Abstract
Tumors develop with dysregulated activation of mammalian target of rapamycin (mTOR), the kinase activity of which is kept in an inactive state by a tumor suppressor dimer containing tuberous sclerosis 1 (TSC1) and TSC2. We examined whether conditional deletion of TSC1 by a knock-in allele of the anti-Müllerian hormone type 2 receptor (Amhr2) driving Cre expression and subsequent activation of mTOR in granulosa cells and in oviductal and uterine stromal cells affects fertility in female mice. Increased phosphorylation of ribosomal protein S6, a downstream target of activated mTOR, was observed in all AMHR2-expressing tissues examined, indicating loss of TSC1 activity. TSC1 deletion in granulosa cells led to the detection of significantly fewer primordial follicles in mutant mice at 12 wk, suggesting premature ovarian insufficiency, which might be related to the significantly increased time mutant mice spent in estrus. Although the number of good-quality ovulated oocytes was not significantly different compared with controls, there was a significantly higher number of degenerated oocytes after normal and superovulation, suggesting compromised oocyte quality, as well. Natural mating also showed severalfold higher numbers of degenerate bodies in the mutants that collected in bilateral swellings resembling hydrosalpinges that formed in all mice examined because of occlusion of the proximal oviduct. Attempts to transfer control embryos into mutant uteri also failed, indicating that implantation was compromised. Endometrial epithelial cells continued to proliferate, and quantitative RT-PCR showed that mucin 1 expression persisted during the window of implantation in mutant uteri, without any changes in progesterone receptor mRNA expression, suggesting a mechanism that does not involve disrupted estradiol-regulated progesterone receptor expression. Homozygous deletion of TSC1 in reproductive tract somatic tissues of mice rendered females completely infertile, which is likely due to these pleiotropic effects on follicle recruitment, oviductal development, and blastocyst implantation.
Compromised female fertility is caused by many mechanisms, including ovarian dysfunction, tubal obstruction or adhesions, uterine malformations, implantation failure, and polycystic ovary syndrome (1). Mouse models with deletion of specific genes have provided clues to the genetic causes of some of these (2). However, there are still substantial numbers of patients with infertility of unknown etiology.
The phosphoinositide-3 kinase (PI3K) pathway plays an important role in many biological functions including metabolic control, immunity, and cancer. Although the basic framework of PI3K signaling is well understood, much remains to be learned about its role in reproductive biology. FSH, which normally regulates granulosa cell differentiation and follicular development after antrum formation via the G protein-coupled receptor/protein kinase A signaling pathway, also activates PI3K to activate AKT, which in turn phosphorylates and regulates forkhead box O1 (FOXO1) and RAS to control granulosa cell function and differentiation (3). Additionally, the LH surge stimulates not only protein kinase A but also PI3K/AKT and RAS signaling cascades, and activation of each of these is essential for ovulation (4). Studies using tissue-specific deletion have further implicated the PI3K/AKT signaling pathway in the regulation of primordial follicle activation (5–7). The PI3K/AKT pathway is also required for 17β-estradiol (E2)-induced vascular endothelial growth factor A (VEGF-A) expression in rat uterus to increase vascular permeability in preparation for implantation (8). Additionally, activation of AKT by loss of phosphatase and tensin homolog (PTEN) in uterine epithelium is sufficient for initiation of endometrial cancer (9).
The tuberous sclerosis complex (TSC)/mammalian target of rapamycin (mTOR) signaling pathway is a critical downstream effector of PI3K/AKT activity (10). The activity of mTOR complex I, a serine/threonine kinase that regulates cell growth and proliferation in response to growth factors and nutrients, is negatively regulated by a heterodimeric complex consisting of TSC1 (or hamartin) and TSC2 (or tuberin). Patients with inactivating mutations in either of these genes present with multiple benign tumors in the brain, heart, kidney, lung, and skin (11, 12). In granulosa cells, FSH has been shown to activate mTOR by the PI3K signaling pathway, which in turn leads to up-regulation of hypoxia-inducible factor 1α (HIF1α) and VEGF expression (13, 14) and suggests that dysregulated activation of mTOR might have deleterious effects on folliculogenesis. Oocyte-specific deletion of either Tsc1 or Tsc2 leads to global activation of the primordial follicle pool resulting in follicle depletion and subfertility in female mice (6, 15, 16). More recent studies have also shown that mTOR is activated in granulosa/luteal cells by LH by mechanisms that are independent of PI3K/AKT (17), suggesting an unidentified role for mTOR in the corpus luteum as well.
We have previously shown that constitutive activation of β-catenin leads to induction of mTOR expression in female reproductive tract cells, which can lead to tumor development (18). However, the results of TSC1 or TSC2 deletion with subsequent dysregulation of mTOR activation in ovarian somatic cells or in the uterus have not been reported. We speculated that deletion of TSC1 might disrupt proliferative processes required for normal reproduction such as granulosa cell expansion during folliculogenesis. To investigate whether TSC1 is required for normal reproductive physiology, we have generated conditional Tsc1 knockout mice and show that deletion of TSC1 in somatic cells of the reproductive tract result in complete female infertility. Our results suggest that several potential etiologies could be responsible for the sterility in Tsc1 conditional knockout mice, including defects in ovarian folliculogenesis, compromised oocyte/embryo integrity, inhibited transit of embryos through the oviduct, and failure of implantation.
Materials and Methods
Animal husbandry
The mice used in this study were housed under standard animal housing conditions. All protocols involving animal experimentation were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital. Mice were maintained on a C57BL/6;129/SvEv mixed genetic background. Amhr2tm3(cre)Bhr (Amhr2-Cre) mice (19) (kindly provided by Dr. Richard Behringer, M. D. Anderson, Houston, TX) were mated with Tsc1loxP/loxP mice (20) (The Jackson Laboratory, Bar Harbor, ME) to generate Amhr2-Cre/+;Tsc1Δ/Δ mice (hereafter referred to as Tsc1CKO). The DNA from tail biopsies was used to genotype mice using standard PCR protocols as described for Amhr2-Cre (21). The wild-type and flox alleles of Tsc1 were detected with PCR primers 5′-GTCACGACCGTAGGAGAAGC-3′ and 5′-GAATCAACCCCACAGAGCAT-3′ and 35 cycles of 94 C for 30 sec, 65 C for 1 min, and 72 C for 1 min. The deleted Tsc1 allele was detected as described (22).
Fertility and estrous cycle analyses
To evaluate reproductive performance, five individually housed 6-wk-old Tsc1loxP/loxP and Tsc1CKO females were bred to Tsc1loxP/loxP males with known fertility. The numbers of litters and pups were recorded over a 13- to 30-wk period. Cycling was determined over a 3-wk period with vaginal smears collected each day between 1300 and 1500 h from control and mutant mice that were at least 6 wk old. The smears were classified into one of four phases of the estrous cycle (proestrus, estrus, metestrus, and diestrus) by criteria described elsewhere (23). Data are presented as the average number of days spent in each phase of the estrous cycle ± sem. E2 and progesterone (P4) measurement was performed on serum samples from mice in diestrus (P4, n = 5 each controls and mutants; E2, n = 3 mutants, n = 5 controls) and estrus (n = 3 mutants and controls for both P4 and E2) at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core.
Western blotting
Ovaries were collected from five Tsc1loxP/loxP and Tsc1CKO age-matched mice for Western blotting analyses using antibodies to ribosomal protein S6 (RPS6; clone 54D2), phosphorylated RPS6 (p-RPS6; clone 91B2), and hamartin, all from Cell Signaling Technology (Danvers, MA) as previously described (24). Actin (Thermo Fisher Scientific, Fremont, CA) was used as a loading control. Bands were quantitated by scanning, averaged, and plotted.
Natural ovulation, superovulation, and embryo analyses
Oocytes were collected from 3 -month-old mice after natural ovulation at estrus. Briefly, the female mice were checked for estrous cycle every day as described above. When females were in estrus, they were mated with a vasectomized male. The next morning, the females were euthanized, and the oviducts were dissected to collect and analyze oocytes after treatment with 80 IU/ml hyaluronidase (Irvine Scientific, Santa Ana CA) in HEPES buffer to remove cumulus cells. For superovulation, 6- to 7-wk-old mutant and control mice were treated with ip injections of 7.5 IU equine chorionic gonadotropin (Sigma Chemical Co., St. Louis, MO) followed by 7.5 IU human chorionic gonadotropin (hCG) (Sigma) after 48 h. Fourteen hours after hCG injection, females were euthanized, and their oviducts were removed for oocyte retrieval and analysis as described for natural ovulation. For embryo analyses, mice were checked in the morning for evidence of a seminal plug, which was considered embryonic d 0.5 (E0.5). Mice were euthanized to collect embryos for analysis, which were classified as four-cell (E1.5) to morula (E3), early blastocyst (E3.5), and expanded blastocyst (E4.5). Photos were taken with a Nikon TE 2000-S microscope equipped with a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI).
Histology, immunohistochemistry (IHC), and immunofluorescence
For quantification of ovarian follicles, murine ovaries were fixed in Dietrick's solution for 10–12 h and then transferred to 70% ethanol until processing. For hematoxylin and eosin (H&E) staining and IHC, murine uteri and ovaries were fixed by immersion in 4% paraformaldehyde for 10–12 h. The fixed tissues were dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin wax. Embedded tissue samples were sectioned at 6 μm (8 μm for quantification of ovarian follicles) thickness and mounted on slides. The number of nonatretic or atretic primordial, primary, and preantral follicles was determined as described previously (25). Serial sections of tissues were deparaffinized with xylene and rehydrated with graded series of ethanol, followed by two washes of 5 min each in PBS containing 0.05% Tween 20 (PBS-T). Tissue sections were then incubated for 10 min in 3% (vol/vol) hydrogen peroxide in methanol to block endogenous peroxidase activity. After a wash with PBS-T, antigen retrieval was performed by boiling the tissue sections in 10 mm citrate buffer (pH 6) for 20 min. Sections were then washed for 5 min in PBS-T, blocked at room temperature for 1 h with 2% normal donkey serum, 2% BSA, and 0.1% Triton X-100 in PBS, and then incubated in a humidified chamber overnight at 4 C with p-RPS6 or TSC1 primary antibodies (Cell Signaling). Sections were subsequently washed with PBS-T, incubated at room temperature for 1 h with biotinylated secondary antibody, washed, and incubated with ready-to-use streptavidin peroxidase (Thermo Fisher Scientific) for 10 min at room temperature. Signal detection was performed using the Vectastain ABC kit (Vector Laboratories, Burlingame, CA). Sections were counterstained with hematoxylin. For immunofluorescence, methods used have been previously described in detail (18) using anti-mouse vasa homolog (MVH) and anti-c-KIT (Abcam, Cambridge, MA), anti-paired box 8 (PAX8) (ProteinTech Group, Chicago, IL), Cy3-labeled anti-α-smooth muscle actin (α-SMA) (Sigma), anti-phospho-histone H3 (Millipore, Billerica, MA), and biotinylated proliferating cell nuclear antigen (PCNA) (Zymed, Carlsbad, CA) with either streptavidin-conjugated Alexa Fluor 488 or Alexa Fluor second antibodies (Invitrogen, Carlsbad, CA). Terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling staining was performed per the manufacturer's instructions (Roche, Indianapolis, IN). Images were photographed using a Nikon TE 2000-S microscope equipped with a Spot digital camera (Diagnostic Instruments).
Implantation and embryo transfer
Implantation sites were detected by iv injection of 200 μl 0.1% Evans blue dye after anesthesia and 5 min before euthanasia. Uterine transfer of embryos was performed using conventional methods. For donors, 6-wk-old CD1 female mice were injected with 7.5 IU equine chorionic gonadotropin and 48 h later with 7.5 IU hCG and mated with males known to be fertile. Mating was confirmed by the presence of a vaginal plug the next morning. At E2.5, the embryos were collected from the oviducts and cultured in potassium simplex optimized medium (Millipore) for 24 h. Blastocyst transfers were performed at E3.5 with blastocysts of similar morphology (n = 9 per horn) of three each control and mutant pseudopregnant recipients. At 6 d after blastocyst transfer, the recipient mice were euthanized, and the number of implantations was confirmed visually. Gross photos were taken with a Nikon D60 digital camera.
Quantitative real-time PCR
Tissues were collected and stored in RNAlater (Invitrogen) for subsequent RNA extraction using the RNeasy mini kit (QIAGEN, Valencia, CA). cDNA was prepared using oligo-deoxythymidine with a kit from Invitrogen according to the manufacturer's instructions. Real-Time PCR was performed with gene-specific primers (Origene, Rockville, MD) in a CFX96 Real-Time System (Bio-Rad, Hercules, CA) using standard cycling conditions. Threshold values (Ct) were measured in duplicate from n = 3 mice per group, averaged, and subtracted from the actin Ct values, then plotted as 2−(ΔCt).
Statistical analyses
All experiments were repeated at least three times for statistical analyses with Prism version 5.0 (GraphPad Software, La Jolla, CA). For comparisons, differences between the two groups were calculated with Student's t test, and the difference was considered to be significant if P < 0.05.
Results
TSC1 deletion in the ovary leads to premature follicular depletion
To determine whether conditional deletion of TSC1 in ovarian somatic cells affected female fertility, mice with floxed alleles of Tsc1 (Tsc1CKO) were generated by mating Tsc1loxP/loxP mice (20) with Amhr2-Cre mice (19). Anti-Müllerian hormone type 2 receptor (AMHR2) is the receptor for anti-Müllerian hormone, also known as Müllerian-inhibiting substance, and is expressed in the fetal Müllerian duct mesenchyme, in the Sertoli and Leydig cells of adult testes, and in both the ovarian surface epithelium and in the granulosa cells of developing follicles in adult ovaries (18, 26–30). Female control Tsc1loxP/loxP and Tsc1CKO mice were bred to Tsc1loxP/loxP males with known fertility. Seminal plugs were observed in both mutant and control mice, suggesting normal female mating behavior after the exposure to male mice. Control mice exhibited normal fecundity, whereas mutant mice were found to be completely infertile (Table 1). Female Tsc1CKO mice failed to produce any offspring over a 5-month breeding period, indicating that conditional deletion of TSC1 in AMHR2-expressing cells completely blocks female fertility. In contrast, male Tsc1CKO mice were able to sire multiple litters at normal frequency through 12 wk postnatally (data not shown).
Table 1.
Tsc1CKO mice are infertile
| Total litters | Total pups | Pups/litter | Litters/mouse | |
|---|---|---|---|---|
| Tsc1loxP/loxP (n = 4) | 18 | 320 | 6.7 ± 1.9 | 4.5 ± 0.6 |
| Tsc1CKO (n = 5) | 0 | 0 | 0 | 0 |
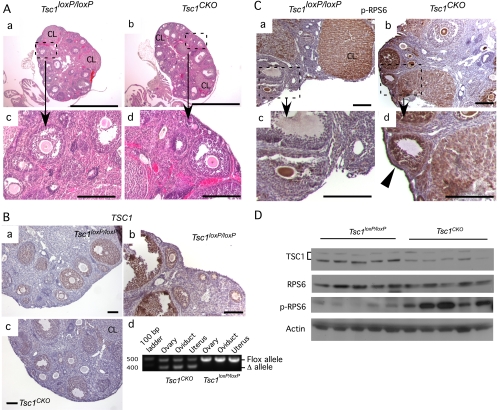
We first assessed whether the infertility in female Tsc1CKO mice could be due to disrupted folliculogenesis by examining the histology of Tsc1CKO mutant ovaries (Fig. 1). Histology of the Tsc1CKO ovaries appeared normal with follicles of every size and corpora lutea (Fig. 1A, a–d). To ensure that TSC1 expression was diminished in the ovarian somatic cells of Tsc1CKO mice, we compared the level of TSC1 expression in control and mutant ovaries (Fig. 1B, a–c). TSC1 expression in the control ovaries was strongest in the granulosa cells of developing follicles by IHC (Fig. 1B, a and b). In Tsc1CKO ovaries, TSC1 was diminished or absent from developing follicles (Fig. 1B, 4). PCR analysis of genomic DNA isolated from control and mutant ovaries, oviducts, and uterus confirms that AMHR2-Cre induces recombination of the flox alleles (Fig. 1B, d). Semiquantitative Western analyses confirmed that significantly higher levels of TSC1 protein (3-fold) are expressed in control ovaries compared with mutants (Fig. 1D). Phosphorylation of RPS6, a substrate for S6 kinase, which is itself a downstream target of mTor kinase activity and should be enhanced in the absence of TSC1-mediated inhibition of mTor, was also analyzed. Elevated levels of p-RPS6 in the granulosa cells of developing follicles, as well as in the surface epithelial cells, were detected (Fig. 1C, a–d). Although there was no significant difference in the expression of RPS6 protein in mutant and control ovaries, significantly higher levels of p-RPS6 (2-fold) were observed in the mutant ovaries by Western analyses (Fig. 1D).
Fig. 1.
Ovaries from Tsc1CKO mice show induced phosphorylation of RPS6. A, H&E of typical ovarian sections from 8-wk-old control Tsc1loxP/loxP (a and c) and Tsc1CKO mice (b and d) showing the presence of follicles in various stages of development. B, In control ovaries, TSC1 expression by IHC shows strongest expression in developing follicles (a and b); in Tsc1CKO ovaries, TSC1 expression is much lower in developing follicles (c); PCR analysis of genomic DNA from the indicated sources shows the deleted allele is amplified in tissues expressing Amhr2-Cre (d). C, The p-RPS6 levels were high in oocytes and corpus luteum (CL) but low or absent in granulosa cells of developing follicles in Tsc1loxP/loxP ovaries by IHC (a and c); in Tsc1CKO ovaries, p-RPS6 levels were high in granulosa cells of developing follicles of Tsc1CKO ovaries (b and d) and also appeared higher in the surface epithelium (d, arrowhead). Photos are representative of n = 3 for each group. D, Western analysis shows lower levels of Tsc1 and higher levels of p-RPS6 in the mutant ovaries. Each lane corresponds to an ovary from n = 5 control and mutant mice. Semiquantitative analysis shows that TSC1 levels are 3-fold greater in control ovaries (56.3 ± 0.5 vs. 18.3 ± 0.4, P < 0.01) and p-RPS6 levels are 2-fold greater in Tsc1CKO ovaries than in controls (10.8 ± 0.2 vs. 5.6 ± 0.2, P < 0.01). In A and C, boxed areas in a and b are shown at higher magnification in c and d, respectively. CL, Corpus luteum. Bars, 100 μm except for those in A, a and b, which are 1 mm.
Fig. 4.
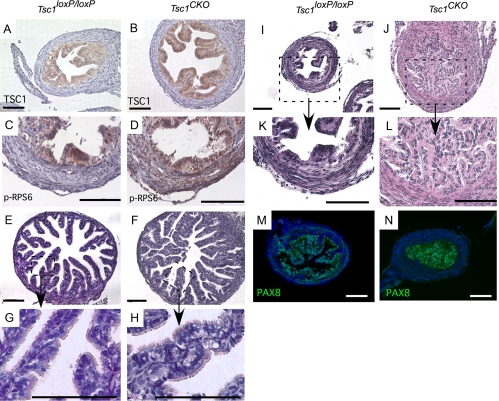
Occlusion of the proximal oviduct in Tsc1CKO mice. A and B, TSC1 expression was detected by IHC in the epithelial cells of both control Tsc1loxP/loxP and Tsc1CKO mice. C and D, IHC for p-RPS6 was used to show evidence for TSC1 deletion in the stroma of Tsc1CKO oviducts. Nuclei are stained with hematoxylin in A–D. E–H, H&E of sections from typical distal portion of oviducts isolated from control and mutant Tsc1CKO mice (E and F) show that the ciliated and secretory epithelial cells of control and mutant distal oviducts are intact (G and H). I–L, Disorganization and dysplasia of secretory epithelial cells of the proximal portion of the oviduct in the mutant females was observed that appeared to occlude the lumen, which might disturb proper transit of embryos. G, H, K, and L are higher magnification images of the boxed areas of E, F, I, and J, respectively. M and N, Immunofluorescence detection of PAX8 in the epithelial cells is shown in the control and the occluded mutant proximal oviducts. Nuclei are stained with 4′,6-diamidino-2-phenylindole. Photos are representative of at least n = 3 for controls and mutants. Bars, 100 μm.
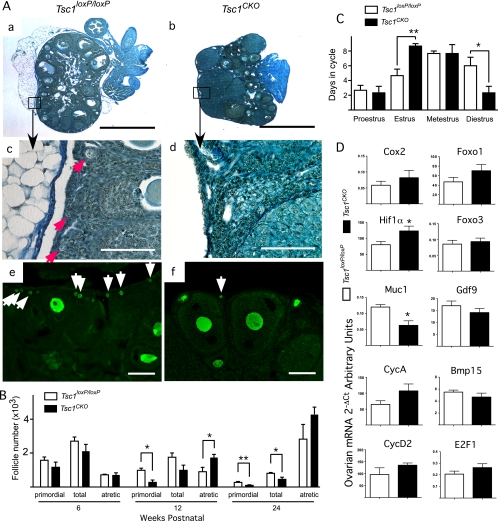
More detailed analyses of the ovaries using Dietrick's staining to highlight follicle structure showed fewer primordial follicles in over 12-wk-old Tsc1CKO ovaries compared with controls (Fig. 2A, a–d). Immunofluorescence staining with the oocyte-specific marker MVH confirmed that fewer primordial follicles were observed in the cortex of Tsc1CKO ovaries (Fig. 2A, e and f). Total immature (primordial, primary, and small preantral) follicles and atretic follicles in Tsc1loxP/loxP ovaries and Tsc1CKO ovaries were counted to quantify the difference in follicle numbers (Fig. 2B). At 6 wk of age, the number of total follicles, primordial follicles, and atretic follicles were not significantly different in Tsc1CKO ovaries and Tsc1loxP/loxP ovaries. However, by 12 wk of age, Tsc1CKO ovaries had significantly fewer primordial follicles and more atretic follicles than Tsc1loxP/loxP ovaries, a difference that was accentuated at 24 wk. These data suggest that TSC1 in granulosa cells helps maintain dormancy of primordial follicles and that its absence results in premature follicular depletion. The number of atretic follicles, although not significantly different at 24 wk, was higher, continuing the trend observed at 12 wk and suggesting that follicle depletion might involve induction of atresia.
Fig. 2.
Ovarian function in Tsc1CKO mice. A, Hematoxylin-picric acid staining of sections from typical ovaries isolated from 12-wk-old control Tsc1loxP/loxP (a and c) and Tsc1CKO mice (b and d); boxed areas in a and b are shown at higher magnification in c and d, respectively; red arrows indicate primordial follicles (c); MVH immunofluorescence in control (e) and Tsc1CKO (f) ovaries; many small MVH-positive oocytes are seen in Tsc1loxP/loxP ovaries (white arrows in e), whereas few MVH-positive oocytes are seen in Tsc1CKO ovaries (white arrow in f). Bars, 100 μm except for a and b, which are 1 mm. Photos are representative of n = 3 for each group. B, Numbers of primordial, total immature (primordial, primary, and small preantral), and atretic follicles in mouse ovaries from Tsc1loxP/loxP and Tsc1CKO ovaries were counted, averaged, and plotted. C, Estrous cycling in 6-wk-old Tsc1loxP/loxP and Tsc1CKO mice was determined daily by vaginal lavage cytology over a period of 21 d and cumulatively plotted. D, Quantitative real-time PCR was performed with cDNA from control and mutant ovaries in estrus to determine the effect of TSC1 deletion on the expression of key genes involved in ovarian function. Graphs represent the mean ± sem; n = 3 for each group. *, P < 0.05; **, P < 0.01.
To examine whether the accelerated rate of folliculogenesis in Tsc1CKO females might be related to estrous cycling, stages were determined daily over a period of 21 d and cumulatively summarized in Fig. 2C. The estrous stage was prolonged in Tsc1CKO females compared with Tsc1loxP/loxP females. In contrast, the duration of diestrus was shortened in Tsc1CKO females. Additionally, no significant difference in serum E2 and P4 levels determined at diestrus and estrus was observed between the two groups of mice (data not shown). Quantitative RT-PCR was performed to determine whether the levels of expression for genes known to be involved in normal ovarian function (31) are affected in the Tsc1CKO ovaries at estrus (Fig. 2D). Only marginal changes were observed in these mRNA, except for mucin 1 (MUC1), which was significantly down-regulated in the mutant ovaries to half the expression in the control ovaries, and HIF1α, which was significantly up-regulated by 50% in the mutant ovaries compared with controls.
Reduced capacity of oocytes from Tsc1CKO ovaries
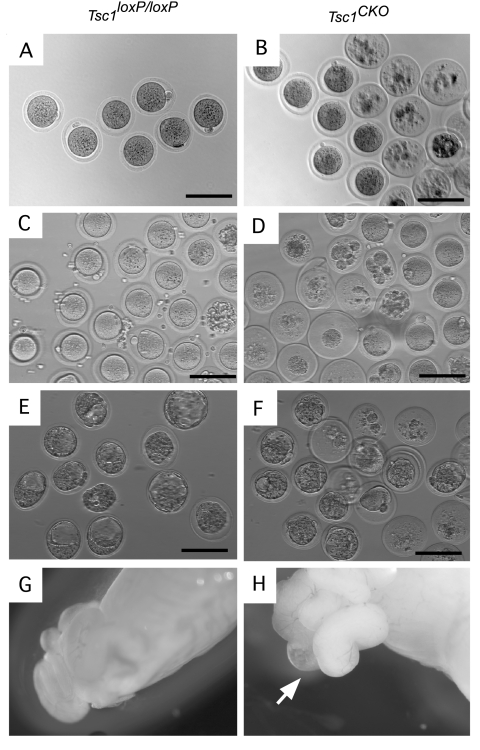
We next compared the number of normally ovulated and superovulated oocytes in control and Tsc1CKO mice to determine whether premature follicle depletion was correlated with ovulation (Fig. 3, A–D, and Table 2). Despite having significantly more normal ovulated oocytes from Tsc1CKO mice, the number of healthy oocytes was not significantly different from controls. In Tsc1CKO mice, 83% of the oocytes collected were atretic compared with only 4% in control mice. Similarly, in superovulated mice, whereas the number of healthy oocytes was not significantly different between mutant and control mice, the number of atretic oocytes in mutant mice was 7-fold higher in the mutant mice. The ability of ovulated oocytes from mutant ovaries to be fertilized was assessed by natural mating. More E2.5 embryos were detected in the oviducts of Tsc1CKO mice compared with controls (Table 2), but almost two thirds were degenerated bodies, which was significantly higher compared with controls. At E3.5 after normal mating, blastocysts were detected in the control uteri after flushing (Fig. 3E), but no embryos were detected in the Tsc1CKO uteri. However, bilateral swelling of the ampullas of E3.5 Tsc1CKO mice was observed in all mice examined (n = 4) (Fig. 3H), which was not observed in controls (Fig. 3G). After the ampullas were punctured, blastocysts and many degenerated bodies (approximately 75%) were released (Fig. 3F). At both E2.5 and E3.5, the absolute number of good-quality embryos found in the ampullas was not significantly different between Tsc1flox/flox and Tsc1CKO mice, suggesting that the good-quality oocytes produced by Tsc1CKO mice can develop into blastocysts (Table 2).
Fig. 3.
Oocyte quality is compromised in Tsc1CKO mice. A–D, Oocytes were collected from the oviducts during normal estrus (A and B) or from superovulated Tsc1loxP/loxP and Tsc1CKO mice (C and D). A majority of the oocytes in Tsc1CKO mice were degenerated. Control Tsc1loxP/loxP Tsc1CKO females were mated with Tsc1loxP/loxP males with known fertility. E, At E3.5, blastocysts were detected in the control uteri after flushing, but no blastocysts were detected in the Tsc1CKO uteri. F, Blastocysts and many degenerated bodies were released from the swollen ampulla (F). G and H, Swelling of the ampullas was observed in E3.5 Tsc1CKO females (H, arrow) but not in E3.5 controls (G). For each analysis, n ≥ 3. Bars, 100 μm.
Table 2.
Reproductive profiles on Tsc1loxP/loxP and Tsc1CKO mice
| Tsc1loxP/loxP | Tsc1CKO | P value | |
|---|---|---|---|
| Total normal ovulation oocytes | 8.7 ± 1.3 (n = 3) | 42.0 ± 1.5 (n = 3) | <0.0001 |
| Healthy oocytes (%) | 8.3 ± 1.3 (95) | 7.3 ± 0.3 (17) | 0.50 |
| Degenerated oocytes (%) | 0.3 ± 0.3 (4.2) | 34.7 ± 0.9 (83) | <0.0001 |
| Total superovulated oocytes | 37.8 ± 5.7 (n = 4) | 56.5 ± 7.6 (n = 4) | 0.10 |
| Healthy oocytes (%) | 34.3 ± 5.7 (91) | 20.8 ± 2.8 (37) | 0.08 |
| Degenerated oocytes (%) | 3.5 ± 2.0 (9.7) | 35.8 ± 5.0 (63) | <0.0001 |
| Total embryos (d 2.5) | 11.0 ± 1.0 (n = 3) | 27.0 ± 9.2 (n = 3) | 0.16 |
| Healthy embryos (%) | 10.3 ± 1.3 (94) | 6.7 ± 0.7 (25) | 0.03 |
| Degenerated embryos (%) | 0.7 ± 0.3 | 20.3 ± 9.0 (75) | 0.02 |
| Total embryos (d 3.5) | 11.0 ± 0 (n = 3) | 32 ± 5.2 (n = 4) | 0.02 |
| Healthy embryos (%) | 9.3 ± 0.3 (85) | 7.0 ± 2.0 (25) | 0.38 |
| Degenerated embryos (%) | 1.7 ± 0.3 (15) | 25.0 ± 0.1 (74) | 0.0047 |
| Implantation (embryo transfer) | 6.5 ± 1.6 (n = 3) | 0 (n = 3) |
Results are shown as mean ± sem.
Tsc1 deletion in the Müllerian duct mesenchyme results in proximal oviductal occlusion
During urogenital ridge development, Amhr2-Cre is expressed in the mesenchyme of the Müllerian ducts, the anlagen of the oviducts, uterus, and cervix. To determine whether defective differentiation of the oviduct might be contributing to the infertility of the Tsc1CKO females, we examined oviductal histology. Expression of TSC1 appeared restricted to the epithelial cells throughout control and Tsc1CKO oviducts (Fig. 4, A and B). Müllerian duct epithelial cells do not express AMHR2-Cre, and TSC1 deletion in those cells was not expected. Because the level of TSC1 in the oviductal stroma was below the level of detection, we used phosphorylation of RPS6 as a marker for TSC1 deletion and detected pRPS6 in the stroma of Tsc1CKO oviducts but not in control oviducts, confirming TSC1 deletion had occurred in the mutant oviducts (Fig. 4, C and D). The epithelial cells of the distal portion of the oviduct appeared relatively normal by H&E, with clearly identified secretory and ciliated cells (Fig. 4, E–H). In contrast to the normal appearance of sections from the distal oviduct, epithelial cell hyperplasia in the proximal portion of the oviduct in the Tsc1CKO mice was observed, which appeared to occlude the lumen (Fig. 4, I–L). Nuclear PAX8 expression, a secretory cell marker (32), confirmed that the cells in the lumen were derived from the oviduct (Fig. 4, M and N). Immunofluorescence of α-SMA and c-Kit showed no noticeable differences in expression between control and Tsc1CKO mice (data not shown), suggesting that neither the contractility nor the pacemaker activities in the oviducts were compromised in the mutants (33). These results suggest that transit through the oviduct is blocked in Tsc1CKO mice, contributing to their infertility.
Tsc1 deletion in endometrial stroma blocks implantation
Because we observed some normal embryos in the mutant oviducts, we investigated whether implantation might also be disrupted in the mutants. Control Tsc1loxP/loxP females and mutant Tsc1CKO females were mated with fertile males. At E4.5 after normal mating, implantation sites were visualized by Evans blue dye in control Tsc1loxP/loxP females (Fig. 5A, a), but none were detected in Tsc1CKO mice (Fig. 5A, b). These results suggest that either embryo transfer through the oviduct is completely blocked or that implantation might also be affected by TSC1 deletion in the endometrial stroma, which is also derived from the AMHR2-expressing Müllerian duct mesenchyme (34). Embryo transfer experiments were performed to examine whether implantation was affected in mutant mice. When E4.5 embryos from wild-type mice were collected and transferred to control mice, embryo implantation was readily observed after 4–6 d (Fig. 5A, c). In contrast, implantation of embryos transferred to Tsc1CKO uteri was not observed (Fig. 5A, d), suggesting that deletion of TSC1 in the endometrial stroma inhibits one or more of the signaling pathways required for embryo implantation.
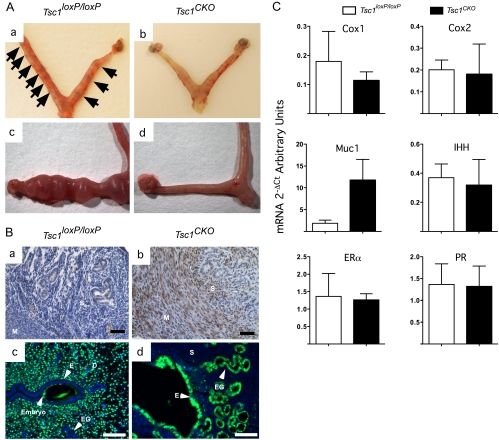
Fig. 5.
Failure of implantation in Tsc1CKO mice. Panel A, Tsc1loxP/loxP and mutant Tsc1CKO mice were mated, and at E4.5, implantation sites were visualized by iv injections of Chicago Blue dye in control Tsc1loxP/loxP uteri (n = 3) (a, arrows) but not in Tsc1CKO uteri (n = 3) (b); E3.5 embryos were collected from control mice and transferred to pseudopregnant control Tsc1loxP/loxP (n = 3) and mutant Tsc1CKO (n = 3) mice, and successful implantation was achieved 6 d later in control mice (c) but not in mutant uteri (d). Panel B, IHC for p-RPS6 was used to show evidence for TSC1 deletion in the stroma (S) and myometrium (M) of Tsc1CKO oviducts (b), which was not observed in control uteri (a); immunofluorescence was performed to detect PCNA (green) in the endometrial epithelium of E4.5 uteri of control (c) and Tsc1CKO mice (d); endometrial (E) and (EG) glandular epithelial cells show no proliferation in the controls, whereas abundant proliferation of the decidual (D) cells was observed (c). In contrast, epithelial cells were highly proliferative in mutant uteri, and the stroma (S) cells were not. Photos are representative of at least three mice per experimental group, and 4′,6-diamidino-2-phenylindole was used to detect nuclei. Panel C, Quantitative real-time PCR was performed with cDNA from control and mutant uteri at E4.5 to determine the effect of TSC1 deletion on the expression of the indicated genes. Graphs represent the mean ± sem; n = 3 or 4 for each group.
In the uterus, two reports have shown that expression of TSC1 at low levels is restricted to the glandular and luminal epithelium of both rats (35) and sheep (36) and that TSC1 doesn't appear to be regulated by estrous cycling or pregnancy (36). We also observed that TSC1 expression was limited to the uterine epithelial cells (data not shown). However, deletion of TSC1 in Müllerian duct mesenchyme does induce p-RPS6 in both the myometrium and uterine stroma compared with controls (Fig. 5B, a and b), indicating activation of mTOR downstream signaling in cycling mutant uteri. One of the major functions attributed to the stroma is the control of endometrial epithelial proliferation, the attenuation of which is controlled by E2-induced stromal P4 receptor (PR)-activated expression of secreted factors and is a hallmark of embryo implantation (37, 38). Analysis of PCNA immunofluorescence in control and mutant uteri at E4.5 was performed and showed that the endometrial epithelium in the Tsc1CKO mice continued to proliferate (Fig. 5B, c and d), indicating that TSC1 deletion disrupted this important stromal function. We next examined the expression of some of the enzymes (cyclooxygenase 1 and 2), receptors [estrogen receptor-α (ERα) and PR], and secreted factors (MUC1 and Indian Hedgehog) known to be involved in implantation (38). In Fig. 5C, we show that only the expression of MUC1 is significantly affected by TSC1 deletion at E4.5. MUC1 is secreted by the endometrial epithelium and serves as a protective layer covering the apical surface of the cells, which also prevents the embryo from accessing the uterine luminal epithelium (39). MUC1 down-regulation by PR is essential for uterine receptivity in most species (40–44). These results showing continued endometrial epithelial cell proliferation and MUC1 expression by the endometrial epithelium of Tsc1CKO mice in the presence of normal levels PR expression suggest that mechanisms downstream of PR expression have been disrupted by TSC1 deletion.
Discussion
TSC is an autosomal dominant, multisystem disorder that affects patients with a rate of one in 6000 live births with mutations in either the hamartin (TSC1) or tuberin (TSC2) genes (11, 45, 46). TSC is characterized by the growth of benign tumors or hamartomas in a variety of organs, such as cardiac rhabdomyomas and subependymal or cortical tubers and nodules that are often detected in early childhood. These characteristic growths are presumed to be the result of dysregulated mTOR activity in the absence of a functional TSC1/TSC2 heterodimer. Although there are several reports indicating that fetal TSC can complicate pregnancy outcomes or even lead to the demise of the fetus (47, 48), to date, there has been no report directly associating TSC with infertility or reproductive tract abnormalities in humans. What is known about the effect of TSC mutations on the reproductive tract has been derived from animal models, notably the Eker rat (49), which has a germline mutation in the Tsc2 gene and develops uterine leiomyomas with approximately 60% frequency by 12 months. Human uterine leiomyomas are the most common gynecological tumor, with an estimated greater than 50% incidence (50, 51). Although the tumors form in the myometrium and are benign, they are often associated with pelvic pain, dysmenorrhea, spontaneous abortion, and infertility and thus present a significant healthcare burden. Because the Müllerian duct mesenchyme, which differentiates into the endometrial stroma and myometrium, expresses AMHR2, we predicted that uterine leiomyomas would form in the Tsc1CKO mice. We have detected significant myometrial hyperplasia in Tsc1CKO mice as they age (data not shown) and are performing additional analyses to determine whether they develop uterine leiomyomas with characteristics of human leiomyoma.
Although ovulation and subsequent fertilization occurs in Tsc1CKO mice, many of the ovulated oocytes were not competent, suggesting that oocyte maturation in Tsc1CKO mice is affected by deletion of TSC1 in ovarian cells. Analyses of granulosa cell proliferation by phospho-histone H3 expression or apoptosis by terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling assays suggest that granulosa cell expansion itself is not significantly affected by TSC1 deletion during folliculogenesis (data not shown), which was surprising because mTor is a master regulator of cellular proliferation (52). Activation of mTOR by PI3K signaling in granulosa cells in response to FSH has been shown to induce expression of HIF1α, which the authors speculated could have an impact on follicular maturation (13, 14). Thus, we predicted that the dysregulated mTOR activity we observed in the ovaries of Tsc1CKO mice might also negatively impact follicular maturation by mechanisms that involve HIF1α, and our real-time PCR results showing a significant 50% increase in HIF1α mRNA expression (Fig. 2D) support their hypothesis. Alternatively, a trivial explanation for the abundance of atretic ovulated oocytes in the mutants would be that oocytes from previous ovulation cycles were accumulating in the blocked oviducts and undergoing degeneration. The similarity in numbers of healthy oocytes and E2.5 embryos between mutants and controls in the oviducts strongly suggest this might be the true. As an interesting aside, the number of good-quality embryos in the mutants would also argue that, although oocytes and embryos cannot exit the oviduct in the mutants, sperm is able to penetrate and fertilize the oocytes.
In vitro analyses have shown that addition of rapamycin, which selectively inhibits the mTOR complex I subunit of mTOR, did not affect the ability of bovine granulosa/luteal cells to produce P4 after treatment with LH and mTOR activation (17). That study also showed that LH-mediated activation of mTOR could be occurring through mechanisms that involve inactivation of AMP-activated protein kinase (AMPK) or glycogen synthase kinase 3β (GSK3β), both of which phosphorylate and activate TSC2 (53). Additional studies will be needed to determine whether corpus luteum function in Tsc1CKO mice is affected by dysregulation of mTor in the absence of the tumor suppressor activity of TSC1.
In addition to disrupted folliculogenesis and oocyte maturation, mutant mice also presented with occlusion of the oviduct, which has a major implication for human fertility as well. In humans, over 98% of ectopic pregnancies occur in the fallopian tubes (54), and the major risk factors for tubal pregnancy is tubal damage caused by surgery, tubal infection such as Chlamydia trachomatis, and assisted reproduction (54). The impairment of fallopian tube development or function could also cause ectopic pregnancy, but most of the mechanisms involved still remain to be identified. Hydrosalpinx, or accumulation of fluid in the distal fallopian tube caused by isthmic occlusion, is a major cause of tubal factor infertility and is usually associated with a history of pelvic inflammatory disease (55). However, up to 10% of hydrosalpinges are of unknown etiology. Our results show that dysregulation of PI3K/AKT/TSC1/mTOR signaling cascade in the mesenchyme of murine oviducts causes retention of embryos by occlusion of the proximal portion of the oviduct with dysplastic epithelial cells (Fig. 4), and these cells are Pax8 positive, which is a marker of secretory epithelial cells. These results are consistent with the fact that more secretory cells than ciliated cells are present in proximal oviduct in mice (56, 57). Our results also suggest that TSC1 in the mesenchyme of the fallopian tube are important for tubal epithelial morphology and function, suggesting epithelial-stromal cell interaction of fallopian tube. The mechanisms involved in oviductal epithelial dysplasia in response to TSC1-deleted stroma have yet to be determined.
Blastocyst attachment to the endometrial epithelium normally takes place within a restricted period, referred to as the window of receptivity (58), which in mice usually takes place between E4 and E5. After attachment of the embryo, a process called decidualization occurs in which the endometrial stromal cells differentiate into support cells that sustain the embryo before development of a placenta and is also essential for successful implantation (38). The whole process requires precise coordination between a competent embryo and a receptive uterus, with estrogen and P4, and their receptors, ERα and PR, playing a central role in this process (38, 59, 60). MUC1, a major component of the protective glycocalyx layer on the apical surface of uterine epithelium, is abundantly expressed in both luminal and glandular epithelia, and its expression is down-regulated by PR during embryo attachment in mice and other species (40, 41, 61). Whereas our quantitative RT-PCR results did not show any difference in ERα or PR at E4.5 in Tsc1CKO uteri, MUC1 expression remained elevated, suggesting that its continued expression during the implantation window might be a mechanism contributing to failure of implantation. These data also suggest that the continued MUC1 expression might be through a post-PR mechanism disrupted by TSC1 deletion. Alternatively, two isoforms of PR, PRA and PRB, have been shown to have differential effects on MUC1 expression in different settings (62), suggesting that their expression ratio, which has not been determined, might be important in Tsc1CKO uterine stroma. The decidualization process was not investigated in Tsc1CKO uteri, but its dysregulation could also contribute to the failure of implantation.
We have recently shown that endometrial stromal deletion of another tumor suppressor, adenomatous polyposis coli, triggers development of endometrial epithelial hyperplasia and endometrial cancer and that stromal factors restricting epithelial proliferation were down-regulated in the mutant stroma (34). Although we observed sporadic evidence for endometrial hyperplasia by deletion of TSC1 in the endometrial stroma (data not shown), we did not observe endometrial cancer, suggesting that mutation of the tumor suppressor TSC1 alone does not play a major role in the pathogenesis of endometrial cancer. We also did not observe any evidence of tumorigenesis in the mutant ovaries, suggesting that TSC1 does not act as a tumor suppressor in that setting. Supporting this speculation was the reduced expression of MUC1, which is associated with cancer progression, in the mutant ovaries (Fig. 2D). However, whether deletion of TSC1 tumor suppressor activity accelerates tumor development or progression in combination with activation of an oncogene in ovarian somatic cells has not been determined.
These results indicate that TSC1 plays various important roles in ovarian and reproductive tract somatic cells, presumably by dysregulated activation of mTOR, a master regulator of cellular proliferation. We did not observe any evidence of tumorigenesis in the tissues described in this study and predict that, in addition to activation of mTOR, other disrupted functions of TSC1 caused by its deletion might be contributing to the infertile reproductive phenotype we have observed in this model.
Acknowledgments
This work was supported by funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (HD052701) and by Vincent Memorial Research Funds to J.M.T. The University of Virginia Center for Research in Reproduction Ligand Core Laboratory is supported by NICHD (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD28934.
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- AMHR2
- Anti-Müllerian hormone type 2 receptor
- E0.5
- embryonic d 0.5
- E2
- 17β-estradiol
- ERα
- estrogen receptor-α
- hCG
- human chorionic gonadotropin
- H&E
- hematoxylin and eosin
- HIF1α
- hypoxia-inducible factor 1α
- IHC
- immunohistochemistry
- mTOR
- mammalian target of rapamycin
- MUC1
- mucin 1
- MVH
- mouse vasa homolog
- P4
- progesterone
- PAX8
- paired box 8
- PBS-T
- PBS with Tween 20
- PCNA
- proliferating cell nuclear antigen
- PI3K
- phosphatidylinositide-3 kinase
- p-RPS6
- phosphorylated ribosomal protein S6
- PR
- P4 receptor
- RPS6
- ribosomal protein S6
- α-SMA
- α-smooth muscle actin
- TSC
- tuberous sclerosis complex.
References
- 1. Cahill DJ, Wardle PG. 2002. Management of infertility. BMJ 325:28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matzuk MM, Lamb DJ. 2008. The biology of infertility: research advances and clinical challenges. Nat Med 14:1197–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wayne CM, Fan HY, Cheng X, Richards JS. 2007. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol 21:1940–1957 [DOI] [PubMed] [Google Scholar]
- 4. Fan HY, Liu Z, Cahill N, Richards JS. 2008. Targeted disruption of Pten in ovarian granulosa cells enhances ovulation and extends the life span of luteal cells. Mol Endocrinol 22:2128–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hämäläinen T, Peng SL, Lan ZJ, Cooney AJ, Huhtaniemi I, Liu K. 2008. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 319:611–613 [DOI] [PubMed] [Google Scholar]
- 6. Reddy P, Adhikari D, Zheng W, Liang S, Hämäläinen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. 2009. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet 18:2813–2824 [DOI] [PubMed] [Google Scholar]
- 7. John GB, Shirley LJ, Gallardo TD, Castrillon DH. 2007. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction 133:855–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kazi AA, Koos RD. 2007. Estrogen-induced activation of hypoxia-inducible factor-1α, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology 148:2363–2374 [DOI] [PubMed] [Google Scholar]
- 9. Memarzadeh S, Zong Y, Janzen DM, Goldstein AS, Cheng D, Kurita T, Schafenacker AM, Huang J, Witte ON. 2010. Cell-autonomous activation of the PI3-kinase pathway initiates endometrial cancer from adult uterine epithelium. Proc Natl Acad Sci USA 107:17298–17303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wullschleger S, Loewith R, Hall MN. 2006. TOR signaling in growth and metabolism. Cell 124:471–484 [DOI] [PubMed] [Google Scholar]
- 11. Kwiatkowski DJ, Manning BD. 2005. Tuberous sclerosis: a GAP at the crossroads of multiple signaling pathways. Hum Mol Genet 14(Spec No. 2):R251–R258 [DOI] [PubMed] [Google Scholar]
- 12. Orlova KA, Crino PB. 2010. The tuberous sclerosis complex. Ann NY Acad Sci 1184:87–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alam H, Weck J, Maizels E, Park Y, Lee EJ, Ashcroft M, Hunzicker-Dunn M. 2009. Role of the phosphatidylinositol-3-kinase and extracellular regulated kinase pathways in the induction of hypoxia-inducible factor (HIF)-1 activity and the HIF-1 target vascular endothelial growth factor in ovarian granulosa cells in response to follicle-stimulating hormone. Endocrinology 150:915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alam H, Maizels ET, Park Y, Ghaey S, Feiger ZJ, Chandel NS, Hunzicker-Dunn M. 2004. Follicle-stimulating hormone activation of hypoxia-inducible factor-1 by the phosphatidylinositol 3-kinase/AKT/Ras homolog enriched in brain (Rheb)/mammalian target of rapamycin (mTOR) pathway is necessary for induction of select protein markers of follicular differentiation. J Biol Chem 279:19431–19440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. 2010. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet 19:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. 2009. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod 15:765–770 [DOI] [PubMed] [Google Scholar]
- 17. Hou X, Arvisais EW, Davis JS. 2010. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 151:2846–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanwar PS, Lee HJ, Zhang L, Zukerberg LR, Taketo MM, Rueda BR, Teixeira JM. 2009. Constitutive activation of β-catenin in uterine stroma and smooth muscle leads to the development of mesenchymal tumors in mice. Biol Reprod 81:545–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. 2002. Requirement of Bmpr1a for Mullerian duct regression during male sexual development. Nat Genet 32:408–410 [DOI] [PubMed] [Google Scholar]
- 20. Kwiatkowski DJ, Zhang H, Bandura JL, Heiberger KM, Glogauer M, el-Hashemite N, Onda H. 2002. A mouse model of TSC1 reveals sex-dependent lethality from liver hemangiomas, and up-regulation of p70S6 kinase activity in Tsc1 null cells. Hum Mol Genet 11:525–534 [DOI] [PubMed] [Google Scholar]
- 21. Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. 2005. Conditional deletion of β-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol 288:276–283 [DOI] [PubMed] [Google Scholar]
- 22. Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP. 2009. The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 18:151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. 1999. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology 140:5789–5796 [DOI] [PubMed] [Google Scholar]
- 24. Renlund N, Pieretti-Vanmarcke R, O'Neill FH, Zhang L, Donahoe PK, Teixeira J. 2008. c-Jun N-terminal kinase inhibitor II (SP600125) activates Mullerian inhibiting substance type II receptor-mediated signal transduction. Endocrinology 149:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. 1999. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet 21:200–203 [DOI] [PubMed] [Google Scholar]
- 26. Teixeira J, Maheswaran S, Donahoe PK. 2001. Mullerian inhibiting substance: an instructive developmental hormone with diagnostic and possible therapeutic applications. Endocr Rev 22:657–674 [DOI] [PubMed] [Google Scholar]
- 27. Szotek PP, Chang HL, Brennand K, Fujino A, Pieretti-Vanmarcke R, Lo Celso C, Dombkowski D, Preffer F, Cohen KS, Teixeira J, Donahoe PK. 2008. Normal ovarian surface epithelial label-retaining cells exhibit stem/progenitor cell characteristics. Proc Natl Acad Sci USA 105:12469–12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanwar PS, Kaneko-Tarui T, Zhang L, Rani P, Taketo MM, Teixeira J. 2010. Constitutive WNT/β-catenin signaling in murine Sertoli cells disrupts their differentiation and ability to support spermatogenesis. Biol Reprod 82:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baarends WM, Uilenbroek JT, Kramer P, Hoogerbrugge JW, van Leeuwen EC, Themmen AP, Grootegoed JA. 1995. Anti-mullerian hormone and anti-mullerian hormone type II receptor messenger ribonucleic acid expression in rat ovaries during postnatal development, the estrous cycle, and gonadotropin-induced follicle growth. Endocrinology 136:4951–4962 [DOI] [PubMed] [Google Scholar]
- 30. Rouiller-Fabre V, Carmona S, Merhi RA, Cate R, Habert R, Vigier B. 1998. Effect of anti-Müllerian hormone on Sertoli and Leydig cell functions in fetal and immature rats. Endocrinology 139:1213–1220 [DOI] [PubMed] [Google Scholar]
- 31. Richards JS, Pangas SA. 2010. The ovary: basic biology and clinical implications. J Clin Invest 120:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bowen NJ, Logani S, Dickerson EB, Kapa LB, Akhtar M, Benigno BB, McDonald JF. 2007. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol 104:331–337 [DOI] [PubMed] [Google Scholar]
- 33. Hutchings G, Williams O, Cretoiu D, Ciontea SM. 2009. Myometrial interstitial cells and the coordination of myometrial contractility. J Cell Mol Med 13:4268–4282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tanwar PS, Zhang L, Roberts DJ, Teixeira JM. 2011. Stromal deletion of the APC tumor suppressor in mice triggers development of endometrial cancer. Cancer Res 71:1584–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fukuda T, Kobayashi T, Momose S, Yasui H, Hino O. 2000. Distribution of Tsc1 protein detected by immunohistochemistry in various normal rat tissues and the renal carcinomas of Eker rat: detection of limited colocalization with Tsc1 and Tsc2 gene products in vivo. Lab Invest 80:1347–1359 [DOI] [PubMed] [Google Scholar]
- 36. Gao H, Wu G, Spencer TE, Johnson GA, Bazer FW. 2009. Select nutrients in the ovine uterine lumen. VI. Expression of FK506-binding protein 12-rapamycin complex-associated protein 1 (FRAP1) and regulators and effectors of mTORC1 and mTORC2 complexes in ovine uteri and conceptuses. Biol Reprod 81:87–100 [DOI] [PubMed] [Google Scholar]
- 37. Li Q, Kannan A, DeMayo FJ, Lydon JP, Cooke PS, Yamagishi H, Srivastava D, Bagchi MK, Bagchi IC. 2011. The antiproliferative action of progesterone in uterine epithelium is mediated by Hand2. Science 331:912–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang H, Dey SK. 2006. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet 7:185–199 [DOI] [PubMed] [Google Scholar]
- 39. Thathiah A, Carson DD. 2002. Mucins and blastocyst attachment. Rev Endocr Metab Disord 3:87–96 [DOI] [PubMed] [Google Scholar]
- 40. Hild-Petito S, Fazleabas AT, Julian J, Carson DD. 1996. Mucin (Muc-1) expression is differentially regulated in uterine luminal and glandular epithelia of the baboon (Papio anubis). Biol Reprod 54:939–947 [DOI] [PubMed] [Google Scholar]
- 41. Surveyor GA, Gendler SJ, Pemberton L, Das SK, Chakraborty I, Julian J, Pimental RA, Wegner CC, Dey SK, Carson DD. 1995. Expression and steroid hormonal control of Muc-1 in the mouse uterus. Endocrinology 136:3639–3647 [DOI] [PubMed] [Google Scholar]
- 42. Julian J, Enders AC, Fazleabas AT, Carson DD. 2005. Compartmental distinctions in uterine Muc-1 expression during early pregnancy in cynomolgous macaque (Macaca fascicularis) and baboon (Papio anubis). Hum Reprod 20:1493–1503 [DOI] [PubMed] [Google Scholar]
- 43. DeSouza MM, Mani SK, Julian J, Carson DD. 1998. Reduction of mucin-1 expression during the receptive phase in the rat uterus. Biol Reprod 58:1503–1507 [DOI] [PubMed] [Google Scholar]
- 44. Bowen JA, Bazer FW, Burghardt RC. 1996. Spatial and temporal analyses of integrin and Muc-1 expression in porcine uterine epithelium and trophectoderm in vivo. Biol Reprod 55:1098–1106 [DOI] [PubMed] [Google Scholar]
- 45. Crino PB, Nathanson KL, Henske EP. 2006. The tuberous sclerosis complex. N Engl J Med 355:1345–1356 [DOI] [PubMed] [Google Scholar]
- 46. Curatolo P, Bombardieri R, Jozwiak S. 2008. Tuberous sclerosis. Lancet 372:657–668 [DOI] [PubMed] [Google Scholar]
- 47. Isaacs H. 2009. Perinatal (fetal and neonatal) tuberous sclerosis: a review. Am J Perinatol 26:755–760 [DOI] [PubMed] [Google Scholar]
- 48. King JA, Stamilio DM. 2005. Maternal and fetal tuberous sclerosis complicating pregnancy: a case report and overview of the literature. Am J Perinatol 22:103–108 [DOI] [PubMed] [Google Scholar]
- 49. Everitt JI, Wolf DC, Howe SR, Goldsworthy TL, Walker C. 1995. Rodent model of reproductive tract leiomyomata. Clinical and pathological features. Am J Pathol 146:1556–1567 [PMC free article] [PubMed] [Google Scholar]
- 50. Flake GP, Andersen J, Dixon D. 2003. Etiology and pathogenesis of uterine leiomyomas: a review. Environ Health Perspect 111:1037–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walker CL, Stewart EA. 2005. Uterine fibroids: the elephant in the room. Science 308:1589–1592 [DOI] [PubMed] [Google Scholar]
- 52. Sabatini DM. 2006. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer 6:729–734 [DOI] [PubMed] [Google Scholar]
- 53. Inoki K, Ouyang H, Zhu T, Lindvall C, Wang Y, Zhang X, Yang Q, Bennett C, Harada Y, Stankunas K, Wang CY, He X, MacDougald OA, You M, Williams BO, Guan KL. 2006. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell 126:955–968 [DOI] [PubMed] [Google Scholar]
- 54. Shaw JL, Dey SK, Critchley HO, Horne AW. 2010. Current knowledge of the aetiology of human tubal ectopic pregnancy. Hum Reprod Update 16:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ajonuma LC, Ng EH, Chan HC. 2002. New insights into the mechanisms underlying hydrosalpinx fluid formation and its adverse effect on IVF outcome. Hum Reprod Update 8:255–264 [DOI] [PubMed] [Google Scholar]
- 56. Yamanouchi H, Umezu T, Tomooka Y. 2010. Reconstruction of oviduct and demonstration of epithelial fate determination in mice. Biol Reprod 82:528–533 [DOI] [PubMed] [Google Scholar]
- 57. Okada A, Ohta Y, Brody SL, Watanabe H, Krust A, Chambon P, Iguchi T. 2004. Role of foxj1 and estrogen receptor α in ciliated epithelial cell differentiation of the neonatal oviduct. J Mol Endocrinol 32:615–625 [DOI] [PubMed] [Google Scholar]
- 58. Psychoyos A. 1986. Uterine receptivity for nidation. Ann NY Acad Sci 476:36–42 [DOI] [PubMed] [Google Scholar]
- 59. DeMayo FJ, Zhao B, Takamoto N, Tsai SY. 2002. Mechanisms of action of estrogen and progesterone. Ann NY Acad Sci 955:48–59; discussion 86–88, 396–406 [DOI] [PubMed] [Google Scholar]
- 60. Dey SK, Lim H, Das SK, Reese J, Paria BC, Daikoku T, Wang H. 2004. Molecular cues to implantation. Endocr Rev 25:341–373 [DOI] [PubMed] [Google Scholar]
- 61. Hoffman LH, Olson GE, Carson DD, Chilton BS. 1998. Progesterone and implanting blastocysts regulate Muc1 expression in rabbit uterine epithelium. Endocrinology 139:266–271 [DOI] [PubMed] [Google Scholar]
- 62. Brayman MJ, Julian J, Mulac-Jericevic B, Conneely OM, Edwards DP, Carson DD. 2006. Progesterone receptor isoforms A and B differentially regulate MUC1 expression in uterine epithelial cells. Mol Endocrinol 20:2278–2291 [DOI] [PubMed] [Google Scholar]