Abstract
Synthetic selective thyroid hormone (TH) receptor (TR) modulators (STRM) exhibit beneficial effects on dyslipidemias in animals and humans and reduce obesity, fatty liver, and insulin resistance in preclinical animal models. STRM differ from native TH in preferential binding to the TRβ subtype vs. TRα, increased uptake into liver, and reduced uptake into other tissues. However, selective modulators of other nuclear receptors exhibit important gene-selective actions, which are attributed to differential effects on receptor conformation and dynamics and can have profound influences in animals and humans. Although there are suggestions that STRM may exhibit such gene-specific actions, the extent to which they are actually observed in vivo has not been explored. Here, we show that saturating concentrations of the main active form of TH, T3, and the prototype STRM GC-1 induce identical gene sets in livers of euthyroid and hypothyroid mice and a human cultured hepatoma cell line that only expresses TRβ, HepG2. We find one case in which GC-1 exhibits a modest gene-specific reduction in potency vs. T3, at angiopoietin-like factor 4 in HepG2. Investigation of the latter effect confirms that GC-1 acts through TRβ to directly induce this gene but this gene-selective activity is not related to unusual T3-response element sequence, unlike previously documented promoter-selective STRM actions. Our data suggest that T3 and GC-1 exhibit almost identical gene regulation properties and that gene-selective actions of GC-1 and similar STRM will be subtle and rare.
Native hormones elicit beneficial and deleterious effects when used as therapeutics, and investigators have been trying to develop strategies to capture beneficial effects for many years (1). Thyroid hormones (TH) regulate heart rate, serum lipids, metabolic rate, and multiple steps of pathways involved in carbohydrate, lipid, and protein metabolism (1, 2). Although excess TH lead to reductions in low-density lipoprotein (LDL) cholesterol and lipoprotein (a) in serum and reduced liver and body fat, beneficial effects are offset by deleterious effects on heart, muscle, and bone (1, 3). Thus, TH cannot be used safely to treat dyslipidemias, nonalcoholic fatty liver disease, and obesity.
We developed selective TH receptor (TR) modulators (STRM) with improved profiles relative to TH (1, 2). There are two TR (TRα and TRβ) encoded by different genes, and TRβ mediates beneficial effects of TH on serum cholesterol, whereas TRα mediates harmful effects on heart (1, 2, 4). We used TR x-ray structure-based chemical biology approaches to obtain TRβ-selective ligands (1). Our prototype compound, GC-1 ({4-[4-Hydroxy-3-(1-Methylethyl)benzyl]-3,5-Dimethylphenoxy}acetic acid), is a potent TR agonist that binds TRβ with modest (4- to 10-fold) selectivity, and we and others developed other TRβ-selective ligands (1). Some STRM, including GC-1, also accumulate preferentially in liver, and uptake into other tissues, including heart and muscle, is low relative to T3 (5). This is likely to be beneficial; TH influence liver gene expression to lower serum cholesterol and reduced ligand uptake into heart, and muscle probably helps to eliminate harmful side effects (1). STRM reduce serum total and LDL cholesterol and other atherogenic lipids, enhance aspects of reverse cholesterol transport, reduce body fat, clear liver fat in mouse models of nonalcoholic fatty liver disease, and reduce blood glucose and improve insulin sensitivity in rodent models of type 2 diabetes (5–11). There are no adverse effects on heart, muscle, and bone at clinically relevant doses. Some STRM have moved into clinical trials; GC-1 and a similar compound, KB2115, reduce serum LDL cholesterol, lipoprotein (a), and triglyceride levels without harmful effects on heart or muscle in humans (1, 12). KB2115 also works additively with another cholesterol-lowering therapy, statins, to produce greater reductions in serum cholesterol (13).
Although beneficial effects of STRM are commonly attributed to combinations of liver uptake and TRβ subtype-selective binding, contributions of another mode of selectivity, gene specificity, have not been explored. TR are part of the nuclear receptor (NR) family of ligand-gated transcription factors, which modulate gene expression by binding specific DNA elements and recruiting coregulators (2, 14). Interestingly, there are cases in which apparently similar NR ligands exert distinct effects on gene expression (15). Selective estrogen receptor modulators exhibit distinct partial and mixed agonist/antagonist profiles (16, 17), and similar peroxisome proliferator-activated receptor and glucocorticoid receptor agonists regulate distinct and only partially overlapping gene sets (18, 19). Gene-selective ligand actions are usually explained by one of two mechanisms. First, different NR subtypes can exhibit gene-specific activity, and naturally, NR subtype-specific genes exhibit distinct responses to subtype-selective ligands. Second, closely related NR ligands can exert different effects on the same gene in the presence of the same receptor; this is attributed to subtle ligand-specific effects on NR conformation and dynamics.
Presently, the extent to which STRM exhibit gene-specific actions is not clear. Liver, the main target of GC-1 action, expresses mostly TRβ with some TRα (4:1 ratio), and current evidence suggests that both TR contribute proportionately to regulation of T3-target genes with no obvious pure TR subtype-specific genes (20, 21). Thus, modest GC-1 TRβ selectivity, alone, does not seem likely to translate into significant gene selectivity. The extent to which STRM-specific effects on TR conformation result in gene-specific responses are less clear. Although TR-GC-1 complexes appear indistinguishable from TR-T3 complexes in x-ray structural studies (22), GC-1 and T3 exert distinct and opposite influences on reporters with natural variant inverted palindromic (IP) T3-response elements (TRE) derived from the sarco/endoplasmic reticulum Ca2+-ATPase 1 promoter, unlike reporters with classical direct repeat (DR)-4 TRE (23). Moreover, purified TRβ-GC-1 and TRβ-T3 complexes exhibit different affinities for a subset of 20 NR interacting coactivator peptides, raising the possibility that the two ligands could recruit different cofactors to target genes (24). Conversely, however, quantitative PCR (Q-PCR) analysis of gene profiles in liver and other tissues after treatment of rodent models with T3 and the TRβ-selective ligand GC-24 have failed to reveal any significant gene-specific ligand actions (25–27).
Here, we compared T3 and GC-1 responses in mouse liver and a human liver cell line with low levels of endogenous TRβ (HepG2). T3 and GC-1 exhibit virtually identical gene regulation profiles in both settings. We find one case in which T3 is slightly more potent than GC-1 at one positively regulated gene [angiopoietin-like factor 4 (ANGPTL4)] in HepG2 cells. We propose that gene-specific actions of GC-1 will be subtle and rare and discuss the significance of this finding for clinical ligand development.
Materials and Methods
Treatment of mice
Experiments were approved by Methodist Hospital Institutional Animal Care and Use Committee. C57B/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 9 wk of age. Animals were fed standard diet with water ad libitum and were divided into three groups (n = 5): control, T3, and GC-1. Animals were treated for 1 d by oral gavage ± 1 mg/kg T3 or GC-1. The morning after, animals were killed and tissues collected. TH deficiency was induced by feeding with iodine-deficient diet supplemented with 0.15% propylthiouracil for 14 d (Harlan Laboratories, Madison, WI). Hypothyroidism was confirmed by measuring total T4 in blood serum by ELISA (ALPCO Diagnostics, Salem, NH). Animals were treated overnight with vehicle, 1 mg/kg T3 or GC-1 via oral gavage (n = 4).
Cell culture
HepG2 cells were grown in DMEM-H21 supplemented with 10% fetal bovine serum, 100 U/ml of penicillin, 0.1 g/liter of streptomycin, and 4 mmol/liter glutamine, under 95% air and 5% CO2 at 37 C. For gene expression analysis, cells were grown to 60–70% confluence, washed with PBS three times, and incubated with DMEM supplemented with 10% TH-depleted serum ± T3 or GC-1 for various times (28).
RNA purification
Frozen liver samples were homogenized under liquid nitrogen with Mikro-Dismembrator S (Sartorius, Goettingen, Germany) at 3000 rpm for 2- to 20-sec pulses. RNA was isolated with acid guanidinium thiocyanate/phenol/chloroform by employing TRIzol Reagent (Invitrogen, Carlsbad, CA) following manufacture's instruction. To separate total RNA from genomic DNA and protein, a phase lock gel (heavy; Eppendorf, Westbury, NY) was used in the phase separation step; 700 μl of cell lysate-TRIzol-chloroform mix was added to prespun phase lock gel followed by centrifuging at 13,000 rpm for 10 min at 4 C. The top aqueous phase was separated from the organic phase containing genomic DNA and protein. Total RNA was isopropanol precipitated and further purified using a RNeasy Mini kit (QIAGEN, Valencia, CA) following manufacture's instruction. RNA quality and quantity of purified RNA was determined by NanoDrop 1000 (Thermo Scientific, Wilmington, DE), with 260/280 and 260/230 ratios equal or more than or equal to 2.0. For RNA samples sent for Affymetrix microarray analysis, RNA integrity was analyzed by Bioanalyzer (Agilent Technologies, Santa Clara, CA) applying Agilent RNA 6000 Nano kit (Agilent Technologies). Only samples with 28S/18S rRNA ratio equal or more than or equal to 1.8 were used.
Polymerase chain reaction
First strand cDNA synthesis was carried out using SuperScript III First-Strand Synthesis System for RT-PCR kit (Invitrogen). Oligo(dT)20 and Random Hexamers (1:1 mixture) were used to synthesize first-strand cDNA for PCR and Q-PCR, respectively. PCR was performed using PlatinumTaq DNA polymerase (Invitrogen). PCR products were subjected to agarose gel electrophoresis and visualized under UV. Q-PCR was performed by using Prism TH 7900 sequence detection system (Applied Biosystems, Foster City, CA) with default PCR program [94 C, 10 min; 94 C, 15 sec; 60 C, 60 sec (40 cycles)] using TaqMan Gene Expression or Power SYBR Green PCR Master Mix (Applied Biosystems). Primers that spanned exons were designed with Primer3 to avoid genomic DNA contamination. Sequences of primers and TaqMan Assay identifications (ID) are in Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://endo.endojournals.org. HepG2 primers were from ProbeFinder for Human Universal ProbeLibrary (http://www.universalprobelibrary.com). Dissociation curves were run routinely after Q-PCR to confirm single product. PCR results were evaluated by the comparative cycle time (Ct) method (ΔΔCt method Applied Biosystems' bulletin, Guide to performing relative quantitation of gene expression using real-time quantitative PCR, section VII.3). Results are expressed as an average of at least two independent assays or experiments as specified in figure legends.
Microarrays and data analysis
Datasets have been made publicly available in Gene Expression Omnibus (superseries GSE32445). For Illumina microarray analysis, biotin-labeled cRNA were generated from total RNA using Illumina TotalPrep-96 RNA Amplification kit (Ambion, Austin, TX); incubation time for all in vitro transcription reactions was 14 h at 37 C. Biotinylated cRNA samples were hybridized to MouseWG-6v2 BeadChip (Illumina, San Diego, CA) for 18 h at 58 C. BeadChips were imaged using the Illumina BeadArray Reader. Image files were processed with BeadStudio (Illumina). Only probes aligning to a single gene as defined by Ensembl (93.7%, 33939/36226) had their signal values background corrected and quantile normalized using lumi and differential expression determined with limma with Benjamini-Hochberg FDR correction in R (http://www.r-project.org/). Probes with more than or equal to 1.7-fold change in the ligand vs. vehicle and a Benjamini Hochberg-adjusted P ≤ 0.05 were considered significant.
For Affymetrix microarray analysis, biotin-labeled cRNA were generated from total RNA by using MessageAmp II-Biotin Enhanced Single Round aRNA Amplification kit (Ambion); incubation time for all in vitro transcription reactions was 14 h at 37 C. Biotinylated cRNA were purified and quality and integrity analyzed by NanoDrop 1000 (Thermo Scientific) and Bioanalyzer (Agilent Technologies), respectively. Before array hybridization, biotin-cRNA was fragmented by magnesium-potassium at 94 C to generate 35- to 200-nucleotide cRNA fragments. Subsequently, fragmented cRNA were hybridized to Human Genome U133A 2.0 Array chips (Affymetrix, Santa Clara, CA) at University of California San Francisco Genomic Core Facility. Initial statistical analysis of array data was performed by the University of California San Francisco statistics group: Student's t test followed by Benjamini Hochberg multiple hypothesis testing correction, after which further analysis was conducted at The Methodist Hospital Research Institute. Probe sets determined to be significantly regulated by ligand were further analyzed for associated functions and biological process through the Ingenuity Pathways Analysis (IPA) (version 7.5; Ingenuity Systems, Redwood City, CA).
Western blotting
HepG2 cells were harvested by trypsinization, washed with PBS, and extracts obtained using Pierce NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, IL). Extracts (50 μl of approximately 0.2 μg of protein) were mixed with the same amount of sodium dodecyl sulfate loading buffer [0.0625 m Tris (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerol, 1% 2-mercaptoethanol, and 0.001% Bromophenol blue] and separated by SDS-PAGE in a Bio-Rad Mini-Protean 3 apparatus (Bio-Rad, Hercules, CA), followed by electro-transfer onto a polyvinylidene fluoride membrane at 0.16 A in transfer buffer (40 mm Tris, 150 mm glycine, and 20% of methanol) for 16 h at 4 C. Transfer membranes were incubated in blocking buffer, 5% BSA-[20 mm Tris, 150 mm NaCl (pH 7.6), and 0.05% Tween 20] (TBST) for 2 h at room temperature with shaking. Membranes were then washed with TBST three times and incubated with TR-specific antibody at 1:2500 dilution in 1% BSA-TBST for 16 h at 4 C. TR antibodies were PA1–211A polyclonal (rabbit) anti-THα 1 (TRα 403-410; Affinity Bioreagents, Inc., Rockford, IL) and polyclonal (rabbit) anti-THβ 1 (TRβ 72-93; BabCO, Berkeley Antibody Co., now Covance, Richmond, CA). The membrane was washed three times with TBST and incubated with goat antirabbit IgG-horseradish peroxidase (sc-2004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) at a 1:25,000 dilution in TBST for 45 min at room temperature. Blots were visualized by applying ECL Plus (GE Healthcare, Piscataway, NJ) and exposing to film.
Short interfering RNA (siRNA) transfection
TR specific siRNA oligonucleotides were purchased from Ambion. Silencer Select siRNA THRA (ID no. s14116) targets TRα1 and Silencer Validated siRNA THRB (ID no. 3918) TRβ1. siRNA were transfected into HepG2 cells using Lipofectamine RNAiMAX (Invitrogen) using the reverse transfection procedure; siRNA-Lipofectamine RNAiMAX complexes were generated by mixing TRα1 siRNA (10 nm/well) or TRβ1 siRNA (100 nm/well) with Lipofectamine RNAiMAX (5 μl/well) in Opti-MEM I (Invitrogen). Mixtures were incubated at room temperature for 30 min and aliquoted in a six-well plate, and HepG2 cells were plated at a density of 0.5 × 10−6 cells/well. Finally, culture media containing serum were added and cells incubated for about 48 h. Ligands were added and cells incubated overnight and total RNA extracted for Q-PCR analysis. Remaining RNA levels are expressed as percentage of control values that represent the cells transfected with Silencer Negative Control #1 siRNA (Ambion).
Identification of TRE and reporter construction
A custom python script was created to scan genomic DNA sequences for degenerate canonical half-sites, (AG)GGT(GCA)A (both orientations), and spacing between half-sites equal or less than six base pairs (bp). This procedure allows identification of DR, inverted repeats (IP), and palindromic repeats (Pal). 20-kb bp upstream, through ANGPTL4 and the flanking 20-kb bp downstream, was analyzed. Oligonucleotides to potential TRE were generated and cloned into pGL3 or pGL4 expression vectors using standard methods. For promoter (−5 kb/+1 bp) and enhancer (−12/−11 kb) fragment cloning, regions of interest were amplified with custom PCR primers containing sequences that are homologous to target vector sequences, gel purified, and cloned into reporter genes using a modified QuikChange Site-Directed Mutagenesis procedure (Stratagene, La Jolla, CA). Transfection of HepG2 cells used TransFectin Lipid Reagent (Bio-Rad) and luciferase/β-galactosidase assays carried out by standard methods.
Results
Ligand treatment of mice
We compared effects of T3 and GC-1 on gene expression in mouse liver. We treated 9-wk-old male C57/Bl6 mice ± vehicle or ligand administered by a single oral gavage (n = 5 per group) and performed a similar study, in which mice were made hypothyroid by 2 wk feeding on iodine-deficient diet (n = 4 per group). For the latter, hypothyroid status was confirmed by determination of serum total T4 levels (Supplemental Fig. 1). To best guarantee saturation of liver TR, ligands were administered at supraphysiologic dose (1 mg/kg), 30-fold above that needed for optimal cholesterol lowering in euthyroid mice (data not shown and Ref. 29) (see Discussion). The day after, we isolated livers, prepared mRNA, and compared gene expression profiles.
Metabolic effects of TR ligands on mice were consistent with previous studies (Refs. 5, 6 and Lin, J.H.Z., P. Webb, and K. Philips, unpublished observations). Mice appeared healthy after overnight ligand treatment, and there were no changes in temperature, body weight, blood glucose, and serum lipids; these effects emerge after 2–3 d (6). Thus, changes in gene expression described below are likely to reflect actions of ligand and not secondary effects of altered metabolism.
T3-regulated genes in mouse liver
Large numbers of genes responded to T3 in livers of euthyroid and hypothyroid mice. For this part of the study, we focused on genes that exhibit more than or equal to 2.0- or 1.7-fold change in response to T3 or GC-1 ligand with adjusted P ≤ 0.05. In euthyroid mice, 81 genes exhibited more than or equal to 2-fold responses, and 171 genes exhibited more than or equal to 1.7-fold responses to T3 (adjusted P ≤ 0.05) (Table 1). This increased to 216 (≥2-fold) and 373 (≥1.7-fold) in hypothyroid mice, in keeping with previous estimates of the size of the T3-regulated transcriptome in hypothyroid mouse liver (20). Most T3-regulated genes (60%) exhibited positive responses to hormone in euthyroid mice, whereas proportions of negatively regulated genes increased to 55% in hypothyroid mice, similar to a previous analysis (20).
Table 1.
Number of genes regulated by T3 and GC-1 in mouse liver with expression changes more than or equal to 1.7-fold (adjusted P ≤ 0.05)
| Ligand | Euthyroid |
Hypothyroid |
||
|---|---|---|---|---|
| Induced | Suppressed | Induced | Suppressed | |
| T3 | 103 (52) | 68 (29) | 166 (122) | 207 (94) |
| GC-1 | 97 (53) | 70 (35) | 242 (156) | 256 (101) |
Numbers in parenthesis indicate the subset of these genes with expression changes more than or equal to 2-fold.
T3-regulated genes displayed expected identities. Inspection of gene function (ingenuity pathway analysis) confirms that T3 regulates expected biological pathways, including lipid synthesis, amino acid metabolism, and molecular transport (Supplemental Tables 2–5 and data not shown). In euthyroid mice, the largest inductions were a gene of unknown function (target ID B430219N15RIK) and CYP17A1, and enzyme involved in steroidogenesis (19.8- and 10.2-fold, respectively). The largest repression (7-fold) was obtained with PPP1R3C, an inhibitor of protein phosphatase 1. In hypothyroid mice, the most prominent inductions were with deiodinase 1 (DIO1) (31.1-fold) and CYP17A1 (15.8-fold), and the largest repression was with AC113514.1, a gene with unknown function (25-fold). The target gene list also included well characterized T3-activated genes; including spot 14 (THRSP), malic enzyme, glycerol phosphate dehydrogenase 2, and repressed genes, including sterol- response element-binding factor 1c. In general, T3 target genes were regulated similarly in euthyroid and hypothyroid mice, but there were gene-specific differences in the magnitude of induction/repression; for example, T3 induced DIO1 about 2-fold in euthyroid mice and 30-fold in hypothyroid mice, whereas more similar levels of induction were obtained with CYP17A1 in both systems (10.2- and 15.8-fold, respectively).
T3 and GC-1 regulate identical mouse liver genes
Comparisons of T3- and GC-1-responsive genes that met fold-change cut-offs revealed extensive similarity between gene sets (Tables 1 and 2). In hypothyroid mice, 498 genes responded more than or equal to 1.7-fold to GC-1 (242 induced and 256 repressed) and 257 genes responded more than or equal to 2.0-fold (156 up and 101 down). Of these, 303 genes were shared with T3 at more than or equal to 1.7-fold cut-off (145 induced and 158 repressed) and 174 at more than or equal to 2.0-fold cut-off (106 induced and 68 repressed). In total, only 42 genes were flagged as responding uniquely to T3 and 83 to GC-1 at more than or equal to 2-fold cut-off and 70 (T3) and 195 (GC-1) at 1.7-fold cut-off.
Table 2.
Number of genes flagged as shared between T3 and GC-1 or apparently unique at 1.7- and 2.0-fold cut-offs in hypothyroid mice (adjusted P ≤ 0.05)
| Shared | T3 | GC-1 | |
|---|---|---|---|
| 1.7-Fold cut-off | 303 | 70 | 195 |
| 2.0-Fold cut-off | 174 | 42 | 83 |
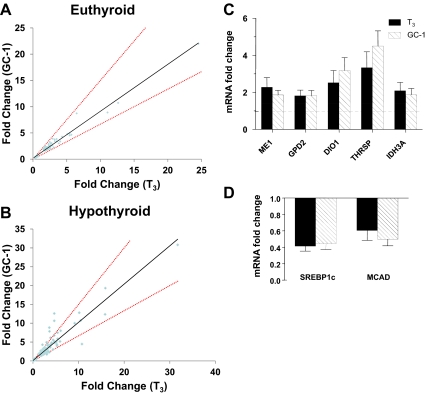
Analysis of genes initially flagged as unique T3 or GC-1 responders suggests that there is even greater similarity between datasets than indicated above. Most genes that are apparently unique at more than or equal to 2.0-fold cut-off appear in the shared set at more than or equal to 1.7-fold cut-off, and new examples of uniquely regulated genes appear in the latter dataset; visual inspection of gene expression levels at both cut-off levels suggests that apparently unique genes are, in fact, similarly regulated with appropriate fold change and/or statistical significance only achieved in one of the two treatments (data not shown). Three separate analyses convince us that overall responses to both ligands are virtually identical. First, comparisons of fold-change for genes that displayed significant ligand response revealed no more than ±30% difference between T3 and GC-1 in euthyroid mice (Fig. 1, A and B). Only two (uridine phosphorylase-2; upp2 and crystallin, beta B3; crybb3) exhibited greater than 30% variance in the hypothyroid mice (Fig. 1B), but these effects could not be verified with follow up Q-PCR (data not shown). Second, we observed high correlation between responses to probe sets corresponding to genes regulated by T3 and GC-1 [judged by Spearman's rank order correlation coefficient (rs) = 0.912, P < 0.0001 for euthyroid mice and rs = 0.948, P < 0.0001 for hypothyroid mice, close to a perfect value of 1 in both cases (Supplemental materials for methods)]. Finally, ligand effects were identical at selected TR target genes involved in carbohydrate and lipid metabolism in Q-PCR assays of RNA from euthyroid mice (Fig. 1, C and D, and data not shown) and hypothyroid mice (data not shown).
Fig. 1.
T3 and GC-1 regulate identical gene sets in mouse liver. A, Comparisons of gene regulation by T3 and GC-1; fold change of genes whose expression was altered by GC-1 was plotted against fold change observed with T3 in euthyroid mice. The red dotted line represents a subjective 33% difference threshold, for example, if gene X is 6-fold induced by T3, then 4- to 8-fold induction by GC-1 would fall between the lines or vice versa. B, Comparison of fold change observed in T3 vs. GC-1-treated mice using Q-PCR analysis of selected T3-inducible genes. The cluster of points that displays greater response to GC-1 than T3 corresponds to multiple probes that map to upp2, the point that shows greater response to T3 than GC-1 corresponds to Crybb3. Neither change was verified with Q-PCR. C, Q-PCR verification of selected positively reregulated genes from euthyroid mouse liver. D, As for C, with repressed genes. ME, Malic enzyme; GPD2, glycerol phosphate dehydrogenase 2; IDH3A, isocitrate dehydrogenase 3 (NAD+) alpha; SREBP1c, sterol regulatory element-binding protein 1c; MCAD, medium-chain acyl-CoA dehydrogenase.
T3 and GC-1 regulate the same genes in HepG2 cells
We also compared T3 and GC-1 action in a human liver cell line (HepG2). This cell line expresses transcripts for both TR (Supplemental Fig. 2) but only contained detectable TRβ protein by Western blotting; thus, this cell line permits us to analyze ligand actions through endogenously expressed TRβ in the absence of TRα (Fig. 2A). We prepared mRNA from cells treated with vehicle or saturating ligands (10 nm) for 24 h (n = 3) and analyzed gene expression. Here, we focused on statistically significant changes between ligand and vehicle-treated samples (adjusted P ≤ 0.05).
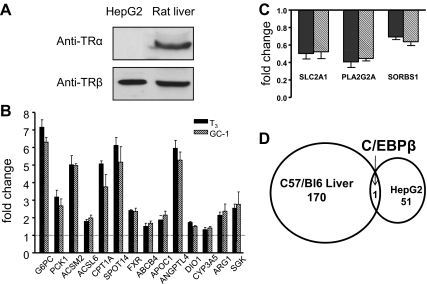
Fig. 2.
T3 and GC-1 induce identical gene sets in HepG2 cells. A, Western blot analysis of HepG2 cell and rat liver extracts reveals detectable TRβ1 in HepG2 cells. B, Comparison of fold change observed in T3 vs. GC-1-treated HepG2 using Q-PCR analysis of selected T3-inducible genes. ABCG4, ATP-binding cassette, sub-family B (MDR/TAP), member 4, APOC1, apolipoprotein C-I; CYP3A5, cytochrome P450, family 3, subfamily A, polypeptide 5; ARG1, arginase, liver; sgk1, serum/glucocorticoid regulated kinase 1 C, As B, with selected repressed genes. SLC2A1, solute carrier family 2 (facilitated glucose transporter), member 1; PLA2G2A, phospholipase A2, group IIA; SORBS1, sorbin and SH3 domain containing 1. D, Lack of overlap between euthyroid mouse liver and HepG2 datasets. Venn diagram of significantly changed genes in both systems reveals only one overlapping gene, C/EBPβ.
More than 100 genes responded to T3 (Supplemental Table 6). Unlike mouse livers, nearly all were positively regulated: 104 genes were induced by T3 and 10 repressed. Of the positively regulated genes, 42 exhibited more than or equal to 1.7-fold change. The largest induction (5-fold) was obtained with glucose 6 phosphatase (G6Pc), and the largest repression (60%) was with solute carrier family 2 member 1, implicated in facilitated glucose transport into cells.
Again, T3 and GC-1 effects were effectively identical. T3 regulated more genes (104 up and 10 down) than GC-1 (70 up and five down) (Supplemental Table 6). However, visual inspection revealed that all T3 induced and repressed genes that were not identified as GC-1 targets, are weakly regulated genes, for which GC-1 effects were detectable but failed to reach statistical significance (Supplemental Table 7 and data not shown). Further, comparisons of gene expression detected by complete probe sets or the subgroup of probe sets that recognize ligand-regulated genes confirms that T3 and GC-1 effects are effectively identical (entire list rs = 0.54, significant probe sets rs = 0.95, both P < 0.0001) (Supplemental materials). We also verified that T3 and GC-1 display identical effects at selected positively (Fig. 2B) and negatively (Fig. 2C) regulated genes by Q-PCR.
Interestingly, there is little overlap between TR target genes in mouse liver and HepG2 cells (Fig. 2D, Supplemental Fig. 4, and Supplemental Tables 7 and 8). One gene (CCAAT enhancer binding protein C/EBPβ) exhibited more than or equal to 1.7-fold response to T3 in both arrays. Q-PCR analysis revealed that THRSP and DIO1 were also similarly regulated in both settings (Fig. 2B). However, the HepG2 dataset did include relevant TR target genes that have previously been shown to respond to T3 (Supplemental IPA analysis), including genes whose products are implicated in gluconeogenesis, G6PC and PCK1 and fatty acid oxidation, CPT1A, Acyl Co-A synthtase M2, and Acyl CoA synthtase L6 (Fig. 2B and Supplemental Table 7). T3 also induced genes involved in other known TR-regulated pathways, including cholesterol/bile acid metabolism and blood coagulation (Supplemental Tables 7 and 8). We confirmed that T3 target genes that were not significantly regulated in mouse liver (including gluconeogenesis genes and others) or HepG2 cells (including lipogenic genes and others) were expressed and not affected by T3 using Q-PCR (Supplemental Fig. 2 and data not shown). Thus, TR regulates distinct sets of relevant target genes in livers of chow-fed mice and HepG2 cells, but importantly, T3 and GC-1 actions are almost identical in both contexts.
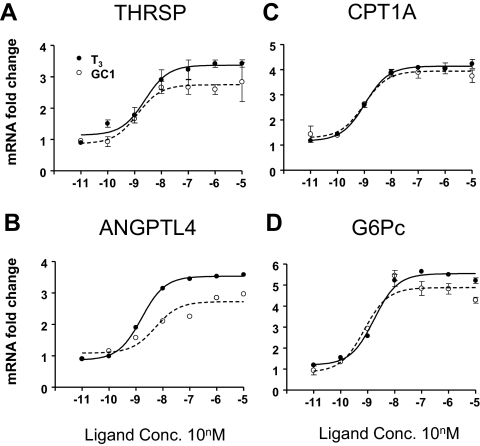
Gene-specific variation in GC-1 potency
We examined effects of T3 and GC-1 at representative positively regulated genes in HepG2 in more detail and found one difference between ligands. Although GC-1 and T3 induction of THRSP, CPT1A, G6Pc, and other transcripts exhibited similar concentration dependencies (Fig. 3, A, C, and D, and data not shown), GC-1 displayed a 3-fold rightward shift in EC50 relative to T3 at ANGPTL4 (Fig. 3B and Supplemental Table 9).
Fig. 3.
Differences in ligand dose responses at one gene in HepG2. Results of Q-PCR analysis with RNA prepared from HepG2 cells treated with indicated doses of ligands. THRSP (A), CPT1A (B), ANGPTL4 (C), and G6Pc (D).
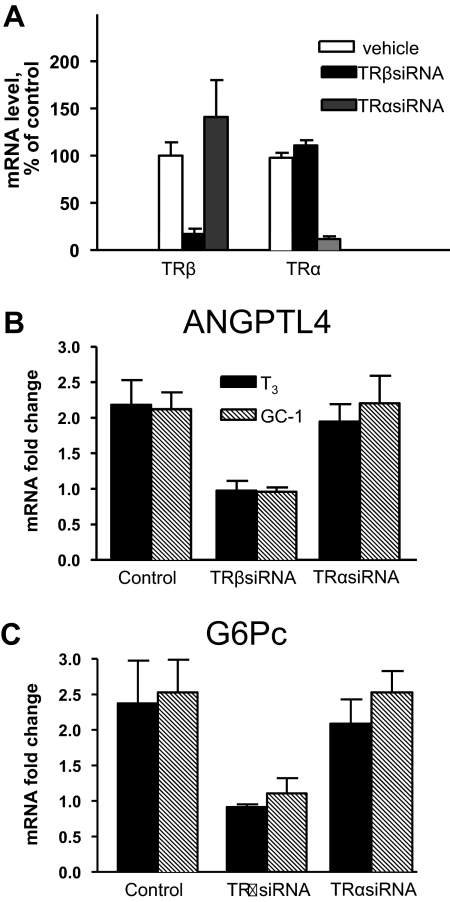
Because GC-1 induction of TRE-regulated reporters is rightward shifted by half an order of magnitude with transfected TRα vs. TRβ in HepG2 cells (Supplemental Fig. 4 and data not shown) (30), we investigated the possibility that the rightward shift in ANGPTL4 dose response is a consequence of selective responses to vanishingly low levels of endogenous TRα. Control experiments with siRNA confirmed that TRα and TRβ-specific siRNA reduce expression of cognate TR transcripts (Fig. 4A). In parallel, the same siRNA directed against TRβ inhibited T3 response at ANGPLT4 (Fig. 4B), G6Pc (Fig. 4C), and other regulated genes (data not shown), whereas TRα siRNA had no effect on any tested TR target gene. This confirms that TRβ mediates T3 responses in HepG2.
Fig. 4.
siRNA treatment reveals that TRβ mediates the T3 response of ANGPLT4 and G6PC in HepG2 cells. Q-PCR analysis with different TR siRNA or scrambled control. A, Q-PCR analysis of TR subtype expression in cells treated with siRNA relative to control. Results represent average of three independent experiments, each with three individual points. Actual average Ct values in each point are: TRβ mRNA; control siRNA 22.82, TRβ siRNA 25.7, and TRα siRNA 22.61; and TRα mRNA control siRNA 24.8, TRβ siRNA 24.76, and TRα siRNA 27.58. B, ANGPLT4; data are presented as fold induction with ligands and represents average of three separate experiments. C, As in B, with G6PC. For the latter two analyses, there was no change in Ct values after TR siRNA treatment in the absence of hormone (ANGPTL4, 28.8; G6Pc, 26.2) relative to control siRNA, implying that unliganded TR do not exert detectable effects on these genes.
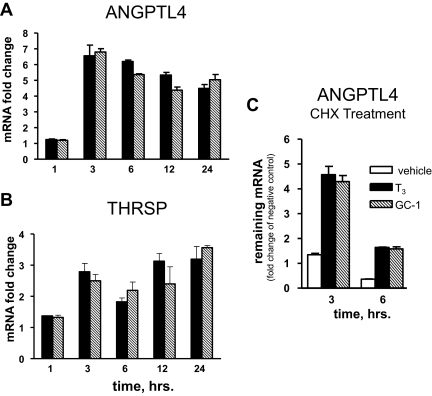
ANGPTL4 is a direct target for TRβ. T3 and GC-1 induction was detectable within 1 h and peaked at 3 h (Fig. 5A). This is comparable with another verified direct TR target, THRSP (Fig. 5B), and faster than other TR targets, such as CPT1A and G6Pc, where maximal induction required extended ligand treatment (data not shown). Interestingly, there were no differences between T3 and GC-1 induction kinetics in these cases or any other genes that we investigated in this study (including CPT1A, G6Pc, Farnesoid X Receptor, and others) (data not shown). The protein synthesis inhibitor cycloheximide (CHX) did not block ANGPTL4 induction; ligand responses persisted after 3- and 6-h CHX treatments (Fig. 5C). Thus, T3 and GC-1 induction of ANGPLT4 is rapid and does not require novel protein synthesis.
Fig. 5.
ANGPTL4 is a direct TRβ target. A and B, Time course of ANGPTL4 and thrsp response to T3. Fold induction as a function of ligand incubation time (hours). C, CHX treatment does not abrogate ligand responsiveness of ANGPTL4. Q-PCR analysis of mRNA fold changes, with samples compared with mRNA obtained from nontreated cells at the same time slots.
A potential TRE near ANGPTL4 does not confer differential ligand response
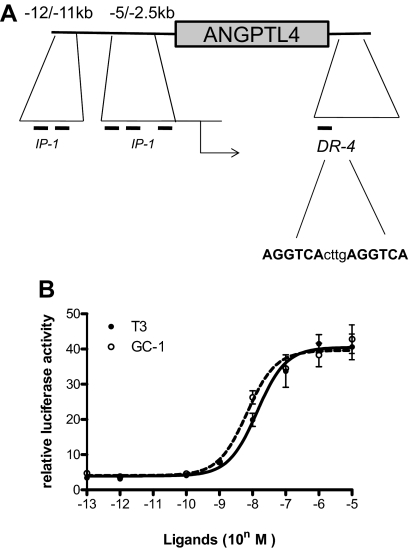
To understand whether differential effects of T3 and GC-1 at ANGPTL4 are related to TRE sequence, as reported for sarco/endoplasmic reticulum CA2+-ATPase TRE (23), we used computer-aided analysis to search for paired degenerate TRE half-sites in the vicinity of the ANGPTL4 gene (Supplemental Table 10). We found several IP-1 elements in the promoter (−5 kbp/+1 bp) and the upstream enhancer (12/−11 kbp) regions. Cloning of both fragments and isolated IP-1 failed to confer response to T3 or GC-1 on luciferase reporters, and isolated IP-1 elements did not yield detectable TR complexes in gel shifts (data not shown). However, a reporter driven by a consensus DR-4 located immediately downstream of the 3′ end of the gene exhibited T3 and GC-1 response in HepG2 cells in the presence of exogenous TRβ (Fig. 6B) and also bound Retinoid X Receptor-TR and TR in gel shifts (data not shown). Although T3 and GC-1 responses were significant, there were no differences in magnitude or potency of response. Thus, altered GC-1 response is not related to the sequence composition of the only TRE that we could detect within the ANGPTL4 locus (Discussion).
Fig. 6.
ANGPTL4 TRE does not confer differential ligand response. A, Schematic of ANGPLT4 gene with approximate locations of potential paired AGGTCA half-sites and the consensus DR-4 site marked. B, Comparison of ligand dose responses with an ANGPTL4 DR-4 TRE-driven reporter in HepG2 cells that contain exogenously expressed TRβ.
Discussion
Selective actions of STRM are attributed to preferential liver uptake and TR subtype selectivity, but there has been little exploration of contributions of gene-target specificity (23, 24). Here, we compared effects of saturating doses of T3 and GC-1 in livers of euthyroid and hypothyroid mice and HepG2 cells with endogenous TRβ to test the extent of these effects. We find that T3 and GC-1 regulate highly similar gene sets in all three systems, as judged by microarray and Q-PCR. Statistical correlation and pathway analysis of T3 and GC-1 underscores the idea that ligand responses are identical (Supplemental data); these approaches provide an even stronger indication of similarity between T3 and GC-1, given that they do not rely on an arbitrary set threshold for individual genes, but instead, organize regulated genes by fold-change and biological function. We also failed to detect differences between T3 and GC-1 induction kinetics for more than 10 tested genes in HepG2 and only observed modest variation in concentration dependence at one gene in HepG2 (ANGPTL4, discussed below). Thus, despite early suggestions that T3 and GC-1 will regulate different gene sets (23, 24), we find no evidence in favor of this idea. We propose instead that differential effects of STRM and T3 arise mostly from tissue uptake and TR subtype selectivity, and contributions of gene specificity are likely to be limited.
Our findings fit well with analysis of androgen receptor ligands on protein interaction profiles in vitro and gene signatures in prostate cells (31); this revealed a continuum of androgen receptor ligand activities from antagonist to superagonist and showed that similar ligands group into classes with very similar effects on receptor protein interaction profile and gene expression. In this framework, we suspect that previous studies that showed different effects of apparently similar NR ligands on gene expression profiles (see introductory section) employed ligands from different activity classes and that T3 and GC-1 fall into a similar TR agonist class.
Our experiments were designed to test the hypothesis that T3 and GC-1 might display fundamentally different gene regulation properties. To achieve this, we used supraphysiologic ligand doses to maximize the likelihood that liver TR (TRβ with small amounts of TRα) (20) would be occupied with T3 or GC-1 and minimize the possibility that we would detect differences that are consequences of partial receptor occupancy; a possible result of differential tissue uptake, pharmacodynamics, or lower affinity of GC-1 for TR relative to T3. Thus, we did not adopt a classic strategy, in which effects of equimolar physiologic ligand doses are compared, and, instead, chose ligand doses (1 mg/kg) based entirely on functional considerations; we used a comparable (30-fold) excess over amounts of ligands required for optimal cholesterol lowering (29). We recognize that this strategy could mean that we missed instances of gene specificity that emerge at subsaturating ligand doses and that this could be important, human trials with KB2115 and GC-1 used low drug doses to minimize possible side effects (12) and would highlight any such effects. However, limited analysis of gene expression in HepG2 suggests that gene-specific effects on ligand potency are likely to be rare; we found one case (of 10 genes tested) (Fig. 4 and data not shown), in which GC-1 showed reduced potency vs. T3, at ANGPTL4 in HepG2 cells. We also think that it is not likely that modest TRβ selectivity of GC-1 will result in significant gene selectivity in liver. The preponderance of TRβ in liver suggests that T3 responses will be strongly dependent on this subtype in this tissue; our preliminary results suggest that liver T3 responses are almost abrogated in TRβ−/− mice, and we have not identified any genes that retain strong T3 induction with TRα alone (Xia, X., and P. Webb, unpublished observations). Moreover, TR regulate similar genes in mouse liver (20) and cell culture (21). Thus, differential responses of GC-1 that relate to TR subtype selectivity would only be observed within very limited dose ranges (half an order of magnitude for GC-1, see Supplemental Fig. 4) and, given modest contributions of TRα to liver gene expression, are not likely to be prominent.
We do not understand why GC-1 exhibits reduced potency at ANGPTL4 in HepG2 cells. TRβ mediates T3 and GC-1 effects at this and other genes in HepG2 (as judged by use of siRNA), and ANGPTL4 is a direct target of TRβ action. We defined one possible functional TRE within the ANGPTL4 locus, a consensus DR-4 element downstream of the coding region. Although it is conceptually difficult to link downstream binding events to regulation of particular genes, several lines of evidence suggest that this element represents a bona fide TRE (including transfection results in Fig. 6, gel shift analysis, data not shown, and our unpublished studies, which reveal a peak of TRβ binding at the 3′ region of ANGPTL4 close to the putative TR-binding site in chromatin immunoprecipitation sequencing assays; Ayers S.D., and P. Webb, unpublished observations). However, this element did not confer differential responses to ligands, suggesting that reduced GC-1 potency is not related to this specific TRE sequence, as seen previously (23), and that other aspects of gene context must influence ligand potency.
Finally, because HepG2 cells express detectable TRβ1 and therefore appear to be a reasonable model for studies of T3 response, it is worth remarking on two aspects of HepG2 as an experimental system. First, TR levels in HepG2 are low, approximately 200 receptors per cell based on T3 binding analysis and comparative Western blottings vs. extracts of HeLa cells with defined amounts of TR as a control (data not shown). Even with low TR, more than 100 genes exhibit significant T3 response and some are highly induced. Thus, it is important to distinguish responses that arise from transfected and endogenous TRβ in studies of T3 responses in HepG2 cells that are stably transfected with TR (21, 32), for example, studies in Ref. 21 clearly differentiate contributions of exogenous and endogenous TR. Second, we note few overlaps between T3-regulated gene sets in mouse liver and HepG2. In principle, differences could arise from human/mouse species variation (33) or cancer cell/in vitro culture context. We note, however, that TR-regulated genes in HepG2 are known T3-targets involved in fasting responses, such as gluconeogenesis and fatty acid β-oxidation (G6Pc, PEPCK, and CPT1A). Such genes may respond to T3 in mouse liver in appropriate conditions, and we suggest that the HepG2 profile may reflect TR responses that predominate in a particular physiologic state. For example, we killed mice in the morning and homogenized whole liver, and TR expression is under circadian rhythm control and zonated; thus, shared target genes may not be appropriately regulated at some times of the day or expressed in particular zones of expression and missed in our analysis, which would likely dilute out signs of expression of these genes (34, 35). Clearly, this issue will require investigation.
Supplementary Material
Acknowledgments
We thank Chandi Griffiths for advice on microarray experiments.
Present address for C.Y.: Department of Nutrition, University of California, Berkeley, California 94720.
This work was supported by NIH RC4 and NIH R01 Grants DK090849-01 and DK61468 (to J.D.B.).
Disclosure Summary: The estate of J.D.B. has stock holdings in KaroBio AB, a company with interests in this area of research. All other authors have nothing to disclose.
Footnotes
- ANGPTL4
- Angiopoietin-like factor 4
- bp
- base pair
- CHX
- cycloheximide
- CPT
- carnitine palmitoyl transferase
- Ct
- cycle time
- DIO1
- deiodinase 1
- DR
- direct repeat
- GC-1
- {4-[4-Hydroxy-3-(1-Methylethyl)benzyl]-3,5-Dimethylphenoxy}acetic acid G6Pc, glucose 6 phosphatase
- ID
- identification
- IP
- inverted palindromic
- IPA
- Ingenuity Pathways Analysis
- LDL
- low-density lipoprotein
- NR
- nuclear receptor
- Q-PCR
- quantitative PCR
- rs
- Spearman's rank order correlation coefficient
- siRNA
- short interfering RNA
- STRM
- selective TR modulator
- TBST
- 20 mm Tris, 150 mm NaCl (pH 7.6), and 0.05% Tween 20
- TH
- thyroid hormone
- TR
- TH receptor
- TRE
- T3-response element.
References
- 1. Baxter JD, Webb P. 2009. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov 8:308–320 [DOI] [PubMed] [Google Scholar]
- 2. Yen PM. 2001. Physiological and molecular basis of thyroid hormone action. Physiol Rev 81:1097–1142 [DOI] [PubMed] [Google Scholar]
- 3. Baxter JD, Webb P, Grover G, Scanlan TS. 2004. Selective activation of thyroid hormone signaling pathways by GC-1: a new approach to controlling cholesterol and body weight. Trends Endocrinol Metab 15:154–157 [DOI] [PubMed] [Google Scholar]
- 4. Forrest D, Vennström B. 2000. Functions of thyroid hormone receptors in mice. Thyroid 10:41–52 [DOI] [PubMed] [Google Scholar]
- 5. Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. 2000. The thyroid hormone receptor-β-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology 141:3057–3064 [DOI] [PubMed] [Google Scholar]
- 6. Grover GJ, Egan DM, Sleph PG, Beehler BC, Chiellini G, Nguyen NH, Baxter JD, Scanlan TS. 2004. Effects of the thyroid hormone receptor agonist GC-1 on metabolic rate and cholesterol in rats and primates: selective actions relative to 3,5,3′-triiodo-L-thyronine. Endocrinology 145:1656–1661 [DOI] [PubMed] [Google Scholar]
- 7. Grover GJ, Mellström K, Ye L, Malm J, Li YL, Bladh LG, Sleph PG, Smith MA, George R, Vennström B, Mookhtiar K, Horvath R, Speelman J, Egan D, Baxter JD. 2003. Selective thyroid hormone receptor-β activation: a strategy for reduction of weight, cholesterol, and lipoprotein (a) with reduced cardiovascular liability. Proc Natl Acad Sci USA 100:10067–10072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, Dong G, Singh R, Shapses S, Malm J, Webb P, Baxter JD, Grover GJ. 2008. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor β subtype selective agonist KB-141. J Steroid Biochem Mol Biol 111:262–267 [DOI] [PubMed] [Google Scholar]
- 9. Perra A, Simbula G, Simbula M, Pibiri M, Kowalik MA, Sulas P, Cocco MT, Ledda-Columbano GM, Columbano A. 2008. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J 22:2981–2989 [DOI] [PubMed] [Google Scholar]
- 10. Cable EE, Finn PD, Stebbins JW, Hou J, Ito BR, van Poelje PD, Linemeyer DL, Erion MD. 2009. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 49:407–417 [DOI] [PubMed] [Google Scholar]
- 11. Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, Linemeyer DL. 2007. Targeting thyroid hormone receptor-β agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci USA 104:15490–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berkenstam A, Kristensen J, Mellström K, Carlsson B, Malm J, Rehnmark S, Garg N, Andersson CM, Rudling M, Sjöberg F, Angelin B, Baxter JD. 2008. The thyroid hormone mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA 105:663–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, Klein I, Baxter JD, Angelin B. 2010. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. New Engl J Med 362:906–916 [DOI] [PubMed] [Google Scholar]
- 14. Glass CK, Rosenfeld MG. 2000. coregulator exchange in transcriptional functions of nuclear receptors. Gene Dev 14:121–141 [PubMed] [Google Scholar]
- 15. Togashi M, Borngraeber S, Sandler B, Fletterick RJ, Webb P, Baxter JD. 2005. Conformational adaptation of nuclear receptor ligand binding domains to agonists: potential for novel approaches to ligand design. J Steroid Biochem Mol Biol 93:127–137 [DOI] [PubMed] [Google Scholar]
- 16. Katzenellenbogen BS, Montano MM, Ediger TR, Sun J, Ekena K, Lazennec G, Martini PG, McInerney EM, Delage-Mourroux R, Weis K, Katzenellenbogen JA. 2000. Estrogen receptors: selective ligands, partners, and distinctive pharmacology. Recent Prog Horm Res 55:163–193; discussion 194–165 [PubMed] [Google Scholar]
- 17. Nilsson M, Dahlman-Wright K, Gustafsson JA. 2004. Nuclear receptors in disease: the oestrogen receptors. Essays Biochem 40:157–167 [DOI] [PubMed] [Google Scholar]
- 18. Sears DD, Hsiao A, Ofrecio JM, Chapman J, He W, Olefsky JM. 2007. Selective modulation of promoter recruitment and transcriptional activity of PPARγ. Biochem Biophys Res Commun 364:515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang JC, Shah N, Pantoja C, Meijsing SH, Ho JD, Scanlan TS, Yamamoto KR. 2006. Novel arylpyrazole compounds selectively modulate glucocorticoid receptor regulatory activity. Gene Dev 20:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yen PM, Feng X, Flamant F, Chen Y, Walker RL, Weiss RE, Chassande O, Samarut J, Refetoff S, Meltzer PS. 2003. Effects of ligand and thyroid hormone receptor isoforms on hepatic gene expression profiles of thyroid hormone receptor knockout mice. EMBO Rep 4:581–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chan IH, Privalsky ML. 2009. TR isoform-specific gene regulation. Mol Endocrinol 23:1758–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. 2001. Hormone selectivity in thyroid hormone receptors. Mol Endocrinol 15:398–410 [DOI] [PubMed] [Google Scholar]
- 23. Gloss B, Giannocco G, Swanson EA, Moriscot AS, Chiellini G, Scanlan T, Baxter JD, Dillmann WH. 2005. Different configurations of specific thyroid hormone response elements mediate opposite effects of thyroid hormone and GC-1 on gene expression. Endocrinology 146:4926–4933 [DOI] [PubMed] [Google Scholar]
- 24. Moore JM, Galicia SJ, McReynolds AC, Nguyen NH, Scanlan TS, Guy RK. 2004. Quantitative proteomics of the thyroid hormone receptor-coregulator interactions. J Biol Chem 279:27584–27590 [DOI] [PubMed] [Google Scholar]
- 25. Amorim BS, Ueta CB, Freitas BC, Nassif RJ, Gouveia CH, Christoffolete MA, Moriscot AS, Lancelloti CL, Llimona F, Barbeiro HV, de Souza HP, Catanozi S, Passarelli M, Aoki MS, Bianco AC, Ribeiro MO. 2009. A TRβ-selective agonist confers resistance to diet-induced obesity. J Endocrinol 203:291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Castillo M, Freitas BC, Rosene ML, Drigo RA, Grozovsky R, Maciel RM, Patti ME, Ribeiro MO, Bianco AC. 2010. Impaired metabolic effects of a thyroid hormone receptor β-selective agonist in a mouse model of diet-induced obesity. Thyroid 20:545–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grijota-Martínez C, Samarut E, Scanlan TS, Morte B, Bernal J. 2011. In vivo activity of the thyroid hormone receptor β- and α-selective agonists GC-24 and CO23 on rat liver, heart, and brain. Endocrinology 152:1136–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Samuels HH, Stanley F, Casanova J. 1979. Depletion of L-3,5,3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology 105:80–85 [DOI] [PubMed] [Google Scholar]
- 29. Johansson L, Rudling M, Scanlan TS, Lundåsen T, Webb P, Baxter J, Angelin B, Parini P. 2005. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA 102:10297–10302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martínez L, Nascimento AS, Nunes FM, Phillips K, Aparicio R, Dias SM, Figueira AC, Lin JH, Nguyen P, Apriletti JW, Neves FA, Baxter JD, Webb P, Skaf MS, Polikarpov I. 2009. Gaining ligand selectivity in thyroid hormone receptors via entropy. Proc Natl Acad Sci USA 106:20717–20722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Norris JD, Joseph JD, Sherk AB, Juzumiene D, Turnbull PS, Rafferty SW, Cui H, Anderson E, Fan D, Dye DA, Deng X, Kazmin D, Chang CY, Willson TM, McDonnell DP. 2009. Differential presentation of protein interaction surfaces on the androgen receptor defines the pharmacological actions of bound ligands. Chem Biol 16:452–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shih CH, Chen SL, Yen CC, Huang YH, Chen CD, Lee YS, Lin KH. 2004. Thyroid hormone receptor-dependent transcriptional regulation of fibrinogen and coagulation proteins. Endocrinology 145:2804–2814 [DOI] [PubMed] [Google Scholar]
- 33. Drover VA, Agellon LB. 2004. Regulation of the human cholesterol 7α-hydroxylase gene (CYP7A1) by thyroid hormone in transgenic mice. Endocrinology 145:574–581 [DOI] [PubMed] [Google Scholar]
- 34. Zandieh Doulabi B, Platvoet-ter Schiphorst M, van Beeren HC, Labruyere WT, Lamers WH, Fliers E, Bakker O, Wiersinga WM. 2002. TR(β)1 protein is preferentially expressed in the pericentral zone of rat liver and exhibits marked diurnal variation. Endocrinology 143:979–984 [DOI] [PubMed] [Google Scholar]
- 35. Yang X, Downes M, Yu RT, Bookout AL, He W, Straume M, Mangelsdorf DJ, Evans RM. 2006. Nuclear receptor expression links the circadian clock to metabolism. Cell 126:801–810 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.