Abstract
Osteoclasts are key players in the maintenance of bone, which is an endocrine target and organ. Bisphosphonates, used for the management of metastatic bone diseases and osteoporosis, suppress osteoclasts. However, the impact of continuously suppressed osteoclasts is unknown. In this study, mice received zoledronic acid (ZA) for 13 months, nearly half the lifespan of mice, and the effects of continual osteoclast suppression on the bone environment and oral wound healing were determined. ZA therapy suppressed osteoclasts, resulting in significantly more bone mass compared with control. Despite continuous and intense suppression of bone loss in mice receiving ZA, serum calcium levels were maintained in the normal range. No differences were noted in serum tartrate-resistant acid phosphatase (TRAP) 5b levels between ZA-treated and control mice. Histomorphometric analyses of bones revealed that ZA therapy significantly decreased osteoclasts on the bone surface but, instead, substantially increased TRAP+ mononuclear cells and osteoclasts that were not on the bone surface. When oral trauma was induced, such TRAP+ mononuclear and nonattached osteoclasts increased considerably with increased inflammatory cell infiltration in the wounds. As a result, oral wound healing was hindered at the connective tissue level. Healing of the epithelium was unaffected. These findings indicate that the continual suppression of osteoclasts does not affect serum calcium levels and that long-term ZA therapy stimulates nonattached osteoclast and TRAP+ mononuclear cell formation that are expanded rapidly in response to oral trauma. Caution should be exercised when using the serum TRAcP5b to estimate the efficacy of antiresorptive therapy.
Bone is a reservoir of calcium and phosphorous, in which its deposition and mobilization by osteoblasts and osteoclasts are under hormonal control in the mature skeleton. Bone is also an endocrine organ where osteoblasts secrete osteocalcin, which plays a role in insulin metabolism (1), and osteocytes secrete fibroblast growth factor 23, which acts on the kidney to decrease phosphate levels (2). Bone is an important hematopoietic organ as well; hence, the proper maintenance of bone by osteoblasts and osteoclasts is crucial for homeostasis of the body. Osteoclasts, in particular, are of hematopoietic origin, share several functions with macrophages, and are capable of activating T cells (3). Therefore, in osseous inflammation, osteoclasts would be involved in immune responses by communicating with other phagocytes (4). Moreover, osteoclasts play a major role in hematopoietic cell trafficking, calcium and phosphorous mobilization, and the maintenance of the skeleton, which provides structural support for the body (5–7). Chronic suppression of osteoclasts by commonly prescribed antiresorptives; therefore, could have a negative impact on the bone-related endocrine system and the cellular environment in bone and may hinder local immune responses required for proper osseous wound healing (8, 9). However, the effect of long-term osteoclast suppression by antiresorptive therapy in the bone cellular environment is not well understood. Recent reports show that the long-term use of nitrogen-containing bisphosphonate (N-BP) is associated with the formation of large osteoclasts that are not attached to bone surfaces (10, 11). The biological significance of these nonattached large osteoclasts is unknown, but because osteoclasts have proteases, such as tartrate-resistant acid phosphatase (TRAP) and cathepsin-K, they could play a role in osseous inflammation.
Osteonecrosis of the jaw (ONJ), an emerging condition associated with the use of potent antiresorptives, has come to medical attention dominantly in the setting of N-BP use for the management of metastatic bone diseases and osteoporosis (12). N-BP, such as alendronate, and zoledronic acid (ZA), possess a strong affinity for bone, and their half-life is estimated to be greater than 10 yr (13). The pharmacological effect of N-BP to suppress osteoclasts persists many years once N-BP are administered. The risk to develop ONJ appeared to be associated with the duration of therapy: the longer the duration, the higher the chances for developing ONJ (14). It has been reported that antiresorptive therapy, such as iv ZA administration greater than 2 yr in oncology patients, would considerably increase the risk of ONJ. For patients with osteoporosis, the risk to develop ONJ increases substantially after 5 yr of oral bisphosphonate therapy (15). Because the chronic suppression of osteoclasts impacts the bone cellular environment, the development of ONJ may be linked to affected cell populations by long-term N-BP therapy.
In this study, ZA was administered to young adult mice for 13 months, and oral osseous wounds were created. We first examined the impact of long-term ZA therapy on the bone cellular environment and next investigated how affected cell populations by ZA play a role in oral osseous wound healing. It was discovered that chronic ZA therapy increased TRAP+ nonattached multinucleated and mononuclear cells (MNC) near bone surfaces. When oral osseous trauma was induced, such TRAP+ nonattached multinucleated cells and MNC significantly increased in the wounds. Healing of the oral wounds was impeded. This study also validated that long-term ZA therapy has no detectable effects on epithelial regeneration and new blood vessel formation in oral wounds.
Materials and Methods
Animals and bisphosphonate therapy
Fourteen C57BL/6J mice (8-wk-old males) were obtained from The Jackson Laboratory (Bar Harbor, ME) and maintained in a temperature controlled room with a 12-h light, 12-h dark cycle. Mice were allowed access to water and standard diet ad libitum. ZA monohydrate (Zometa, Novartis, Stein, Switzerland) was administered sc for 13 months at 0.1 mg/kg · wk (ZA group, n = 7). Injections were performed twice a week. An equivalent volume of vehicle (saline) was injected as control [vehicle control (VC) group, n = 7]. This administration regimen was based on previous studies (8, 16) reported to induce osteoclast suppression in long bones (17) as well as in the jaw bones of mice (18). The experimental protocol was approved, and all animals were treated in accordance with the guidelines of the University Committee on Use and Care of Animals.
Palatal bone denudation
A previously established rodent model of oral wound healing was employed in this study with modifications (8). At 3 wk before euthanasia, the oral mucosa was excised to evaluate wound healing. Mice were anesthetized with ip injections of ketamine (90 mg/kg) and xylazine (5 mg/kg). The palatal mucosa between the left molars and great palatine complex was excised with a scalpel to expose the palatine process of the maxilla. The excised surgical sites were left denuded to evaluate spontaneous wound healing/regeneration.
Microcomputed tomography (μCT)
The femurs were dissected, fixed in 10% formalin, measured for their lengths, and analyzed using μCT (μCT-100; Scanco Medical AG, Bruttisellen, Switzerland) to determine the accumulative effect of antiresorptive therapy for 13 months on the skeleton. The femurs were scanned for trabecular microarchitecture in the metaphyseal region of the distal femur and the midshaft cortical microarchitecture at 10-μm voxel resolution with an energy level of 70 kV; 300 cross-sectional slices were made at 10-μm intervals at the distal end beginning at the edge of the growth plate and extending in a proximal direction, and 100 contiguous slices starting 0.05 mm proximal to the growth plate were selected for analysis of trabecular microarchitecture. The scans for midshaft cortical microarchitecture were obtained by 50 slices at the exact midpoint of the femur. The trabecular and the cortical bone compartments in the distal femurs and the midshaft, respectively, were segmented by the semimanual contouring method and analyzed using built-in Scanco software.
Histomorphometric analyses of tibiae and oral wound healing
The tibiae and maxillae were isolated and fixed in 10% formalin at euthanasia. The bones were demineralized in 10% EDTA, paraffin embedded, and sectioned at 5 μm. Masson's trichrome and TRAP staining were performed to visualize collagen fibers and osteoclasts, respectively, with commercial kits following manufacturer's instructions (HT15 and 386A; Sigma-Aldrich, St. Louis, MO). Hematoxylin and eosin (HE) staining were performed with a standard staining protocol. Immunohistochemical staining to visualize blood vessels and macrophages was conducted as follows. Sections were deparaffinized, rehydrated, and subjected to antigen retrieval. Nonspecific protein was blocked. The sections were incubated with primary antibodies overnight at 4 C. Rabbit antimouse Von Willebrand factor (ab6994; Abcam, Cambridge, MA) at 1:800 dilution and rat antimouse F4/80 antibody (ab6640; Abcam) at 1:100 dilution were used. After incubation, endogenous peroxidase activity was blocked with 0.3% H2O2. Secondary antibodies were applied for 1 h at room temperature. Goat antirabbit IgG-horseradish peroxidase (AP307P; Millipore, Billerica, MA) at 1:400 dilution and goat antirat IgG-horseradish peroxidase (ab97057; Abcam) at 1:200 dilution were used. Staining was developed using 3,3-diaminobenzidine (Vector Laboratories, Burlingame, CA) followed by counterstaining with Mayer's hematoxylin. Stained sections were histomorphometrically analyzed using Image-Pro Plus version 4 (Media Cyberrnetics, Bethesda, MD). Bone histomorphometric analyses, including osteoclast number, osteoclast surface, osteoblast surface, and bone lining cell surface, were performed. Nonattached osteoclasts and osteoclast precursors were assessed both in oral wounds and tibiae. Nonattached osteoclasts were TRAP+ multinucleated cells that were away from the bone surface. Nonattached osteoclasts and TRAP+ MNC were counted in the connective tissue and tibial bone marrow within 100 μm of the bone surface. Bone lining cells were defined morphologically as long, flattened cells covering bone surfaces with no obvious stained cytoplasm (19).
Oral wound healing was assessed by quantifying collagen fibers, inflammatory cell infiltration, and F4/80+ macrophages in regenerated connective tissues. Contralateral intact mucosa served as an internal control. Collagen fibers were quantified in the connective tissue (∼200 × 500 μm) near the tooth. Inflammatory cell infiltration was assessed by quantifying inflammatory cell numbers in the connective tissue within 100 μm of the bone surface [area of interest (AOI), ∼100 × 500 μm]. The numbers of macrophages were measured in the connective tissue (AOI, ∼200 × 500 μm) near the tooth. The ratio of empty osteocyte lacunae per total osteocyte lacunae was quantified in the alveolar bone at the surface, which was once denuded (AOI, ∼100 × 500 μm). Furthermore, the sections of the maxillae were stained with a modified Brown and Brenn (BB) technique to assess bacteria invasions into the oral wounds and colonization on the mucosal epithelium (20). HE- and BB-stained sections were submitted for the assessment of oral wound healing to an oral pathologist not otherwise involved in this study.
To clarify the long-term ZA therapy on blood vessel formation in oral wounds, Von Willebrand factor positive vessels were quantified in the connective tissue in the jaw. Vessel numbers and perimeter were analyzed histomorphometrically using Image-Pro. Vessels with a diameter smaller than or equal to 6 μm were considered capillaries, and diameters between 6 and 16 μm were considered microvessels (21, 22).
Three serial sections per mouse were scored and averaged in all histomorphometric analyses.
Serum tartrate-resistant acid phosphatase isoform 5b (TRAcP5b) and calcium levels
Blood was collected by cardiac puncture at euthanasia in anesthetized mice. Serum samples were prepared and kept at −80 C until use. To assess the systemic effect of ZA therapy on osteoclasts and calcium, serum TRAcP5b and calcium levels were measured using the mouse TRAP assay (IDS, Boldon, UK) and C7503 (Pointe Scientific, Canton, MI), respectively.
Flow cytometry
Bone marrow was isolated from femurs by centrifugation (23). Red blood cells were lysed, and MNC (1 × 106) were incubated with a combination of fluorescein isothiocyanate antimouse cluster of differentiation (CD34) (RAM34), phycoerythrin (PE) antimouse vascular endothelial growth factor receptor (VEGFR)2 (Avas12a1), and allophycocyanin (APC) antimouse CD133 (13A4). Bone marrow MNC were also incubated with PE antimouse δγ T cell receptor (TCR) (GL3). Fluorescein isothiocyanate rat IgG2a, PE rat IgG2a, APC rat IgG2b, and APC rat IgG1 were used for isotype controls. Cell analysis of CD34+, VEGFR2+, CD133+, and δγTCR+ was performed using the C6 Flow Cytometer (Accuri, Ann Arbor, MI). All antibodies used for flow cytometry were purchased from eBioscience (San Diego, CA).
Statistics
Data were analyzed with the Shapiro-Wilk test for normality. For parametric data, independent t tests for two groups and ANOVA for multiple groups were performed. Tukey's test was used as a post hoc test. For nonparametric data, Kruskal-Wallis test was used. All statistical analysis were conducted with SYSTAT 12 (Systat Software, Chicago, IL). An α-level of 0.05 was used for statistical significance. Results are presented as mean ± sem unless specified.
Results
Treatment effect on bone mass in femurs, μCT findings
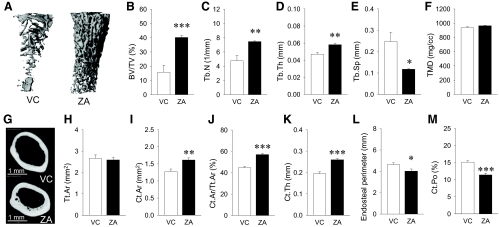
ZA therapy for 13 months significantly counteracted age-associated physiologic trabecular bone loss (Fig. 1A). The average ZA-treated trabecular bone mass was more than twice that in control (Fig. 1B). This large trabecular bone mass in the ZA group was characterized by significantly higher trabecular number and thickness and significantly lower trabecular separation than in control (Fig. 1, C–E). No differences were noted in tissue mineral density between groups (Fig. 1F). ZA therapy resulted in a structural change in the diaphyseal cortical bone at the midshaft. Representative reconstructed images of the middiaphyseal cortical bone are shown in Fig. 1G. In the ZA group, the smaller size of the medullary cavity was apparent compared with control, and numerous small cavities were noted near the endosteum. No differences were found in the total cross-sectional area inside the periosteal envelope between groups (Fig. 1H). However, the cortical area and its fraction were significantly higher in the ZA group than control (Fig. 1, I and J). Indeed, the average cortical thickness was significantly larger in the ZA group, and the average endosteal perimeter was significantly smaller in the ZA group than control (Fig. 1, K and L). Because increased size of the medullary cavity and concomitant decrease in cortical thickness occur in aged C57BL/6 mice (24, 25), these results indicate that long-term ZA therapy negated physiological endocortical bone loss but did not interfere with pericortical bone growth. Interestingly, the cortical porosity was significantly lower in the ZA group than control (Fig. 1M) even though numerous cavities were visible in the ZA group. This indicates higher cortical bone density in the ZA-treated mice than in control.
Fig. 1.
Effect of ZA therapy on bone mass, μCT measurements. A, Representative three-dimensional reconstructions of the metaphyseal trabecular bone of the distal femurs. B–E, The femurs from ZA-treated mice had significantly higher trabecular bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and significantly lower trabecular separation (Tb.Sp) compared with VC-treated mice. F, Tissue mineral density (TMD) was similar between the VC and ZA groups. G, Cross-sectional images of the diaphyseal cortical bone. Numerous cavities devoid of mineral were noted near the endosteum in the ZA group. H, No difference was found in the total cross-sectional area inside the periosteal envelop (Tt.Ar) between groups. I–K, The femurs in the ZA group had significantly higher cortical bone area (Ct.Ar), cortical area fraction (Ct.Ar/Tt.Ar), and cortical thickness (Ct.Th) than the VC group. L, Endosteal perimeters were significantly smaller in the ZA than VC group. M, The cortical bone in the ZA group had significantly less cortical porosity (Ct.Po) than the VC group. n = 7/group; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ZA therapy suppressed osteoclasts but did not alter serum TRAcP5b levels
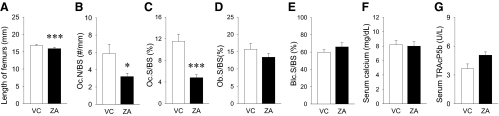
Consistent with the μCT findings for the femurs, histological sections of tibiae in control mice showed sparse and thin trabecular bone, whereas thick and highly connected trabecular structures were observed in the ZA group (data not shown). Femur lengths were significantly shorter in the ZA group compared with control, although the absolute differences were less than 1 mm (Fig. 2A). Histomorphometric analysis of the metaphysis in the proximal tibia revealed that osteoclast numbers and osteoclast surface were significantly lower in the ZA group compared with control (Fig. 2, B and C). No differences were noted in osteoblast surface and bone lining cell surface between groups (Fig. 2, D and E). These results suggest that osteoclasts, but not osteoblasts, were primarily affected by ZA therapy and that suppressed osteoclasts attributed the large bone mass observed in the ZA group. Despite the significant decrease in osteoclast numbers on the bone surfaces, serum calcium levels were maintained in the ZA group (Fig. 1F). Interestingly, no statistical differences were found in the serum TRAcP5b levels between groups (Fig. 1G). Rather, a trend was observed that the ZA group had slightly higher serum TRAcP5b levels than in control.
Fig. 2.
Histomorphometric assessment of the proximal tibiae and serum chemistry. A, The lengths of femurs were plotted as mean ± sd (n = 7/group). The ZA-treated mice had significantly shorter femurs than control. B and C, Osteoclast numbers per linear perimeter (Oc.N/BS) and osteoclast surface (Oc.S/BS) were significantly less in the metaphysis of the proximal tibiae of the ZA-treated mice than control (n = 7/group). D and E, No differences were noted in osteoblast surface (Ob.S/BS) and bone lining cell surface (Blc.S/BS) between the VC and ZA groups (n = 7/group). F, Serum calcium levels were similar between groups (n = 5/group). G, No statistical differences were noted in the serum TRAcP5b levels between groups (n = 5/group). *, P < 0.05; ***, P < 0.001.
ZA therapy increased osteoclast precursors and nonattached osteoclasts
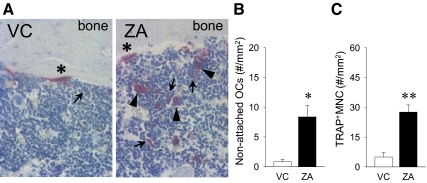
When osteoclast numbers per linear perimeter of bone were assessed in the TRAP-stained sections of the tibiae, we noticed quite a few numbers of TRAP+ multinucleated cells and MNC near the trabecular bone but not on the bone surface. Such TRAP+ multinucleated cells were not artifacts, because MNC were observed between those cells and the bone surface (Fig. 3A). Because there was no evidence that those TRAP+ multinucleated cells were once on the bone surface and then detached in the histological sections, they were designated as nonattached osteoclasts. However, possible explanations are that they could be detached as recently reported in literature (11) or fused in the bone marrow space and then failed to attach to bone. Histomorphometric analyses of the bone marrow area within 100 μm of the bone surface revealed that ZA therapy significantly increased the number of nonattached osteoclasts compared with control (Fig. 3B). Furthermore, we found that TRAP+ MNC, prefusion osteoclasts, were significantly great in number in the ZA vs. VC group (Fig. 3C). Thus, long-term ZA therapy stimulated a cell population in the myeloid lineage to express TRAP in the bone marrow.
Fig. 3.
Nonattached osteoclasts and TRAP+ MNC in the tibiae. A, Representative photomicrographs of TRAP-stained sections of the metaphysis of the proximal tibiae show attached osteoclasts (asterisk), nonattached osteoclasts (arrowheads), and TRAP+ MNC (arrows). ZA therapy for 13 months significantly increased the numbers of nonattached osteoclasts (B) and TRAP+ MNC (C) in the proximal tibiae (n = 7/group). *, P < 0.05; **, P < 0.01.
Impeded bone repair in the maxillae by ZA therapy
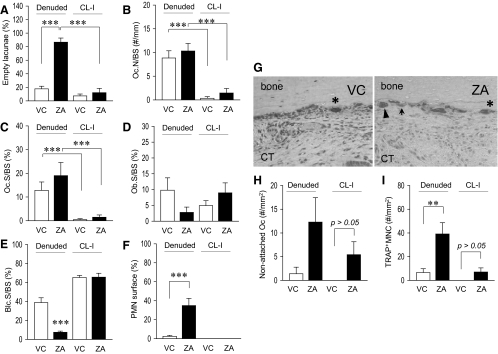
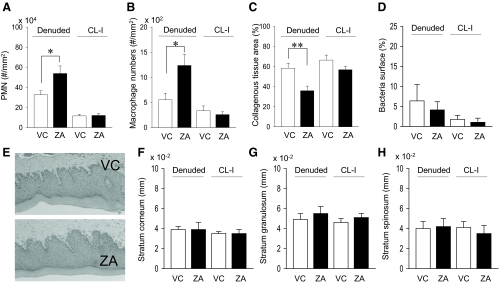
Oral mucosa next to the maxillary left molars was excised to denude a portion of the maxillary bone. Gross healing at 3 wk after surgery was normal in both groups. The denuded bone sites were covered with regenerated epithelium and appeared healed. We have previously demonstrated that osteocytes in bone near the denuded surface died, and the involved lacunae became empty when a portion of the maxillae was denuded (8). When bone contains large numbers of empty lacunae locally, the bone is necrotic and attracts osteoclasts for repair. As anticipated, the denuded maxilla in the ZA group exhibited a significantly higher percentage of empty lacunae compared with the contralateral intact side, whereas the percentage of empty lacunae was low in the denuded maxilla in control and was similar to the contralateral intact side (Fig. 4A). This indicates that during 3 wk of healing, considerable bone repair had occurred in control, but bone repair was impeded in the ZA group. However, when the bone surfaces were assessed for osteoclasts, both groups showed increased numbers of osteoclasts compared with their contralateral intact side (Fig. 4, B and C). Thus, bone repair lead by osteoclasts might not be efficient in the ZA group. To further assess bone repair, cells lining the bone surfaces were morphologically analyzed. Although no statistical difference was detected, osteoblast surface was lower in the denuded side of the ZA-treated mice compared with that in the denuded side in control (Fig. 4D). Moreover, a significantly lower percentage of bone lining cell surface was found in the denuded side of the ZA-treated mice compared with those in the denuded side of the control mice (Fig. 4E). In the ZA group, nearly half of the bone surface appeared to juxtapose to polymorphonuclear leukocytes (PMN) histologically, whereas in control little bone surface had a proximity to PMN (Fig. 4F); instead, most of the surface appeared lined with osteoblasts or bone lining cells. This confirms persistent inflammation and exceedingly delayed bone repair in the ZA group.
Fig. 4.
Bone histomorphometric assessment of the maxillae. A, Numbers of empty osteocyte lacunae per area next to the bone surfaces were plotted. In the denuded side, there was a significant increase in empty osteocyte lacunae in the ZA vs. VC group. B and C, Osteoclast numbers per linear perimeter (Oc.N/BS) and osteoclast surface (Oc.S/BS) were significantly increased in the denuded side than the contralateral intact (CL-I) side regardless of therapy. No differences were found between groups in the denuded side. D, In the denuded side, osteoblast surface (Ob.S/BS) was smaller in the ZA group than control, but no statistical differences were reached between groups. E, Significantly smaller bone lining cell surface (Blc.S/BS) was noted in the denuded side of ZA group vs. control. No differences were noted in the contralateral intact side between groups. F, In the denuded side, significantly higher numbers of PMN cells were juxtaposed to the bone surfaces in the ZA group vs. control. No PMN cells were observed on the bone surfaces in the contralateral intact side regardless of therapy. G, Representative photomicrographs of TRAP-stained sections of the maxillae show attached osteoclasts (asterisk), nonattached osteoclasts (arrowheads), and TRAP+ MNC (arrows). ZA therapy for 13 months significantly increased the numbers of nonattached osteoclasts (H) and TRAP+ MNC (I) in the denuded side as well as in the contralateral intact palate. n = 7/group; **, P < 0.01; ***, P < 0.001.
As in tibiae, increased numbers of nonattached osteoclasts and TRAP+ MNC were observed in the maxillae in the ZA group (Fig. 4G). In the contralateral intact side, nonattached osteoclasts were not detected in control, but more than half of the samples in the ZA group had nonattached osteoclasts (Fig. 4H). In the denuded side of the ZA-treated mice, increased numbers of nonattached osteoclasts were found. In the contralateral intact side, TRAP+ MNC were not observed in control but small numbers of TRAP+ MNC detected in the ZA group (Fig. 4I). In the denuded side, however, TRAP+ MNC increased tremendously in the ZA group but a minor increase in control. Thus, the bone denudation induced expansion of nonattached osteoclasts and TRAP+ MNC in the ZA-treated mice but not in control.
ZA therapy hindered oral wound healing
Inflammatory cell infiltration was assessed by counting aggregated PMN in the connective tissue area within 100 μm of the bone surface. The ZA group had significantly more inflammatory cells than control in the denuded side (Fig. 5A). To identify macrophages in oral wounds, the regenerated connective tissues were examined for F4/80+ MNC. Macrophage numbers were significantly increased in the ZA than control in the denuded side (Fig. 5B). Collagen synthesis was significantly suppressed in the denuded side of the ZA group compared with control (Fig. 5C). Collectively, oral wound healing was hampered in the ZA group at the connective tissue level. To determine whether this hindered wound healing was affected by infection from oral bacteria, BB staining was performed. No bacterial invasions were found in the regenerated connective tissues regardless of therapy. Because ZA therapy might influence bacterial ecology in the mouth and affected wound healing, gram (+) and (−) bacteria surface on the regenerated epithelium was assessed in BB-stained sections. No differences were noted in bacteria surface between groups (Fig. 5D). Furthermore, the integrity of the regenerated epithelium was assessed morphologically. No differences were noted in thickness of stratum cornium, granulosum, and spinosum (Fig. 5, E–H), indicating that the oral epithelium was regenerated normally both groups.
Fig. 5.
Histomorphometric findings in the oral mucosa. Significantly more inflammatory (PMN) cells (A) and macrophages (B) were found in the connective tissues of the denuded side in ZA-treated mice vs. control mice. The numbers of inflammatory cells (A) and macrophages (B) were low and similar between groups in the connective tissues of the contralateral intact side. C, Collagenous tissue areas were assessed in Masson's trichrome-stained sections of the maxillae. The collagenous tissue area in the denuded side was significantly smaller in ZA-treated mice vs. control mice. D, Gram (+) and (−) bacteria were stained, and the bacterial surface per epithelial surface was quantified. No differences were detected between groups. E, Representative photomicrographs of HE-stained regenerated oral mucosa. F and H, The epithelium were histomorphometrically analyzed. No differences were noted in the thickness of stratum corneoum, granulosum, and spinosum between groups. n = 7/group; *, P < 0.05; **, P < 0.01.
No alteration in blood vessel formation by ZA therapy
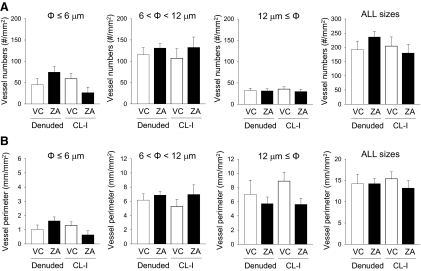
Blood vessels were visualized in the histological sections of the oral mucosa by the immunohistochemical staining of Von Willebrand factor and analyzed histomorphometrically. There were no statistical differences in the numbers of capillaries (φ ≤ 6 μm), microvessels (6 μm < φ < 12 μm), and vessels (φ ≥ 12 μm) between groups in the denuded side as well as in the contralateral intact side (Fig. 6A). Furthermore, the vessel perimeter was analyzed and compared (Fig. 6B). No statistical differences were noted between groups in the denuded or contralateral intact side.
Fig. 6.
Histomorphometric findings of blood vessels in the maxillae. Capillaries (φ ≤ 6 μm), microvessels (6 μm < φ < 12 μm), and vessels (φ ≥ 12 μm) were analyzed in Von Willebrand factor-stained sections of the maxillae for their vessel numbers (A) and perimeters (B). No statistical differences were found between groups in the denuded side or in the contralateral intact side. n = 7/group.
Flow cytometric findings
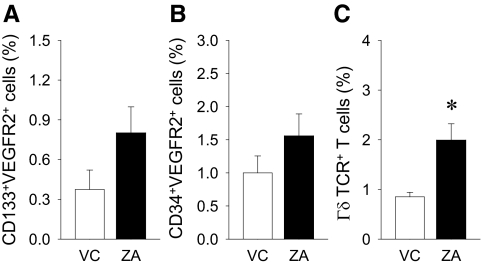
To examine the antiangiogenic effect of ZA therapy, flow cytometric analysis of endothelial progenitor cells in the bone marrow was performed. No statistical differences were found between groups in endothelial progenitor cells (CD133+VEGFR2+ or CD34+VEGFR2+ cells). However, there was a trend toward more endothelial progenitor cells in the ZA than control (Fig. 7, A and B). No CD34+CD133+VEGFR2+ cells were detected after 200,000 events in this study. The effect of ZA therapy on γδTCR positive T cells was investigated in the bone marrow. A significantly greater number of γδ T cells was detected in the ZA group vs. VC (Fig. 7C).
Fig. 7.
Flow cytometric findings. Flow cytometric analysis of bone marrow MNC was performed to assess γδ T cells and endothelial progenitor cells. No statistical differences were found in CD133+VEGFR2+ (A) or CD34+VEGFR2+ (B) cell populations in the bone marrow between the ZA-treated and control mice. C, Significantly more γδ T cells were found in the ZA-treated vs. control mice. n = 7/group; *, P < 0.05.
Discussion
Male C57BL/6J mice reach their skeletal maturity by 3 months of age and is maintained thereafter (24). After 42 wk, bone resorption outpaces formation and age-associated bone loss begins, resulting in a net decrease of bone mass, increased size of medullary cavity, and decreased cortical thickness. Metaphyseal trabecular bone volume decreases by 50% from 1.5 to 15 months of age (25). In this study, the ZA-treated mice showed significantly thicker cortical bone and 2.5-fold higher trabecular bone volume than control. ZA therapy for 13 months, therefore, counteracted age-associated physiologic bone loss. Although the magnitude of suppression in bone resorption is unknown, because bone resorption was not monitored during therapy, a remarkably high bone mass phenotype, as revealed in the μCT assessment, and considerably decreased osteoclast surface in the histomorphometric analysis suggest that bone resorption was continually suppressed in the ZA group over the 13 months. Indeed, the average length of femurs was shorter in the ZA-treated mice than control (Fig. 2A). This is because ZA therapy likely hindered osteoclastic resorption of terminal hypertrophic chondrocytes and blunted proper endochondral bone growth. Thus, bone resorption was hampered from the early stage of the therapy and seemingly continued for 13 months. Although bone resorption was suppressed in the ZA-treated mice, serum calcium levels were maintained in the normal range. In fact, ZA therapy suppressed osteoclastic bone resorption considerably but not completely, because there were a few osteoclasts present on the bone surface. It has been reported that bone turnover is suppressed to a normal premenopausal level after the initiation of bisphosphonate therapy in humans and remained this way over the treatment period (26, 27). Skeletal retention of bisphosphonates decreases gradually, and small amounts of bisphosphonates are excreted in urine as a result of osteoclastic bone resorption (28). Therefore, diminished but constant bone resorption likely continued in the ZA-treated mice.
N-BP inhibit osteoclastic bone resorption by blocking prenylation of small GTPases (29). Deregulation of small GTPase signaling causes disruption of cytoskeletal organization, vesicle transport, loss of the sealing zone, and ruffled boarder (30, 31), resulting in the detachment of osteoclasts from bone surfaces (32). Therefore, osteoclast activity on bone surfaces would be short and bone resorption inefficient in the ZA-treated mice. To counteract such inefficient bone resorption, one mechanism would be to augment osteoclastogenesis. We found numerous nonattached osteoclasts and TRAP+ MNC near the bone surface in the ZA-treated mice. Furthermore, the ZA-treated mice showed higher serum TRAcP5b levels than control, although statistical significance was not reached. Because serum TRAcP5b levels reflect total osteoclast numbers (33, 34), a trend of higher serum TRAcP5b levels in the ZA group reflects a combined outcome of a few osteoclasts on the bone surface, numerous nonattached osteoclasts, and many TRAP+ MNC. Because osteoclast number is generally considered a reliable index of bone resorption, the circulating levels of TRAP5b are often used as a marker of systemic bone resorption (35–37). Indeed, the use of TRAP5b has been proposed as a specific and sensitive marker for monitoring antiresorptive therapy (38, 39) and bone loss in hemodialysis patients (40). TRAP5b is also reported to be a useful marker for bone metastasis in breast cancer patients (41). In this study, the TRAcP5b levels did not reflect the suppressed bone resorption status. This may suggest that TRAcP5b is not a trustworthy osteoclast marker to estimate the efficacy of antiresorptive therapy, especially when N-BP are used. It has been reported that in human biopsies where patients received 3-yr bisphosphonate (alendronate) therapy, a greater total number of osteoclasts was seen despite significantly decreased bone resorption in bisphosphonate-treated patients than placebo-received patients (11). Approximately one third of such osteoclasts were detached osteoclasts. Although no assessment on TRAP+ MNC was provided in the study, their major finding of increased detached osteoclast number in the group receiving bisphosphonates over 3 yr is consistent with our finding. It should be noted that multinucleated macrophages occur in chronic inflammatory conditions, such as tuberculosis, foreign body reactions, and Langerhans cell histiocytosis (42–44). The retention of acellular damaged bone for extended time due to osteoclast suppression may induce chronic inflammatory responses in tissues nearby.
It has been reported that ZA therapy is associated with the development of ONJ (45), and the high cumulative dose of ZA is a major risk factor (45, 46). The administration of ZA for 13 months in the study was long enough to clarify its effect on oral osseous wound healing. When oral mucosa is denuded, involved bone becomes necrotic and attracts osteoclasts for repair (8). This study successfully modeled that denuded bone sites exhibited bone necrosis and were subjected to repair. Osteoclastic bone resorption was seen in both the ZA and VC groups with no significant differences. This observation was somewhat surprising, because in our previous study, where rats received ZA therapy for 14 wk, bone resorption was markedly suppressed in oral osseous wounds (8). This inconsistency could be explained by the use of different animals (mice vs. rats), different healing time (3 vs. 2 wk), and/or different treatment time (13 months vs. 14 wk). In the current study, nonattached osteoclasts and TRAP+ MNC increased considerably in the denuded side of the ZA-treated mice. In the contralateral intact side, nonattached osteoclasts and TRAP+ MNC were not detected in control mice, whereas they were found in the ZA-treated mice. Hence, ZA therapy increased nonattached osteoclasts and TRAP+ MNC not only in long bones but also in the jaw. Interestingly, TRAP+ cells in the connective tissue, especially TRAP+ MNC, were expanded substantially in the denuded side in the ZA group. This may suggest that myeloid cells were marginally hypersensitive by ZA therapy and inflammation induced by oral trauma was augmented. In fact, significantly more inflammatory cell infiltration and macrophages were found in the oral wounds of the ZA-treated mice than in control. Connective tissue maturation assessed by collagen contents was significantly hindered in the ZA-treated mice. As a result, oral wound healing was impeded in mice receiving ZA. In accordance with this, γδ T cells increased significantly in the bone marrow of the ZA-treated mice. γδ T cells play an important role in innate immunity. The increased numbers of γδ T cells have been described in a variety of inflammatory diseases, such as type 1 diabetes and rheumatoid arthritis (47, 48). Therefore, elevated γδ T cells would be parallel to the marginally hypersensitive state of myeloid cells in the ZA group. Numerous microorganisms inhabit the oral cavity, and the thin oral mucosal lining is a potential portal of entry for pathogenic organisms. To oppose such opportunistic bacterial challenges, host defense mechanisms should function properly. When local immune responses are deregulated due to osteoclast suppression, oral osseous wounds are more likely to get opportunistic infections and, hence, hinder healing. Therefore, the marginally hypersensitive state of myeloid cells by long-term ZA therapy could be associated with the development of ONJ. Because N-BP is known to have soft tissue toxicity in the gastrointestinal tract (49), whether the impaired wound healing in the ZA-treated mice was caused by disrupted epithelial regeneration and/or bacterial infections was examined (Fig. 5, D–H). We found well-regenerated epithelium and no bacterial invasions. Hence, soft tissue toxicity of ZA as a cause of the inflammation is unlikely in this study.
The antiangiogenic effect of N-BP therapy in tumors has been demonstrated in animal studies (50, 51). However, it is not clear whether bisphosphonates suppress angiogenesis in tissues other than tumors (52, 53). Bisphosphonates are used to treat the avascular necrosis of the femoral head with promising results (54, 55). In fracture healing N-BP therapy did not have negative influences on the repair of endochondral bone (56) but showed a 50% decrease in spine fusion (57). Although the correct explanation for such an inconsistency in the effect of N-BP on fracture healing is unknown, it could be explained by the use of different bones (distal radius vs. spine) between them. To our knowledge, no angiogenesis-related adverse events of long-term bisphosphonate therapy, such as delayed wound healing, have been described on osteoporotic patients. In the current study, no differences were found in the numbers or diameters of newly formed capillary, microvessels, and relatively large vessels in the denuded side of the oral mucosa between the ZA-treated and control mice (Fig. 6A). Likewise, no differences in blood vessel numbers or perimeters were noted in the contralateral intact side. Therefore, although the antiangiogenic effect of ZA therapy may be significant in tumors, this is not so in normal tissues. Suppressed angiogenesis has been discussed as an etiology of ONJ (58). However, the results of recent three large clinical trials investigating bevacizumab (an angiogenic inhibitor) in the treatment of oncology patients revealed no association between bevacizumab and risk of ONJ (59). Therefore, the role of suppressed angiogenesis in the development of ONJ may not be considerable. Because ONJ occurs in the jaw that does not bear a tumor, our finding supports that suppressed angiogenesis is not important in the etiology of ONJ.
In summary, we demonstrated that ZA therapy for 13 months, nearly half the lifespan of mice, suppressed bone resorption but increased nonattached osteoclasts and TRAP+ MNC in both the jaw and long bones. The serum TRAcP5b levels were high despite the considerable suppression of bone resorption. Oral wound healing was impeded due to persistent inflammation in the connective tissues. Substantially increased nonattached osteoclasts and TRAP+ MNC were associated with the persistent inflammation in the oral wounds. The false negative reading of the serum TRAcP5b levels in the study indicates that caution should be exercised when using the serum TRAcP5b to estimate the efficacy of antiresorptive therapy.
Acknowledgments
We thank Nisha D'Silva, B.D.S., M.S.D., Ph.D., for the histopathological diagnosis of mucosal wound healing and Christopher Strayhorn with help in histology preparation.
This work was supported by the National Institute of Dental and Craniofacial Research Grant R03DE018923.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AOI
- Area of interest
- APC
- allophycocyanin
- BB
- Brown and Brenn
- CD
- cluster of differentiation
- μCT
- microcomputed tomography
- HE
- hematoxylin and eosin
- MNC
- mononuclear cells
- N-BP
- nitrogen-containing bisphosphonate
- ONJ
- osteonecrosis of the jaw
- PE
- phycoerythrin
- PMN
- polymorphonuclear leukocytes
- TCR
- T cell receptor
- TRAcP5b
- tartrate-resistant acid phosphatase isoform 5b
- TRAP
- tartrate-resistant acid phosphatase
- VC
- vehicle control
- VEGFR
- vascular endothelial growth factor receptor
- ZA
- zoledronic acid.
References
- 1. Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. 2007. Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T. 2006. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444:770–77417086194 [Google Scholar]
- 3. Kiesel JR, Buchwald ZS, Aurora R. 2009. Cross-presentation by osteoclasts induces FoxP3 in CD8+ T cells. J Immunol 182:5477–5487 [DOI] [PubMed] [Google Scholar]
- 4. Herman S, Müller RB, Krönke G, Zwerina J, Redlich K, Hueber AJ, Gelse H, Neumann E, Müller-Ladner U, Schett G. 2008. Induction of osteoclast-associated receptor, a key osteoclast costimulation molecule, in rheumatoid arthritis. Arthritis Rheum 58:3041–3050 [DOI] [PubMed] [Google Scholar]
- 5. Shu L, Ji J, Zhu Q, Cao G, Karaplis A, Pollak MR, Brown E, Goltzman D, Miao D. 2011. The calcium-sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J Bone Miner Res 26:1057–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. 2006. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med 12:657–664 [DOI] [PubMed] [Google Scholar]
- 7. Yao Z, Xing L, Qin C, Schwarz EM, Boyce BF. 2008. Osteoclast precursor interaction with bone matrix induces osteoclast formation directly by an interleukin-1-mediated autocrine mechanism. J Biol Chem 283:9917–9924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamashita J, Koi K, Yang DY, McCauley LK. 2011. Effect of zoledronate on oral wound healing in rats. Clin Cancer Res 17:1405–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S. 2011. Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 26:1871–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheung MS, Glorieux FH, Rauch F. 2009. Large osteoclasts in pediatric osteogenesis imperfecta patients receiving intravenous pamidronate. J Bone Miner Res 24:669–674 [DOI] [PubMed] [Google Scholar]
- 11. Weinstein RS, Roberson PK, Manolagas SC. 2009. Giant osteoclast formation and long-term oral bisphosphonate therapy. N Engl J Med 360:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Khosla S, Burr D, Cauley J, Dempster DW, Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, McCauley LK, McGowan J, McKee MD, Mohla S, Pendrys DG, Raisz LG, Ruggiero SL, Shafer DM, Shum L, Silverman SL, Van Poznak CH, Watts N, Woo SB, Shane E. 2007. Bisphosphonate-associated osteonecrosis of the jaw: report of a task force of the American society for bone and mineral research. J Bone Miner Res 22:1479–1491 [DOI] [PubMed] [Google Scholar]
- 13. Gertz BJ, Holland SD, Kline WF, Matuszewski BK, Porras AG. 1993. Clinical pharmacology of alendronate sodium. Osteoporos Int 3(Suppl 3):S13–S16 [DOI] [PubMed] [Google Scholar]
- 14. Vahtsevanos K, Kyrgidis A, Verrou E, Katodritou E, Triaridis S, Andreadis CG, Boukovinas I, Koloutsos GE, Teleioudis Z, Kitikidou K, Paraskevopoulos P, Zervas K, Antoniades K. 2009. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol 27:5356–5362 [DOI] [PubMed] [Google Scholar]
- 15. Barasch A, Cunha-Cruz J, Curro FA, Hujoel P, Sung AH, Vena D, Voinea-Griffin AE, Beadnell S, Craig RG, DeRouen T, Desaranayake A, Gilbert A, Gilbert GH, Goldberg K, Hauley R, Hashimoto M, Holmes J, Latzke B, Leroux B, Lindblad A, Richman J, Safford M, Ship J, Thompson VP, Williams OD, Yin W. 2011. Risk factors for osteonecrosis of the jaws: a case-control study from the CONDOR dental PBRN. J Dent Res 90:439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Croucher PI, De Hendrik R, Perry MJ, Hijzen A, Shipman CM, Lippitt J, Green J, Van Marck E, Van Camp B, Vanderkerken K. 2003. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res 18:482–492 [DOI] [PubMed] [Google Scholar]
- 17. Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S. 2010. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res 25:1668–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubek DJ, Burr DB, Allen MR. 2010. Ovariectomy stimulates and bisphosphonates inhibit intracortical remodeling in the mouse mandible. Orthod Craniofac Res 13:214–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Everts V, Delaissé JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W. 2002. The bone lining cell: its role in cleaning Howship's lacunae and initiating bone formation. J Bone Miner Res 17:77–90 [DOI] [PubMed] [Google Scholar]
- 20. Taylor RD. 1966. Modification of the Brown and Brenn gram stain for the differential staining of gram-positive and gram-negative bacteria in tissue sections. Am J Clin Pathol 46:472–474 [DOI] [PubMed] [Google Scholar]
- 21. Hao Q, Su H, Marchuk DA, Rola R, Wang Y, Liu W, Young WL, Yang GY. 2008. Increased tissue perfusion promotes capillary dysplasia in the ALK1-deficient mouse brain following VEGF stimulation. Am J Physiol Heart Circ Physiol 295:H2250–H2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. MacDonald IC, Aharinejad S, Schmidt EE, Groom AC. 1995. Luminal constrictions due to endothelial cells in capillaries of mouse exocrine pancreas. Microvasc Res 49:64–77 [DOI] [PubMed] [Google Scholar]
- 23. Dobson KR, Reading L, Haberey M, Marine X, Scutt A. 1999. Centrifugal isolation of bone marrow from bone: an improved method for the recovery and quantitation of bone marrow osteoprogenitor cells from rat tibiae and femurae. Calcif Tissue Int 65:411–413 [DOI] [PubMed] [Google Scholar]
- 24. Ferguson VL, Ayers RA, Bateman TA, Simske SJ. 2003. Bone development and age-related bone loss in male C57BL/6J mice. Bone 33:387–398 [DOI] [PubMed] [Google Scholar]
- 25. Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S. 2002. Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res 17:1044–1050 [DOI] [PubMed] [Google Scholar]
- 26. Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA. 2004. Ten years' experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199 [DOI] [PubMed] [Google Scholar]
- 27. Devogelaer JP, Broll H, Correa-Rotter R, Cumming DC, De Deuxchaisnes CN, Geusens P, Hosking D, Jaeger P, Kaufman JM, Leite M, Leon J, Liberman U, Menkes CJ, Meunier PJ, Reid I, Rodriguez J, Romanowicz A, Seeman E, Vermeulen A, Hirsch LJ, Lombardi A, Plezia K, Santora AC, Yates AJ, Yuan W. 1996. Oral alendronate induces progressive increases in bone mass of the spine, hip, and total body over 3 years in postmenopausal women with osteoporosis. Bone 18:141–150 [DOI] [PubMed] [Google Scholar]
- 28. Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, Beneton MN, Gertz BJ, Sciberras DG, Holland SD, Orgee J, Coombes GM, Rogers SR, Porras AG. 1997. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res 12:1700–1707 [DOI] [PubMed] [Google Scholar]
- 29. Coxon FP, Helfrich MH, Van't Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ. 2000. Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 15:1467–1476 [DOI] [PubMed] [Google Scholar]
- 30. Coxon FP, Thompson K, Rogers MJ. 2006. Recent advances in understanding the mechanism of action of bisphosphonates. Curr Opin Pharmacol 6:307–312 [DOI] [PubMed] [Google Scholar]
- 31. Halasy-Nagy JM, Rodan GA, Reszka AA. 2001. Inhibition of bone resorption by alendronate and risedronate does not require osteoclast apoptosis. Bone 29:553–559 [DOI] [PubMed] [Google Scholar]
- 32. Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J. 2011. Biochemical and molecular mechanisms of action of bisphosphonates. Bone 49:34–41 [DOI] [PubMed] [Google Scholar]
- 33. Janckila AJ, Neustadt DH, Yam LT. 2008. Significance of serum TRACP in rheumatoid arthritis. J Bone Miner Res 23:1287–1295 [DOI] [PubMed] [Google Scholar]
- 34. Rissanen JP, Suominen MI, Peng Z, Halleen JM. 2008. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int 82:108–115 [DOI] [PubMed] [Google Scholar]
- 35. Schneider A, Kalikin LM, Mattos AC, Keller ET, Allen MJ, Pienta KJ, McCauley LK. 2005. Bone turnover mediates preferential localization of prostate cancer in the skeleton. Endocrinology 146:1727–1736 [DOI] [PubMed] [Google Scholar]
- 36. Samadfam R, Richard C, Nguyen-Yamamoto L, Bolivar I, Goltzman D. 2009. Bone formation regulates circulating concentrations of fibroblast growth factor 23. Endocrinology 150:4835–4845 [DOI] [PubMed] [Google Scholar]
- 37. Chang J, Wang Z, Tang E, Fan Z, McCauley L, Franceschi R, Guan K, Krebsbach PH, Wang CY. 2009. Inhibition of osteoblastic bone formation by nuclear factor-κB. Nat Med 15:682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Halleen JM, Alatalo SL, Suominen H, Cheng S, Janckila AJ, Väänänen HK. 2000. Tartrate-resistant acid phosphatase 5b: a novel serum marker of bone resorption. J Bone Miner Res 15:1337–1345 [DOI] [PubMed] [Google Scholar]
- 39. Nenonen A, Cheng S, Ivaska KK, Alatalo SL, Lehtimaki T, Schmidt-Gayk H, Uusi-Rasi K, Heinonen A, Kannus P, Sievanen H, Vuori I, Vaananen HK, Halleen JM. 2005. Serum TRACP 5b is a useful marker for monitoring alendronate treatment: comparison with other markers of bone turnover. J Bone Miner Res 20:1804–1812 [DOI] [PubMed] [Google Scholar]
- 40. Shidara K, Inaba M, Okuno S, Yamada S, Kumeda Y, Imanishi Y, Yamakawa T, Ishimura E, Nishizawa Y. 2008. Serum levels of TRAP5b, a new bone resorption marker unaffected by renal dysfunction, as a useful marker of cortical bone loss in hemodialysis patients. Calcif Tissue Int 82:278–287 [DOI] [PubMed] [Google Scholar]
- 41. Chao TY, Yu JC, Ku CH, Chen MM, Lee SH, Janckila AJ, Yam LT. 2005. Tartrate-resistant acid phosphatase 5b is a useful serum marker for extensive bone metastasis in breast cancer patients. Clin Cancer Res 11:544–550 [PubMed] [Google Scholar]
- 42. Shen Z, Crotti TN, McHugh KP, Matsuzaki K, Gravallese EM, Bierbaum BE, Goldring SR. 2006. The role played by cell-substrate interactions in the pathogenesis of osteoclast-mediated peri-implant osteolysis. Arthritis Res Ther 8:R70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu XW, Price NM, Gilman RH, Recarvarren S, Friedland JS. 2007. Multinucleate giant cells release functionally unopposed matrix metalloproteinase-9 in vitro and in vivo. J Infect Dis 196:1076–1079 [DOI] [PubMed] [Google Scholar]
- 44. Coury F, Annels N, Rivollier A, Olsson S, Santoro A, Speziani C, Azocar O, Flacher M, Djebali S, Tebib J, Brytting M, Egeler RM, Rabourdin-Combe C, Henter JI, Arico M, Delprat C. 2008. Langerhans cell histiocytosis reveals a new IL-17A-dependent pathway of dendritic cell fusion. Nat Med 14:81–87 [DOI] [PubMed] [Google Scholar]
- 45. Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, Nooka A, Sayegh G, Guarneri V, Desrouleaux K, Cui J, Adamus A, Gagel RF, Hortobagyi GN. 2008. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res 23:826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kyrgidis A, Vahtsevanos K, Koloutsos G, Andreadis C, Boukovinas I, Teleioudis Z, Patrikidou A, Triaridis S. 2008. Bisphosphonate-related osteonecrosis of the jaws: a case-control study of risk factors in breast cancer patients. J Clin Oncol 26:4634–4638 [DOI] [PubMed] [Google Scholar]
- 47. Harrison LC, Dempsey-Collier M, Kramer DR, Takahashi K. 1996. Aerosol insulin induces regulatory CD8 γδ T cells that prevent murine insulin-dependent diabetes. J Exp Med 184:2167–2174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Holoshitz J. 1999. Activation of γδ T cells by mycobacterial antigens in rheumatoid arthritis. Microbes Infect 1:197–202 [DOI] [PubMed] [Google Scholar]
- 49. Lufkin EG, Argueta R, Whitaker MD, Cameron AL, Wong VH, Egan KS, O'Fallon WM, Riggs BL. 1994. Pamidronate: an unrecognized problem in gastrointestinal tolerability. Osteoporos Int 4:320–322 [DOI] [PubMed] [Google Scholar]
- 50. Fournier P, Boissier S, Filleur S, Guglielmi J, Cabon F, Colombel M, Clézardin P. 2002. Bisphosphonates inhibit angiogenesis in vitro and testosterone-stimulated vascular regrowth in the ventral prostate in castrated rats. Cancer Res 62:6538–6544 [PubMed] [Google Scholar]
- 51. Bäuerle T, Merz M, Komljenovic D, Zwick S, Semmler W. 2010. Drug-induced vessel remodeling in bone metastases as assessed by dynamic contrast enhanced magnetic resonance imaging and vessel size imaging: a longitudinal in vivo study. Clin Cancer Res 16:3215–3225 [DOI] [PubMed] [Google Scholar]
- 52. Deckers MM, Van Beek ER, Van Der Pluijm G, Wetterwald A, Van Der Wee-Pals L, Cecchini MG, Papapoulos SE, Löwik CW. 2002. Dissociation of angiogenesis and osteoclastogenesis during endochondral bone formation in neonatal mice. J Bone Miner Res 17:998–1007 [DOI] [PubMed] [Google Scholar]
- 53. Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. 2008. Effect of zoledronic acid on serum angiogenic factors in patients with bone metastases. Med Oncol 25:346–349 [DOI] [PubMed] [Google Scholar]
- 54. Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT, Lin RM. 2005. The use of alendronate to prevent early collapse of the femoral head in patients with nontraumatic osteonecrosis. A randomized clinical study. J Bone Joint Surg Am 87:2155–2159 [DOI] [PubMed] [Google Scholar]
- 55. Ramachandran M, Ward K, Brown RR, Munns CF, Cowell CT, Little DG. 2007. Intravenous bisphosphonate therapy for traumatic osteonecrosis of the femoral head in adolescents. J Bone Joint Surg Am 89:1727–1734 [DOI] [PubMed] [Google Scholar]
- 56. Rozental TD, Vazquez MA, Chacko AT, Ayogu N, Bouxsein ML. 2009. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J Hand Surg Am 34:595–602 [DOI] [PubMed] [Google Scholar]
- 57. Huang RC, Khan SN, Sandhu HS, Metzl JA, Cammisa FP, Jr, Zheng F, Sama AA, Lane JM. 2005. Alendronate inhibits spine fusion in a rat model. Spine 30:2516–2522 [DOI] [PubMed] [Google Scholar]
- 58. Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T. 2010. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 28:165–175 [DOI] [PubMed] [Google Scholar]
- 59. Guarneri V, Miles D, Robert N, Diéras V, Glaspy J, Smith I, Thomssen C, Biganzoli L, Taran T, Conte P. 2010. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat 122:181–188 [DOI] [PubMed] [Google Scholar]