Abstract
Propolis is a resinous substance produced by honeybees as defense against intruders. It has relevant therapeutic properties that have been used since ancient times. Nowadays, propolis is of increasing importance as a therapeutic, alone or included in many medicines and homeopathic products or in cosmetics. Propolis is produced worldwide and honeybees use the flora surrounding their beehives for its production. Therefore its chemical composition may change according to the flora. The phenolic and volatile fractions of propolis have been revised in the present study, as well as some of the biological properties attributed to this natural product. An alert is given about the need to standardize this product, with quality control. This has already been initiated by some authors, mainly in the propolis from the poplar-type. Only this product can constitute a good complementary and alternative medicine under internationally acceptable quality control.
KEY WORDS: Biological properties, phenols, propolis, volatiles
Propolis or bee glue (CAS number 9009-62-5) is a generic name for a resinous substance obtained from beehives, used traditionally as an antimicrobial. It is a heterogeneous mixture of many substances collected, transformed, and used by bees to seal holes in their honeycombs, smooth out the internal walls, and protect the entrance against intruders.[1] In fact, propolis means ‘defense of the city’. The word propolis is derived from the Greek words ‘pro’ (meaning ‘in front of”), and ‘polis’ (meaning ‘the city’). In this manner, propolis might serve as a means for colonies to better maintain homeostasis of the nest environment through the reduction of microbial growth on hive walls, prevention of uncontrolled airflow into the nest, waterproofing of walls against external moisture, and protection against invaders.[2] Therefore, propolis could simply mean defense of the hive.[3] However, more recently, some studies have revealed that propolis in honey bee colonies may play a more subtle role in colony level immunity than direct defense against parasites and pathogens.[2]
Bees make use of the mechanical properties of propolis and of its biological action. This action against microorganisms has also been used by human beings since ancient times.[4] Nevertheless, other biological properties have been attributed to this natural product: hepatoprotective, antitumor, antioxidative, and anti-inflammatory.[1,5–8] In fact, propolis, along with other honeybee products such as honey, royal jelly, and pollen, have relevant therapeutic properties, being used since 300 years B.C. in folk medicine worldwide.[9] Research regarding the chemical composition of propolis of diverse origins, their pharmacological properties, and relation between its composition and biological activities has increased in the last few years.[10] This product has, therefore, gained popularity as an alternative medicine for health amelioration and disease prevention.[11] Some examples include its utilization for increasing the body's natural resistance to infections and lowering blood pressure and cholesterol levels. It has been also used in mouthwash products and toothpastes to prevent caries and treat gingivitis and stomatitis,[12] in cough syrups, oral pills, lozenges, ointments, lotions, and vitamins.[13] Holistic therapists often use propolis for the relief of some inflammations, viral diseases, fungal infections, ulcers, and superficial burns along with acupuncture, ayurveda, and homeopathy.[13]
Among the chemical substances found in propolis are waxes, resins, balsams, aromatic and ethereal oils, pollen, and other organic matter.[14] Typically propolis is comprised of resin (50%), which is composed of flavonoids and related phenolic acids, generally called as the polyphenolic fraction, waxes (30%), essential oil (10%), pollen (5%), and other organic compounds (5%).[15,16] However, this chemical composition may change according to the plant source, which is related to the regional vegetation and to the season in which it is collected by the bees.[17] Therefore, the standardization of this product is difficult on account of the inherent difficulties associated with the analysis of complex mixtures from different vegetal sources.[18]
The present study is focused on the chemical variability of the phenols and volatiles of propolis and its repercussion in the biological activities.
Chemical Variability of the Phenols
Propolis cannot be used as a crude material. It must be purified by extraction with adequate solvents, to remove the unwanted material, preserving the active components, for example, the polyphenolic fractions. Several solvents have been used such as water, ethanol, methanol, hexane, acetone, and chloroform, with ethanol being the most common, particularly at concentration of 70%.[12,19]
The compounds present in the propolis resin have three origins: substances actively secreted by plants and substances exuded from wounds in plants (lipophilic materials on leaves and leaf buds, resins, mucilage, gums, lattices, among others) collected by bees, secreted substances from bee metabolism, and materials which are introduced during propolis elaboration.[10,20] As such compounds have a plant origin, the composition of the plant source determines the chemical composition of propolis.
The chemical composition of propolis depends on the local floral at the site of collection, being therefore, highly variable.[4,10] In spite of this diversity and according to the most studied propolis types, Bankova[20] cited at least six main chemical types of propolis: (1) Poplar propolis found in Europe, North America, and the non-tropical regions of Asia, for which the plant source is Populus spp. of section Aigeiros, most often P. nigra. (2) Birch propolis from Russia, for which the plant source is Betula verrucosa Ehrh.; (3) Green propolis from Brazil, mainly from Baccharis spp., predominantly B. dracunculifolia DC.; (4) Red propolis from Cuba and Venezuela, from Clusia spp.; (5) ‘Pacific’ propolis from Okinawa and Taiwan, for which the plant source is unidentified yet; and (6) ‘Canarian’ propolis from Canary Islands, for which the plant source is also unknown [Table 1].
Table 1.
Types of propolis, their origins and chemical compositions (Adapted from[20])
The main biologically active substances in these types of propolis are different. Flavones, flavanones, phenolic acids, and their esters predominate in the poplar propolis [Figure 1], while flavones and flavonols dominate in the birch propolis [Figure 2]. In green and red propolis, prenylated p-coumaric acids, diterpenic acids [Figure 3], and polyprenylated benzophenones [Figure 4] dominate. C-prenylflavanones [Figure 5] and furofuran lignans [Figure 6] predominate in the ‘Pacific’ and ‘Canarian’ propolis, respectively.[20] However, particularly due to the richness of the flora in some countries, different types of propolis have been reported for the same region.
Figure 1.

Some chemical compounds detected in poplar propolis from Europe
Figure 2.

Some chemical compounds detected in birch propolis from Russia
Figure 3.

Some chemical compounds detected in green propolis from Brazil
Figure 4.
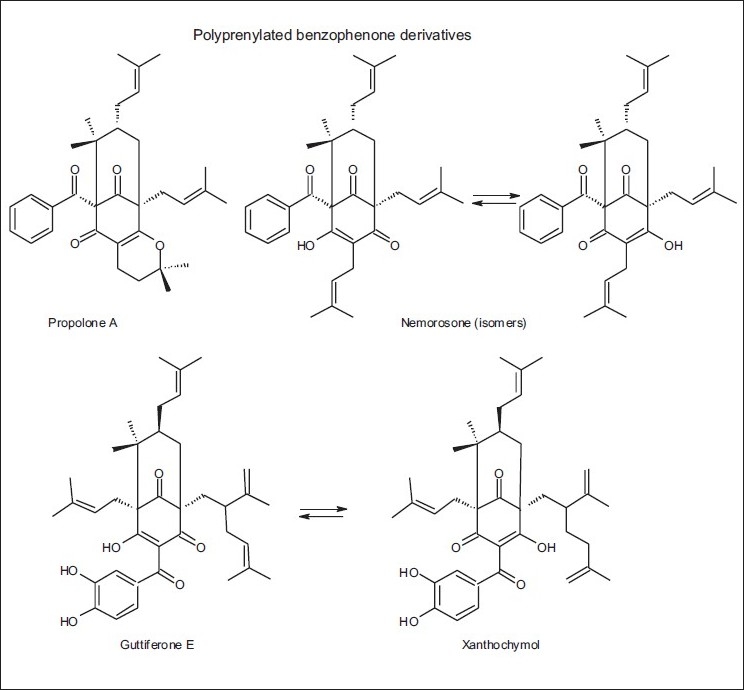
Some chemical compounds detected in red propolis from Cuba and Venezuela
Figure 5.
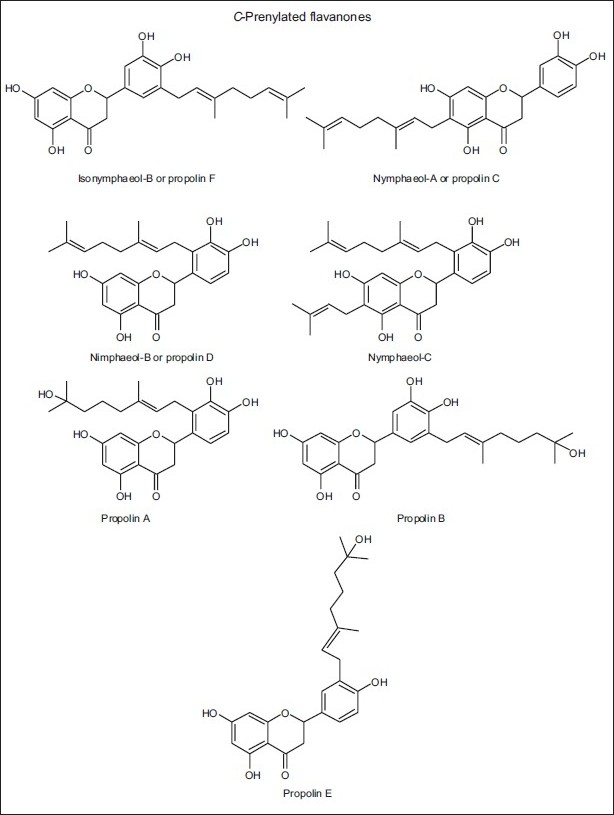
Some C-prenylated flavanones detected in propolis from Okinawa and Taiwan (‘Pacific’ propolis)
Figure 6.
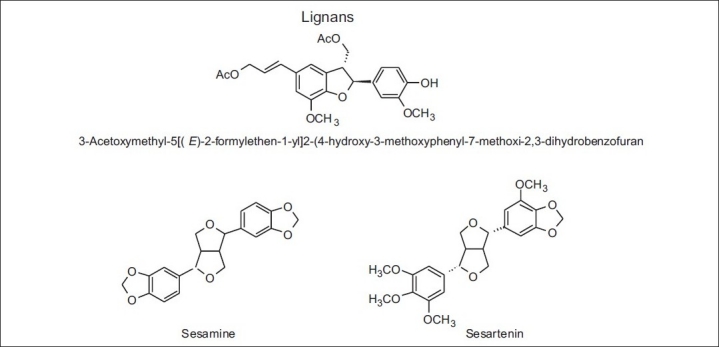
Some lignans detected in propolis from Canary Islands
Propolis from Europe, China, and North America
In Europe, China, and North America, propolis is generally considered to be of the poplar-type; nevertheless, several authors have demonstrated that other types can be found, owing to the flora specificity of each region. In Europe, the Mediterranean propolis from diverse places in Greece contains mainly diterpenes and almost no phenolics.[21] Such a profile has been described for propolis from Sicily and northwestern Greece,[22,23] and can also be found in Croatia and Malta [Table 2 and Figure 7].[21–24] The botanical origin of diterpenes in the propolis samples from Greece is yet unidentified, but on the basis of the diterpenic profile, the source plant should be the conifer species of the Cupressaceae family, in which the flora of the region is very rich.[21] In addition, in Malta, there are other propolis samples also, wherein another compound group is observed: mono- and sesquiterpenyl esters of substituted benzoic acids [Table 2 and Figure 8],[24] which are attributed to a genus Ferula plant found in this region. Mono- and sesquiterpene esters of benzoic acids have also been found in propolis samples from Iran, along with others in which flavonoids and caffeate ester compounds predominate.[25,26] Such results may be due to the simultaneous presence of Populus spp. and Ferula spp.
Table 2.
Some components present in the Mediterranean propolis
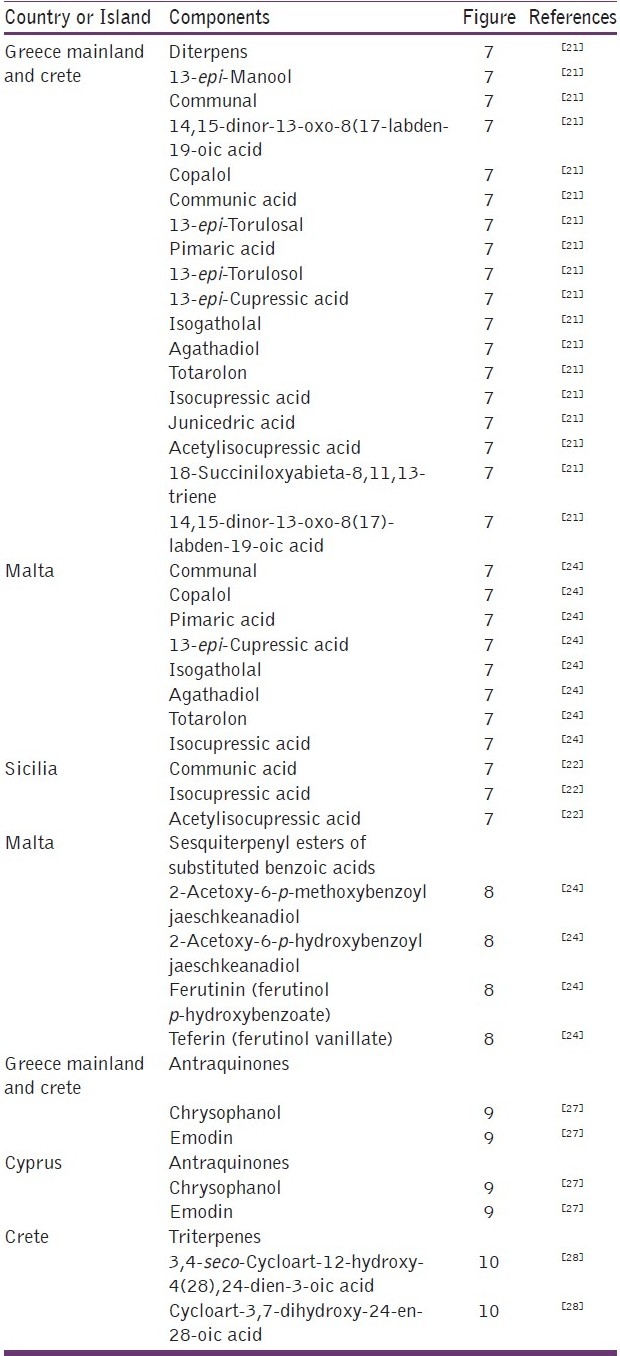
Figure 7.
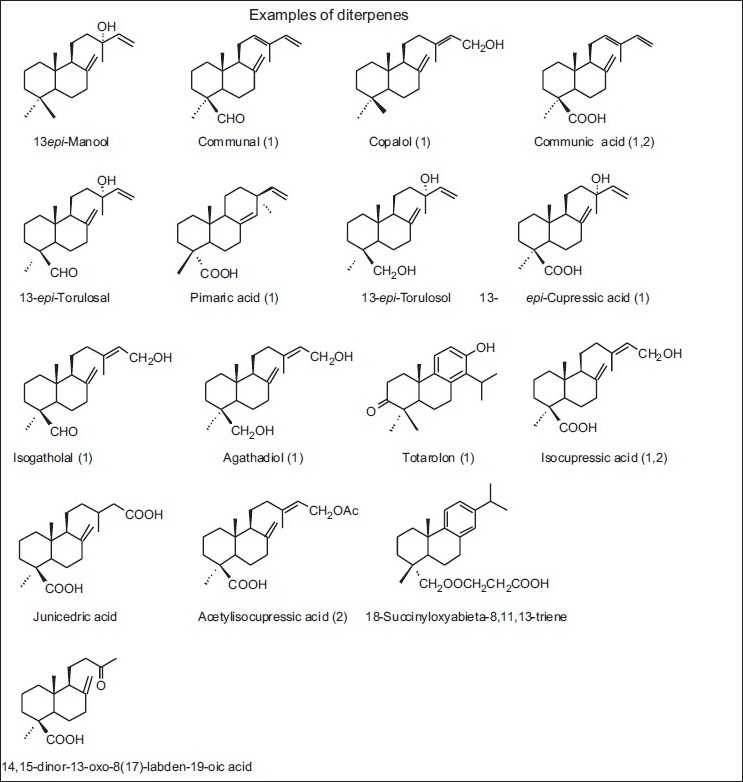
Some diterpenes detected in propolis of some regions of Greece, Malta (1) and Sicilia (2)
Figure 8.
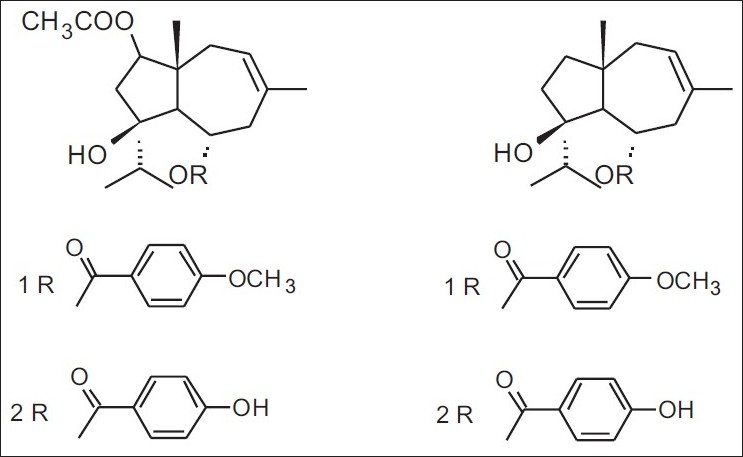
Terpenyl esters in Maltese propolis: 1. 2-acetoxy-6-p-methoxybenzoyl jaeschkeanadiol; 2. 2- acetoxy-6-p-hydroxybenzoyl jaeschkeanadiol; 3. ferutinin (ferutinol p-hydroxybenzoate); 4. teferin (ferutinol vanillate)
In another study, Kalogeropoulos et al.,[27] also reported that the Greek (mainland Greece and Greek islands) and east Cypriot propolis samples shared characteristics that were different from typical European propolis, because those samples presented anthraquinones (mainly emodin and chrysophanol) [Table 2 and Figure 9] and terpenes and/or flavonoids in significant amounts, and had low abundance of phenolic acids and their esters.
Figure 9.

Antraquinones found in some propolis from East Cypriot, mainland Greece, and Greek Islands
In Cretan propolis, five new terpenes were isolated: the diterpenes 14,15-di(nor)-13-oxo-8(17)-labden-19-oic acid and a mixture of labda-8(17),13E-dien-19-carboxy-15-yl oleate and palmitate, as well as the triterpenes, 3,4-seco-cycloart-12-hydroxy-4(28),24-dien-3-oic acid and cycloart-3,7-dihydroxy-24-en-28-oic acid [Table 2 and Figure 10].[28] These cycloartane triterpenes were isolated only from Brazilian propolis (tropical type), Mangifera indica (Anacardiaceae) being the plant source [Table 3 and Figure 11].[29]
Figure 10.
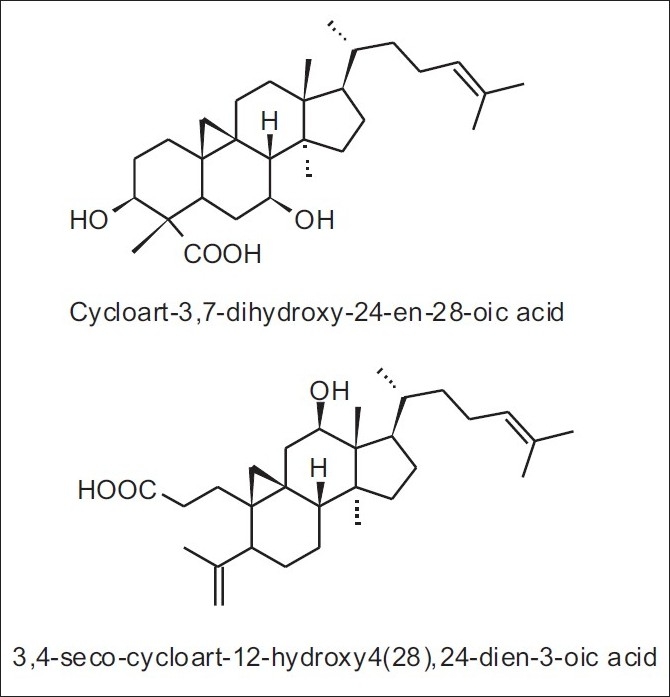
Cycloartane triterpenoids found in some Cretan propolis
Table 3.
Some components present in green and red propolis from Brazil

Figure 11.
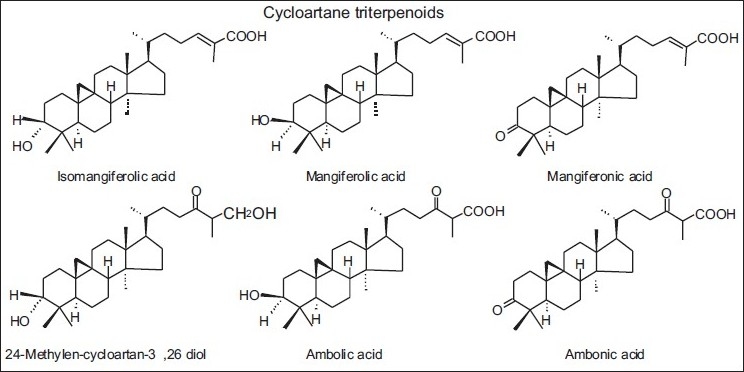
Some cycloartane triterpenes isolated only from Brazilian propolis (tropical propolis)
The main sources of phenolic compounds found in Turkish propolis were the poplar bud exudates. Nevertheless some authors were able to classify the samples collected in different regions of Turkey in four main groups: the typical poplar samples from Central and Western Anatolia; a sample from Adana (Central Anatolia), in which the poplar type compounds were present in lower amounts, the main ones being cinnamyl cinnamate and diterpenic acids (dihydroabietic, abietic, and isopimaric); a sample from Eastern Anatolia that presented significant amounts of hydroxyl fatty acids (hydroxypalmitic, hydroxystearic) and triterpenoid alcohols besides the poplar phenolics in lower amounts; and finally, also from Eastern Anatolia, a sample with low concentrations of flavonoids and very high levels of p-coumaric and ferulic acids, as well as a series of phenolic glycerides.[30]
In recent times, some authors found in the propolis samples from North Portugal, phenolic acids and their esterified and / or methylated derivatives, as well as dihydroflavonols, flavones, flavanones and flavonols, either as free from or with methylated/esterified forms, similar to those of the European-type. Nevertheless, new compounds could also be found: methylated and/or esterified or hydroxylated derivatives of common poplar flavonoids as well as a p-coumaric ester derivative dimmer [Table 4 and Figure 12].[31]
Table 4.
Some new components of propolis from North of Portugal

Figure 12.
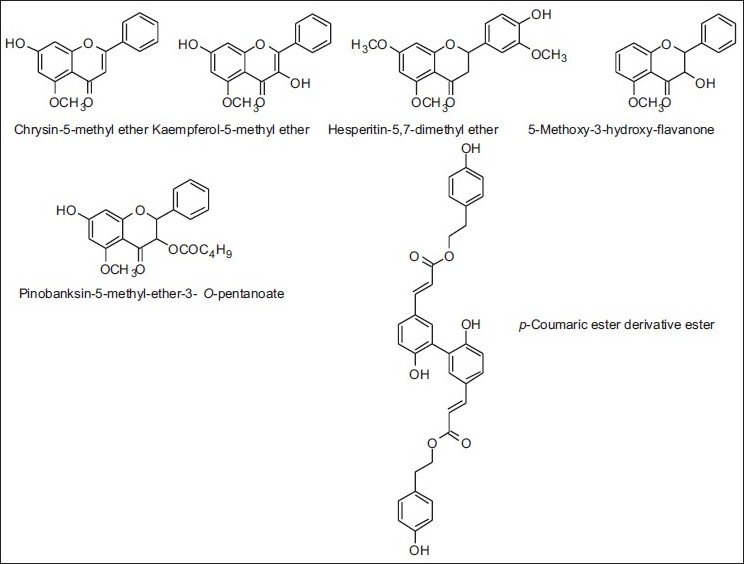
Some compounds found in propolis from Portugal
With regard to China, the propolis of this region is also of the poplar-type [Table 5 and Figure 13].[20,32–35] However in the Chinese propolis some authors also have described some new compounds that had not yet been referred [Table 5 and Figure 14].[33,35] Continuing in the Asian Continent, propolis samples from Myanmar have cycloartane-type triterpenoids and prenylated flavanones, practically dissimilar from those of its neighboring China.[36]
Table 5.
Some components present in Chinese propolis
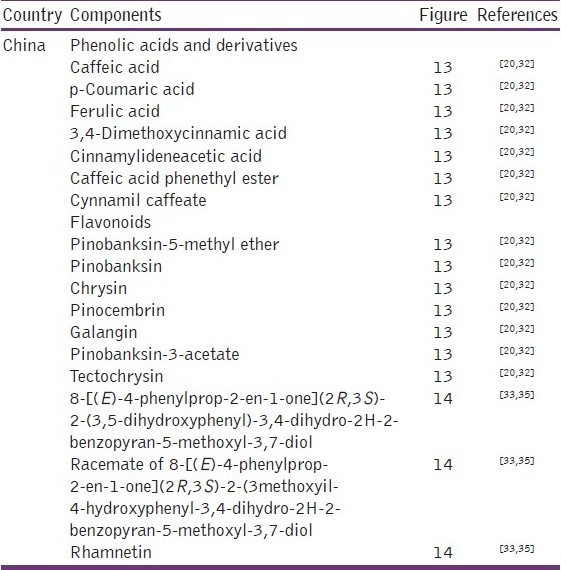
Figure 13.
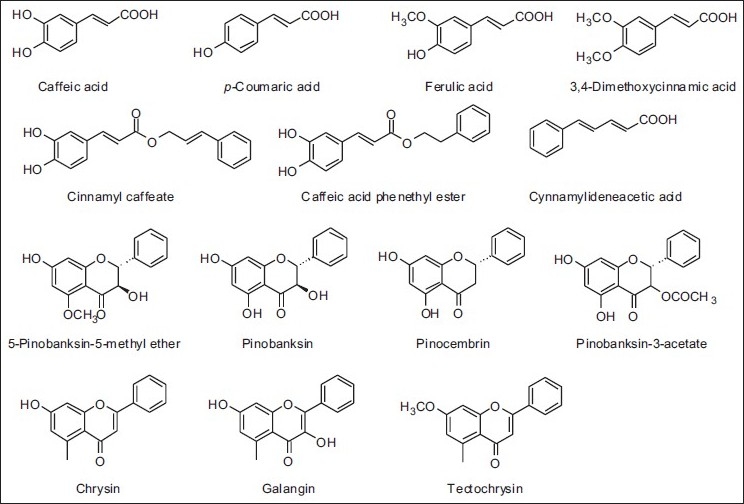
Structures of some constituents of propolis from China
Figure 14.
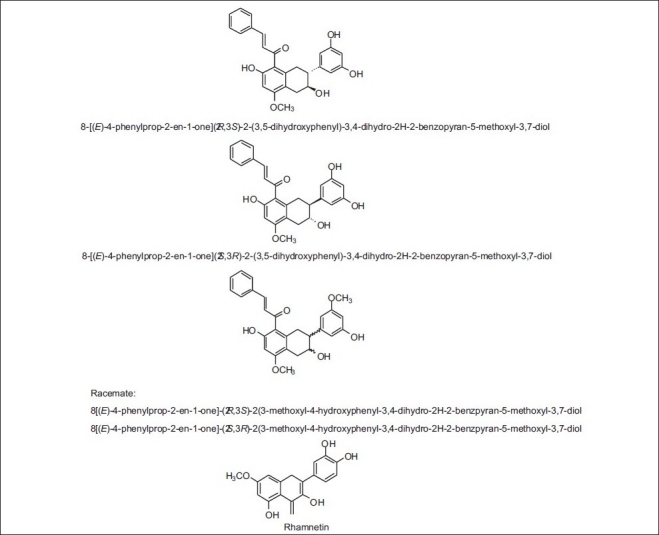
New compounds found in propolis from China (cytotoxic)
In Korea, studies performed by some authors allowed to classify the propolis from several places of this country into poplar-types, such as those of Europe and China.[37] Also, for the first time in Jeju, a southern island of Korea with a subtropical climate, a new chalcone 4‘-methoxy-bavachromanol as well as new khellactone derivatives (laserpitin and isolaserpitin) were found [Table 6 and Figure 15].[38]
Table 6.
Some components present in Korean propolis

Figure 15.
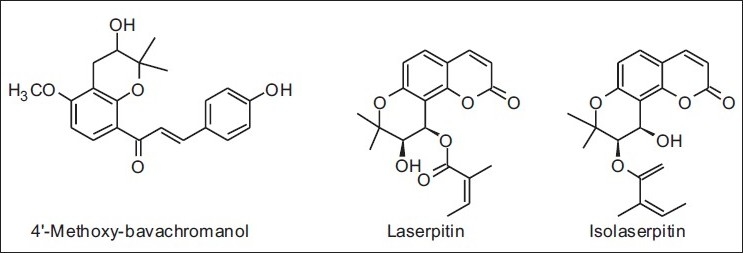
Some new compounds isolated from propolis of Korea
In the same continent, and for the first time, open-chain neoflavonoids were identified in propolis samples from Nepal, as also new chalcone, flavanone, flavan-3-ol, isoflavonoids, and flavanonol compounds [Table 7 and Figure 16].[39–41]
Table 7.
Some components present in propolis from Nepal

Figure 16.
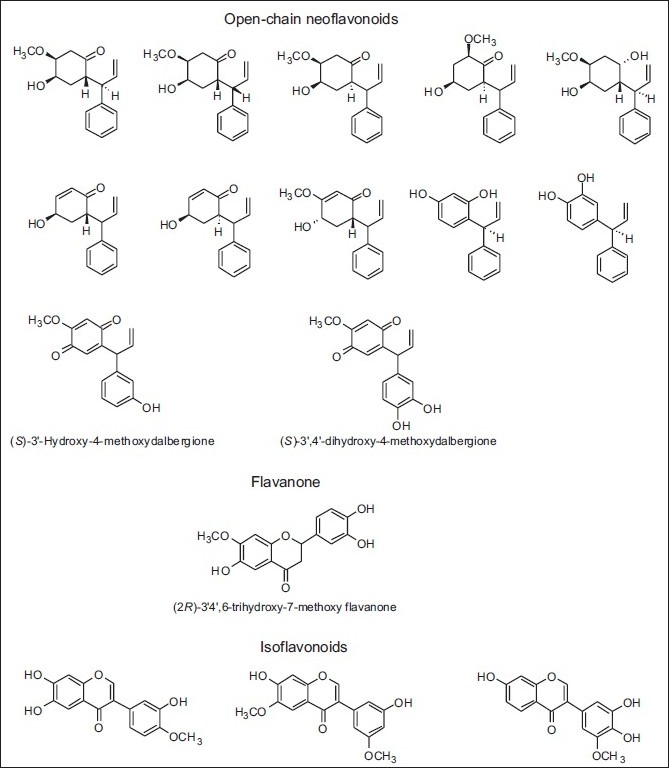
Some new compounds belonging to a diverse group of compounds isolated from propolis of Nepal
Bud exudates of poplar trees are the main source of propolis in the temperate zones and chemical data show a clear preference for the Populus species belonging to the section Aigeiros, as is seen in Europe and North America.[42] In fact, in North America, mainly in the propolis from Ontario (Canada), most studies demonstrate that propolis also originates from the Populus section Aigeiros. Nevertheless, the compounds found in the propolis from Canada in which Aigeiros poplars are absent (Boreal forest and Pacific Coastal forest regions), are different: in Victoria, the components found in propolis are dihydrochalcones, considered to be characteristic of bud exudates of poplars from Populus section Tacamahaca and in the Richmond region, are found large amounts of p-coumaric and cinnamic acids, typical of poplars of section Leuce.[43]
However, in South America, concretely the Uruguayan propolis components were similar to those from Europe and China, suggesting that there is a similar plant origin in the Uruguayan propolis also.[44]
Propolis from Russia
Significant amounts of phenolic glycerides such as dicoumaroyl acetyl glycerol, diferuloyl acetyl glycerol, feruloyl coumaroyl acetyl glycerol, and caffeoyl coumaroyl acetyl glycerol have been isolated from the propolis obtained in Northern Russia.[42] These compounds are attributed to Populus tremula, in which the exudates are rich in such components. Birch propolis (Betula verrucosa Ehrh) predominates in Russia and its main biologically active substances are flavones and flavonols, other than those reported for European propolis.[20,42,45]
Propolis from Brazil
The Brazilian propolis represents 10 – 15% of the worldwide production, Brazil being the third world producer, behind Russia and China.[46] Among the types produced in Brazil, green propolis (from greenish-yellow to deep green) prevails, gaining preference in the world propolis market.[47] Prenylated derivatives of p-coumaric, artepillin C (4-hydroxy-3,5-diprenyl cinnamic acid), dupranin (4-hydroxy-3-prenyl cinnamic acid), (E)-3-prenyl-4-(dihydroxicinnamoyloxy)-cinnamic acid, and diterpenic acids were detected in green propolis as well as in the buds of Baccharis dracunculifolia, an Asteraceae family from southeast and western-central Brazil.[11,18,47,48]
Nevertheless, other types of Brazilian propolis have been reported. For example, a new type of red propolis, in which isoflavonoids[49,50] instead of prenylated p-coumaric acids predominated has been reported.[51–53] The red propolis is mainly collected in north east Brazil where Baccharis spp are practically absent, in this case, Dalbergia ecastophyllum (L.) Taub. (Leguminosae) is the main source of isoflavonoids.[49,50] Flavonoids, flavonol, isoflavones, isoflavanones, isoflavans, chalcones, auronol, pterocarpans, 2-arylbenzofuran, neoflavonoids, and lignans in the red propolis from Brazil have also been referred by Awale et al. to possess cytotoxic activities [Table 3 and Figure 17].[54]
Figure 17.
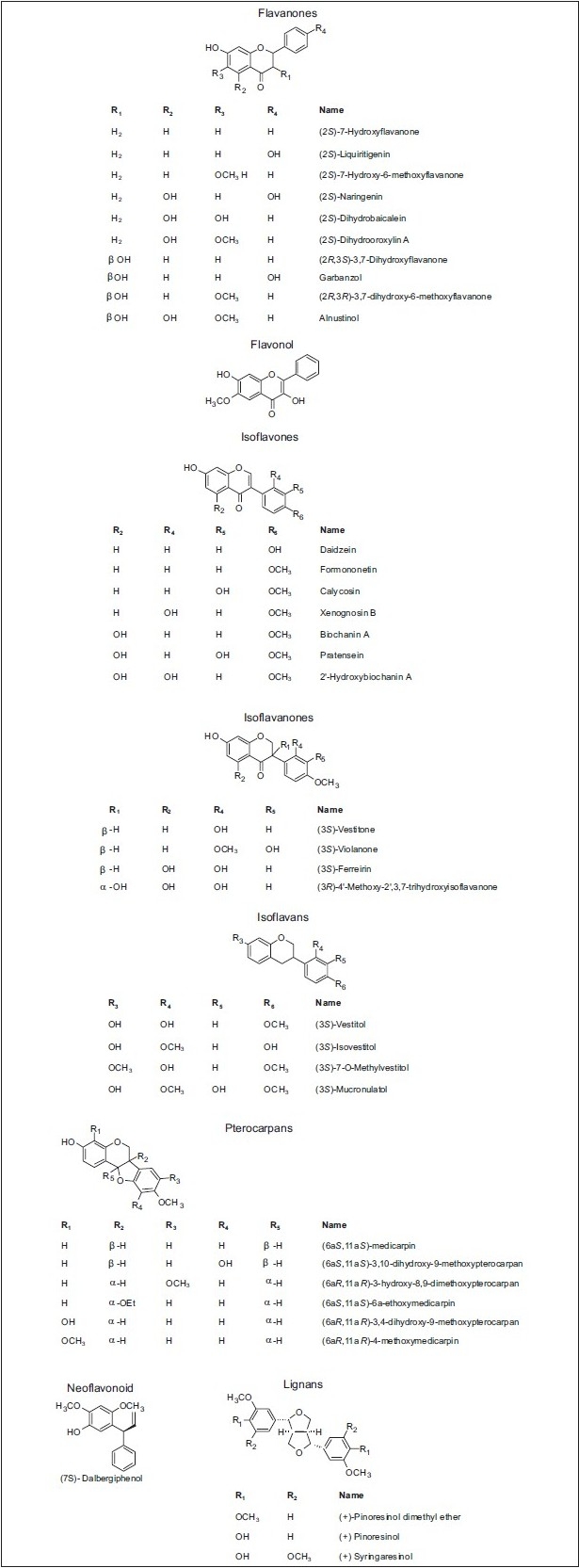
Some compounds present in red propolis from Brazil
Red propolis from Cuba and Venezuela
Red propolis from Cuba was reported to be particularly rich in polyprenylated benzophenones (propolone A, nemorosone, guttiferone, E, xanthochymol, propolones B-D, garcinielliptone I, and hyperibone), due to the presence of Clusia spp.[55–58] Nevertheless, the chemical composition of other propolis samples from the same country did not present prenylated benzophenones. In the samples from Pinar del Rio province, isoflavonoids predominated.[59] Of late, some authors classified the Cuban propolis into three types: (1) Brown Cuban propolis mainly comprised of polyisoprenylated benzophenone derivatives; (2) Red Cuban propolis, characterized by the presence of isoflavonoids; and (3) Yellow Cuban propolis, in which 1H- and 13C-NMR spectral data suggested the presence of aliphatic compounds (terpenoids and sterols) as the main constituents, but these compounds were not identified.[60] Only recently, and by GC-MS, the yellow Cuban propolis, collected from different regions of Cuba, was classified into two main types: type A, rich in triterpenic alcohols [Table 8 and Figure 18] with polymethoxylated flavonoids [Table 8 and Figure 19] as minor compounds, and type B, containing acetyl triterpenes as the main constituents.[61] According to these authors the botanical sources of the two types of yellow Cuban propolis were not yet identified.
Table 8.
Some components present in yellow propolis from Cuba

Figure 18.
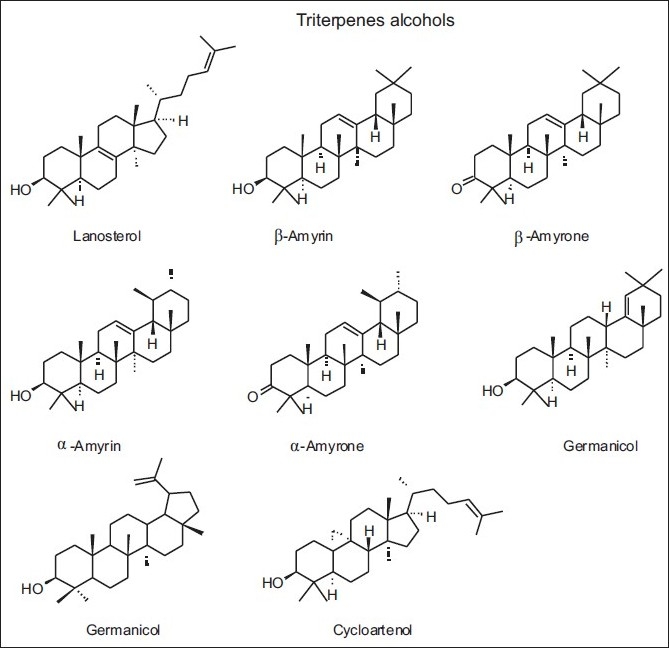
Some triterpenic alcohols and derivatives found in yellow propolis from Cuba
Figure 19.
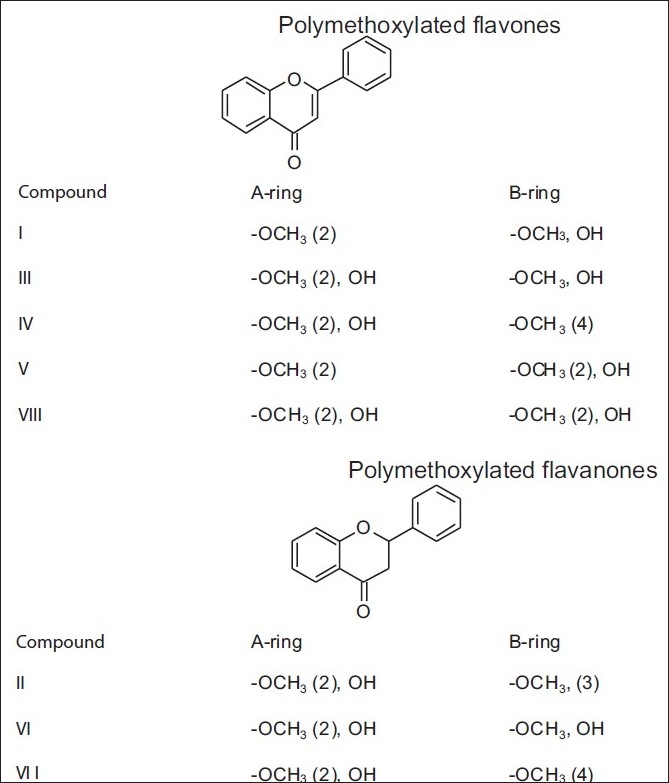
Some polymethoxylated flavonoids found in yellow propolis from Cuba
Some authors suggested that Clusia minor and C. major (Guttiferae) were the source of the main phenolics present in the propolis from Venezuela. Their resins exuded polyprenylated benzophenones as found in propolis. Only few samples presented flavonoids and in each case they were found to be lipophilic methylated 6-oxygenated flavones (eupatorin, hispidulin, among others).[62] Later on, Trusheva et al.[63] found two new polyisoprenylated benzophenones: 18-ethyloxy-17-hydroxy-17,18-dihydroscrobiculatone A and 18-ethyloxy-17-hydroxy-17,18-dihydroscrobiculatone B, together with the known scrobiculatones A and B, in the propolis samples from Venezuela [Table 9 and Figures 4 and 20]. These polyisoprenylated benzophenones were only found in the floral resin of C. scrobiculata. According to the authors, this plant material was used by honeybees as a source for propolis, in the tropical rain forest of the Truhillo state in Venezuela.
Table 9.
Some components present in red propolis from Cuba and Venezuela

Figure 20.
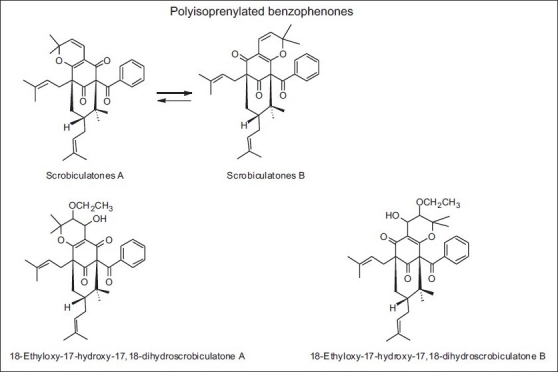
Polyisoprenylated benzophenones found in propolis from Venezuela
In the red-type Mexican propolis, samples of flavanones, isoflavans, and pterocarpans were found to be in agreement with the chemical profiles of the Cuban and Brazilian red propolis, but three new isoflavonoids were also found along with the presence of compounds with a 1,3-diaryl-propane and 1,3-diaryl propene carbon skeleton, which was related to the presence of Dalbergia genus [Table 10 and Figure 21].[64]
Table 10.
Some components present in red propolis from Mexico
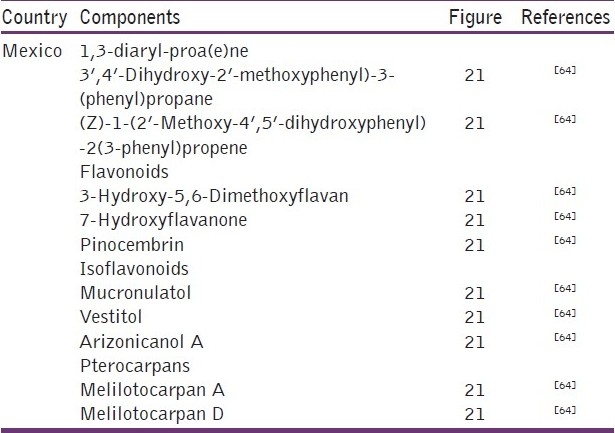
Figure 21.
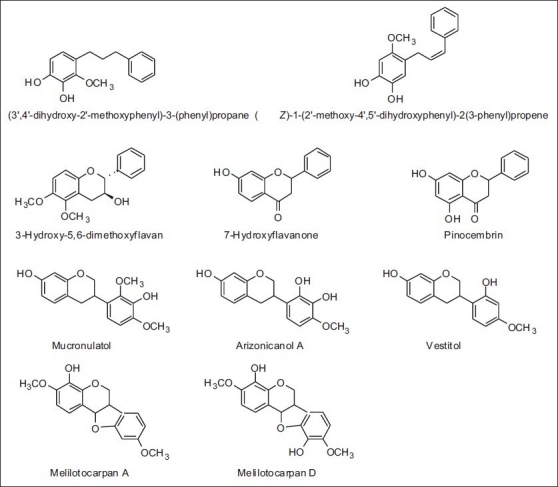
Some components present in red-type Mexican propolis
‘Pacific’ propolis from Okinawa and Taiwan
In the ‘Pacific’ propolis from Okinawa and Taiwan, the structures of two prenylflavanones were elucidated by the NMR spectral technique in the Taiwanese propolis. They were reported for the first time and called propolins.[65] Further studies allowed the finding of other similar structures within the propolin group in the Taiwanese propolis, whose concentrations were closely dependent on the locations and collection seasons [Table 11 and Figure 5].[66,67] These propolins (prenylated flavonoids) had Macaranga tanarius as the plant source.[68]
Table 11.
Some components present in propolis from Japan (Okinawa) and Taiwan

Fujimoto et al.,[69] analyzing several propolis samples from all over the world, considered that samples from Japan were similar to the European and Chinese ones (European-type). However, in some places in Japan, some variability was reported.[70,71] These authors considered such diversity was due to the great diversity of climate and vegetation in the islands of Japan, which extended from north (Hokkaido) to south (Okinawa). This place was located in the subtropical zone and the vegetation was quite different from the other areas.[70] Studies conducted by some authors[72] revealed that not all propolis from the Okayama Prefecture (central Japan) considered as ‘poplar type’ were effectively of this type. Propolis from Takebe-cho had constituents not present in the propolis of other places of the same Prefecture. Such results were attributed to a plant grown mainly in this region (Rhus javanica var. chinensis).
In the Southern Hemisphere, the chemical composition of propolis from different regions in Java were also evaluated.[73] The authors found some variability of constituents in the samples of propolis from three different places, in spite of that, all of them contained phenolic acids. The samples from Batany showed the presence of aromatic acids groups, terpenes, and 3,4-dimethylthioquinoline and 3-quinolinecarboxamine, two new compounds. In the Lawang sample, high amounts of aromatic acids were found and three new compounds were also reported (4-oxo-2-thioxo-3-thiazolidinepropionic acid, glucofuranuronic acid, and patchoulene). In the Sukabumi propolis sample, only very low amounts of aromatic acids were found and silanol was reported for the first time. The presence of this compound in the propolis samples was explained by the presence of rubber plants in that area and the extract latex product from the Hevea rubber plants was polydimathylsiloxane elastomer.[73]
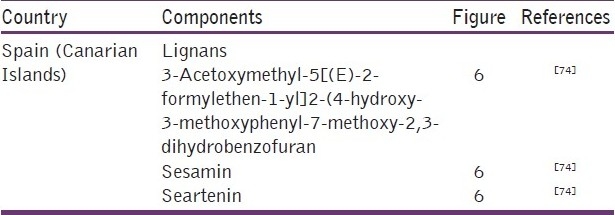
‘Canarian’ Propolis from Canary Islands
Only one study was found about the chemical composition of the propolis from Canary Islands. The authors who studied the samples of this region detected that they were significantly different from the remaining ones studied so far in the world. Lignans [Table 12 and Figure 6], mainly of the furofuran type, and carbohydrates (pentoses, hexoses, and dissacharide) were found to be the main components.[74] According to the authors, two plant species could be responsible for that composition: one of them would produce resinous exudates rich in lignans of the furofuran type and a second plant source from which most sugars were used by honeybees for creating propolis.
Table 12.
Some components present in propolis from Canarian Islands
Volatile Compounds
Very few studies on the volatile fraction of worldwide propolis were found. This was not surprising, as essential oils constituted generally 10% of the samples against 50% of the resinous substances.
Volatile compounds are in fact found in low concentrations in propolis, but their aroma and biological activity make them significant for the characterization of propolis.[4] Mono- and sesquiterpenoids constitute the most important group of compounds found in propolis samples from diverse regions of Europe. Prenylated acetophenones can also be found in some samples from Brazil.[4] Similar to reports on the extracts, the volatile chemical composition of propolis is also dependent on the flora. Some authors have reported that the volatile composition of propolis is also dependent on the bee species, because Apis mellifera and Melipona beechei in the same region of Yucatán (Mexico) have produced propolis with different volatile compositions.[75,76] The same has occurred in Turkey where phenols and terpenes present in the propolis samples depend on the race of honeybees.[77]
There are variations in the chemical composition of the volatile fraction of propolis from the temperate and tropical zones. Although propolis from the temperate climate zones is generally classified into two types: eudesmol and benzyl benzoate, in the tropical zones there is great chemical variability.[76] However, propolis collected at five different locations in Greece revealed a predominance of a-pinene, except in samples from one location, in which junipene existed. The presence of junipene along with trans-b-terpineol, manool or manoyl oxide in these samples was reported for the first time as a propolis constituent.[78] However, in Europe, -pinene does not predominate in the volatile fraction of propolis. For example, the major volatile components of Dalmatian (Croatia) propolis were benzyl alcohol, benzoic acid, and benzyl benzoate (49%), along with terpenes (30%). In the Canary Islands, the main components appeared to be terpenoids, mainly sesquiterpene hydrocarbons and alcohols. Spathulenol was the major sesquiterpene in the Canary Island samples, and benzoyl benzoate was also found.[74]
With regard to terpenoids obtained by extraction with 70% ethanol, it was found that propolis from Anatolia (Turkey) had a-cedrol, b-eudesmol, a-eudesmol, -bisabolol, b-caryophyllene, d-cadinene, caryophyllene oxide, b-selinene, a-cadinol, aromadendrene 2, and germacrene A. These compounds were not present in all samples, but depended on the region from where they were collected.[79]
The volatile components of 23 propolis samples from 17 provinces of China were analyzed by dynamic headspace sampling, with gas chromatography and mass spectrometry.[80] The major components were a-cedrene, g-eudesmol, 3-methyl-3-buten-l-ol, g-terpineol, acetic acid, benzyldehyde, benzyl alcohol, cedrol, butanoic acid, ethyl ester, g-cadinene, phenylethyl alcohol, and styrene. Acetic acid was already found in higher content in other Chinese propolis.[81] Luo et al.[80] suggested that the volatile chemical composition detected in the propolis samples could be related to different climates and plant origins.
Several studies concerning the essential oils isolated from Brazilian propolis were found, and they demonstrated the diversity of the chemical composition. The essential oil isolated from the green propolis of Minas Gerais was for the first time studied and the authors reported that (E)-nerolidol, b-caryophyllene, and selina-3,7(11-diene) constituted the most important components of the essential oil of propolis.[82] Green propolis collected in southeastern Brazil, was mainly composed of nerolidol, a-pinene, 1-phenyl-ethanone, linalool, trans-caryophyllene, d-cadinene, Spathulenol, and globulol.[83] Generally all the compounds were also present in the essential oil of Baccharis dracunculifolia.
The red propolis from Maceio city, Alagoas State, had trans-anethole, methyl eugenol, trans-methylisoeugenol, elemicin, and trans-isoelemicin. These components were partially responsible for the unusual anis-like odor of this red propolis.[84] Red propolis from Goiana, Pernambuco State, had major volatile components like trans-anethole, a-copaene, and methyl cis-isoeugenol. The authors observed also that some compounds could be present or absent according to the collection season, although trans-anethole was always the major one.[85] Also, in October they found, d-cadinol, b-gurjunene, isocaryophyllene, and d-cadinene, which were absent in the remaining collection seasons, whereas, in June, 1,8-cineole and a-selinene were the main components found, in contrast to the remaining collection seasons.
Nerolidol, spathuelenol, aliphatic hydrocarbons, and alkylbenzenes and alkylnaphtalenes constituted the major components or the group of components present in samples of propolis from Piaui State and Parana State (Brazil).[86] Kusumoto et al.,[87] found in the Brazilian essential oils of propolis from the virgin forests of Alecrim, in the Southern part of Minas Gerais State, the following components: 2,2-dimethyl-8-prenyl-6-vinylchromene and 2,6-diprenyl-4-vinylphenol (two new compounds), along with 2,2-dimethyl-6-vinylchromene, acetophenone, 2-prenyl-4-vinylphenol, 3,4-dimethoxy-styrene, 3,4-dimethoxy-allylbenzene, 4-hydroxy-3,5-diprenylbenzaldehyde, and (-)-spathulenol.
These findings show the variability of the chemical composition of the volatile fraction of propolis. Another example is that in which propolis from five different places of Brazil is shown to be qualitatively diverse: 1,8-cineole, exo-fenchol, terpin-4-ol, and fenchone (Teresina); α-pinene, caryophyllene oxide, α-pinene, and α-copaene (Pio IX); (E)-caryophyllene, α-copaene, α-pinene, caryophyllene oxide, and α-cadinene (Campo Maior); (E)-caryophyllene, α-gurjunene, and α-selinene (Pedro II); and (E)-caryophyllene, α-gurjunene, α-cadinene, and α-copaene (Lagoa de Sao Francisco). All these places belong to the Piaui State.[76] More examples can be found, the most abundant components of propolis from Rio de Janeiro collected in July were: b-caryophyllene, acetophenone, linalool, g-elemene, g-cadinene, and g-muurolene.[88]
Biological Properties of Propolis
Despite the great chemical variability of propolis, there are hundreds of studies dealing with the biological activity of propolis, sometimes from different origins, but with similar properties.[89,134]
The distinct chemistry of propolis from diverse origins sometimes does not mean dissimilar properties.[20] Propolis, being a defense system of bees against infections, they have to find components that possess such properties. Bees can find them in the flora that surrounds their beehives. In Europe, such compounds include flavanones, flavones, phenolic acids, and their esters from poplars, whereas in Brazil the anti-infectious compounds are prenylated p-coumaric acids and diterpenes from B. dracunculifolia or prenylflavanones from ‘Pacific’ propolis.[20,115]
Some authors[135] compared the antibacterial activity of three groups of propolis: European, Brazilian, and Central American. Propolis from Brazil and Europe possessed similar activities despite the dissimilarity in the composition, and the propolis from Central America had lower antibacterial activity. In the same study, no significant correlation was found between geographic origin and potential cytotoxicity.
However, it is also true that different components can give diverse antimicrobial properties. Seidel et al.[101] comparing the antibacterial activity of propolis from different geographical origins, including countries from tropical, subtropical, and temperate zones, found that propolis extracts were mostly active against Gram positive bacteria. They also observed that propolis from the wet-tropical, rainforest-type climate had the highest antibacterial activity.[101] The methodology used was the same for all analyses (broth microdilution assay). Other studies demonstrated that propolis from Brazil, Peru, The Netherlands, and China, generally possessed hepatoprotective activity and scavenging activity against DPPH free radicals, whereas, those from China and The Netherlands had the strongest cytotoxic activity.[136]
In vitro antioxidant activity (b-carotene bleaching test and DPPH free radical scavenging assays) of the ethanol extracts of propolis from diverse regions (Argentina, Austria, Brazil, Bulgaria, Chile, China, Hungary, New Zealand, South Africa, Thailand, Ukraine, Uruguay, the USA, and Uzbekistan) was evaluated by Kumazawa et al.[137] and compared with the chemical composition. This was performed by HPLC analysis with photo-diode array (PDA) and mass spectrometry (MS). The most active propolis was that obtained from Argentina, Australia, China, Hungary, and New Zealand, which also possessed the highest total polyphenol and flavonoid contents. The activity correlated well with the levels of kaempferol and phenethyl caffeate present in these samples of propolis. Practically the same compounds were present in the propolis obtained from various places in China (caffeic acid, ferulic acid, and caffeic acid phenethyl ester), presenting in all of them a strong antioxidant activity.[35] Similar results were observed in the propolis from different locations of Korea, which means, samples with high amounts of caffeic acid, kaempferol, and phenethyl caffeate possessed the best antioxidant activity, in contrast to a sample from a more distant place, in which the levels of those components were lower.[37] Some authors,[70] reported a relationship between high phenolic content and antioxidant activity in the propolis from different zones of Japan. The samples with the best activities were those from Akita (Minamiakita) and Okinawa. However, the constituents of the phenolic fraction were different. In fact, in the samples from Akita, caffeic acid and phenethyl caffeate were predominant. In Okinava, the antioxidant activity was due to the prenylated flavonoids. Kumazawa et al.[138] showed that the position of the geranyl or prenyl groups on the flavonoid skeleton played an important role on the antioxidant activity. Also Chen et al.[66] reported that some propolins had better activity than the others.
Both propolis from Europe and Brazil, with diverse chemical compositions, possessed anti-inflammatory activities. In both cases the mechanism was due to the inhibition of NO production.[108,139] The open-chained neoflavonoids from Nepal, also had the capacity for inhibiting NO production, mainly those having a ketone carbonyl at C-1 in ring A, instead of those having a methoxyl substituent.[39]
The caffeic acid phenethyl ester and its related polyphenolic acid esters elicited an important effect on erythrocyte membrane lipid peroxidation, cellular strand breakage, and protein fragmentation. According to these results, the authors considered that caffeic acid phenethyl ester could be used as a template for designing novel drugs to combat diseases induced by oxidative stress components, such as, diverse types of cancer.[140] Propolis from Brazil, China, Peru, and The Netherlands also possessed strong cytotoxicity toward murine colon 26-L5 carcinoma and human HT-1080 fibrosarcoma cells, although those from The Netherlands and China possessed the strongest cytotoxicity toward murine colon 26-L5 carcinoma cells, which may because these samples had the highest presence of phenolic compounds.[136]
Despite the chemical difference among the three types of propolis studied by Messerli et al.,[132] the caffeic acid phenethyl ester-based propolis in Europe, Far East, and New Zealand, the artepillin C-based Brazilian green propolis, and the Brazilian red propolis, they found that all of them possessed anticancer activity. They blocked selective oncogenic PAK1 signaling and suppressed the growth of neurofibromatosis tumors in mice.
Conclusions
Propolis is a heterogeneous product constituted by several groups of compounds. Moreover, the chemical composition depends strongly on the phytogeographic characteristics of the collection site, as honey bees can only use the plant species existing in their habitats. Their chemical variability can give rise to diverse types of biological activities or diverse structures may present similar properties. Therefore, to make a standardization and quality control of this product is very difficult, particularly if we take into account the quantification of the active substances. Popova et al.[67] have proposed to specify multiple standards for different propolis types according to their plant source and corresponding chemical profile. Popova et al.[141] has already made a standardization for the poplar-type propolis from Europe, Asia, and Americas. More recently, Popova et al.[67] have validated a spectrophotometric method for the quantification of prenylated flavanones in the ‘Pacific’ propolis from Taiwan. In addition, it is necessary to connect a particular chemical propolis type to a specific type of biological activity for formulating recommendations for the practitioners. Only by following this scheme will it be possible for people to choose and make more efficient use of the beneficial properties of propolis, in respect to complementary and alternative medicine.[142]
In spite of propolis being commonly used in cosmetic and medicinal preparations owing to its antiseptic, anti-inflammatory, and anesthesic properties, it is not completely innocuous because 1.2 to 6.6 patients who were patch-tested for dermatitis were sensitive to propolis. The main allergens were 3-methyl-2-butenyl caffeate and phenylethyl caffeate, that is, components present in the poplar-type propolis.[143] Clinical allergy in humans is presented as contact dermatitis or oral mucositis, beekeepers being the most affected. Nevertheless there has been a recent rise in this incidence among biocosmetic users, on account of the increasing popularity of natural products such as propolis.[144] According to these authors, patients with an allergy to propolis may be at risk of cross-sensitization with balsam of Peru, a common allergen found in flavoring agents, perfumed products, certain spices, and products that contain the peel of citrus fruit.
Therefore, propolis is a complex natural product with a great diversity of chemical structures and subsequent biological activities, nevertheless, it is not completely innocuous and care must been taken, mainly when such a product has a great diversity of origins. An absence of quality control may be pernicious to human health.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–63. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 2.Simone-Finstrom M, Spivak M. Propolis and bee health: The natural history and significance of resine use by honey bees. Apidologie. 2010;41:295–311. [Google Scholar]
- 3.Ghisalberti EL. Propolis: A review. Bee World. 1979;60:59–84. [Google Scholar]
- 4.Bankova VS, de Castro SL, Marucci MC. Propolis: Recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 5.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–71. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 6.Sforcin JM. Propolis and immune system: a review. J Ethnopharmacol. 2007;113:1–14. doi: 10.1016/j.jep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Ramos AF, Miranda JL. Propolis: A review of its anti-inflammatory and healing actions. J Venom Anim Incl Trop Dis. 2007;13:697–710. [Google Scholar]
- 8.Viuda-Martos M, Ruiz-Navajas Y, Fernández-López J, Pérez-Álvarez JA. Functional properties of honey, propolis, and royal jelly. J Food Sci. 2008;73:R117–24. doi: 10.1111/j.1750-3841.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- 9.Bankova VS, Popov SS, Markov NL. Isopentenyl cinnamates from poplar buds and propolis. Phytochemistry. 1989;28:871–3. [Google Scholar]
- 10.Marcucci MC. Propolis: Chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 11.Teixeira EW, Message D, Negri G, Salatino A, Stringheta PC. Seasonal variation, chemical composition and antioxidant activity of Brazilian propolis samples. Evid Based Complement Alternat Med. 2010;7:307–15. doi: 10.1093/ecam/nem177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gómez-Caravaca AM, Gómez-Romero M, Arráez-Román D, Segura-Carretero A, Fernández-Gutiérrez A. Advances in the analysis of phenolic compounds in products derived from bees. J Pharm Biomed Anal. 2006;41:1220–34. doi: 10.1016/j.jpba.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Scheman A, Jacob S, Zirwas M, Warshaw E, Nedorost S, Katta R, et al. Contact allergy: Alternatives for the 2007 North American contact dermatitis group (NACDG) Standard Screening Tray. Dis Mon. 2008;54:7–156. doi: 10.1016/j.disamonth.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Ghisalberti EL, Jefferies PR, Lanteri R, Matisons J. Constituents of propolis. Experientia. 1978;34:157–8. [Google Scholar]
- 15.Juliano C, Pala CL, Cossu M. Preparation and characterisation of polymeric films containing propolis. J Drug Deliv Sci Technol. 2007;17:177–80. [Google Scholar]
- 16.Popova M, Chen CN, Chen PY, Huang CY, Bankova V. A validated spectrophotometric method for quantification of prenylated flavanones in pacific propolis from Taiwan. Phytochem Anal. 2010;21:186–91. doi: 10.1002/pca.1176. [DOI] [PubMed] [Google Scholar]
- 17.de Sousa JP, Bueno PC, Gregório LE, da Silva Filho AA, Furtado NA, de Sousa ML, et al. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. J Sep Sci. 2007;30:2656–65. doi: 10.1002/jssc.200700228. [DOI] [PubMed] [Google Scholar]
- 18.Chang R, Piló-Veloso D, Morais SA, Nascimento EA. Analysis of a Brazilian green propolis from Baccharis dracunculifolia by HPLC-APCI-MS and GC-MS. Braz J Pharmacogn. 2008;18:549–56. [Google Scholar]
- 19.Miguel MG, Nunes S, Dandlen SA, Cavaco AM, Antunes MD. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem Toxicol. 2010;48:3418–23. doi: 10.1016/j.fct.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 20.Bankova V. Chemical diversity of propolis and the problem of standardization. J Ethnopharmacol. 2005;100:114–7. doi: 10.1016/j.jep.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Popova MP, Graikou K, Chinou I, Bankova VS. GC-MS profiling of diterpene compounds in Mediterranean propolis from Greece. J Agric Food Chem. 2010;58:3167–76. doi: 10.1021/jf903841k. [DOI] [PubMed] [Google Scholar]
- 22.Trusheva B, Popova M, Bankova V, Tsvetkova I, Naydensky H, Sabatini AG. A new type of European propolis, containing bioactive labdanes. Riv Ital EPPOS. 2003;36:3–7. [Google Scholar]
- 23.Melliou E, Chinou I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Medica. 2004;70:515–9. doi: 10.1055/s-2004-827150. [DOI] [PubMed] [Google Scholar]
- 24.Popova M, Trusheva B, Antonova D, Cutajar S, Mifsud D, Farrujia C, et al. The specific chemical profile of Mediterranean propolis from Malta. Food Chem. 2011;126:1431–5. [Google Scholar]
- 25.Trusheva B, Todorov I, Ninova M, Najdenski H, Daneshmand A, Bankova V. Antibacterial mono- and sesquiterpene esters of benzoic acids from Iranian propolis. [Last accessed on 2011 Feb 15];Chem Cent J. 2010 4:8. doi: 10.1186/1752-153X-4-8. Available from: http://journal.chemistrycentral.com/content/4/1/8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tukmechi A, Ownagh A, Mohebbat A. In vitro antibacterial activities of ethanol extract of Iranian propolis (EEIP) against fish pathogenic bacteria (Aeromonas hydrophila, Yersinia ruckeri and Streptococcus iniae) Braz J Microbiol. 2010;41:1086–92. doi: 10.1590/S1517-838220100004000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116:452–61. [Google Scholar]
- 28.Popova MP, Chinou IB, Marekov IN, Bankova VS. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry. 2009;70:1262–71. doi: 10.1016/j.phytochem.2009.07.025. [DOI] [PubMed] [Google Scholar]
- 29.da Silva MS, Citó MG, Chaves MH, Lopes JA. Triterpenóides tipo cicloartano de própolis de Teresina-PI. Quím Nova. 2005;28:801–4. [Google Scholar]
- 30.Popova M, Silici S, Kaftanoglu O, Bankova V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine. 2005;12:221–8. doi: 10.1016/j.phymed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Falcão SI, Villas-Boas M, Estevinho LM, Barros C, Domingues MR, Cardoso SM. Phenolic characterization of Northeast Portuguese propolis: Usual and unsual compounds. Anal Bioanal Chem. 2010;396:887–97. doi: 10.1007/s00216-009-3232-8. [DOI] [PubMed] [Google Scholar]
- 32.Sha N, Huang HL, Zhang JQ, Chen GT, Tao SJ, Yang M, et al. Simultaneous quantification of eight major bioactive phenolic compounds in Chinese propolis by high-performance liquid chromatography. Nat Prod Commun. 2009;4:813–8. [PubMed] [Google Scholar]
- 33.Sha N, Guan SH, Lu ZQ, Chen GT, Huang HL, Xie FB, et al. Cytotoxic constituents of chinese propolis. J Nat Prod. 2009;72:799–801. doi: 10.1021/np900118z. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y-W, Sun S-Q, Zhao J, Li Yi, Zhou Q. Rapid discrimination of extracts of Chinese propolis and poplar buds by FT-IR and 2D IR correlation spectroscopy. J Mol Struct. 2008;883(4):48–54. [Google Scholar]
- 35.Ahn MR, Kumazawa S, Usui Y, Nakamura J, Matsuka M, Zhu F, et al. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007;101:1383–92. [Google Scholar]
- 36.Li F, Awale S, Tezuka Y, Kadota S. Cytotoxic constituents of propolis from Myanmar and their structure-activity relationship. Biol Pharm Bull. 2009;32:2075–8. doi: 10.1248/bpb.32.2075. [DOI] [PubMed] [Google Scholar]
- 37.Ahn MR, Kumazawa S, Hamasaka T, Bang KS, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Korea. J Agric Food Chem. 2004;52:7286–92. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- 38.Kumazawa S, Suzuki S, Ahn MR, Kamihira M, Udagawa Y, Bang KS, et al. A new chalcone from propolis collected on Jeju Island, Korea. Food Sci Technol Res. 2006;12:67–9. [Google Scholar]
- 39.Awale S, Shrestha SP, Tezuka Y, Ueda J, Matsushige K, Kadota S. Neoflavonoids and related constituents from Nepalese propolis and their nitric oxide production inhibitory activity. J Nat Prod. 2005;68:858–64. doi: 10.1021/np050009k. [DOI] [PubMed] [Google Scholar]
- 40.Shrestha SP, Narukawa Y, Takeda T. Chemical constituents of Nepalese propolis: isolation of new dalbergiones and related compounds. J Nat Med. 2007;61:73–6. doi: 10.1248/cpb.55.926. [DOI] [PubMed] [Google Scholar]
- 41.Shrestha SP, Narukawa Y, Takeda T. Chemical constituents of Nepalese propolis (II) Chem Pharm Bull (Tokyo) 2007;55:926–9. doi: 10.1248/cpb.55.926. [DOI] [PubMed] [Google Scholar]
- 42.Bankova V, Popova M, Bogdanov S, Sabatini AG. Chemical composition of European propolis: expected and unexpected results. Z Naturforsch C. 2002;57:530–3. doi: 10.1515/znc-2002-5-622. [DOI] [PubMed] [Google Scholar]
- 43.Christov R, Trusheva B, Popova M, Bankova V, Bertrand M. Chemical composition of propolis from Canada, its antiradical activity and plant origin. Nat Prod Res. 2006;20:531–6. doi: 10.1080/14786410500056918. [DOI] [PubMed] [Google Scholar]
- 44.Kumazawa S, Hayashi K, Kajiya K, Ishii T, Hamasaka T, Nakayama T. Studies of the constituents of Uruguayan propolis. J Agric Food Chem. 2002;50:4777–82. doi: 10.1021/jf020279y. [DOI] [PubMed] [Google Scholar]
- 45.Vardar-Ünlü G, Silici S, Ünlü M. Composition and in vitro antimicobial activity of Populus buds and poplar-type propolis. World J Microbiol Biotechnol. 2008;24:1011–7. [Google Scholar]
- 46.Paviani LC, Dariva C, Marcucci MC, Cabral FA. Supercritical carbon dioxide selectivity to fractionate phenolic compounds from the dry ethanolic extract of propolis. J Food Process Eng. 2010;33:15–27. [Google Scholar]
- 47.Salatino A, Teixeira EW, Negri G, Message D. Origin and chemical variation of Brazilian propolis. Evid Based Complement Alternat Med. 2005;2:33–8. doi: 10.1093/ecam/neh060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teixeira EW, Negri G, Meira RM, Message D, Salatino A. Evid Based Complement Alternat Med. 2005;2:85–92. doi: 10.1093/ecam/neh055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva BB, Rosalen PL, Cury JA, Ikegaki M, Souza VC, Esteves A, et al. Chemical composition and botanical origin of red propolis, a new type of Brazilian propolis. Evid Based Complement Alternat Med. 2008;5:313–6. doi: 10.1093/ecam/nem059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daugsch A, Moraes CS, Fort P, Park YK. Brazilian red propolis-chemical composition and botanical origin. Evid Based Complement Alternat Med. 2008;5:435–41. doi: 10.1093/ecam/nem057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aga H, Shibuya T, Sugimoto T, Kurimoto M, Nakajima S. Isolation and identification of antimicrobial compounds in Brazilian propolis. Biosci Biotechnol Biochem. 1994;58:945–6. [Google Scholar]
- 52.Marcucci MC, Ferreres F, García-Viguera C, Bankova VS, De Castro SL, Dantas AP, et al. Phenolic compounds from Brazilian propolis with pharmacological activities. J Ethnopharmacol. 2001;74:105–12. doi: 10.1016/s0378-8741(00)00326-3. [DOI] [PubMed] [Google Scholar]
- 53.Park YK, Paredes-Guzman JF, Aguiar CL, Alencar SM, Fujiwara FY. Chemical constituents in Baccharis dracunculifolia as the main botanical origin of southeastern Brazilian propolis. J Agric Food Chem. 2004;52:1100–3. doi: 10.1021/jf021060m. [DOI] [PubMed] [Google Scholar]
- 54.Li F, Awale S, Tezuka Y, Kadota S. Cytotoxic constituents from Brazilian red propolis and their structure-activity relationship. Bioorg Med Chem. 2008;16:5434–40. doi: 10.1016/j.bmc.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 55.Rubio OC, Cuellar Cuellar A, Rojas N, Castro HV, Rastrelli L, Aquino R. A polyisoprenylated benzophenone from Cuban propolis. J Nat Prod. 1999;62:1013–5. doi: 10.1021/np980339n. [DOI] [PubMed] [Google Scholar]
- 56.Porto AL, Machado SM, de Oliveira CM, Bittrich V, Amaral MC, Marsaioli AJ. Polyisoprenylated benzophenones from Clusia floral resins. Phytochemistry. 2000;55:755–68. doi: 10.1016/s0031-9422(00)00292-2. [DOI] [PubMed] [Google Scholar]
- 57.Cuesta-Rubio O, Frontana-Uribe BA, Ramírez-Apan T, Cárdenas J. Polyisoprenylated benzophenones in cuban propolis; biological activity of nemorosone. Z Naturforsch C. 2002;57:372–8. doi: 10.1515/znc-2002-3-429. [DOI] [PubMed] [Google Scholar]
- 58.Hernández IM, Fernandez MC, Cuesta-Rubio O, Piccinelli AL, Rastrelli L. Polyprenylated benzophenone derivatives from Cuban propolis. J Nat Prod. 2005;68:931–4. doi: 10.1021/np0495884. [DOI] [PubMed] [Google Scholar]
- 59.Piccinelli AL, Campo Fernandez M, Cuesta-Rubio O, Márquez Hernández I, De Simone F, Rastrelli L. Isoflavonoids isolated from Cuban propolis. J Agric Food Chem. 2005;53:9010–6. doi: 10.1021/jf0518756. [DOI] [PubMed] [Google Scholar]
- 60.Cuesta-Rubio O, Piccinelli AL, Fernandez MC, Hernández IM, Rosado A, Rastrelli L. Chemical characterization of Cuban propolis by HPLC-PDA, HPLC-MS, and NMR: The brown, red and yellow Cuban varieties of propolis. J Agric Food Chem. 2007;55:7502–9. doi: 10.1021/jf071296w. [DOI] [PubMed] [Google Scholar]
- 61.Márquez Hernández I, Cuesta-Rubio O, Campo Fernández M, Rosado Pérez A, Montes de Oca Porto R, Piccinelli AL, et al. Studies on the constituents of yellow Cuban propolis: GC-MS determination of triterpenoids and flavonoids. J Agric Food Chem. 2010;58:4725–30. doi: 10.1021/jf904527n. [DOI] [PubMed] [Google Scholar]
- 62.Tomás-Berberan FA, García-Viguera C, Vit-Olivier P, Ferreres F, Tomás-Lorente F. Phytochemical evidence for the botanical origin of tropical propolis from Venezuela. Phytochemistry. 1993;34:191–6. [Google Scholar]
- 63.Trusheva B, Popova M, Naydenski H, Tsvetkova I, Gregorio Rodriguez J, Bankova V. New polyisoprenylated benzophenones from venezuelan propolis. Fitoterapia. 2004;75:683–9. doi: 10.1016/j.fitote.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Lotti C, Campo Fernandez M, Piccinelli AL, Cuesta-Rubio O, Márquez Hernández I, Rastrelli L. Chemical composition of red Mexican propolis. J Agric Food Chem. 2010;58:2209–13. doi: 10.1021/jf100070w. [DOI] [PubMed] [Google Scholar]
- 65.Chen CN, Wu CL, Shy HS, Lin JK. Cytotoxic prenylflavanones from Taiwanese propolis. J Nat Prod. 2003;66:503–6. doi: 10.1021/np0203180. [DOI] [PubMed] [Google Scholar]
- 66.Chen YW, Wu SW, Ho KK, Lin SB, Huang CY, Chen CN. Characterization of Taiwanese propolis collected from different locations and seasons. J Sci Food Agric. 2008;88:412–9. [Google Scholar]
- 67.Popova M, Chen CN, Chen PY, Huang CY, Bankova V. A validated spectrophotometric method for quantification of prenylated flavanones in pacific propolis from Taiwan. Phytochem Anal. 2010;21:186–91. doi: 10.1002/pca.1176. [DOI] [PubMed] [Google Scholar]
- 68.Kumazawa S, Nakamura J, Murase M, Miyagawa M, Ahn MR, Fukumoto S. Plant origin of Okinawan propolis: Honeybee behaviour observation and phytochemical analysis. Naturwissenschaften. 2008;95:781–6. doi: 10.1007/s00114-008-0383-y. [DOI] [PubMed] [Google Scholar]
- 69.Fujimoto T, Nakamura J, Matsuka M. Diversity of propolis. Part 1. Propolis from the world. Honeybee Sci. 2001;22:9–16. [Google Scholar]
- 70.Hamasaka T, Kumazawa S, Fujimoto T, Nakayama T. Antioxidant activity and constituents of propolis collected in various areas of Japan. Food Sci Technol Res. 2004;10:86–92. doi: 10.1021/jf048726s. [DOI] [PubMed] [Google Scholar]
- 71.Kumazawa S, Goto H, Hamasaka T, Fukumoto S, Fujimoto T, Nakayama T. A new prenylated flavonoid from propolis collected in Okinawa, Japan. Biosci Biotechnol Biochem. 2004;68:260–2. doi: 10.1271/bbb.68.260. [DOI] [PubMed] [Google Scholar]
- 72.Murase M, Kato M, Sun A, Ono T, Nakamura J, Sato T, et al. Rhus javanica var. chinensis as a new plant origin of propolis from Okayama, Japan. Biosci Biotechnol Biochem. 2008;72:2782–4. doi: 10.1271/bbb.80339. [DOI] [PubMed] [Google Scholar]
- 73.Syamsudin SW, Simanjuntak P, Heffen WL. Chemical composition of propolis from different regions in Java and their cytotoxic activity. Am J Biochem Biotechnol. 2009;5:180–3. [Google Scholar]
- 74.Bankova VS, Christov RS, Tejera AD. Lignans and other constituents of propolis from the Canary Islands. Phytochemistry. 1998;49:1411–5. [Google Scholar]
- 75.Pino JA, Marbot R, Delgado A, Zumárraga C, Sauri E. Volatile constituents of propolis from honey bees and stingless bees from Yucatán. J Essent Oil Res. 2006;18:53–6. [Google Scholar]
- 76.Torres RN, Lopes JA, Neto JM, Cito AM. Constituintes voláteis de própolis Piauiense. Quim Nova. 2008;31:479–85. [Google Scholar]
- 77.Silici S, Kuthuca S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J Ethnopharmacol. 2005;99:69–73. doi: 10.1016/j.jep.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 78.Melliou E, Stratis E, Chinou I. Volatile constituents of propolis from various regions of Greece - antimicrobial activity. Food Chem. 2007;103:375–80. [Google Scholar]
- 79.Sahinler N, Kaftanoglu O. Natural product propolis: chemical composition. Nat Prod Res. 2005;19:183–8. doi: 10.1080/14786410410001704877. [DOI] [PubMed] [Google Scholar]
- 80.Luo LP, Fu YX, Xu YJ, Chen B, Li Y. Peoples Republic of China. 1 and 2. Xian: NW University; 2009. Volatile components of propolis collected from different areas of China. Proceedings of 2009 International Conference of Natural Products and Traditional Medicine; pp. 507–11. [Google Scholar]
- 81.Yang C, Luo L, Zhang H, Yang X, Lv Y, Song H. Common aroma-active components of propolis from 23 regions of China. J Sci Food Agric. 2010;90:1268–82. doi: 10.1002/jsfa.3969. [DOI] [PubMed] [Google Scholar]
- 82.Albuquerque IL, Alves LA, Lemos TL, Dorneles CA, de Morais MO. Constituents of the essential oil of Brazilian green propolis from Brazil. J Essent Oil Res. 2008;20:1–2. [Google Scholar]
- 83.Marcóstica Junior MR, Daugsch A, Moraes CS, Queiroga CL, Pastore GM, Park YK. Comparison of volatile and polyphenolic compounds in Brazilian green propolis and its botanical origin Baccharis dracunculifolia. Ciência e Tecnologia de Alimentos. 2008;28:178–81. [Google Scholar]
- 84.Trusheva B, Popova M, Bankova V, Simova S, Marcucci MC, Miorin PL, et al. Bioactive constituents of Brazilian red propolis. Evid Based Complement Alternat Med. 2006;3:249–54. doi: 10.1093/ecam/nel006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nunes LC, Galindo AB, de Deus AS, Rufino DA, Randau KP, Xavier HS, et al. Variabilidade sazonal dos constituintes da própolis vermelha e bioactividade em Artemia salina. Braz J Pharmacogn. 2009;19:524–9. [Google Scholar]
- 86.Bankova V, Christov R, Popov S, Marcucci MC, Tsvetkova I, Kujumgiev A. Antibacterial activity of essential oils from Brazilian propolis. Fitoterapia. 1999;70:190–93. [Google Scholar]
- 87.Kusumoto T, Miyamoto THiguchi R, Doi S, Sugimoto H, Yamada H. Isolation and structures of two new compounds from the essential oil of Brazilian propolis. Chem Pharm Bull (Tokyo) 2001;49:1207–9. doi: 10.1248/cpb.49.1207. [DOI] [PubMed] [Google Scholar]
- 88.Oliveira AP, França HS, Kuster RM, Teixeira LA, Rocha LM. Chemical composition and antibacterial activity of Brazilian propolis essential oil. J Venom Anim Incl Trop Dis. 2009;16:121–30. [Google Scholar]
- 89.Tosi B, Donini A, Romagnoli C, Bruni A. Antimicrobial activity of some commercial extracts of propolis prepared with different solvents. Phytother Res. 1996;10:335–6. [Google Scholar]
- 90.Kujumgiev A, Tsevtkova I, Serkedjieva Yu, Bankova V, Christov R, Popov S. Antibacterial, antifungal and antiviral activity of propolis of different origin. J Ethnopharmacol. 1999;64:235–40. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 91.Özcan M. Antifungal properties of propolis. Grasas y Aceites. 1999;50:395–8. [Google Scholar]
- 92.Martins RS, Péreira ES, Jr, Lima SM, Senna MI, Mesquita RA, Santos VR. Effect of commercial ethanol propolis extract on the in vitro growth of Candida albicans collected from HIV-seropositive and HIV-seronegative Brazilian patients with oral candidiasis. J Oral Sci. 2002;44:41–8. doi: 10.2334/josnusd.44.41. [DOI] [PubMed] [Google Scholar]
- 93.D’Auria FD, Tecca M, Scazzocchio F, Renzini V, Strippoli V. Effect of propolis on virulence factors of Candida albicans. J Chemometr. 2003;15:454–60. doi: 10.1179/joc.2003.15.5.454. [DOI] [PubMed] [Google Scholar]
- 94.Fernandes FF, Dias AL, Ramos CL, Ikegaki M, de Siqueira AM, Franco MC. The in vitro antifungal activity evaluation of propolis G12 ethanol extract on Cryptococcus neoformans. Rev Inst de Med Trop Sao Paulo. 2007;49:93–5. doi: 10.1590/s0036-46652007000200005. [DOI] [PubMed] [Google Scholar]
- 95.Dota KF, Consolaro ME, Svidzinski TI, Bruschi ML. Antifungal activity of Brazilian propolis microparticles against yeast isolated from vulvovaginal candidiasis. Evid Based Complement Alternat Med. 2011;2011:201953. doi: 10.1093/ecam/neq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Özdemira AE, Çandir EE, Kaplankiran M, Soylu EM, Şahinler N, Gül A. The effects of ethanol-dissolved propolis on the storage of grapefruit cv. Star Rubi. Turk J Agric For. 2010;34:155–62. [Google Scholar]
- 97.Ramanauskiene K, Inkeniene AM, Savickas A, Masteikova R, Brusokas V. Analysis of the antimicrobial activity of propolis and lysozime in semisolid emulsion systems. Acta Pol Pharm. 2009;66:681–8. [PubMed] [Google Scholar]
- 98.Najmadeen HH, Kakamand FA Kh. Antimicrobial activity of propolis collected in different regions of Sulaimani province-Kurdistan region/Iraq. J Duhok Univ. 2009;12:233–9. [Google Scholar]
- 99.Rezende GP, Pimenta FC, Costa LR. Antimicrobial activity of two Brazilian commercial propolis extracts. Braz J Oral Sci. 2006;5:967–70. [Google Scholar]
- 100.Awawdeh L, Al-Beitawi M, Hammad M. Effectiveness of propolis and calcium hydroxide as a short-term intracanal medicament against Enterococcus faecalis: a laboratory study. Aust Endod J. 2009;35:52–8. doi: 10.1111/j.1747-4477.2008.00125.x. [DOI] [PubMed] [Google Scholar]
- 101.Seidel V, Peyfoon E, Watson DG, Fearnley J. Comparative study of the antibacterial activity of propolis from different geographical and climate zones. Phytother Res. 2008;22:1256–63. doi: 10.1002/ptr.2480. [DOI] [PubMed] [Google Scholar]
- 102.Shimizu T, Akane H, Tsutsumi A, Park YK, Watanabe W, Kurokawa M. Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir Chem Chemother. 2008;19:7–13. doi: 10.1177/095632020801900102. [DOI] [PubMed] [Google Scholar]
- 103.Ayres DC, Marcucci MC, Giorgio S. Effects of Brazilian propolis on Leishmania amazonensis. Mem Inst Oswaldo Cruz. 2007;102:215–20. doi: 10.1590/s0074-02762007005000020. [DOI] [PubMed] [Google Scholar]
- 104.Topalkara A, Vural A, Polat Z, Toker MI, Arici MK, Ozan F, et al. In vitro amoebicidal activity of propolis on Acanthamoeba castellanii. J Ocul Pharmacol Ther. 2007;23:40–5. doi: 10.1089/jop.2006.0053. [DOI] [PubMed] [Google Scholar]
- 105.Dantas AP, Salomão K, Barbosa HS, de Castro SL. The effect of Bulgarian propolis against Trypanosoma cruzi and during its interaction with host cells. Mem Inst Oswaldo Cruz. 2006;101:207–11. doi: 10.1590/s0074-02762006000200013. [DOI] [PubMed] [Google Scholar]
- 106.Geckil H, Ates B, Durmaz G, Erdogan S, Yilmaz I. Antioxidant free radical scavenging and metal chelating characteristics of propolis. Am J Biochem Biotechnol. 2005;1:27–31. [Google Scholar]
- 107.Naito Y, Yasumuro M, Kondou K, Ohara N. Antiinflammatory effect of topically applied propolis extract in carrageenan-induced rat hind paw edema. Phytother Res. 2007;21:452–6. doi: 10.1002/ptr.2093. [DOI] [PubMed] [Google Scholar]
- 108.Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, et al. Anti-inflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull. 2006;29:96–9. doi: 10.1248/bpb.29.96. [DOI] [PubMed] [Google Scholar]
- 109.Orsatti CL, Missima F, Pagliarone AC, Sforcín JM. Th1/Th2 cytokines’ expression and production by propolis-treated mice. J Ethnopharmacol. 2010;129:314–8. doi: 10.1016/j.jep.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 110.Ishihara M, Naoi K, Hashita M, Itoh Y, Suzui M. Growth inhibitory activity of ethanol extracts of Chinese and Brazilian propolis in four human colon carcinoma cell lines. Oncol Rep. 2009;22:349–54. [PubMed] [Google Scholar]
- 111.Kunimasa K, Ahn MR, Kobayashi T, Eguchi R, Kumazawa S, Fujimori Y, et al. Brazilian Propolis Suppresses Angiogenesis by inducing Apoptosis in Tube-forming Endothelial Cells through Inactivation of Survival Signal ERK1/2. Evid Based Complement Alternat Med. 2011 doi: 10.1093/ecam/nep024. DOI: 10.1093/ecam/nep024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kang L-J, Lee HB, Bae H-J, Lee S-G. Antidiabetic effect of propolis: reduction of expression of glucose-6-phosphatase through inhibition of Y279 and Y216 autophosphorylation of GSK-3a/b in HEPG2 cells. Phytother Res. 2010;24:1554–15561. doi: 10.1002/ptr.3147. [DOI] [PubMed] [Google Scholar]
- 113.El-Sayed E-S, Abo-Salem OM, Aly HA, Mansour AM. Potential antidiabetic and hypolipidemic-induced diabetic rats. Pak J Pharm Sci. 2009;22:168–174. [PubMed] [Google Scholar]
- 114.Quiroga EN, Sampietro DA, Sobéron JR, Sgariglia MA, Vattuone MA. Propolis from the northwest of Argentina as a source of antifungal principles. J Appl Microbiol. 2006;101:103–10. doi: 10.1111/j.1365-2672.2006.02904.x. [DOI] [PubMed] [Google Scholar]
- 115.Casaroto AR, Lara VS. Phytomedicines for Candida-associated denture stomatitis. Fitoterapia. 2010;81:323–8. doi: 10.1016/j.fitote.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 116.Herrera CL, Alvear M, Barrientos L, Montenegro G, Salazar LA. The antifungal effect of six commercial extracts of Chilean propolis on Candida spp. Cien Inv Agr. 2010;37:75–384. [Google Scholar]
- 117.Raghukumar R, Vali L, Watson D, Fearnley J, Seidel V. Antimethicilin-resistant Staphylococcus aureus (MRSA) activity of “Pacific propolis” and isolated prenylflavanones. Phytother Res. 2010;24:1181–7. doi: 10.1002/ptr.3096. [DOI] [PubMed] [Google Scholar]
- 118.Yaghoubi SM, Ghorbani GR, Soleimanian ZS, Satari R. Antimicrobial activity of Iranian propolis and its chemical composition. DARU. 2007;15:45–8. [Google Scholar]
- 119.Campana R, Patrone V, Franzini IT, Diamantini G, Vittoria E, Baffone W. Antimicrobial activity of two propolis samples against human Campylobacter jejuni. J Med Food. 2009;12:1050–6. doi: 10.1089/jmf.2008.0173. [DOI] [PubMed] [Google Scholar]
- 120.Castro ML, Vilela WR, Zauli RC, Ikegaki M, Rehder VL, Foglio MA, et al. Bioassay guided purification of the antimicrobial fraction of a Brazilian propolis from Bahia state. BMC Complement Altern Med. 2009;9:25. doi: 10.1186/1472-6882-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katircioğlu H, Mercan N. Antimicrobial activity and chemical composition of Turkish propolis from different regions. Afr J Biotechnol. 2006;5:1151–3. [Google Scholar]
- 122.Díaz-Carballo D, Ueberla K, Kleff V, Ergun S, Malak S, Freistuehler M, et al. Antiretroviral activity of two polyisoprenylated acylphloroglucinols, 7-epi-nemorosone and plukenetione A, isolated from Caribbean propolis. Int J Clin Pharmacol Ther. 2010;48:670–7. doi: 10.5414/cpp48670. [DOI] [PubMed] [Google Scholar]
- 123.Schnitzler P, Neuner A, Nolkemper S, Zundel C, Nowack H, Sensch KH, et al. Antiviral activity and mode of action of propolis extracts and selected compounds. Phytother Res. 2010;24(Suppl 1):S20–8. doi: 10.1002/ptr.2868. [DOI] [PubMed] [Google Scholar]
- 124.Freitas SF, Shinohara L, Sforcin JM, Guimarães S. In vitro effects of propolis on Giardia duodenalis trophozoites. Phytomedicine. 2006;13:170–5. doi: 10.1016/j.phymed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 125.Russo A, Longo R, Vanella A. Antioxidant activity of propolis: role of caffeic acid phenethyl ester and galangin. Fitoterapia. 2002;73(Suppl 1):S21–9. doi: 10.1016/s0367-326x(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 126.Gómez-Romero M, Arráez-Román D, Moreno-Torres R, García-Salas P, Segura-Carretero A, Fernández-Gutiérrez A. Antioxidant compounds of propolis determined by capillary electrophoresis-mass spectrometry. J Sep Sci. 2007;30:595–603. doi: 10.1002/jssc.200600354. [DOI] [PubMed] [Google Scholar]
- 127.Mohammadzadeh S, Sharriatpanahi M, Hamedi M, Amanzadeh Y, Ebrahimi SE, Ostad SN. Antioxidant power of Iranian propolis extract. Food Chem. 2007;103:729–33. [Google Scholar]
- 128.Gregoris E, Stevanato R. Correlations between polyphenolic composition and antioxidant activity of Venetian propolis. Food Chem Toxicol. 2010;48:76–82. doi: 10.1016/j.fct.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 129.Kumazawa S, Ahn MR, Fujimoto T, Kato M. Radical-scavenging activity and phenolic constituents of propolis from different regions of Argentina. Nat Prod Res. 2010;24:804–12. doi: 10.1080/14786410802615270. [DOI] [PubMed] [Google Scholar]
- 130.Sosa S, Bornancin A, Tubaro A, Loggia RD. Topical anti-inflammatory activity of an innovative aqueous formulation of actichelatedâ propolis vs. two commercial propolis formulations. Phytother Res. 2007;21:823–6. doi: 10.1002/ptr.2206. [DOI] [PubMed] [Google Scholar]
- 131.Li F, Awale S, Tezuka Y, Kadota S. Cytotoxicity of constituents from Mexican propolis against a panel of six different cancer cell lines. Nat Prod Commun. 2010;5:1601–6. [PubMed] [Google Scholar]
- 132.Messerli SM, Ahn MR, Kunimasa K, Yanagihara M, Tatefuji T, Hashimoto K, et al. Artepillin (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother Res. 2009;23:423–7. doi: 10.1002/ptr.2658. [DOI] [PubMed] [Google Scholar]
- 133.Szliszka E, Czuba ZP, Domino M, Mazur B, Zydowicz G, Krol W. Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules. 2009;14:738–54. doi: 10.3390/molecules14020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Demestre M, Messerli SM, Celli N, Shahhossini M, Kluwe L, Mautner V, et al. CAPE (caffeic acid phenetyl ester)-based propolis extract (Bio 30) suppresses the growth of human neurofibromatosis (NF) tumor xenografts in mice. Phytother Res. 2009;23:226–30. doi: 10.1002/ptr.2594. [DOI] [PubMed] [Google Scholar]
- 135.Popova M, Bankova V, Naydensky C, Tsvetkova I, Kujumgiev A. Comparative study of the biological activity of propolis from different geographic origin: A statistical approach. Maced Pharm Bull. 2004;50:9–14. [Google Scholar]
- 136.Banskota AH, Tezuka Y, Adnyana IK, Midorikawa K, Matsushige K, Message D, et al. Cytotoxic, hepatoprotective and free radical scavenging effects of propolis from Brazil, Peru, the Netherlands and China. J Ethnopharmacol. 2000;72:239–46. doi: 10.1016/s0378-8741(00)00252-x. [DOI] [PubMed] [Google Scholar]
- 137.Kumazawa S, Hamasaka T, Nakayama T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004;84:329–39. [Google Scholar]
- 138.Kumazawa S, Ueda R, Hamasaka T, Fukumoto S, Fujimoto T, Nakayama T. Antioxidant prenylated flavonoids from propolis collected in Okinawa, Japan. J Agric Food Chem. 2007;55:7722–5. doi: 10.1021/jf071187h. [DOI] [PubMed] [Google Scholar]
- 139.Nagaoka T, Banskota AH, Tezuka Y, Midorikawa K, Matsushige K, Kadota S. Caffeic acid phenetyl ester (CAPE) analogues: potent nitric oxide inhibitors from the Netherlands propolis. Biol Pharm Bull. 2003;26:487–91. doi: 10.1248/bpb.26.487. [DOI] [PubMed] [Google Scholar]
- 140.Wang T, Chen L, Wu W, Long Y, Wang R. Potential cytoprotection: antioxidant defense by caffeic acid phenethyl ester against free radical-induced damage of lipids, DNA, and proteins. Can J Physiol Pharmacol. 2008;86:279–87. doi: 10.1139/y08-029. [DOI] [PubMed] [Google Scholar]
- 141.Popova MP, Bankova VS, Bogdanov S, Tsvetkova I, Naydenski C, Marcazzan GL, et al. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie. 2007;38:306–11. [Google Scholar]
- 142.Bankova V. Recent trends and important developments in propolis research. eCAM. 2005;2:29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Walgrave SE, Warshaw EM, Glesne LA. Allergic contact dermatitis from propolis. Dermatitis. 2005;16:209–15. [PubMed] [Google Scholar]
- 144.Lieberman HD, Fogelman JP, Ramsay DL, Cohen DE. Allergic contact dermatitis to propolis in a violin maker. J Am Acad Dermatol. 2002;46(2 Suppl):S30–1. doi: 10.1067/mjd.2002.106349. [DOI] [PubMed] [Google Scholar]


