Abstract
A wide variety of endocrine, physiological, and metabolic functions follow daily oscillations. Most of these regulations are controlled at the level of gene expression by the circadian clock, a remarkably coordinated transcription-translation machinery that exerts its function in virtually all mammalian cells. A large fraction of the genome is under control of the circadian clock, a regulation that is achieved through dynamic changes in chromatin states. Recent findings have demonstrated intimate connections between the circadian clock and epigenetic control. The case of nicotinamide adenine dinucleotide, which modulates the circadian activity of the deacetylase sirtuin 1, constitutes a paradigmatic example of the link between cyclic cellular metabolism and chromatin remodeling. Indeed, the clock transcriptional feedback loop is interlocked with the enzymatic loop of the nicotinamide adenine dinucleotide salvage pathway.
The circadian clock governs many biological functions in most living organisms. Accumulating evidence demonstrates that the circadian system regulates a large array of metabolic functions and that variations in metabolic cycles feed back to the clock (1, 2). The circadian system in mammals is organized in a network of circadian oscillators. The master or central clock is located in the hypothalamic suprachiasmatic nucleus (SCN), about 20,000 neurons whose activity oscillates in synchrony. The rhythmic function of SCN neurons is adjusted through light input originated from retinal cells and via the retinohypothalamic tract (3). The SCN operates in concert with a coordinated network of circadian oscillators present in other brain areas and in peripheral organs (3, 4). Although the physiological pathways and molecular mechanisms that allow synchronization of all clocks within an organism are yet ill defined, evidence exists that availability of metabolites or food intake is critical, especially for peripheral tissues (1, 2). Several studies have indicated that more than 10% of the transcripts oscillate in a circadian manner in each given tissue (5). Because only a limited fraction of the circadian transcripts is common among tissues, it appears that cell-specific molecular mechanisms interplay with the clock machinery in the SCN and in peripheral tissues (6). It is our prediction that variations in the metabolic state ultimately regulate distinct patterns of gene expression through the establishment of unique links between a specific transcription node and a corresponding metabolic node. We propose that disruption of rhythmic life patterns, as for example through restricted feeding or altered sleep-wake cycles, coordinately impinges on specific nodes, leading to alterations in the body's clock and metabolism. It is well demonstrated that disruption of the circadian clock may indeed lead to a variety of pathological states, including depression, insomnia, metabolic syndromes, inflammation, and cancer (7).
The molecular gears of the clock machinery have been identified. Central to the core clock mechanism are the CLOCK and BMAL1 basic-helix-loop-helix-PAS [Per (period circadian protein) Arnt (aryl hydrocarbon nuclear translocator protein) and Sim (single-minded protein)] transcription activators that heterodimerize to induce the expression of circadian clock-controlled genes (CCG) that contain E-box elements (CACGTG) in their promoters. Additional gene promoters containing E-box, D-box, and retinoic acid-responsive elements consensus sequences are also clock regulated and make up a large number of sequences within the circadian genome. Among the CCG, Per and Cry genes encode additional elements of the core clock machinery, which in the classical view function to form heterodimeric complexes that translocate to the nucleus and inhibit circadian transcription through direct interaction with circadian locomotor output cycles kaput/brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like (CLOCK:BMAL1) (3, 7). This classical view of interconnected transcriptional-translational feedback loops is useful but also deceivingly simple. Several other components contribute to the tightness and plasticity of circadian transcription, including micro-RNA (8), posttranslational modifications of clock proteins (9, 10), and their stability through specific F-box proteins (11–13). A critical level of control is exerted by chromatin remodeling, which needs to be cyclically coordinated to accommodate the transcriptional oscillation of a significant fraction of the genome (6).
Linking Chromatin Remodeling to Metabolism
The nucleosome is the building block of chromatin. Each nucleosome is comprised by two of each of the four canonic histones H2A, H2B, H3, and H4. Histones have a C-terminal globular domain in the core of the nucleosome, and an N-terminal unstructured domain that overhangs from the surface of the nucleosome and is the base of posttranslational modifications, such as acetylation, methylation, phosphorylation, and ubiquitination (14, 15). These modifications contribute to most genomic functions, specifically transcriptional activation or silencing, by recruiting specific protein complexes with enzymatic activity (16). Although the transmission through generations of these modifications still awaits convincing demonstration, these events of chromatin remodeling are loosely referred to as epigenetic changes. This is how this term is used throughout this article.
Ten years after the first evidence directly linking circadian regulation and chromatin remodeling (17), much more information has been accumulated. That first experiment demonstrated that exposing mice to short light pulses in the middle of the night triggers phosphorylation at Ser10 of histone H3 in SCN clock neurons. This modification is associated with transcriptional activation and in SCN neurons is tightly paralleled by light-induced activation of the circadian gene Per1 (17). Subsequent studies have shown that additional histone modifications, including H3 K9 and K14 acetylation, H3 K27 methylation, and H3 K4 methylation, all oscillate following a circadian cycle at promoters of CCG (18–20). Intriguingly, cyclic CLOCK:BMAL1 recruitment to CCG promoters accompanies oscillatory histone modifications, suggesting that this event could be a prerequisite for chromatin remodeling. A molecular interpretation of this notion came with the discovery that CLOCK itself functions as a chromatin remodeling enzyme, being endowed of histone acetyltransferase (HAT) activity (21). The HAT function of CLOCK is specific for H3, targeting mostly K14 and to a lesser extent K9, both modifications being intimately coupled to transcriptional activation (21). As other HAT do, CLOCK is also able to acetylate nonhistone protein substrates; CLOCK acetylates its heterodimeric partner BMAL1, on a unique, highly conserved Lys 537 residue, an event that appears critical for circadian regulation (22). It is tempting to speculate that CLOCK may target other nonhistone proteins. The search for these targets could reveal connections with cellular metabolism, cell cycle, or other physiological processes. An additional report shows that CLOCK acetylates the glucocorticoid receptor (23).
Because most HAT are counterbalanced by specific histone deacetylases (HDAC), the search for the HDAC that would operate in concert with CLOCK initiated. The result of this search generated much interest because it revealed a critical relationship between the circadian system and cellular metabolism. Indeed, sirtuin 1 (SIRT1), a sirtuin of the HDAC class III family, was found to be associated with CLOCK (24). SIRT1 is the mammalian ortholog of yeast Sir2, and has been found to control the metabolic processes in various organisms (25). Additional studies suggest that the beneficial effects of caloric restriction require SIRT1, which is also implicated in the control of inflammation and proliferation (25). There are seven members of the sirtuin family, whose intracellular distribution can be nuclear, cytoplasmic, and mitochondrial. A critical feature of the sirtuins is that their deacetylase activity is nicotinamide (NAM) adenine dinucleotide (NAD+) dependent, establishing a direct link between their function and energy metabolism. Our interpretation is that SIRT1 acts as a rheostat of the circadian clock, exerting its control predominantly on the amplitude of CCG expression (25, 26).
NAD+, an Oscillating Metabolite
The involvement of SIRT1 in circadian regulation demonstrated a direct link between cyclic rhythms and energy metabolism in the cell (24, 27). Yet, an analysis along the circadian cycle in various cell types demonstrated that the expression levels of SIRT1 gene and protein are noncyclic (24). On the contrary, the HDAC activity of the enzyme is circadian, indicating that some other sort of regulation, unrelated to protein levels, had to intervene to regulate SIRT1 function. Subsequent studies revealed that NAD+ levels oscillate in a circadian fashion in all cell types analyzed and that it is through the cyclic availability of its own coenzyme that SIRT1 HDAC activity is circadian (28, 29). The circadian regulation of NAD+ synthesis is itself conceptually remarkable.
A major enzymatic loop that operates in most cells is the NAD+ salvage pathway. Enzymes such as SIRT1 and poly(ADP-ribose) polymerase-1 (PARP-1) heavily use NAD+ as coenzyme, risking depletion of the intracellular stores, which can lead to cell death. Thus, levels of NAD+ need to be controlled even in the absence of de novo biosynthesis through nutritional pathways. The NAD+ salvage pathway allows NAM, the by-product of enzymes that use NAD+ as coenzyme, to be reconverted into NAD+ via the use of a group of NAM mononucleotide adenylyl-transferases, and the NAM phosphoribosyltransferase (NAMPT) enzyme. Importantly, NAMPT is the rate-limiting enzyme within the NAD+ salvage pathway (1, 28, 29). Thus, changes in NAMPT activity will directly dictate the levels of intracellular NAD+.
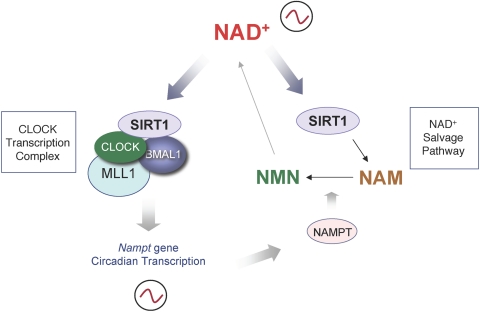
The rhythmicity of NAD+ levels parallels the antiphasic oscillation in NAM, both oscillations being abolished in cells with a mutation in the circadian machinery (28). Thus, the clock directs the oscillation of critical metabolites. Because SIRT1 associates with and modulates CLOCK:BMAL1 (24, 26), this suggested the presence of an enzymatic feedback loop, in which CLOCK:BMAL1 would control their own activity by directing the oscillatory synthesis of NAD+. This was demonstrated to be indeed the case. The regulatory region of the Nampt gene contains two E-box promoter elements, known to bind CLOCK:BMAL1. Additional experiments demonstrated that the expression of the Nampt gene is indeed controlled by CLOCK:BMAL1 in a complex that contains SIRT1. Thus, SIRT1 is present in both the transcriptional regulatory loop of the Nampt gene as well as in the NAD+ enzymatic salvage pathway. This two-way control results in the circadian expression of the Nampt gene, a circadian function of the NAD+ salvage pathway, and thereby in a circadian synthesis of NAD+ (Fig. 1). Importantly, the use of FK866, a highly specific NAMPT pharmacological inhibitor, abolished NAD+ circadian oscillations and thereby SIRT1 cyclic activity (28). This finding is of interest because FK866 is used to control cell death in human cancer tissues. Thus, in addition of revealing a critical enzymatic circadian cycle, these results suggest that a direct molecular coupling exists between circadian clock, energy metabolism, and cell survival. Intriguingly, the protein Sir2 appears to control aging in yeast by mediating transcriptional silencing at telomeres (30).
Fig. 1.
By activating SIRT1, NAD+ conjoins two feedback loops necessary for cross talk between the circadian clock and metabolite production. The levels of the metabolite NAD+ were shown to oscillate in a circadian manner in various cell types, suggesting that it could determine the cyclic function of NAD+-dependent enzymes, such as SIRT1. It has been shown that the salvage pathway is critical to regulate intracellular NAD+ levels. After the conversion of NAM into NAM mononucleotide (NMN) by NAMPT, NMN is further modified into NAD+ by the NAM mononucleotide adenylyl transferases (Nmnat-1, -2, and -3). Although NAM inhibits SIRT1 activity, NAD+-activated SIRT1 feeds back into the NAD+ salvage pathway by directly regulating Nampt gene expression in a CLOCK:BMAL1-dependent manner (28). By this mechanism, NAD+ conjoins the two feedback loops, contributing to the fine tuning necessary for achieving energy balance.
Thus, the circadian clock is directly implicated in controlling the intracellular levels of critical metabolites, generating an interlocking of the transcriptional feedback clock loop with the enzymatic feedback loop of the NAD+ salvage pathway. This view is confirmed by recent results using mice deficient in CD38, a NAD+ hydrolase, which display NAD+ levels elevated during most of the circadian cycle. CD38-null mice show altered circadian rhythmicity, CCG expression, and aberrant metabolism (31).
More Epigenetic Control, More Circadian Metabolites?
In addition to acetylation, a critical histone posttranslational modification (PTM) tightly linked to control of gene expression is methylation. Because specific combinations of histone PTM correspond to distinct nuclear functions and physiological responses (14, 15), it is critical to understand what are the time-specific combinations of PTM leading to circadian transcription. Lysine methylation of histones occupies a pivotal position in chromatin remodeling, and it is mostly associated with transcriptional repression (15). However, methylation of histone H3 at K4 is outstanding because it has been intimately linked to transcriptional activation (32). Lysine residues can be mono-, di-, or trimethylated at the ε-amino group, with each state correlating with a distinct functional effect (14). Dimethylation of H3 K4 occurs at both inactive and active euchromatic genes, whereas trimethylation is present exclusively at actively transcribed genes (32) and is widely accepted as a unique epigenetic mark defining an active chromatin state in most eukaryotes (14, 15). Importantly, H3 K4 methylation has been shown to be often associated with site-specific histone acetylation, such as H3 K9/14 and H4 K16, both ‘marks’ associated with active gene expression (26). We have recently identified a histone methyltransferase (HMT), mixed-lineage leukemia (MLL1), that is critically involved in oscillatory methylation of H3 K4 at circadian gene promoters. This event is necessary for subsequent H3 K9/14 acetylation, thereby establishing a permissive state for circadian transcription (32).
MLL1 is a HMT that specifically trimethylates histone H3 at K4 and regulates transcriptional activation (33). MLL1 is a mammalian homolog of the Drosophila trithorax gene, with which it shares several functional domains, including the most conserved C-terminal region. The most C-terminal 250 amino acids of MLL1 include a Drosophila suppressor of variegation Su(var)3-9, the enhancer of zeste E(z) and thrithorax (SET) domain, which displays histone H3 K4 methyltransferase activity (33). MLL1 was first reported as a transcriptional coactivator involved in the maintenance of selected Hox gene expression in morphogenesis (33). MLL1 is an element of a large chromatin remodeling complex that includes other critical regulators, including WDR5 and Rbbp5 (34). MLL1 has been shown also to interact with other regulators, including HAT, ATP-dependent chromatin remodeling factors, and RNA polymerase II (34, 35). We have demonstrated that MLL1 is critical for circadian gene expression and H3 K4 cyclic methylation. MLL1 exerts this striking function by directing the circadian recruitment to CCG promoters of CLOCK:BMAL1 (33).
Finally, the metabolite used by HMT as source of methyl groups is mainly S-adenosylmethionine. It is yet unknown whether S-adenosylmethionine levels oscillate to then feed into MLL1 and control its activity in a circadian manner. This is a fascinating possibility that would establish another important link between intracellular metabolic pathways and epigenetic control. Alternatively, because the recruitment to circadian promoters of CLOCK:BMAL1 and SIRT1 is MLL1 dependent (34), it could be envisaged that these proteins may operate in a sort of enzymatic interplay. In this scenario, the activity of MLL1 could be modulated by SIRT1, and thereby NAD+, and vice versa. Although this remains speculation at present, it stresses the possibility that chromatin remodeling enzymes could influence each other's function by sensing changes in the levels of one or another metabolite. Another important concept in this respect is that the circadian clock would not exclusively coordinate a large array of metabolic processes but that metabolites would feed back to the clock machinery, possibly influencing the chromatin remodeling component of the circadian system (31, 36).
Acknowledgments
I thank all the components of my laboratory for discussions and support.
Our studies are supported by the National Institutes of Health, the Institut National de la Santé et de la Recherche Médicale, France), and Sirtris-GSK.
Disclosure Summary: The author has nothing to disclose.
This Minireview is the report of the Roy Greep Award Lecture at the ENDO 2011.
- BMAL 1
- Brain and muscle aryl hydrocarbon receptor nuclear translocator (ARNT)-like
- CCG
- Clock-controlled genes
- CLOCK
- circadian locomotor output cycles kaput
- HAT
- histone acetyltransferase
- HDAC
- histone deacetylase
- HMT
- histone methyltransferase
- MLL1
- mixed-lineage leukemia
- NAD+
- nicotinamide adenine dinucleotide
- NAM
- nicotinamide
- NAMPT
- NAM phosphoribosyltransferase
- PTM
- posttranslational modification
- SCN
- suprachiasmatic nucleus
- SIRT1
- sirtuin 1.
References
- 1. Bellet MM, Sassone-Corsi P. 2010. Mammalian circadian clock and metabolism: the epigenetic link. J Cell Sci 123:3837–3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Green CB, Takahashi JS, Bass J. 2008. The meter of metabolism. Cell 134:728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bell-Pedersen D, Cassone VM, Earnest DJ, Golden SS, Hardin PE, Thomas TL, Zoran MJ. 2005. Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet 6:544–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schibler U, Sassone-Corsi P. 2002. A web of circadian pacemakers. Cell 111:919–922 [DOI] [PubMed] [Google Scholar]
- 5. Hogenesch JB, Ueda HR. 2011. Understanding systems-level properties: timely stories from the study of clock. Nat Rev Genet 12:407–416 [DOI] [PubMed] [Google Scholar]
- 6. Masri S, Sassone-Corsi P. 2010. Plasticity and specificity of the circadian epigenome. Nat Neurosci 13:1324–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahar S, Sassone-Corsi P. 2009. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer 9:886–896 [DOI] [PubMed] [Google Scholar]
- 8. Cheng HY, Papp JW, Varlamova O, Dziema H, Russell B, Curfman JP, Nakazawa T, Shimizu K, Okamura H, Impey S, Obrietan K. 2007. MicroRNA modulation of circadian-clock period and entrainment. Neuron 54:813–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gallego M, Virshup DM. 2007. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8:139–148 [DOI] [PubMed] [Google Scholar]
- 10. Cardone L, Hirayama J, Giordano F, Tamaru T, Palvimo JJ, Sassone-Corsi P. 2005. Circadian clock control by SUMOylation of BMAL1. Science 309:1390–1394 [DOI] [PubMed] [Google Scholar]
- 11. Busino L, Bassermann F, Maiolica A, Lee C, Nolan PM, Godinho SI, Draetta GF, Pagano M. 2007. SCFFbxl3 controls the oscillation of the circadian clock by directing the degradation of cryptochrome proteins. Science 316:900–904 [DOI] [PubMed] [Google Scholar]
- 12. Godinho SI, Maywood ES, Shaw L, Tucci V, Barnard AR, Busino L, Pagano M, Kendall R, Quwailid MM, Romero MR, O'neill J, Chesham JE, Brooker D, Lalanne Z, Hastings MH, Nolan PM. 2007. The after-hours mutant reveals a role for Fbxl3 in determining mammalian circadian period. Science 316:897–900 [DOI] [PubMed] [Google Scholar]
- 13. Siepka SM, Yoo SH, Park J, Song W, Kumar V, Hu Y, Lee C, Takahashi JS. 2007. Circadian mutant Overtime reveals F-box protein FBXL3 regulation of cryptochrome and period gene expression. Cell 129:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berger SL. 2007. The complex language of chromatin regulation during transcription. Nature 447:407–412 [DOI] [PubMed] [Google Scholar]
- 15. Borrelli E, Nestler EJ, Allis CD, Sassone-Corsi P. 2008. Decoding the epigenetic language of neuronal plasticity. Neuron 60:961–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee KK, Workman JL. 2007. Histone acetyltransferase complexes: one size doesn't fit all. Nat Rev Mol Cell Biol 8:284–295 [DOI] [PubMed] [Google Scholar]
- 17. Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. 2000. Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci 3:1241–1247 [DOI] [PubMed] [Google Scholar]
- 18. Etchegaray JP, Lee C, Wade PA, Reppert SM. 2003. Rhythmic histone acetylation underlies transcription in the mammalian circadian clock. Nature 421:177–182 [DOI] [PubMed] [Google Scholar]
- 19. Ripperger JA, Schibler U. 2006. Rhythmic CLOCK-BMAL1 binding to multiple E-box motifs drives circadian Dbp transcription and chromatin transitions. Nat Genet 38:369–374 [DOI] [PubMed] [Google Scholar]
- 20. Curtis AM, Seo SB, Westgate EJ, Rudic RD, Smyth EM, Chakravarti D, FitzGerald GA, McNamara P. 2004. Histone acetyltransferase-dependent chromatin remodeling and the vascular clock. J Biol Chem 279:7091–7097 [DOI] [PubMed] [Google Scholar]
- 21. Doi M, Hirayama J, Sassone-Corsi P. 2006. Circadian regulator CLOCK is a histone acetyltransferase. Cell 125:497–508 [DOI] [PubMed] [Google Scholar]
- 22. Hirayama J, Sahar S, Grimaldi B, Tamaru T, Takamatsu K, Nakahata Y, Sassone-Corsi P. 2007. CLOCK-mediated acetylation of BMAL1 controls circadian function. Nature 450:1086–1090 [DOI] [PubMed] [Google Scholar]
- 23. Nader N, Chrousos GP, Kino T. 2009. Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: potential physiological implications. FASEB J 23:1572–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. 2008. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134:329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bordone L, Guarente L. 2005. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6:298–305 [DOI] [PubMed] [Google Scholar]
- 26. Zocchi L, Sassone-Corsi P. 2010. Joining the dots: from chromatin remodeling to neuronal plasticity. Curr Opin Neurobiol 20:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. 2008. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134:317–328 [DOI] [PubMed] [Google Scholar]
- 28. Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. 2009. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324:654–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, Takahashi JS, Imai S, Bass J. 2009. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324:651–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sauve AA, Wolberger C, Schramm VL, Boeke JD. 2006. The biochemistry of sirtuins. Annu Rev Biochem 75:435–465 [DOI] [PubMed] [Google Scholar]
- 31. Sahar S, Nin V, Barbosa MT, Chini EN, Sassone-Corsi P. 2011. Altered behavioral and metabolic circadian rhythms in mice with disrupted NAD+ oscillation. Aging 3:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Santos-Rosa H, Schneider R, Bannister AJ, Sherriff J, Bernstein BE, Emre NC, Schreiber SL, Mellor J, Kouzarides T. 2002. Active genes are tri-methylated at K4 of histone H3. Nature 419:407–411 [DOI] [PubMed] [Google Scholar]
- 33. Katada S, Sassone-Corsi P. 2010. The histone methyltransferase MLL1 permits the oscillation of circadian gene expression. Nat Struct Mol Biol 17:1414–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, Hess JL. 2002. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell 10:1107–1117 [DOI] [PubMed] [Google Scholar]
- 35. Hsieh JJ, Ernst P, Erdjument-Bromage H, Tempst P, Korsmeyer SJ. 2003. Proteolytic cleavage of MLL generates a complex of N- and C-terminal fragments that confers protein stability and subnuclear localization. Mol Cell Biol 23:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Eckel-Mahan K, Sassone-Corsi P. 2009. Metabolism control by the circadian clock and vice versa. Nat Struct Mol Biol 16:462–467 [DOI] [PMC free article] [PubMed] [Google Scholar]