overexpressing TGF-β (23, 24). These observations have led us to examine whether the suppressive effects of estradiol on adipogenesis are mediated by TGF-β induction. Because connective tissue growth factor (CTGF) has been recognized as a TGF-β target gene (25) and CTGF inhibits adipocyte differentiation (26), we have examined whether CTGF is induced by estradiol through induction of TGF-β to suppress adipogenesis in tripotential ST2 cells that do not overexpress either estrogen receptor.
Materials and Methods
Unless otherwise indicated, all chemicals were from Sigma Chemical Co. (St Louis, MO). TGF-β1, -2, and -3 were purchased from R&D Systems (Minneapolis, MN) and suspended in PBS/0.01% BSA (vehicle). Rosiglitazone was purchased from Cayman Chemical Co. (Ann Arbor, MI) and suspended in dimethylsulfoxide (DMSO). Estradiol was suspended in ethanol.
Cell culture
ST2 cells are mouse bone marrow-derived mesenchymal cells. Early-passage ST2 cells (a gift from Dr. Ryo Okazaki) were plated at 1 × 105 cells per well in six-well plates in α-MEM supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and antibiotic/antimycotic. Twenty-four hours later, cells were treated as per individual experiments as detailed in the figure legends. Estradiol treatments were at 1 × 10−8 m, TGF-β treatments were at 2 ng/ml, TGF-β inhibitor (SB 431542) treatments were at 2 × 10−7 m, and rosiglitazone treatment was at 1.12 × 10−6 m. Cells were re-fed on d 3, and the experiments concluded on d 6 as outlined in the figure legends. The hFOB/ER9 cell line are human osteoblastic cells containing the temperature-sensitive T-antigen expression vector with the neomycin resistance gene and the wild-type estrogen receptor α expression vector with the hygromycin B resistance gene (27). Cells were cultured in a 1:1 mixture of phenol red-free DMEM/F12 containing 10% charcoal-stripped fetal bovine serum supplemented with 300 μg/ml geneticin and 100 μg/ml hygromycin. The cells were maintained at 34 C. For study, the cells were plated at 1 × 105 cells per well in 24-well plates in the above media and cultured at 34 C overnight. The next day, they were fed fresh media as outlined in the figure legend. Cells were re-fed on d 3 and fixed for adipocyte staining and quantitation on d 6.
Adipocyte staining
On d 6, media were aspirated and cells rinsed with PBS before fixing in 10% formalin in PBS at room temperature. The fixative was removed and the wells washed with 60% isopropanol, followed by air drying. Oil Red O working solution (2.1 mg/ml in 40% isopropanol) was added for 10 min. The stain was removed and the cells washed with water before drying and subsequent imaging. The Oil Red O was extracted with 100% isopropanol for at least 10 min. OD was read at an absorbance at 500 nm.
Quantitative real-time PCR
ST2 cells were treated as detailed in the figure legends. Cells were rinsed with PBS and RNA harvested using Trizol reagent according to the product literature (Invitrogen, Carlsbad, CA). After quantitation, cDNA was synthesized, and real-time PCR analysis carried out as we have reported using tubulin expression as the reference gene (28).
The following primers were used for real-time PCR analysis (sense and antisense, respectively): PPARγ, 5′-GAGGAGTCCCTTCCCTCATC-3′ and 5′-TCCTCGAAGGTTAAGGCTGA-3′; C/EBPα, 5′-TGGACAAGAACAGCAACGAG-3′ and 5′-TCACTGGTCAACTCCAGCAC-3′; C/EBPβ, 5′-GTTTCGGGACTTGATGCAAT-3′ and 5′-GCCCGGCTAGACAGTTACAC-3′; LPL, 5′-GTCACAATTGTCCCATGCTG-3′ and 5′-GCTAAGGCAGGATGGTTGAG-3′; adipsin, 5′-CAAGCGATGGTATGATGTGC-3′ and 5′-CTCGTATTGCAAGGGTAGGG-3′; leptin, 5′-GACCAGACTGGATCCTCAGC-3′ and 5′-AGCACCACAAAACCTGATCC-3′; TGF-β1, 5′-GTCCTTGCCCTCTACAACCA-3′ and 5′-GTTGGACAACTGCTCCACCT-3′; TGF-β2, 5′-CATGAGCTACCTGGGTCCAT-3′ and 5′-GACGCAGAAAAGGCTGAAAC-3′; TGF-β3, 5′-CAGAGGTGGTGGGGTAGAGA-3′ and 5′-CATTGCCACTCACAATGTCC-3′; CTGF, 5′-TGCTGTGCAGGTGATAAAGC-3′ and 5′-AAGGCCATTTGTTCACCAAC-3′; estrogen receptor α, 5′-ATGATGAAAGGCGGCATACG-3′ and 5′-TCTGACGCTTGTGCTTCAACAT-3′; estrogen receptor β, 5′-TGCCTCTTCTCACAAGGATTTTT-3′ and 5′-TGGTGAGTTTTTGATCTCCATGTC-3′; activin, 5′-CGGAAGAGTACCTGGCACAT-3′ and 5′-GCCCAGAAGCACTAGACTGG-3′; and tubulin, 5′-CTGCTCATCAGCAAGATCAGAG-3′ and 5′-GCATTATAGGGCTCCACCACAG-3′.
Statistics
Each experiment had at least three replicates and was repeated at least three times. These results are representative of the repeats. Data were analyzed using a one-way ANOVA compared with controls as indicated in each figure legend and are presented as mean ± sem. Significance was determined at P < 0.05 using KaleidaGraph software.
Results
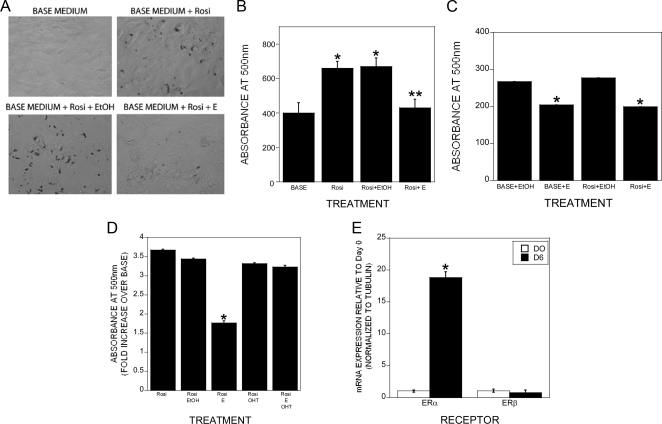
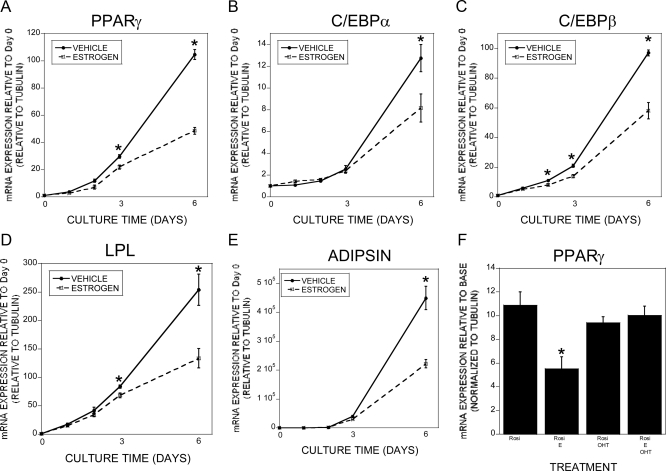
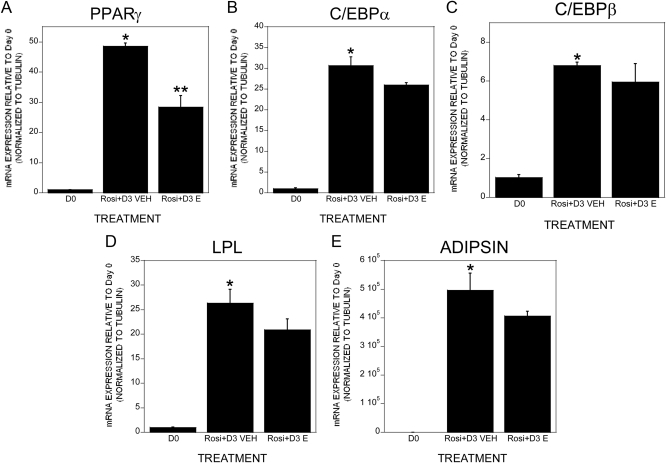
We examined the tripotential ST2 cells for rosiglitazone-induced adipogenesis and estradiol effects on this response. Rosiglitazone-induced adipogenesis as determined by staining with Oil Red O followed by elution was observed within 6 d of treatment and 1 × 10−8 m estradiol significantly impaired this response (Fig. 1, A and B). This response was observed at 1 × 10−9 but not 1 × 10−10 m (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org). We examined the human osteoblastic cell line hFOB/ER9 for rosiglitazone-driven adipogenesis and the effects of estrogen on adipogenesis (Fig. 1C). Surprisingly, there was a low level of spontaneous adipogenesis, and rosiglitazone did not further enhance it. In both base media and rosiglitazone-treated cultures, estrogen significantly suppressed adipogenesis. Because we examined the parental ST2 cell line without overexpression of exogenous estrogen receptors and observed a significant estradiol response, we evaluated the influence of the estrogen receptor antagonist 4-hydroxytamoxifen on adipogenesis and found that estradiol effects on adipogenesis were suppressed by cotreatment with the antagonist (Fig. 1D). We therefore examined the influences of rosiglitazone treatment on estrogen receptor expression. Six days of culture significantly induced expression of estrogen receptor α but not β in the ST2 cells (Fig. 1E). The timing of estradiol influences on rosiglitazone induction of adipogenic genes was examined by quantitative real-time PCR. As expected, rosiglitazone rapidly induced expression of PPARγ, CCAAT enhancer-binding protein α (C/EBPα), C/EBPβ, lipoprotein lipase (LPL), and adipsin (Fig. 2, A–E). Cotreatment with estradiol did not significantly suppress adipogenic gene expression until 2 or 3 d of treatment, indicating that estradiol's suppressive effects could be indirect through induction of other effector genes. Treatment with 4-hydroxytamoxifen blocked estradiol-mediated suppression of PPARγ (Fig. 2F). We did not detect a significant influence of estradiol on leptin expression (rosiglitazone: 2.65 ± 0.44-fold increase; rosiglitazone plus estrogen: 1.73 ± 0.21-fold increase; P = 0.13 between treatments). TGF-β treatment results in a clear bifurcation in gene expression because adipocytic gene expression was suppressed under osteogenic conditions and expression of osteoblastic genes was suppressed under adipogenic conditions (Supplemental Fig. 2). We next examined whether delaying estradiol treatment until the cells were committed to the adipogenic pathway influenced estradiol-suppressive effects. PPARγ expression was somewhat suppressed by delayed estradiol treatment, whereas expression of C/EBPα, C/EBPβ, LPL, and adipsin were not similarly suppressed (Fig. 3).
Fig. 1.
Estrogen repression of adipogenesis as assessed by accumulation of neutral lipid droplets in ST2 cells. A and B, ST2 cells were cultured in base medium with or without the indicated treatments (E, 10 nm 17β-estradiol; EtOH, 0.1% ethanol; Rosi, rosiglitazone). Cultures were fed on d 3 with the same media and fixed after 6 d. Cultures were treated throughout the culture time. Cells were stained for lipid with Oil Red O as outlined. A, Image of stained cultures (×200). B, The stain was extracted and the amount of stain quantitated by measurement at OD 500 as detailed in Materials and Methods. Data are the mean of three replicates. *, P < 0.05 compared with base; **, P < 0.05 compared with rosiglitazone+EtOH. C, hFOB/ER9 cells were cultured and analyzed as outlined for the ST2 cells in A and B. *, P < 0.05 compared with EtOH treatment. D, ST2 cells were treated with base medium with rosiglitazone (Rosi), rosiglitazone and vehicle (Rosi EtOH), rosiglitazone and 10 nm 17β-estradiol (Rosi E), rosiglitazone and 100 nm 4-hydroxytamoxifen (Rosi OHT), or rosiglitazone, 10 nm 17β-estradiol, and 100 nm 4-hydroxytamoxifen (Rosi E OHT). Cells were stained and quantitated as above. *, P < 0.05 compared with Rosi. E, Cells were harvested (D0) or cultured in base plus rosiglitazone medium for 6 d with a refeeding on d 3. RNA was harvested and analyzed by real-time PCR for the indicated estrogen receptor. Expression is relative to d 0 (D0). *, P < 0.05 compared with D0.
Fig. 2.
Estradiol repression of adipogenic gene expression. A–E, ST2 cells were harvested at the beginning of culture (D0) or treated with rosiglitazone. ST2 cells cultured with rosiglitazone were cotreated with vehicle (0.1% EtOH) or 10 nm estradiol for the indicated time. RNA was harvested and analyzed by real-time PCR for the indicated mRNA. The experiment was repeated three times, and these results are representative of these experiments. *, P < 0.05 compared with vehicle at the same time point. F, ST2 cells were treated with base medium (BASE), rosiglitazone (Rosi), rosiglitazone and 10 nm 17β-estradiol (Rosi+E), rosiglitazone and 100 nm 4-hydroxytamoxifen (Rosi+OHT), or rosiglitazone, 10 nm 17β-estradiol, and 100 nm 4-hydroxytamoxifen (Rosi+E+OHT). RNA was harvested and analyzed by real-time PCR for the indicated mRNA. *, P < 0.05 compared with base medium; **, P < 0.05 compared with rosiglitazone treatment; ***, P < 0.05 compared with rosiglitazone and 17β-estradiol treatment.
Fig. 3.
A–E, Delayed estradiol treatment blunts suppression of adipogenic gene expression. ST2 cells were harvested at the beginning of culture (D0) or treated with rosiglitazone. On d 3, the cells were fed with rosiglitazone-containing medium with the addition of either vehicle (0.1% EtOH) or 10 nm 17β-estradiol until d 9 with a refeeding on d 6. RNA was harvested and analyzed by real-time PCR for the indicated mRNA. *, P < 0.05 compared with D0; **, P < 0.05 compared with either D0 or rosiglitazone plus vehicle treatment.
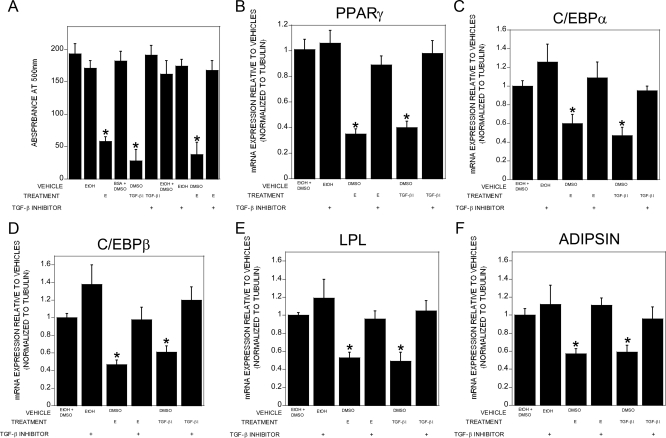
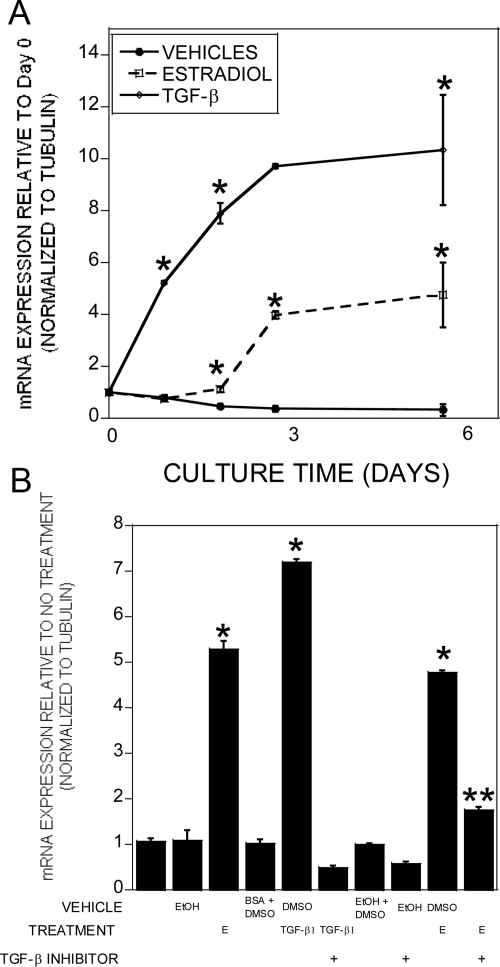
Given the observations that estradiol induces TGF-β expression in bone cells and the studies implicating TGF-β in regulating adipogenesis, we examined whether rosiglitazone-induced adipogenesis in tripotential ST2 cells could be attenuated by TGF-β and conversely whether suppression of TGF-β signaling could potentially reverse estradiol effects on the phenotype. Treatment with either TGF-β or estradiol significantly suppressed adipocyte formation (Fig. 4A). Although the TGF-β signaling inhibitor alone had no influence on adipogenesis, cotreatment with estradiol and the TGF-β inhibitor abrogated estradiol-mediated suppression of adipogenesis. Because these data indicated that estradiol-mediated stimulation of TGF-β was required to block adipocyte differentiation, we examined the influence of blocking TGF-β in the presence of estradiol on expression of adipogenic genes (Fig. 4, B–F). Treatment with the TGF-β inhibitor alone had no impact on expression of PPARγ, C/EBPα, C/EBPβ, LPL, or adipsin. However, cotreatment with estradiol and the TGF-β inhibitor eliminated the suppressive effects of estradiol on gene expression. Estradiol or TGF-β treatment also induced the osteoblastic genes alkaline phosphatase, osteocalcin, osteopontin, and Runx2 (Supplemental Fig. 3). Osterix expression was significantly induced by TGF-β, but estradiol did not significantly induce osterix in DMSO-cotreated cells. These responses were blocked by cotreatment with the TGF-β inhibitor. We were unable to detect any influence of estradiol on alkaline phosphatase staining, perhaps reflecting the inability of estradiol treatment to fully differentiate these cells (data not shown).
Fig. 4.
Estradiol effects on adipogenesis are dependent on TGF-β expression. ST2 cells were cultured in base plus rosiglitazone medium for 6 d with a refeeding on d 3 with or without the indicated treatments: 0.1% ethanol (EtOH), 0.1% DMSO, 10 nm 17β-estradiol (E), 2 ng/ml TGF-β1, and 2 × 10−7 m SB 431542 (TGF-β inhibitor). Cultures were treated throughout the culture time. A, Cultures were fixed and stained for lipid with Oil Red O, extracted, and quantitated as outlined. B–F, RNA was harvested and analyzed by real-time PCR for the indicated mRNA. Expression is relative to EtOH plus DMSO vehicles. *, P < 0.05 compared with EtOH plus DMSO vehicles.
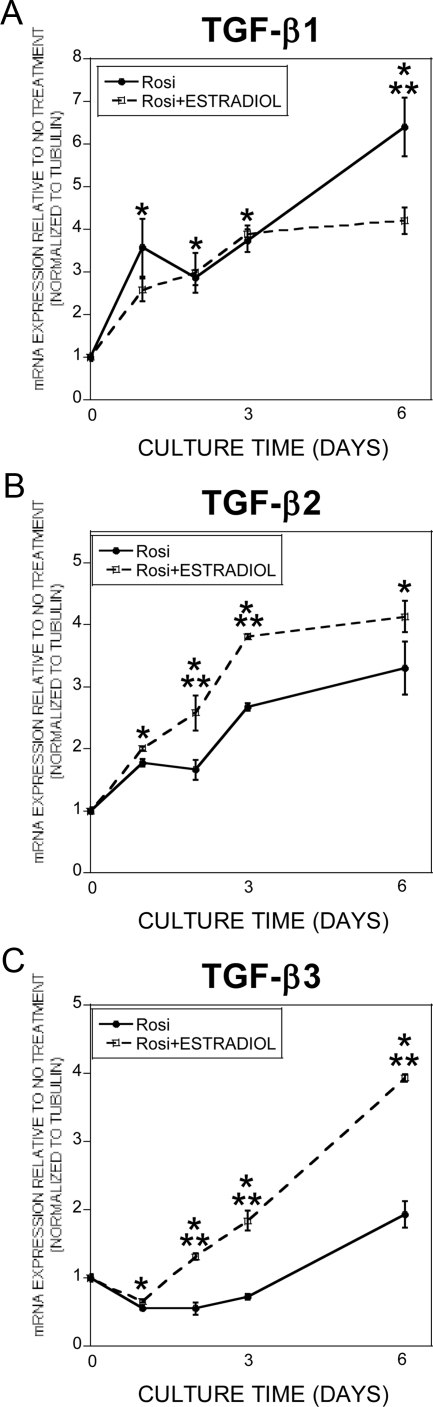
A survey of the literature indicted that CTGF is a TGF-β target gene that also has been shown to inhibit adipogenesis (29–31). We therefore compared the timing of estradiol and TGF-β influences on CTGF gene expression in adipogenic cultures (Fig. 5A). Within 24 h of TGF-β treatment CTGF gene expression was significantly elevated. In contrast, estradiol treatment required 48 h of treatment to induce CTGF gene expression. Once induced, CTGF expression remained elevated through the remaining culture time. The delay in estradiol induction of CTGF led us to examine the influence of blocking TGF-β signaling on estradiol effects on gene expression (Fig. 5B). Cotreatment with estradiol and the TGF-β inhibitor significantly reduced estradiol influences on CTGF gene expression. We next examined the timing of estradiol effects on expression of TGF-β isoforms. Expression of TGF-β1, TGF-β2, and TGF-β3 were elevated during adipogenesis (Fig. 6). Estradiol further increased expression of TGF-β2 and TGF-β3, whereas estradiol treatment suppressed TGF-β1 during the late phase of culture. Because the TGF-β inhibitor also blocks activin signaling, we examined estradiol influences on activin expression and were unable to detect any significant changes in activin expression (data not shown).
Fig. 5.
Estradiol-mediated stimulation of TGF-β leads to increased CTGF expression. A, ST2 cells harvested (d 0) or cultured in base plus rosiglitazone medium for up to 6 d with a refeeding on d 3 with or without the indicated treatments: vehicles with 0.1% ethanol plus 0.1% BSA, 10 nm 17β-estradiol, and 2 ng/ml TGF-β1. Cultures were treated throughout the culture time. RNA was harvested and analyzed by real-time PCR for relative CTGF mRNA expression compared with d 0. *, P < 0.05 compared with vehicles at the same time point. B, ST2 cells were cultured for 6 d in base plus rosiglitazone medium with or without the indicated treatments with a refeeding on d 3: 0.1% ethanol (EtOH), 0.1% DMSO, 10 nm 17β-estradiol (E), 2 ng/ml TGF-β1, and 2 × 10−7 m SB 431542 (TGF-β inhibitor). RNA was harvested and analyzed by real-time PCR for relative CTGF mRNA expression compared with no additional treatment. *, P < 0.05 compared with vehicle; **, P < 0.05 compared with 17β-estradiol without the TGF-β inhibitor.
Fig. 6.
A–C, Estradiol modulates TGF-β gene expression. ST2 cells were cultured in base plus rosiglitazone with 0.1% ethanol (Rosi) or rosiglitazone plus 10 nm 17β-estradiol for the indicated time with a refeeding on d 3. Cultures were treated throughout the culture time. RNA was harvested and analyzed by real-time PCR for the indicated TGF-β isoform. Values are normalized to d 0. *, P < 0.05 compared with d 0 for both treatments; **, P < 0.05 between rosiglitazone and rosiglitazone plus estradiol.
Discussion
Studies have documented that estrogens act through effects on bone-forming osteoblasts and on bone-resorbing osteoclast-lineage cells to control the number of osteoclasts (32, 33). In young adults, bone resorption leads to subsequent bone formation in a process that is termed coupling, and we have found that osteoclasts produce secreted factors that promote bone formation (34). After levels of estrogens drop and bone resorption increases, coupling of bone resorption to bone formation causes increased bone formation, but this increase cannot compensate for the elevated bone resorption, leading to a reduction in bone volume (35). Thus, understanding how estrogens influence bone metabolism may provide new avenues to pursue to maintain bone formation with aging. Although the studies reported by Okazaki et al. (15) used ST2 cells overexpressing either estrogen receptor α or estrogen receptor β, our studies were with the parental cell line, indicating that estradiol is activating endogenous estrogen receptors in the studies reported here. We found that culturing ST2 cells in adipogenic conditions induced expression of estrogen receptor α over 18-fold but had no detectable effect on estrogen receptor β expression. Moreover, the estrogen receptor antagonist 4-hydroxytamoxifen blocked estradiol effects on adipogenesis. It is therefore likely that estradiol influences on these cells is through activation of estrogen receptor α. In this study, we have documented that the estradiol suppression of mesenchymal cell commitment to adipogenesis requires induction of TGF-β, which induces the antiadipogenic growth factor CTGF. Commitment of progenitors to the adipocyte lineage is determined by specific transcription factors. C/EBP and PPARγ are the key transcription factors that drive adipogenesis (reviewed in Ref. 36). Thus, PPARγ and C/EBP cooperatively induce the adipocyte gene expression pattern that includes adipsin and LPL (37–40). In these studies, we have used expression of adipsin and LPL to verify that changes in PPARγ that we observed led to altered expression of responsive genes. Delaying estradiol treatment until PPARγ and C/EBP were already induced suppressed estradiol effects on PPARγ target genes. This supports the conclusion that early suppression of the master regulators is essential to estradiol effects on adipogenesis and, once PPARγ and C/EBP are induced, progression along the adipocytic lineage continues despite delayed estradiol-mediated suppression of PPARγ gene expression.
Many previous studies of regulation of adipogenesis were carried out with populations of primary cells that inherently contain multiple cell lineages. Confirming the existence of committed lineages in mesenchymal populations, Post et al. (41) examined two clonal cell lines derived from mesenchymal stem cells for their ability to differentiate and found that these cell lines were already committed to become either adipocytic or osteogenic but could not be induced to the other phenotype. Because of the existence of multiple cell lineage commitments in mesenchymal cells, we employed the multipotential bone marrow mesenchymal cell line ST2 for this study. The ST2 cell line is capable of differentiation along osteoblast (42), adipocyte (43), or chondrocyte (44) lineages. There are no reports that these cells can commit to other lineages. Okazaki et al. (15) documented that this cell line has the capacity to differentiate along either the osteoblast or adipocyte lineage when treated with BMP2. We have documented here the capacity of the cells to develop into adipocytes when treated with rosiglitazone. We have verified that ST2 treatment in osteogenic medium stimulates osteoblastic gene expression (Supplemental Fig. 1), confirming that these cells are at least bipotential in our hands.
For these studies, we elected to study TGF-β1 responses because of its prevalence in bone and because estradiol stimulates osteoblast TGF-β1 expression (21, 45, 46). These considerations support that TGF-β1 levels are elevated in vivo in the bone milieu in the presence of estradiol. Given our observations that estradiol induces TGF-β1 in osteoblasts, it was unexpected that estradiol induced expression of TGF-β2 and TGF-β3 but not TGF-β1 in ST2 cells. The three TGF-β isoforms most frequently differ in the magnitude of the response, not in the biological influences (46–50), and we have found no consistent differences in ST2 responses between the three TGF-β isoforms (data not shown). We employed an inhibitor to TGF-β and activin signaling in these studies. Combining the inhibitor studies with our observation that estradiol had no impact on activin expression while inducing TGF-β supports the conclusion that TGF-β induction is required for estrogen-mediated suppression of adipogenesis.
In HeLa cells, estrogen receptors bind directly to the PPARγ response element to repress PPARγ-mediated transactivation (51). In our studies, influences of estradiol on adipogenic gene expression was delayed several days and completely inhibited by blocking TGF-β signaling, indicating that bone marrow stromal cell estrogen receptors do not directly target PPARγ transactivation and require TGF-β induction to suppress adipogenesis.
Choy and Derynck (23) examined NIH3T3 cells in which either C/EBP or PPARγ was overexpressed and observed direct interactions between activated SMAD3 and C/EBP that suppressed transcriptional activity of the C/EBP. Although this mechanism may contribute to TGF-β influences on adipogenesis in cells not overexpressing C/EBP such as the ST2 model examined here, the excessive amount of C/EBP in the overexpressing cells in the Choy study may have resulted in interactions that are not physiologically meaningful. In the studies reported here, TGF-β treatment increased expression of CTGF, which has been identified as a physiological mediator of adipogenesis (29). The mechanisms by which TGF-β induces CTGF are complex, involving both SMAD-dependent and SMAD-independent signaling events (31, 52). CTGF interactions with cells are also complex because they involve multiple transmembrane protein interactions including interactions with integrins, heparin sulfate proteoglycans, and tyrosine kinase receptors (53). Overexpression of CTGF in mesenchymal stem cells in vitro caused osteogenic differentiation (54). In contrast, CTGF overexpression driven by the osteocalcin promoter in vivo reduced bone formation and caused osteopenia (55). Delivery of CTGF in a gelatin complex with a collagen scaffold in vivo to an intractable bone defect where it was continually released for 14 d caused significant enhancement of bone regeneration (56). Thus, CTGF is pro-osteogenic in vivo, but perhaps its overexpression may have caused anomalous impacts due to the presence of excess CTGF. A recent study by Canalis et al. (57) may have shed some light on these discrepancies. Cre/lox approaches were used to conditionally eliminate CTGF using mice with Cre driven by either the paired-related homeobox gene 1(Prx1) or the osteocalcin promoter. The Prx1 promoter is active early in embryonic life [embryonic d 10.5 (E10.5)] in mesenchymal cells, whereas the osteocalcin promoter is active later in embryonic life (E18.5) in more committed osteoblastic cells. In the Prx1del mice, there was a transient osteopenia observed in 1-month-old mice, whereas the osteocalcindel mice did not develop osteopenia until 6 months of age. These data suggest that CTGF influences on the skeleton begin early in embryonic development (between E105. and 18.5) whereas postnatal influences develop over time or with maturity of the skeleton. Interestingly, these observations were only in male mice. Our observation that estradiol induces CTGF through stimulation of TGF-β may explain this gender-based differential effect of loss of CTGF. The fact that female mice have higher circulating levels of estrogens would stimulate CTGF expression in cells not expressing Cre within the bone microenvironment in the Canalis study. Thus, paracrine CTGF influences on mesenchymal cells or osteoblasts may compensate for the lack of autocrine CTGF expression in the Cre-expressing cells in the female mice.
In conclusion, our studies indicate that estradiol-mediated suppression of adipogenesis is through TGF-β-mediated CTGF expression. The roles of TGF-β in estradiol-mediated suppression of adipogenesis in aging and with altered sex steroid levels in vivo remain to be defined.
Supplementary Material
Acknowledgments
We are thankful to Dr. Ryo Okazaki for sharing the parental ST2 cell line with us.
This work was supported by National Institutes of Health Grants P01 AG004875 and R01 AG028936 and The Mayo Foundation.
Current address for F.S.: Abbott Bioresearch Center, Worcester, Massachusetts 01545.
Disclosure Summary: F.S. is currently employed by Abbott Laboratories. The other authors have no conflicts of interest.
Footnotes
- BMP2
- Bone morphogenetic protein 2
- C/EBPα
- CCAAT enhancer-binding protein α
- CTGF
- connective tissue growth factor
- DMSO
- dimethylsulfoxide
- LPL
- lipoprotein lipase
- PPARγ
- peroxisome proliferator-activated receptor isoform γ.
References
- 1. Riggs BL, Khosla S, Melton LJ., 3rd 2002. Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 [DOI] [PubMed] [Google Scholar]
- 2. Spelsberg TC, Subramaniam M, Riggs BL, Khosla S. 1999. The actions and interactions of sex steroids and growth factors/cytokines on the skeleton. Mol Endocrinol 13:819–828 [DOI] [PubMed] [Google Scholar]
- 3. Meunier P, Aaron J, Edouard C, Vignon G. 1971. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin Orthop Relat Res 80:147–154 [DOI] [PubMed] [Google Scholar]
- 4. Beresford JN, Bennett JH, Devlin C, Leboy PS, Owen ME. 1992. Evidence for an inverse relationship between the differentiation of adipocytic and osteogenic cells in rat marrow stromal cell cultures. J Cell Sci 102(Pt 2):341–351 [DOI] [PubMed] [Google Scholar]
- 5. Jilka RL, Weinstein RS, Takahashi K, Parfitt AM, Manolagas SC. 1996. Linkage of decreased bone mass with impaired osteoblastogenesis in a murine model of accelerated senescence. J Clin Invest 97:1732–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nuttall ME, Patton AJ, Olivera DL, Nadeau DP, Gowen M. 1998. Human trabecular bone cells are able to express both osteoblastic and adipocytic phenotype: implications for osteopenic disorders. J Bone Miner Res 13:371–382 [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez JP, Garat S, Gajardo H, Pino AM, Seitz G. 1999. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem 75:414–423 [DOI] [PubMed] [Google Scholar]
- 8. Rodríguez JP, Montecinos L, Ríos S, Reyes P, Martínez J. 2000. Mesenchymal stem cells from osteoporotic patients produce a type I collagen-deficient extracellular matrix favoring adipogenic differentiation. J Cell Biochem 79:557–565 [DOI] [PubMed] [Google Scholar]
- 9. Rosen CJ, Bouxsein ML. 2006. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract 2:35–43 [DOI] [PubMed] [Google Scholar]
- 10. Snijder MB, van Dam RM, Visser M, Deeg DJ, Dekker JM, Bouter LM, Seidell JC, Lips P. 2005. Adiposity in relation to vitamin D status and parathyroid hormone levels: a population-based study in older men and women. J Clin Endocrinol Metab 90:4119–4123 [DOI] [PubMed] [Google Scholar]
- 11. Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. 2000. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest 106:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astudillo P, Ríos S, Pastenes L, Pino AM, Rodríguez JP. 2008. Increased adipogenesis of osteoporotic human-mesenchymal stem cells (MSCs) characterizes by impaired leptin action. J Cell Biochem 103:1054–1065 [DOI] [PubMed] [Google Scholar]
- 13. Syed FA, Oursler MJ, Hefferanm TE, Peterson JM, Riggs BL, Khosla S. 2008. Effects of estrogen therapy on bone marrow adipocytes in postmenopausal osteoporotic women. Osteoporos Int 19:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Benayahu D, Shur I, Ben-Eliyahu S. 2000. Hormonal changes affect the bone and bone marrow cells in a rat model. J Cell Biochem 79:407–415 [DOI] [PubMed] [Google Scholar]
- 15. Okazaki R, Inoue D, Shibata M, Saika M, Kido S, Ooka H, Tomiyama H, Sakamoto Y, Matsumoto T. 2002. Estrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) α or β. Endocrinology 143:2349–2356 [DOI] [PubMed] [Google Scholar]
- 16. Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Löwik CW. 2002. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res 17:394–405 [DOI] [PubMed] [Google Scholar]
- 17. Heim M, Frank O, Kampmann G, Sochocky N, Pennimpede T, Fuchs P, Hunziker W, Weber P, Martin I, Bendik I. 2004. The phytoestrogen genistein enhances osteogenesis and represses adipogenic differentiation of human primary bone marrow stromal cells. Endocrinology 145:848–859 [DOI] [PubMed] [Google Scholar]
- 18. Barnes S. 2010. The biochemistry, chemistry and physiology of the isoflavones in soybeans and their food products. Lymph Res Biol 8:89–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robinson JA, Waters KM, Turner RT, Spelsberg TC. 2000. Direct action of naturally occurring estrogen metabolites on human osteoblastic cells. J Bone Miner Res 15:499–506 [DOI] [PubMed] [Google Scholar]
- 20. Heino TJ, Hentunen TA, Väänänen HK. 2002. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: enhancement by estrogen. J Cell Biochem 85:185–197 [DOI] [PubMed] [Google Scholar]
- 21. Oursler MJ, Cortese C, Keeting P, Anderson MA, Bonde SK, Riggs BL, Spelsberg TC. 1991. Modulation of transforming growth factor-β production in normal human osteoblast-like cells by 17β-estradiol and parathyroid hormone. Endocrinology 129:3313–3320 [DOI] [PubMed] [Google Scholar]
- 22. Hawse JR, Subramaniam M, Ingle JN, Oursler MJ, Rajamannan NM, Spelsberg TC. 2008. Estrogen-TGFβ cross-talk in bone and other cell types: role of TIEG, Runx2, and other transcription factors. J Cell Biochem 103:383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Choy L, Derynck R. 2003. Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 278:9609–9619 [DOI] [PubMed] [Google Scholar]
- 24. Ponce ML, Koelling S, Kluever A, Heinemann DE, Miosge N, Wulf G, Frosch KH, Schütze N, Hufner M, Siggelkow H. 2008. Coexpression of osteogenic and adipogenic differentiation markers in selected subpopulations of primary human mesenchymal progenitor cells. J Cell Biochem 104:1342–1355 [DOI] [PubMed] [Google Scholar]
- 25. Igarashi A, Okochi H, Bradham DM, Grotendorst GR. 1993. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell 4:637–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ichijo H, Nishida E, Irie K, ten Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. 1997. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science 275:90–94 [DOI] [PubMed] [Google Scholar]
- 27. Harris SA, Tau KR, Enger RJ, Toft DO, Riggs BL, Spelsberg TC. 1995. Estrogen response in the hFOB 1.19 human fetal osteoblastic cell line stably transfected with the human estrogen receptor gene. J Cell Biochem 59:193–201 [DOI] [PubMed] [Google Scholar]
- 28. Karst M, Gorny G, Galvin RJ, Oursler MJ. 2004. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-β regulation of osteoclast differentiation. J Cell Physiol 200:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan JT, McLennan SV, Song WW, Lo LW, Bonner JG, Williams PF, Twigg SM. 2008. Connective tissue growth factor inhibits adipocyte differentiation. Am J Physiol Cell Physiol 295:C740–C751 [DOI] [PubMed] [Google Scholar]
- 30. Arnott JA, Nuglozeh E, Rico MC, Arango-Hisijara I, Odgren PR, Safadi FF, Popoff SN. 2007. Connective tissue growth factor (CTGF/CCN2) is a downstream mediator for TGF-β1-induced extracellular matrix production in osteoblasts. J Cell Physiol 210:843–852 [DOI] [PubMed] [Google Scholar]
- 31. Arnott JA, Zhang X, Sanjay A, Owen TA, Smock SL, Rehman S, DeLong WG, Safadi FF, Popoff SN. 2008. Molecular requirements for induction of CTGF expression by TGF-β1 in primary osteoblasts. Bone 42:871–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oursler MJ, Pederson L, Fitzpatrick L, Riggs BL, Spelsberg T. 1994. Human giant cell tumors of the bone (osteoclastomas) are estrogen target cells. Proc Natl Acad Sci USA 91:5227–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Spelsberg TC, Riggs BL. 1999. Estrogen stimulates gene expression and protein production of osteoprotegerin in human osteoblastic cells. Endocrinology 140:4367–4370 [DOI] [PubMed] [Google Scholar]
- 34. Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ. 2008. Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci USA 105:20764–20769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. 2009. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med 15:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosen ED. 2005. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids 73:31–34 [DOI] [PubMed] [Google Scholar]
- 37. Clarke SL, Robinson CE, Gimble JM. 1997. CAAT/enhancer binding proteins directly modulate transcription from the peroxisome proliferator-activated receptor γ2 promoter. Biochem Biophys Res Commun 240:99–103 [DOI] [PubMed] [Google Scholar]
- 38. Gonzales AM, Orlando RA. 2007. Role of adipocyte-derived lipoprotein lipase in adipocyte hypertrophy. Nutr Metab (Lond) 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Saladin R, Fajas L, Dana S, Halvorsen YD, Auwerx J, Briggs M. 1999. Differential regulation of peroxisome proliferator activated receptor γ1 (PPARγ1) and PPARγ2 messenger RNA expression in the early stages of adipogenesis. Cell Growth Differ 10:43–48 [PubMed] [Google Scholar]
- 40. Zhang B, Berger J, Hu E, Szalkowski D, White-Carrington S, Spiegelman BM, Moller DE. 1996. Negative regulation of peroxisome proliferator-activated receptor-γ gene expression contributes to the antiadipogenic effects of tumor necrosis factor-α. Mol Endocrinol 10:1457–1466 [DOI] [PubMed] [Google Scholar]
- 41. Post S, Abdallah BM, Bentzon JF, Kassem M. 2008. Demonstration of the presence of independent pre-osteoblastic and pre-adipocytic cell populations in bone marrow-derived mesenchymal stem cells. Bone 43:32–39 [DOI] [PubMed] [Google Scholar]
- 42. Sato T, Hong MH, Jin CH, Ishimi Y, Udagawa N, Shinki T, Abe E, Suda T. 1991. The specific production of the third component of complement by osteoblastic cells treated with 1α,25-dihydroxyvitamin D3. FEBS Lett 285:21–24 [DOI] [PubMed] [Google Scholar]
- 43. Ding J, Nagai K, Woo JT. 2003. Insulin-dependent adipogenesis in stromal ST2 cells derived from murine bone marrow. Biosci Biotechnol Biochem 67:314–321 [DOI] [PubMed] [Google Scholar]
- 44. Robins JC, Akeno N, Mukherjee A, Dalal RR, Aronow BJ, Koopman P, Clemens TL. 2005. Hypoxia induces chondrocyte-specific gene expression in mesenchymal cells in association with transcriptional activation of Sox9. Bone 37:313–322 [DOI] [PubMed] [Google Scholar]
- 45. Roberts AB, Kim SJ, Noma T, Glick AB, Lafyatis R, Lechleider R, Jakowlew SB, Geiser A, O'Reilly MA, Danielpour D, et al. 1991. Multiple forms of TGF-beta: distinct promoters and differential expression. Ciba Found Symp 157:7–15; discussion 15–28 [DOI] [PubMed] [Google Scholar]
- 46. Janssens K, ten Dijke P, Janssens S, Van Hul W. 2005. Transforming growth factor-β1 to the bone. Endocr Rev 26:743–774 [DOI] [PubMed] [Google Scholar]
- 47. Chakravarthy D, Green AR, Green VL, Kerin MJ, Speirs V. 1999. Expression and secretion of TGF-β isoforms and expression of TGF-β-receptors I, II and III in normal and neoplastic human breast. Int J Oncol 15:187–194 [DOI] [PubMed] [Google Scholar]
- 48. Maier R, Schmid P, Cox D, Bilbe G, McMaster GK. 1991. Localization of transforming growth factor-β1, -β2 and -β3 gene expression in bovine mammary gland. Mol Cell Endocrinol 82:191–198 [DOI] [PubMed] [Google Scholar]
- 49. Schmid P, Cox D, Bilbe G, Maier R, McMaster GK. 1991. Differential expression of TGF β1, β2 and β3 genes during mouse embryogenesis. Development 111:117–130 [DOI] [PubMed] [Google Scholar]
- 50. Jacobsen SE, Keller JR, Ruscetti FW, Kondaiah P, Roberts AB, Falk LA. 1991. Bidirectional effects of transforming growth factor β (TGF-β) on colony-stimulating factor-induced human myelopoiesis in vitro: differential effects of distinct TGF-β isoforms. Blood 78:2239–2247 [PubMed] [Google Scholar]
- 51. Bonofiglio D, Gabriele S, Aquila S, Catalano S, Gentile M, Middea E, Giordano F, Andò S. 2005. Estrogen receptor α binds to peroxisome proliferator-activated receptor response element and negatively interferes with peroxisome proliferator-activated receptor γ signaling in breast cancer cells. Clin Cancer Res 11:6139–6147 [DOI] [PubMed] [Google Scholar]
- 52. Zhang X, Arnott JA, Rehman S, Delong WG, Jr, Sanjay A, Safadi FF, Popoff SN. 2010. Src is a major signaling component for CTGF induction by TGF-β1 in osteoblasts. J Cell Physiol 224:691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Morrison BL, Jose CC, Cutler ML. 2010. Connective Tissue Growth Factor (CTGF/CCN2) enhances lactogenic differentiation of mammary epithelial cells via integrin-mediated cell adhesion. BMC Cell Biol 11:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang JJ, Ye F, Cheng LJ, Shi YJ, Bao J, Sun HQ, Wang W, Zhang P, Bu H. 2009. Osteogenic differentiation of mesenchymal stem cells promoted by overexpression of connective tissue growth factor. J Zhejiang Univ Sci B 10:355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durant D, Canalis E. 2008. Skeletal overexpression of connective tissue growth factor impairs bone formation and causes osteopenia. Endocrinology 149:4374–4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kikuchi T, Kubota S, Asaumi K, Kawaki H, Nishida T, Kawata K, Mitani S, Tabata Y, Ozaki T, Takigawa M. 2008. Promotion of bone regeneration by CCN2 incorporated into gelatin hydrogel. Tissue Eng Part A 14:1089–1098 [DOI] [PubMed] [Google Scholar]
- 57. Canalis E, Zanotti S, Beamer WG, Economides AN, Smerdel-Ramoya A. 2010. Connective tissue growth factor is required for skeletal development and postnatal skeletal homeostasis in male mice. Endocrinology 151:3490–3501 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.