Abstract
Gonadotropin-inhibitory hormone (GnIH) is a hypothalamic neuropeptide that inhibits gonadotropin secretion in birds and mammals. To further understand its physiological roles in mammalian reproduction, we identified its precursor cDNA and endogenous mature peptides in the Siberian hamster brain. The Siberian hamster GnIH precursor cDNA encoded two RFamide-related peptide (RFRP) sequences. SPAPANKVPHSAANLPLRF-NH2 (Siberian hamster RFRP-1) and TLSRVPSLPQRF-NH2 (Siberian hamster RFRP-3) were confirmed as mature endogenous peptides by mass spectrometry from brain samples purified by immunoaffinity chromatography. GnIH mRNA expression was higher in long days (LD) compared with short days (SD). GnIH mRNA was also highly expressed in SD plus pinealectomized animals, whereas expression was suppressed by melatonin, a nocturnal pineal hormone, administration. GnIH-immunoreactive (-ir) neurons were localized to the dorsomedial region of the hypothalamus, and GnIH-ir fibers projected to hypothalamic and limbic structures. The density of GnIH-ir perikarya and fibers were higher in LD and SD plus pinealectomized hamsters than in LD plus melatonin or SD animals. The percentage of GnRH neurons receiving close appositions from GnIH-ir fiber terminals was also higher in LD than SD, and GnIH receptor was expressed in GnRH-ir neurons. Finally, central administration of hamster RFRP-1 or RFRP-3 inhibited LH release 5 and 30 min after administration in LD. In sharp contrast, both peptides stimulated LH release 30 min after administration in SD. These results suggest that GnIH peptides fine tune LH levels via its receptor expressed in GnRH-ir neurons in an opposing fashion across the seasons in Siberian hamsters.
The decapeptide GnRH is the primary factor responsible for hypothalamic control of gonadotropin secretion. GnRH was originally isolated from mammals (1, 2) and subsequently from birds (3–5) and other vertebrates. Gonadal sex steroids, follistatin, and inhibin can also modulate gonadotropin secretion via feedback from the gonads, but a neuropeptide inhibitor for gonadotropin secretion was unknown. However, Tsutsui and colleagues (6) identified a novel hypothalamic dodecapeptide (SIKPSAYLPLRF-NH2) that directly inhibited gonadotropin release from the cultured quail anterior pituitary and termed it gonadotropin-inhibitory hormone (GnIH). GnIH is located in neurons of the paraventricular nucleus in birds. These neurons project to the median eminence, thus providing neuroanatomical infrastructure to control anterior pituitary function (6–8). The GnIH precursor mRNA is also expressed only in the paraventricular nucleus in birds (7–10). The cognate G protein-coupled receptor for GnIH was identified in the quail pituitary (11), and GnIH was shown to act on the pituitary to suppress synthesis and release of gonadotropins (12). Consequently, GnIH inhibits the development and maintenance of gonadal functions (13). GnIH neurons also project to GnRH neurons (14, 15), and GnIH receptor mRNA is expressed in GnRH neurons (15). Accordingly, GnIH may inhibit gonadotropin secretion by decreasing the activity of GnRH neurons as well as directly acting on the pituitary gland.
GnIH orthologs are present in the brains of fish, amphibians, and mammals (16, 17) including monkey (18) and humans (19). These peptides belong to the RFamide-related peptide (RFRP) family (20, 21) and possess a characteristic C-terminal LPXRFamide (X = L or Q) motif (16, 17). GnIH and RFRP designate the same peptide from its structure. GnIH precursor mRNA encodes a polypeptide that is possibly cleaved into three mature LPXRFamide peptides in birds (GnIH, GnIH-RP-1, and GnIH-RP-2) and two in mammals (RFRP-1 and RFRP-3) (16, 17). The receptor for GnIH orthologs has also been characterized in vertebrates, which belongs to the GPR147 subfamily of the G protein-coupled receptor super family (11, 15–17, 19–21). It was shown that quail GnIH or rat RFRP-3 inhibits LH secretion in Syrian hamsters (22) and rats (23, 24) in vivo. It was also shown that RFRP-3 inhibits pulsatile gonadotropin release in vivo as well as gonadotropin release from cultured pituitary cells in sheep (25, 26) and cattle (27), suggesting that a hypothalamic gonadotropin-inhibitory system exists in mammals.
Photoperiodic mammals rely on the annual cycle of changes in nocturnal secretion of a pineal hormone, melatonin, to drive their reproductive responses (28). Several lines of evidence indicate that melatonin is involved in the regulation of seasonal processes in birds, including gonadal activity and gonadotropin secretion (29–32). These studies are consistent with our recent findings that melatonin acts directly on GnIH neurons through Mel1c, a melatonin receptor subtype, to stimulate GnIH expression (33) and release (34) in quail, a highly photoperiodic bird species. It was also shown that expression of GnIH is regulated by photoperiods in hamsters (35, 36) and sheep (37). Because the dorsomedial hypothalamic area (DMH), where GnIH-ir neuronal cell bodies exist in mammals, is essential for gonadotropic responses to melatonin (38), it is possible that GnIH neurons mediate melatonin action to control gonadotropin release in mammals. The suprachiasmatic nucleus (SCN) is also a major target responsible for receiving the melatonin message in Siberian hamsters (39), and the SCN projects to the GnIH system (40). Accordingly, it is highly possible that the expression of GnIH is regulated by melatonin in photoperiodic mammals.
To understand the physiological roles of GnIH in the control of reproduction in mammals, we first determined the sequence of GnIH precursor cDNA, and mature endogenous peptides, in the brain of Siberian hamster by PCR cloning and mass spectrometry, respectively. Siberian hamsters are widely used as a model species for studying the photoperiodic control of the hypothalamo-pituitary-gonadal axis. We then investigated the effect of photoperiod, pinealectomy, and melatonin administration on the expression of GnIH precursor mRNA and GnIH peptides (RFRP-1 and RFRP-3). We also investigated the effect of photoperiod on the interaction of GnIH-ir fiber terminals and GnRH-immunoreactive (GnRH-ir) neurons. We found that GnIH receptor was expressed on GnRH-ir neurons. Finally, we tested the effect of central administration of hamster RFRP-1 or RFRP-3 on LH release in hamsters that were housed in different photoperiodic conditions. The results suggest that the expression of hamster GnIH and its interactions with the GnRH system is regulated by photoperiod via melatonin. Together, these findings suggest that GnIH peptides may fine tune LH levels via its receptor expressed on GnRH neurons in an opposing fashion across seasons in Siberian hamsters.
Materials and Methods
Animals
Adult male Siberian hamsters, Phodopus sungorus, were used in this study. They were provided with laboratory chow and tap water ad libitum along with a weekly sunflower seed supplement. They were kept from birth under a long-day photoperiod (LD; 16 h light, 8 h dark) for 3 months before the experiment. The room temperature was maintained at 23 ± 2 C. The experimental protocol was approved in accordance with the guidelines prepared by Waseda University (Tokyo, Japan).
Determination of the Siberian hamster GnIH precursor cDNA
Total RNA of the diencephalon was extracted by using Sepazol-RNA I Super (Nacalai Tesque, Kyoto, Japan) and reverse transcribed using oligo(deoxythymidine) primer and reverse transcriptase. The sequence of GnIH precursor cDNA was determined by our established method (9, 15, 18), which is described in Supplemental Materials and Methods (published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org).
Immunoaffinity purification and mass spectrometry of Siberian hamster GnIH peptides
To identify mature endogenous GnIH peptides in the hamster brain, we carried out affinity purification and immunoassay using the antiserum raised against quail GnIH (6). Nine hamster brains were boiled for 7 min and homogenized in 5% acetic acid as described (41), and affinity chromatography (42) was carried out as described in Supplemental Materials and Methods.
The immunoreactive fraction was assayed by an immunoblot assay, and the molecular mass of the material was analyzed by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry (MALDI-TOF MS) (AXIMA-CFR-plus; Shimadzu, Kyoto, Japan). Immunoreactive samples were deposited on the plate, followed by an addition of a saturated solution of the matrix [10 mg/ml α-cyano-4-hydrocinnamic acid (Aldrich Chemical, Milwaukee, WI) in 50% vol/vol acetonitrile containing 0.1% vol/vol trifluoroacetic acid]. SPAPANKVPHSAANLPLRF-NH2 and TLSRVPSLPQRF-NH2 were predicted as mature peptides from the deduced amino acid sequence of the GnIH precursor cDNA sequence and their theoretical mass numbers. Accordingly, the peptides were synthesized by using a peptide synthesizer (PSSM-8; Shimadzu), and the molecular behavior of the synthetic and native peptides was further compared by HPLC and MALDI-TOF MS.
Effect of photoperiods, pinealectomy, and melatonin administration on the expression of Siberian hamster GnIH precursor mRNA
The pineal gland is the major source of melatonin in hamsters. To investigate the action of melatonin on the expression of Siberian hamster GnIH precursor mRNA, 16 hamsters were pinealectomized (Px) as previously described (33). Eight hamsters were sham operated; i.e. the pineal gland was exposed but not removed. All surgery was performed under Nembutal anesthesia (40 mg/kg). Two days after surgery, Px hamsters were divided into four groups (n = 4 in each group) and sc implanted with a SILASTIC brand (silicone type; Dow Corning, Midland, MI) plate containing melatonin (Sigma Chemical Co., St. Louis, MO) at three different doses (low dose, 10 μg/plate; medium dose, 100 μg/plate; and high dose, 1 mg/plate) or vehicle as described (33). Px plus melatonin or Px plus vehicle hamsters were transferred from LD (16 h light, 8 h dark) to short-day photoperiod (SD; 8 h light, 16 h dark). Four sham-operated hamsters were also transferred to SD, whereas four sham-operated hamsters were maintained in LD. Thirteen weeks later, LD, SD, and Px plus melatonin or vehicle hamsters (n = 4 in each group) were terminated by decapitation in light hours for the collection of diencephalons.
To quantify mRNA encoding the GnIH precursor polypeptide in the diencephalon, competitive PCR analysis was performed according to our established method (8, 33) that is described in Supplemental Materials and Methods. GnIH precursor mRNA level was normalized with the expression level of β-actin mRNA and expressed as a ratio of GnIH precursor mRNA concentration to β-actin mRNA concentration in the corresponding total RNA derived from each diencephalon.
Effect of photoperiods, pinealectomy, and melatonin administration on immunoreactivity in GnIH perikarya and fibers
To investigate the effect of photoperiod, pinealectomy and melatonin administration on GnIH immunoreactivity in the perikarya and fibers, four hamsters were Px, and 12 hamsters were sham operated as described (33). Four Px and four sham-operated hamsters were transferred from LD to SD. The other eight sham-operated hamsters were sc implanted with a SILASTIC plate containing melatonin (n = 4, 50 μg/plate) or vehicle (n = 4) and maintained in LD. Four Px and four sham-operated hamsters that were transferred to SD were implanted with a vehicle plate.
Thirteen weeks after the treatments, LD, LD plus melatonin, SD, and SD plus Px hamsters (n = 4 in each group) were anesthetized by Nembutal (40 mg/kg) and perfused with saline followed by fixative solution (Bouin's fluid). Frontal sections at 30 μm thickness were prepared by using a cryostat at −20 C. The sections were immersed with the antiquail GnIH antibody (6) at a dilution of 1:16,000 for 24 h at 4 C. The primary immunoreaction was followed by a 60-min incubation with biotinylated goat antirabbit IgG at 1:600 dilution and finally by a 60-min incubation with avidin-biotin peroxidase complex (1:300 dilution, Vectastain ABC kit; Vector Laboratories, Burlingame, CA). Immunoreactive products were detected by immersing the sections in a 3,3′-diaminobenzidine solution. The location of GnIH-ir perikarya and fibers was identified by Nissl staining of the adjacent sections. The immunoreactive cell bodies and fibers were studied using a Nikon ECLIPSE E600W microscope (Nikon Corp. Nikon Instech Co., Ltd., Kanagawa, Japan). The images of GnIH-ir perikarya and fibers were captured with a CCD camera (Digital Camera DXM1200F; Nikon, Tokyo, Japan). The immunoreactivity of GnIH in the perikarya was measured as a grayscale value from 0 (white) to 256 (black) by using Scion Image (version 4.02; Scion, Frederick, MD) and expressed as the mean density per cell, obtained by subtracting immediate background gray values as described previously (33). Thirty GnIH-ir perikarya per animal were analyzed, and the data were averaged for each animal. The integrated density of GnIH-ir fibers was also measured as a grayscale value from 0–256 and expressed as the mean density in each brain region, which was obtained by subtracting background gray values in the immediate brain region where there were no GnIH-ir fibers. Three sections per animal were analyzed in each brain region, and the data were averaged for each animal.
Effect of photoperiod on the interaction of GnIH-ir neuronal fiber terminals with GnRH-ir neurons
To investigate the effect of photoperiods on the interaction of GnIH-ir fiber terminals with GnRH-ir neurons, double-labeling immunocytochemistry of GnIH and GnRH was performed with slight modifications of our previous method (14, 15). Briefly, frontal sections at 50 μm thickness were prepared by using a cryostat at −20 C. The sections were immersed with a mouse monoclonal antibody directed against mammalian GnRH (SMI 41; Abcam, Cambridge, MA) at a dilution of 1:10,000 overnight at 4 C. The primary immunoreaction was followed by a 60-min incubation with biotinylated goat antimouse IgG at 1:600 and finally by a 60-min incubation with avidin-biotin peroxidase complex. Immunoreactive products were detected by immersing the sections in a 3,3′-diaminobenzidine solution containing 0.02% NiCl.
The number of GnRH-ir neurons and GnRH-ir neurons with a close apposition of GnIH-ir neuronal fiber terminal was counted at ×200 by two observers, and independent counts were averaged for the statistical analysis. The average number of GnRH-ir neurons analyzed per animal in LD and SD hamsters was 191 ± 35 (mean ± se, n = 4) and 156 ± 22 (mean ± se, n = 4), respectively. A putative contact was scored only if a GnIH-ir bouton-like structure was observed in close proximity to GnRH-ir neurons and the bouton contacted the cell body in the same focal plane.
Double-labeling immunocytochemistry of GnRH and GnIH receptor in the preoptic area (POA)
To investigate the expression of GnIH receptor in GnRH-ir neurons, double-label immunocytochemistry for GnRH and GnIH receptor was performed with slight modifications of our previous method (18). Briefly, frontal sections at 20 μm thickness were prepared by using a cryostat at −20 C. The antibody used to label GnRH neurons was a rabbit polyclonal anti-GnRH antibody (ab5617; Abcam) at a concentration of 1:1000. The antibody used to label GnIH receptor was mouse monoclonal anti-NPFFR1 (GPR147) antibody, clone 2A10 (Abnova, Taipei, Taiwan) at a concentration of 5 μg/ml. After immersing the sections with the primary antibodies overnight, sections were rinsed in PBS and then incubated with fluorophore-conjugated secondary antibodies. The secondary antibodies used were Alexa Fluor 488 goat antirabbit IgG (Invitrogen, San Diego, CA) and Alexa Fluor 555 goat antimouse IgG (Invitrogen) at a concentration of 1:1000. Accordingly, GnRH-ir neurons and GnIH receptor-ir cells were labeled with green and red fluorescence, respectively. No fluorescence was observed in the sections incubated without primary antibodies and with secondary antibodies. Twenty to 40 GnRH-ir neurons per animal were investigated in three male hamsters to calculate the percentage of GnRH-ir neurons expressing GnIH receptor.
Effect of GnIH administration on gonadotropin release
To determine the effect of hamster GnIH on gonadotropin release, GnIH peptides were centrally administered to hamsters. Cannulation was implanted into the third ventricle using a stereotaxic device under Nembutal anesthesia (40 mg/kg) to 40 LD hamsters and 32 hamsters that were transferred from LD to SD and kept in SD for 13 wk. Hamster RFRP-1 or RFRP-3 was administered at different doses (100 or 500 pmol) in 5 μl physiological saline according to our previous study (22). Control hamsters received saline vehicle. Blood was collected into heparinized tubes and centrifuged at 1800 × g for 20 min at 4 C. Plasma was stored at −20 C until assay.
Plasma LH and FSH concentrations were quantified by enzyme immunoassay (EIA) kits according to the manufacturer's instruction (rat LH Biotrak EIA system and rat FSH Biotrak EIA system; Amersham Biosciences, Little Chalfont, UK). The sensitivity of the EIA was 0.41 ng LH and 6.25 ng FSH per milliliter assay buffer. The intraassay coefficients of variation were 6.2 and 7.4%, respectively.
Statistics
Effect of photoperiod, pinealectomy, and melatonin administration on the expression of GnIH precursor mRNA and GnIH peptides and effect of GnIH administration on gonadotropin release were analyzed by one-way ANOVA, followed by Bonferroni's multiple range test. Effect of photoperiod on the interaction of GnIH-ir neuronal fiber terminals with GnRH-ir neurons was analyzed by Student's t test.
Results
Characterization of hamster GnIH precursor cDNA
The full length of the hamster GnIH precursor cDNA was identified by combining nucleotide sequences determined by rapid amplification of cDNA ends experiments (GenBank JF727837). As shown in Fig. 1, the hamster GnIH precursor cDNA was composed of 811 nucleotides containing a short 5′-untranslated sequence of 51 nucleotides, a single open reading frame of 567 nucleotides, and a 3′-untranslated sequence of 193 nucleotides with the addition of various lengths of poly(A) tail. The open reading frame region began with a start codon at position 52–54 and terminated with a TAG stop codon at position 619–621. A single polyadenylation signal (AATAAA) was found in the 3′-untranslated region at position 793–798. As shown in Fig. 1, the deduced precursor polypeptide consisted of 189 amino acid residues, encoding two putative sequences that included LPXRF (X is L or Q) with glycine and arginine as C-terminal amidation signals.
Fig. 1.
Nucleotide sequence and deduced amino acid sequence of hamster GnIH precursor cDNA. The amino acid sequence for hamster GnIH peptides, with glycine (G) as an amidation signal and arginine (R) as an endoproteolytic basic amino acid at the C terminus, are boxed (hamster RFRP-1, amino acids 76–96; hamster RFRP-3, amino acids 114–127). C-terminal LPXRF (X = L or Q) motifs are shown in bold. Stop codon is indicated by an asterisk. The poly(A) adenylation signal AATAAA is underlined.
Detection of mature endogenous hamster GnIH peptides in the brain
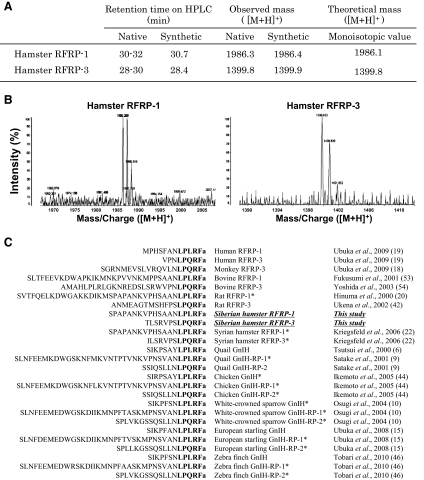
We determined the naturally occurring RFRP in the brain by immunoaffinity purification combined with mass spectrometry. The mass values of predicted peptides were calculated on the basis of the deduced sequence of hamster preproprotein (Fig. 2A). On the MALDI-TOF MS, the ion peak in the spectrum of the substance eluted at 28–30 min was 1399.8 mass to charge ratio ([M + H]+, protonated molecule) and at 30–32 min was 1986.3 mass to charge ratio ([M+H]+) (Fig. 2, A and B). These values were substantially identical to the mass number calculated for TLSRVPSLPQRF-NH2, and SPAPANKVPHSAANLPLRF-NH2. Accordingly, the peptides having the suggested sequences were synthesized, and the retention time on HPLC and the mass number were compared with the purified peptide from the brain. Corresponding purified and synthetic peptides showed a similar retention time on the reversed-phase HPLC and a similar molecular mass (Fig. 2A). The identified Siberian hamster RFRP-1 and -3 showed high homologies to RFRP or GnIH peptides of various mammals and birds and especially to the putative Syrian hamster RFRP-1 and -3, respectively (Fig. 2C).
Fig. 2.
A, Behavior of native and synthetic peptides on HPLC and MS. B, Chromatograms of MALDI-TOF MS of the native Siberian hamster RFRP-1 and RFRP-3. C, Alignment of amino acid sequences of LPXRFa (X = L or Q) peptides in mammals and birds. C-terminal LPXRFa (X = L or Q) motifs are shown in bold. *, Putative LPXRFa peptides hypothesized from their precursor mRNA sequences.
Effects of photoperiod, pinealectomy, and melatonin administration on the expression of hamster GnIH precursor mRNA
GnIH precursor mRNA was significantly lower in SD than in LD (P < 0.05, LD vs. SD, Fig. 3). However, GnIH mRNA levels did not decrease in SD if the hamsters were Px (P < 0.05, SD vs. SD + Px + vehicle, Fig. 3). Melatonin administration for 13 wk to Px hamsters kept in SD decreased GnIH mRNA levels (P < 0.05, SD + Px + vehicle vs. SD + Px + medium melatonin, SD + Px + high melatonin, Fig. 3). The effective dose leading to a decrease in GnIH mRNA in SD plus Px hamsters was estimated to be between low and medium melatonin.
Fig. 3.
Effects of photoperiod and melatonin administration on the expression of hamster GnIH precursor mRNA in the diencephalon. Various doses of melatonin (vehicle, 0 g/plate; low, 10 μg/plate; medium, 100 μg/plate; high, 1 mg/plate) were administered sc by means of a SILASTIC plate to Px hamsters maintained in SD for 13 wk. Other hamsters were sham operated and maintained in LD or SD for 13 wk. Each column and the vertical line represent the mean ± sem (n = 4 samples; one sample from one animal). Different letters (a, b) indicate statistical differences (P < 0.05) by one-way ANOVA, followed by Bonferroni's multiple range test.
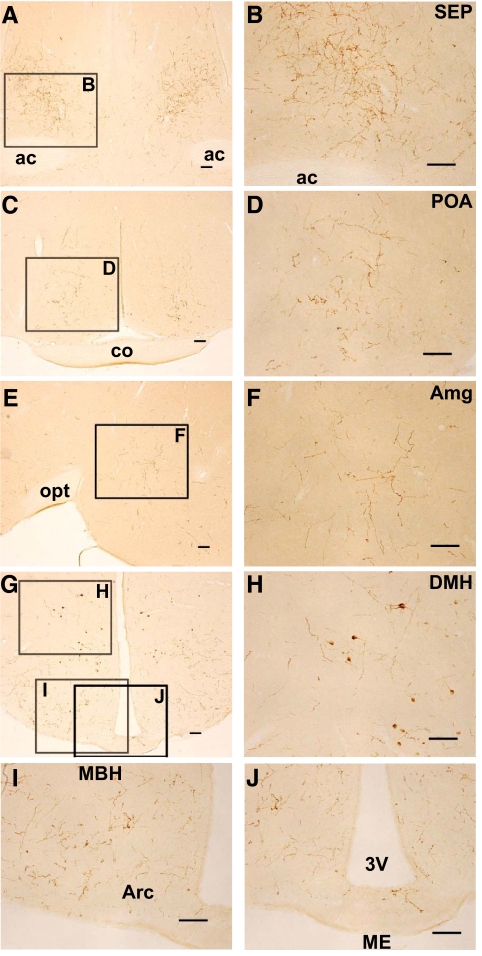
Distribution of GnIH-ir fibers and cell bodies in the Siberian hamster brain
We investigated the histological location of GnIH neurons in the Siberian hamster brain by immunocytochemistry. Hamsters were exposed to LD before euthanasia. The brain regions were identified by Nissl staining of adjacent sections. Dense GnIH-ir fibers were observed in the lateral septal nucleus (SEP) (Fig. 4, A and B), medial POA (Fig. 4, C and D), amygdala (Amg) (Fig. 4, E and F), and arcuate nucleus (Fig. 4, G and I). Moderate GnIH-ir fibers were observed in the paraventricular thalamic nucleus, the paraventricular hypothalamic nucleus, and the central gray. On the contrary, only sparse GnIH-ir fibers were observed in the median eminence (Fig. 4, G and J). GnIH-ir cell bodies were distributed in the medial region of the hypothalamus spanning from the anterior hypothalamic area, DMH (Fig. 4, G and H), and premammillary nucleus.
Fig. 4.
Location of GnIH-ir fibers and cell bodies in the hamster brain. A, GnIH-ir fibers in the septal region. ac, Anterior commissure. Bar, 100 μm. B, Higher magnification of the highlighted area in A showing GnIH-ir fibers in the SEP. Bar, 100 μm. C, GnIH-ir fibers in the anterior hypothalamic region. co, Optic chiasm. Bar, 100 μm. D, Higher magnification of the highlighted area in C showing GnIH-ir fibers in the medial POA. Bar, 100 μm. E, GnIH-ir fibers in the amygdaloid region. Medial is to the left. opt, Optic tract. Bar, 100 μm. F, Higher magnification of the highlighted area in E showing GnIH-ir fibers in the medial amygdaloid nucleus (Amg). Bar, 100 μm. G, GnIH-ir fibers in the medial hypothalamic region. Bar, 100 μm. H, Higher magnification of the highlighted area in G showing GnIH-ir fibers in the DMH. Bar, 100 μm. I, Higher magnification of the highlighted area in G showing GnIH-ir fibers in the MBH. Arc, Arcuate nucleus. Bar, 100 μm. J, Higher magnification of the highlighted area in G showing GnIH-ir fibers in the median eminence (ME). 3V, Third ventricle. Bar, 100 μm.
The specificity of the staining was assessed by preabsorption of the serum (1:16,000) with hamster RFRP-1 or RFRP-3, overnight, at a saturating concentration (10 μg peptide/ml) before use. All of the immunoreactions observed in this study were completely abolished by using the antiserum preabsorbed with an excess amount of hamster RFRP-1 or RFRP-3, whereas the staining was not affected by a saturating concentration of the related RFamide peptides, neruopeptide FF or prolactin-releasing peptide 20.
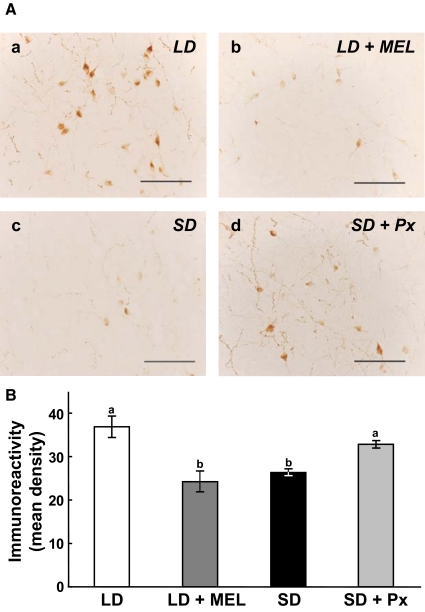
Effects of photoperiod, pinealectomy, and melatonin administration on the density of GnIH-ir cells
Clusters of immunoreactive perikarya were found in the DMH (Fig. 4, G and H). The OD of immunoreactive labeling in GnIH-ir cells tended to decrease with melatonin administration in hamsters maintained in LD (Fig. 5A, a and b). OD also tended to decrease after SD treatment (Fig. 5A, a and c). However, OD was markedly increased in GnIH-ir cells examined in SD hamsters that were Px (Fig. 5A, a, c, and d).
Fig. 5.
Effects of photoperiod, melatonin administration (MEL), and Px on the expression of GnIH in the perikarya. A, a, Expression of GnIH in the perikarya in hamsters kept in LD; b, expression of GnIH in the perikarya in hamsters administered sc with melatonin (50 μg per SILASTIC plate), and maintained in LD for 13 wk; c, expression of GnIH in the perikarya in hamsters maintained in SD for 13 wk; d, expression of GnIH in the perikarya in hamsters Px and maintained in SD for 13 wk. Scale bars, 100 μm. B, The immunoreactivity of GnIH in the perikarya was measured as a grayscale value from 0 (white) to 256 (black) and expressed as the mean density per cell, obtained by subtracting background gray values. Each column and the vertical line represent the mean ± sem (n = 4 samples; one sample from one animal). Different letters indicate statistical differences (P < 0.05) by one-way ANOVA, followed by Bonferroni's multiple range test.
Quantitative analyses show that the OD of immunoreactivity in GnIH perikarya was decreased by melatonin administration in hamsters kept in LD (P < 0.05, LD vs. LD + MEL, Fig. 5B; also compare Fig. 5A, a and b). The OD of immunoreactivity in GnIH perikarya was also decreased by SD treatment (P < 0.05, LD vs. SD, Fig. 5B; also compare Fig. 5A, a and c). In contrast, the OD of immunoreactivity in GnIH perikarya did not decrease in SD hamsters that were Px (P < 0.05, SD vs. SD + Px, Fig. 5B; also compare Fig. 5A, a, c, and d).
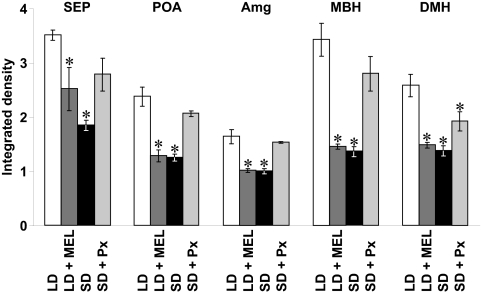
Effects of photoperiod, pinealectomy, and melatonin administration on the density of GnIH-ir fibers
Dense GnIH-ir fibers were observed in the SEP (Fig. 4B), POA (Fig. 4D), Amg (Fig. 4F), and medial basal hypothalamus (MBH) (Fig. 4I). Accordingly, the effects of photoperiods, pinealectomy, and melatonin administration on the density of GnIH-ir fibers were investigated in these brain regions. The OD of GnIH-ir was also measured in the DMH (Fig. 4H), where GnIH-ir cell bodies also exist. Integrated OD of GnIH-ir in SEP, POA, Amg, MBH, and DMH were decreased by melatonin administration in hamsters remaining in LD (P < 0.05, LD vs. LD + MEL, Fig. 6). The integrated OD of GnIH-ir in SEP, POA, Amg, MBH, and DMH were also decreased by SD treatment (P < 0.05, LD vs. SD, Fig. 6). Conversely, the OD of GnIH-ir in SEP, POA, Amg, and MBH were not decreased by SD treatment if the hamsters were Px (Fig. 6).
Fig. 6.
Effects of photoperiod, melatonin administration (MEL), and Px on the expression of GnIH-ir fibers. LD, Integrated density of GnIH-ir fibers in hamsters maintained in LD; LD + MEL, integrated density of GnIH-ir fibers in hamsters administered sc with melatonin (50 μg per SILASTIC plate) and maintained in LD for 13 wk; SD, integrated density of GnIH-ir fibers in hamsters kept in SD for 13 wk; SD + Px, integrated density of GnIH-ir fibers in hamsters Px and maintained in SD for 13 wk. The integrated density of GnIH-ir fibers was measured as a grayscale value from 0 (white) to 256 (black) and expressed as the mean density in each brain region, which was obtained by subtracting background gray values. Each column and the vertical line represent the mean ± sem (n = 4 samples; one sample from one animal). *, P < 0.05 vs. LD by one-way ANOVA, followed by Bonferroni's multiple range test.
Effects of photoperiod on the interaction of GnIH-ir neuronal fiber terminals with GnRH neurons
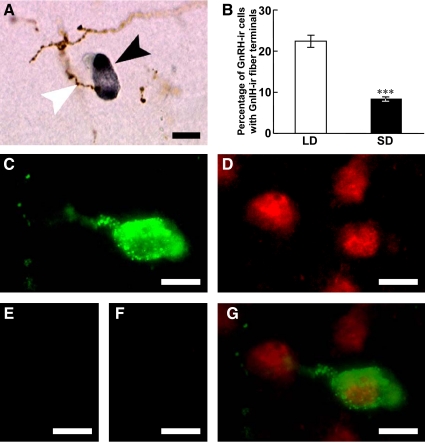
Double immunocytochemistry revealed the occurrence of hamster GnIH-ir neuronal fiber terminals in close proximity to GnRH-ir cell bodies in the POA (Fig. 7A). We accordingly investigated the effect of photoperiod on the interaction of GnIH-ir neuronal fiber terminal-like structure with GnRH-ir neurons. The percentage of GnRH-ir cells with a close apposition to GnIH-ir fiber terminal-like structures was decreased by SD treatment for 13 wk (P < 0.001, LD vs. SD, Fig. 7B).
Fig. 7.
Effects of photoperiod on the interaction of GnIH-ir fibers with GnRH-ir neurons and expression of GnIH receptor in a GnRH-ir neuron. A, GnIH-ir fibers (brown) and a GnRH-ir neuron (dark gray) in the medial POA. A white arrowhead indicates a GnIH-ir fiber in close proximity to a GnRH neuron indicated by a black arrowhead. Bar, 10 μm. B, Percentage of GnRH-ir cells with a close apposition of a GnIH-ir fiber terminal in hamsters kept in LD or SD for 13 wk. Each column and the vertical line represent the mean ± sem (n = 4 samples; one sample from one animal). ***, P < 0.001 vs. LD by Student's t test. C, Fluorescence microscopic image of GnRH-ir neuron (green) in the POA. Bar, 10 μm. D, Fluorescence microscopic image of GnIH receptor (red) in the same section. Bar, 10 μm. E, Immunocytochemistry without anti-GnRH antibody showed no fluorescence. Bar, 10 μm. F, Immunocytochemistry without anti-GnIH receptor antibody also showed no fluorescence. Bar, 10 μm. G, Merged image of C and D showed that GnIH receptor (red) was expressed on GnRH-ir (green) neuron. Bar, 10 μm.
Expression of GnIH receptor in GnRH-ir neurons
GnRH-ir neurons (Fig. 7C) and GnIH receptor-ir cells (Fig. 7D) were expressed in the POA. Double immunocytochemistry revealed that most of GnRH-ir neurons in the POA expressed GnIH receptor (Fig. 7G). The percentage of GnRH-ir neurons that also expressed GnIH receptor was 86.1 ± 1.70% (mean ± se; n = 3). GnIH receptor was also expressed on cells of unknown phenotype that did not express GnRH (Fig. 7G).
Effect of central administration of hamster GnIH on gonadotropin release
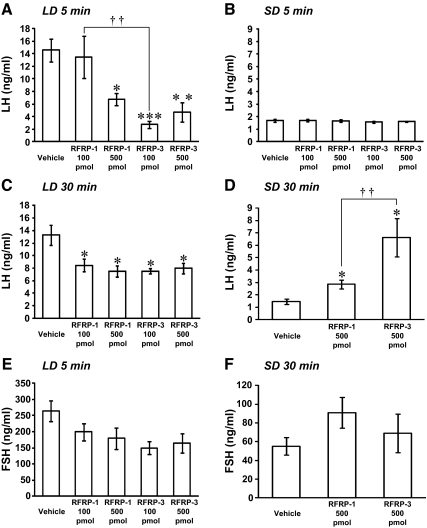
To determine whether hamster GnIH can influence gonadotropin release, we administered hamster GnIH peptides (Siberian hamster RFRP-1, Siberian hamster RFRP-3) intracerebroventricularly (icv) to LD hamsters, and plasma LH and FSH concentrations were quantified by EIA. As shown in Fig. 8A, plasma LH concentrations decreased 5 min after the administration of 500 pmol hamster RFRP-1 (P < 0.05, vehicle vs. 500 pmol RFRP-1) or 500 pmol hamster RFRP-3 (P < 0.01, vehicle vs. 500 pmol RFRP-3). Although administration of 100 pmol hamster RFRP-1 did not decrease LH concentrations, 100 pmol hamster RFRP-3 reliably decreased LH (P < 0.001, vehicle vs. 100 pmol RFRP-3; P < 0.01, 100 pmol RFRP-1 vs. 100 pmol RFRP-3, Fig. 8A). A decrease in plasma LH concentrations was also observed 30 min after the administration of hamster RFRP-1 and RFRP-3 (P < 0.05, vehicle vs. 100 and 500 pmol RFRP-1, 100 and 500 pmol RFRP-3, Fig. 8C). The experimental treatments failed to influence plasma FSH (Fig. 8E).
Fig. 8.
Effects of icv administration of hamster GnIH peptides on plasma LH and FSH concentrations in LD and SD. A, RFRP-1 (100 or 500 pmol), RFRP-3 (100 or 500 pmol), or vehicle was administered icv to hamsters kept in LD, and LH concentration was measured in the plasma at 5 min after administration. Each column and the vertical line represent the mean ± sem (n = 4). ***, P < 0.001; **, P < 0.01; *, P < 0.05 vs. vehicle. ††, P < 0.01, RFRP-1 100 pmol vs. RFRP-3 100 pmol. Statistical differences were analyzed by one-way ANOVA, followed by Bonferroni's multiple range test. B, RFRP-1 (100 or 500 pmol), RFRP-3 (100 or 500 pmol), or vehicle was administered icv to hamsters kept in SD, and LH concentration was measured in the plasma at 5 min after administration. Each column and the vertical line represent the mean ± sem (n = 4). C, RFRP-1 (100 or 500 pmol), RFRP-3 (100 or 500 pmol), or vehicle was administered icv to hamsters kept in LD, and LH concentration was measured in the plasma at 30 min after administration. Each column and the vertical line represent the mean ± sem (n = 4). *, P < 0.05 vs. vehicle. D, RFRP-1 (500 pmol), RFRP-3 (500 pmol), or vehicle was administered icv to hamsters maintained in SD, and LH concentration was measured in the plasma at 30 min after administration. Each column and the vertical line represent the mean ± sem (n = 4). *, P < 0.05 vs. vehicle. ††, P < 0.01, RFRP-1 500 pmol vs. RFRP-3 500 pmol. E, RFRP-1 (100 or 500 pmol), RFRP-3 (100 or 500 pmol), or vehicle was administered icv to hamsters maintained in LD, and FSH concentration was measured in the plasma at 5 min after administration. Each column and the vertical line represent the mean ± sem (n = 4). F, RFRP-1 (500 pmol), RFRP-3 (500 pmol), or vehicle was administered icv to hamsters maintained in SD, and FSH concentration was measured in the plasma at 30 min after administration. Each column and the vertical line represent the mean ± sem (n = 4).
We also investigated the effect of icv administration of hamster GnIH peptides on plasma LH and FSH concentrations in SD hamsters. We could not detect an inhibitory effect of GnIH peptides (RFRP-1 and RFRP-3) on gonadotropin release 5 min after the administration (Fig. 8B). However, as shown in Fig. 8D, plasma LH concentrations increased significantly 30 min after administration of 500 pmol hamster RFRP-1 or RFRP-3 (P < 0.05, vehicle vs. 500 pmol RFRP-1 or 500 pmol RFRP-3, Fig. 8D). The stimulatory effect of 500 pmol RFRP-3 on LH release was higher than 500 pmol RFRP-1 (P < 0.01, 500 pmol RFRP-1 vs. 500 pmol RFRP-3, Fig. 8D). In contrast to the impact of GnIH on LH, icv administration of 500 pmol hamster RFRP-1 or RFRP-3 had no effect on plasma FSH level (Fig. 8F).
Discussion
In cloning the full-length Siberian hamster GnIH precursor cDNA from the hypothalamus, we found that the Siberian hamster GnIH precursor mRNA encoded two LPXRFamide (X = L or Q) motifs, the characteristic C-terminal structure of RFRP (16–21). Mature Siberian hamster GnIH peptides were further isolated by immunoaffinity purification, and their structure was identified as SPAPANKVPHSAANLPLRF-NH2 (hamster RFRP-1) and TLSRVPSLPQRF-NH2 (hamster RFRP-3) by mass spectrometric analyses. This finding is consistent with the results in other mammals in which the GnIH precursor mRNA encodes a polypeptide that is cleaved into two mature RFRP (16, 17). In the human GnIH precursor polypeptide, two LPXRFamide (X = L or Q) peptides are encoded, and we recently identified both of them as endogenous mature peptides by mass spectrometric analyses (19). It is becoming clear that GnIH ortholog is conserved in mammals as well as birds (Fig. 2C).
Immunocytochemistry of GnIH showed that GnIH-ir neuronal cell bodies are distributed in the medial region of the hypothalamus spanning from the anterior hypothalamic area, DMH, and premammillary nucleus in Siberian hamsters. This finding is also consistent with the results in other mammals, where GnIH-ir neuronal cell bodies are distributed in the medial region of the hypothalamus. GnIH-ir neuronal cell bodies are localized in the intermediate periventricular nucleus of the hypothalamus in rhesus macaques (18), in the periventricular nucleus and around the ventromedial nucleus of the hypothalamus in rats (20, 43), in the DMH in Syrian hamsters (22), and in the dorsomedial hypothalamic nucleus and the paraventricular nucleus in sheep (25). Dense GnIH-ir fibers were observed in the lateral SEP, medial POA, Amg, and arcuate nucleus. These regions belong to the limbic system or the hypothalamus, which governs emotional, autonomic, and endocrine functions as described elsewhere. In contrast, only sparse GnIH-ir fibers were observed in the median eminence in Siberian hamsters, suggesting that GnIH may not directly regulate anterior pituitary function in this animal.
The hypothalamic neuropeptide GnRH (1, 2, 45) is the primary factor responsible for the stimulatory control of gonadotropin secretion from the pituitary gland. In the present study, GnIH-ir fibers were found in close proximity to GnRH neurons in the POA. Interactions of GnIH-ir fibers with GnRH-ir neurons are consistent with the results in birds (14, 15, 46), rodents (22), sheep (47), monkeys (18), and humans (19). In starlings, GnIH receptor mRNA is expressed in GnRH neurons (15). It was shown in this study that GnIH receptor is expressed on GnRH-ir neurons. Central administration of GnIH (RFRP) inhibits gonadotropin release in Syrian hamsters (22) and rats (23), as was shown in the present study. Furthermore, it was recently shown that RFRP administration reduces the firing activity (48, 49) and immediate-early gene expression in GnRH neurons (50). According to the neuroanatomical location of GnIH-ir fibers relative to GnRH neurons with more sparse labeling observed in the median eminence, Siberian hamster GnIH may primarily inhibit the secretion of gonadotropins by decreasing the activity of GnRH neurons.
In Siberian hamsters, reproductive status is primarily driven by day length (photoperiod), with this environmental seasonal cue influencing a broad spectrum of physiological parameters including body weight and pelage color and quality. Photoperiodic mammals rely on the annual cycle of changes in nocturnal secretion of a pineal hormone, melatonin, to drive their reproductive responses (28). If GnIH is involved in the control of reproductive functions in Siberian hamsters, the expression of GnIH precursor mRNA and GnIH peptides should be regulated by photoperiod and melatonin. In this study, expression of GnIH precursor mRNA, GnIH-ir in GnIH-ir perikarya and GnIH-ir fiber density decreased under SD photoperiod compared with LD. This inhibitory effect of SD was not seen if the hamsters were Px, and melatonin administration inhibited the expression of GnIH precursor mRNA, GnIH-ir in GnIH-ir perikarya and GnIH-ir fiber density. It was further shown that the percentage of GnRH-ir neurons receiving GnIH-ir fiber terminals decreased in SD photoperiod. These findings are consistent with the possibility that the activity of GnIH neurons decreases in SD by the inhibitory action of pineal melatonin. This result is in line with the results in Syrian hamsters (35, 36) and sheep (37). In quail, melatonin appears to act directly on GnIH neurons through its receptor (Mel1c) to induce GnIH expression (33). It was also shown that melatonin directly induces GnIH (RFRP-3) mRNA expression in the rat GnIH cell line (rHypoE-7; 51). The mechanism of different actions of melatonin on GnIH (RFRP) expression across species is an open question.
To understand the role of hamster GnIH peptides (RFRP-1 and RFRP-3) in reproduction, we tested the effect of central administration of GnIH peptides on gonadotropin release. It was shown in LD hamsters that both RFRP-1 and RFRP-3 inhibited LH release 5 and 30 min after administration. We could not detect an inhibitory effect of GnIH peptides on LH release in SD hamsters at 5 min after administration. However, in SD hamsters both RFRP-1 and RFRP-3 stimulated LH release at 30 min after administration. This finding agrees with those in Syrian hamsters in which central administration of GnIH inhibits LH release in ovariectomized female hamsters 5 and 30 min after injection (22). Importantly, these findings suggest that GnIH peptides may inhibit LH release in LD when the basal LH level is high and stimulate LH release in SD when the basal LH level is low. An effect of hamster RFRP-1 and RFRP-3 on FSH release was not evident in this study. Hamster GnIH may control LH release by regulating the action of GnRH neurons as described above. It is therefore possible that hamster GnIH may inhibit the action of GnRH neurons when the activity of GnRH is high in LD and stimulate the action of GnRH when the activity of GnRH is low in SD.
We tested the effect of icv administration of 100 and 500 pmol Siberian hamster RFRP-1and RFRP-3 on gonadotropin release according to our previous study in Syrian hamsters (22). We tested their effects in both LD and SD hamsters in this study. Because we found that LD hamsters expressed a higher concentration of GnIH than SD animals, GnIH administration to LD animals might have exceeded its physiological range. Alternatively, it is possible that GnIH transport and release is lower in LD than SD, resulting in a higher number of neurons being labeled immunohistochemically. RFRP-1 and RFRP-3 increased LH release 30 min after their administration and not at 5 min in SD animals, suggesting that stimulation with RFRP requires a longer time course than inhibition. Additional studies are required to test various doses of the peptides and times after administration and also quantify the expression of gonadotropins to draw a firm conclusion.
Taken together, the present results indicate that GnIH decreases in SD relative to LD by the actions of pineal melatonin and that GnIH inhibits LH release in LD and stimulates LH release in SD hamsters. This pattern of GnIH expression, and its association with seasonal changes in reproductive status, suggests that short durations of melatonin in LD stimulate GnIH expression to suppress/precisely regulate high concentration of LH and that long durations of melatonin in SD inhibit GnIH expression to prevent GnRH cell stimulation. It is also possible that GnIH is sensitive to daily changes in melatonin and participates in circadian LH secretion and/or the female estrous cycle (39). In addition to the actions of melatonin, it was recently shown that stressful environmental conditions significantly induce the expression of GnIH in rats (52), suggesting that GnIH conveys environmental information, including stressors, to GnRH neurons to fine tune reproductive function across seasons.
Supplementary Material
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture, Japan (18107002, 22132004, and 22227002 to K.T.) and National Institutes of Health Grant HD050470 (to L.J.K.).
The sequence reported in this paper has been deposited in the GenBank database (accession no. JF727837) for the cDNA sequence of Siberian hamster GnIH precursor polypeptide.
Disclosure Summary: The authors of this manuscript have nothing to disclose.
Footnotes
- Amg
- Amygdala
- DMH
- dorsomedial hypothalamic area
- EIA
- enzyme immunoassay
- GnIH
- gonadotropin-inhibitory hormone
- GnRH-ir
- GnRH-immunoreactive
- icv
- intracerebroventricularly
- LD
- long-day photoperiod
- MALDI-TOF MS
- matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry
- MBH
- medial basal hypothalamus
- POA
- preoptic area
- Px
- pinealectomized
- RFRP
- RFamide-related peptide
- SD
- short-day photoperiod
- SEP
- septal nucleus.
References
- 1. Matsuo H, Baba Y, Nair RM, Arimura A, Schally AV. 1971. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun 43:1334–1339 [DOI] [PubMed] [Google Scholar]
- 2. Burgus R, Butcher M, Amoss M, Ling N, Monahan M, Rivier J, Fellows R, Blackwell R, Vale W, Guillemin R. 1972. Primary structure of the ovine hypothalamic luteinizing hormone-releasing factor (LRF). Proc Natl Acad Sci USA 69:278–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. King JA, Millar RP. 1982. Structure of chicken hypothalamic luteinizing hormone-releasing hormone. I. Structural determination on partially purified material. J Biol Chem 257:10722–10728 [PubMed] [Google Scholar]
- 4. Miyamoto K, Hasegawa Y, Minegishi T, Nomura M, Takahashi Y, Igarashi M, Kangawa K, Matsuo H. 1982. Isolation and characterization of chicken hypothalamic luteinizing hormone-releasing hormone. Biochem Biophys Res Commun 107:820–827 [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto K, Hasegawa Y, Nomura M, Igarashi M, Kangawa K, Matsuo H. 1984. Identification of the second gonadotropin-releasing hormone in chicken hypothalamus: evidence that gonadotropin secretion is probably controlled by two distinct gonadotropin-releasing hormones in avian species. Proc Natl Acad Sci USA 81:3874–3878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. 2000. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochem Biophys Res Commun 275:661–667 [DOI] [PubMed] [Google Scholar]
- 7. Ukena K, Ubuka T, Tsutsui K. 2003. Distribution of a novel avian gonadotropin-inhibitory hormone in the quail brain. Cell Tissue Res 312:73–79 [DOI] [PubMed] [Google Scholar]
- 8. Ubuka T, Ueno M, Ukena K, Tsutsui K. 2003. Developmental changes in gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica) hypothalamo-hypophysial system. J Endocrinol 178:311–318 [DOI] [PubMed] [Google Scholar]
- 9. Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K. 2001. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. Biochem J 354:379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Osugi T, Ukena K, Bentley GE, O'Brien S, Moore IT, Wingfield JC, Tsutsui K. 2004. Gonadotropin-inhibitory hormone in Gambel's white-crowned sparrow (Zonotrichia leucophrys gambelii): cDNA identification, transcript localization and functional effects in laboratory and field experiments. J Endocrinol 182:33–42 [DOI] [PubMed] [Google Scholar]
- 11. Yin H, Ukena K, Ubuka T, Tsutsui K. 2005. A novel G protein-coupled receptor for gonadotropin-inhibitory hormone in the Japanese quail (Coturnix japonica): identification, expression and binding activity. J Endocrinol 184:257–266 [DOI] [PubMed] [Google Scholar]
- 12. Ciccone NA, Dunn IC, Boswell T, Tsutsui K, Ubuka T, Ukena K, Sharp PJ. 2004. Gonadotrophin inhibitory hormone depresses gonadotrophin α and follicle-stimulating hormone β subunit expression in the pituitary of the domestic chicken. J Neuroendocrinol 16:999–1006 [DOI] [PubMed] [Google Scholar]
- 13. Ubuka T, Ukena K, Sharp PJ, Bentley GE, Tsutsui K. 2006. Gonadotropin-inhibitory hormone inhibits gonadal development and maintenance by decreasing gonadotropin synthesis and release in male quail. Endocrinology 147:1187–1194 [DOI] [PubMed] [Google Scholar]
- 14. Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. 2003. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. J Neuroendocrinol 15:794–802 [DOI] [PubMed] [Google Scholar]
- 15. Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. 2008. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology [Erratum (2008) 149:4229] 149:268–278 [DOI] [PubMed] [Google Scholar]
- 16. Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. 2010. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Front Neuroendocrinol 31:284–295 [DOI] [PubMed] [Google Scholar]
- 17. Ubuka T, McGuire NL, Calisi RM, Perfito N, Bentley GE. 2008. The control of reproductive physiology and behavior by gonadotropin-inhibitory hormone. Integr Comp Biol 48:560–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. 2009. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. J Comp Neurol 517:841–855 [DOI] [PubMed] [Google Scholar]
- 19. Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. 2009. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PLoS One 4:e8400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. 2000. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nat Cell Biol 2:703–708 [DOI] [PubMed] [Google Scholar]
- 21. Fukusumi S, Fujii R, Hinuma S. 2006. Recent advances in mammalian RFamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides 27:1073–1086 [DOI] [PubMed] [Google Scholar]
- 22. Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. 2006. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proc Natl Acad Sci USA 103:2410–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson MA, Tsutsui K, Fraley GS. 2007. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Horm Behav 51:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. 2008. Hypophysiotropic role of RFamide-related peptide-3 (RFRP-3) in the inhibition of LH secretion in female rats. J Endocrinol 199:105–112 [DOI] [PubMed] [Google Scholar]
- 25. Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. 2008. Potent action of RFRP-3 on pituitary gonadotropes indicative of an hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology 149:5811–5821 [DOI] [PubMed] [Google Scholar]
- 26. Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. 2009. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology 150:5549–5556 [DOI] [PubMed] [Google Scholar]
- 27. Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. 2009. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domest Anim Endocrinol 36:219–224 [DOI] [PubMed] [Google Scholar]
- 28. Bronson FH. 1989. Mammalian reproductive biology. Chicago: University of Chicago Press [Google Scholar]
- 29. Ohta M, Kadota C, Konishi H. 1989. A role of melatonin in the initial stage of photoperiodism in the Japanese quail. Biol Reprod 40:935–941 [DOI] [PubMed] [Google Scholar]
- 30. Bentley GE, Van't Hof TJ, Ball GF. 1999. Seasonal neuroplasticity in the songbird telencephalon: a role for melatonin. Proc Natl Acad Sci USA 96:4674–4679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guyomarc'h C, Lumineau S, Vivien-Roels B, Richard J, Deregnaucourt S. 2001. Effect of melatonin supplementation on the sexual development in European quail (Coturnix coturnix). Behav Processes 53:121–130 [DOI] [PubMed] [Google Scholar]
- 32. Rozenboim I, Aharony T, Yahav S. 2002. The effect of melatonin administration on circulating plasma luteinizing hormone concentration in castrated White Leghorn roosters. Poult Sci 81:1354–1359 [DOI] [PubMed] [Google Scholar]
- 33. Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. 2005. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proc Natl Acad Sci USA 102:3052–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chowdhury VS, Yamamoto K, Ubuka T, Bentley GE, Hattori A, Tsutsui K. 2010. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology 151:271–280 [DOI] [PubMed] [Google Scholar]
- 35. Revel FG, Saboureau M, Pévet P, Simonneaux V, Mikkelsen JD. 2008. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology 149:902–912 [DOI] [PubMed] [Google Scholar]
- 36. Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, Silver R, Kriegsfeld LJ. 2010. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus). J Biol Rhythms 25:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dardente H, Birnie M, Lincoln GA, Hazlerigg DG. 2008. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. J Neuroendocrinol 20:1252–1259 [DOI] [PubMed] [Google Scholar]
- 38. Maywood ES, Bittman EL, Hastings MH. 1996. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biol Reprod 54:470–477 [DOI] [PubMed] [Google Scholar]
- 39. Bartness TJ, Goldman BD, Bittman EL. 1991. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. Am J Physiol 260:R102–R112 [DOI] [PubMed] [Google Scholar]
- 40. Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. 2008. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology 149:4958–4969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ukena K, Koda A, Yamamoto K, Kobayashi T, Iwakoshi-Ukena E, Minakata H, Kikuyama S, Tsutsui K. 2003. Novel neuropeptides related to frog growth hormone-releasing peptide: isolation, sequence, and functional analysis. Endocrinology 144:3879–3884 [DOI] [PubMed] [Google Scholar]
- 42. Ukena K, Iwakoshi E, Minakata H, Tsutsui K. 2002. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS Lett 512:255–258 [DOI] [PubMed] [Google Scholar]
- 43. Yano T, Iijima N, Kakihara K, Hinuma S, Tanaka M, Ibata Y. 2003. Localization and neuronal response of RFamide related peptides in the rat central nervous system. Brain Res 982:156–167 [DOI] [PubMed] [Google Scholar]
- 44. Ikemoto T, Park MK. 2005. Chicken RFamide-related peptide (GnIH) and two distinct receptor subtypes: identification, molecular characterization, and evolutionary considerations. J Reprod Dev 51:359–377 [DOI] [PubMed] [Google Scholar]
- 45. Millar RP. 2005. GnRHs and GnRH receptors. Anim Reprod Sci 88:5–28 [DOI] [PubMed] [Google Scholar]
- 46. Tobari Y, Iijima N, Tsunekawa K, Osugi T, Okanoya K, Tsutsui K, Ozawa H. 2010. Identification of gonadotropin-inhibitory hormone in the zebra finch (Taeniopygia guttata): peptide isolation, cDNA cloning and brain distribution. Peptides 31:816–826 [DOI] [PubMed] [Google Scholar]
- 47. Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. 2008. Variation in kisspeptin and gonadotropin-inhibitory hormone expression and terminal connections to GnRH neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu M, Dumalska I, Morozova E, van den Pol AN, Alreja M. 2009. Gonadotropin inhibitory hormone inhibits basal forebrain vGluT2-gonadotropin-releasing hormone neurons via a direct postsynaptic mechanism. J Physiol 587:1401–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ducret E, Anderson GM, Herbison AE. 2009. RFamide-related peptide-3, a mammalian gonadotropin-inhibitory hormone ortholog, regulates gonadotropin-releasing hormone neuron firing in the mouse. Endocrinology 150:2799–2804 [DOI] [PubMed] [Google Scholar]
- 50. Anderson GM, Relf HL, Rizwan MZ, Evans JJ. 2009. Central and peripheral effects of RFamide-related peptide-3 on luteinizing hormone and prolactin secretion in rats. Endocrinology 150:1834–1840 [DOI] [PubMed] [Google Scholar]
- 51. Gingerich S, Wang X, Lee PK, Dhillon SS, Chalmers JA, Koletar MM, Belsham DD. 2009. The generation of an array of clonal, immortalized cell models from the rat hypothalamus: analysis of melatonin effects on kisspeptin and gonadotropin-inhibitory hormone neurons. Neuroscience 162:1134–1140 [DOI] [PubMed] [Google Scholar]
- 52. Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. 2009. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc Natl Acad Sci USA 106:11324–11329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fukusumi S, Habata Y, Hoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. 2001. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochim Biophys Acta 1540:221–232 [DOI] [PubMed] [Google Scholar]
- 54. Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. 2003. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochim Biophys Acta 1593:151–157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.