Abstract
Context:
An audit of antibiotic prescribing patterns is an important indicator of the quality and standard of clinical practice.
Aims:
To study the (1) antibiotic prescription and consumption patterns at admission into the intensive care unit (ICU); (2) average costs of antibiotics prescribed; and (3) correlation of antibiotic usage and the costs incurred with age, severity of illness, and diagnosis.
Settings and Design:
A 13-bedded tertiary level ICU. A prospective, observational audit.
Materials and Methods:
Two hundred consecutive prescriptions on patients admitted to the ICU from August to October, 2008, were audited. The total number of drugs and antibiotics, the class, dose, route, and cost of antibiotics were noted and the Defined Daily Dose/100 bed-days (DDD/100 bed-days) of the 10 most frequently prescribed antibiotics were calculated. Statistical analysis used: Univariate analysis was performed using Epi Info software (version 8.0).
Results:
A total of 1246 drugs and 418 antibiotics were prescribed in the 200 patients studied, that is, an average of 6.23 (± SD 2.73) drugs/prescription and 2.09 (± SD 1.27) antibiotics/prescription. Antibiotics were prescribed on 190 patients (95%) at admission. There was a significant correlation between the number of patients prescribed three or more antibiotics and mortality rates (53% nonsurvivors vs. 33.5% survivors (P = 0.015). The average cost of the antibiotics was Rupees 1995.08 (± SD 2099.99) per patient and antibiotics expenditure accounted for 73.2% of the total drug costs.
Conclusions:
Antibiotics are commonly prescribed to most ICU patients at admission and contribute significantly to the total drug costs. Antibiotic restriction policies and a multidisciplinary effort to reduce usage are urgently required.
KEY WORDS: Admission, antibiotic usage, intensive care unit, prescriptions
Patients admitted into the intensive care units (ICUs) are often prescribed multiple broad spectrum antibiotics at admission as they are more sick, exposed to multiple invasive procedures, and vulnerable to multidrug-resistant pathogens. However, these prescriptions are often empiric and based on physician comfort and prior experience, often leading to overuse or misuse of antibiotics. This not only increases the burden of antibiotic resistance but also exposes patients to the unnecessary side effects of these drugs besides increasing treatment costs.[1–3] Increasing multidrug resistance with limited availability of newer agents to treat emerging multidrug-resistant clones[4] emphasizes the urgent need for vigilant surveillance, stringent infection control practices, as well as rational antibiotic prescription.[1,2] There is limited data from Indian ICUs on antibiotic prescription, consumption patterns, and cost analysis, especially at admission to the ICU.
Hence, we proposed to study (1) the antibiotic prescription patterns and consumption patterns at admission into the ICU; (2) the average costs of antibiotics prescribed at admission in the ICU; and (3) the correlation of antibiotic usage as well as costs incurred with respect to age, severity of illness, and diagnosis.
Materials and Methods
This was a prospective, observational study of antibiotic prescribing patterns at admission into the ICU. Ours is an open, mixed medical–surgical, adult, 13-bedded ICU in a tertiary care hospital in northern India with approximately 1000 admissions per year. This study was conducted between August and October 2008. The prescription data on 200 consecutive patients at admission into the ICU was audited. All antibiotic prescriptions were made by the primary admitting team and there was no infectious diseases specialist or antibiotic prescription policy in place at our hospital during the study period. Baseline demographic variables on all patients, such as name, age, gender, hospital number, clinical diagnosis, and the APACHE II score (Acute Physiology and Chronic Health Evaluation score, a standard tool used in the ICU to assess the severity of illness) were recorded. Other variables, such as duration of ICU admission, the total number of drugs prescribed on the day of admission, and the total number of antibiotics prescribed were also noted. Additional data included the generic name, dose, duration, and route of administration of the antibiotic. All drugs listed on the first prescription slip at admission to the ICU, namely, antibiotics, aerosolized medications, agents used for stress ulcer prophylaxis and deep vein thrombosis, antihypertensives, anticonvulsants, and so on, were noted. Intravenous fluids, drugs given as infusions, such as vasoactive agents, sedatives and insulin, were not included in the analysis. The cost of antibiotics and total drugs prescribed on each patient was calculated and all patients were followed-up till death or discharge from the ICU.
The antibiotics were classified using the anatomical therapeutic chemical (ATC) Classification System and drug utilization was measured as DDD/100 bed-days. In the ATC classification system, the drugs are divided into different groups according to the organ or system on which they act and their chemical, pharmacological and therapeutic properties.[5] The DDD per 100 bed-days was calculated by the formula:
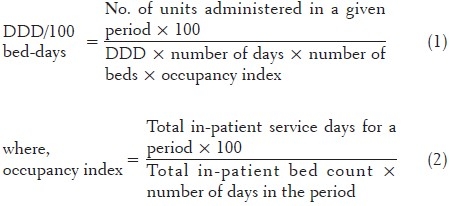
The daily antibiotic cost per patient was calculated by the multiplication of the cost per unit and the number of doses that were used in each patient. The unit price of each antibiotic and drug used were obtained from the hospital pharmacy.
The data were analyzed using Epi Info software (version 8.0). Univariate analysis was performed to compare the patient groups.
Results
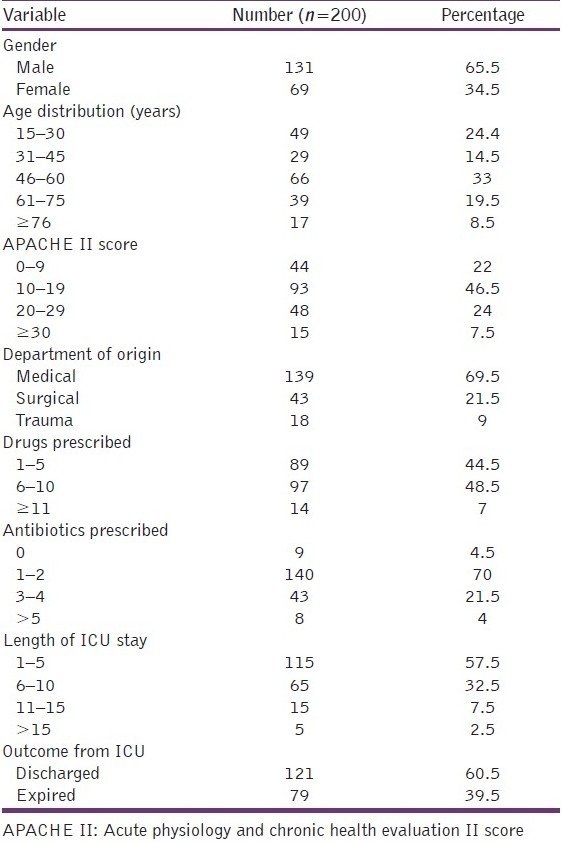
The prescriptions of 200 consecutive patients admitted into the ICU were analyzed. This included 131 male and 69 female patients. The average age of the patients was 49 years (± SD 19.5) and the median APACHE II score was 16. Most of our patients were admitted to the ICU from the medical specialty (69.5%) and the most common diagnosis at admission was sepsis syndrome (61 patients). The average length of stay (LOS) in the ICU was 5.75 days, which was similar in medical 5.67 (±SD 4.07) and surgical patients 5.95 (±SD 3.83). The demographic data and patient characteristics studied are as given in Table 1.
Table 1.
Demographic data and patient characteristics
A total of 1246 drugs were prescribed at admission in 200 patients, that is, an average of 6.23 (±SD 2.73) drugs/prescription. One hundred and ninety patients (95%) were prescribed an antibiotic at the time of admission into the ICU. In all, 418 antibiotics, at an average of 2.09 (±SD 1.27) antibiotics/prescription were ordered, and antibiotics constituted 33.54% of the total drugs prescribed. Figure 1 shows the distribution of patients according to the number of antibiotics prescribed [Figure 1].
Figure 1.
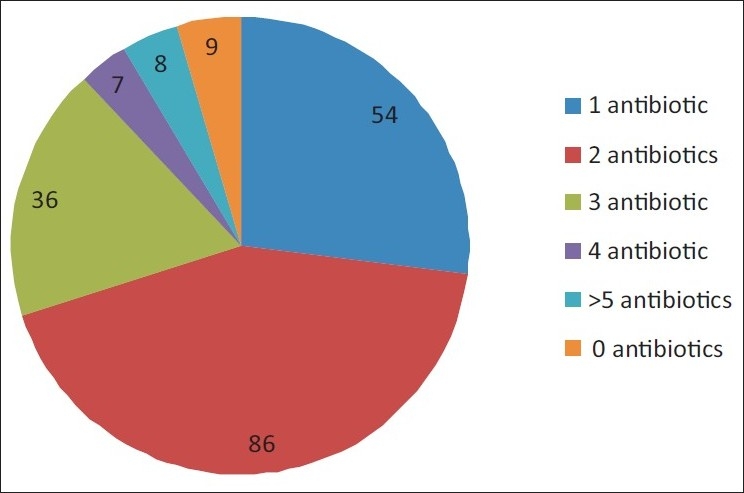
Distribution of patients according to the number of antibiotics prescribed
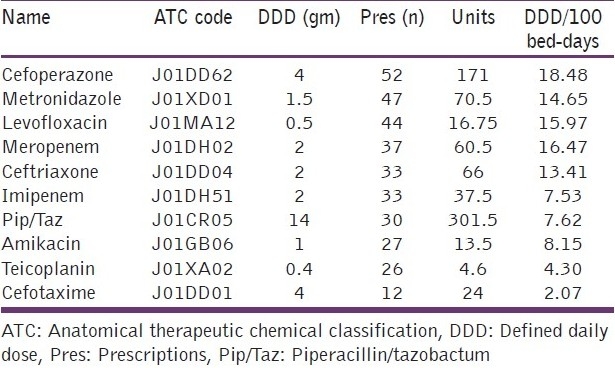
The influence of various clinical factors, such as diagnosis at admission and APACHE II score in determining the number of drugs and antibiotics prescribed was studied. Patients admitted with sepsis syndromes were prescribed the most number of antibiotics [Table 2]. Patients who had higher APACHE II scores (>15) at admission were prescribed significantly more total drugs than their counterparts with lower scores, 6.61 ± SD 2.92 vs 5.73 ± SD 2.40, respectively (P = 0.020). However, the average number of antibiotics prescribed in these patients were similar, 2.13 ± SD 1.18 and 2.00 ± SD 1.37, respectively (P = 0.480). We found that the number of patients who were prescribed 1-2 antibiotics did not vary from those prescribed 3 or more antibiotics with respect to their age, gender, source of admission, and total days of ICU stay. However, the number of antibiotics prescribed significantly correlated with poorer outcomes (P = 0.015). Patients with APACHE II scores, between 15 and 30, and who were prescribed more than 3 antibiotics at admission had a significantly higher risk of mortality (P= 0.0289) [Table 3]. The most commonly prescribed antimicrobial at ICU admission was cefoperazone (26% of all prescriptions) and 171 units of the same were consumed among the patients studied. Table 4 enlists the 10 most commonly prescribed antibiotics along with their DDD per 100 bed-days [Table 4].
Table 2.
Drug prescription data according to patient diagnosis
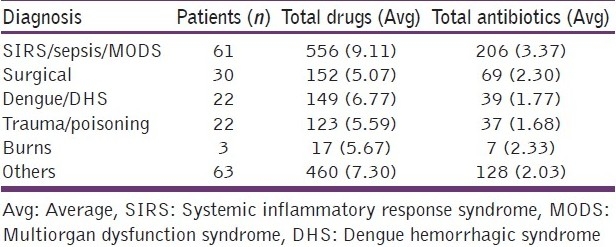
Table 3.
Univariate analysis of the factors affecting the number of antibiotics prescribed in patients
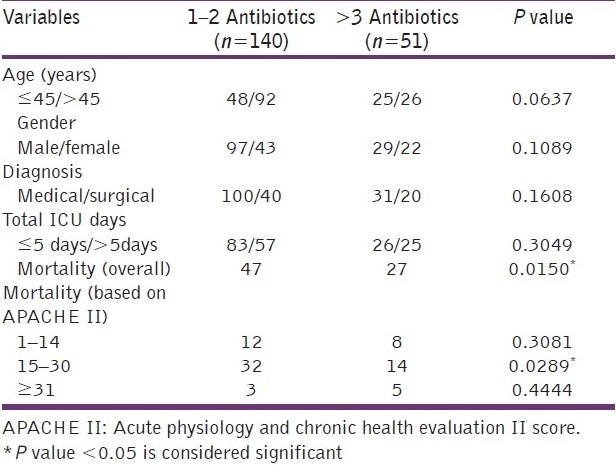
Table 4.
Most frequently used antibiotics (according to the number of prescriptions)
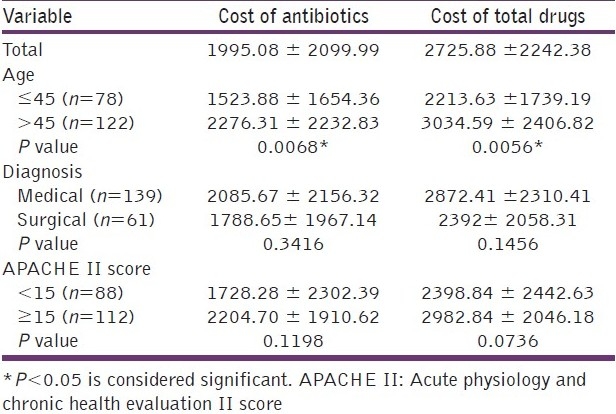
The total cost of antibiotics prescribed at admission varied widely, and ranged from rupees (Rs) 55 to Rs 10,200 while the total drugs prescribed cost between Rs 200 and Rs 11,200. The total cost of antibiotics prescribed in all patients was Rs 3, 99,016, an average of Rs 1995.08/patient (± SD 2099.99). The median cost was Rs 1489 and the 25th to 75th percentile was Rs 500–2016. The total cost of all drugs was Rs 5,45,177, with an average of Rs 2725.88/ patient (± SD 2242.38). The median cost of drugs prescribed was Rs 2100 and 25th to 75th percentile was Rs 1500–2950. Thus the total cost incurred by antibiotics was nearly 73.2% of the total drug costs in these patients. On univariate analysis, we found that the average cost of the total drugs prescribed and antibiotics prescribed were significantly lower in younger patients (<45 years) (P = 0.005 and 0.006, respectively). Although the total cost of antibiotics and drugs was lower in surgical patients and in patients with APACHE scores < 15, this was not a statistically significant difference [Table 5]. Meropenem was the most expensive drug prescribed among our patients (Rs. 1,38,484), accounting for 34.71% of the total cost of antibiotics.
Table 5.
Comparison of the total drug and antibiotic costs with respect to age, diagnosis, and APACHE II scores
Most of the patients included in this study were discharged from the ICU after recovery (121 patients), while 79 patients expired.
Discussion
Prescriptions of 200 consecutive ICU admissions were audited over a 3-month period to study drug utilization patterns in the ICU. Most of the patients were middle aged (46–60 years), and admitted with sepsis syndromes. The median APACHE II score of the patients studied was 16 (range 10–19) and the average ICU length of stay (LOS) was 5.8 days. In a study on drug use patterns from an ICU in Iran,[6] the average age of patients studied was 50 years with an average LOS of 6 days, lower in surgical patients as compared to medical patients. Similarly, in a study by Bergmans et al,[7] the average duration of patient stay in the ICU was 7 days and the most frequent diagnosis at admission was skin and soft tissue infections. Their patients also had similar APACHE II scores as ours.
The average number of drugs per prescription is an important index of a prescription audit. It is recommended that the number of drugs per prescription should be kept as low as possible to minimize the risk of drug interactions, development of bacterial resistance, and hospital costs.[8] In our study, a mean of 6.2 drugs were prescribed per patient, which is comparable to the other data reported in literature, ranging from 5.1 to 12,[9,10] according to the type of patient population and the geographical location studied.
Our study revealed that a large number of patients were prescribed an antibiotic at admission (95%). A survey on antibiotic prescribing patterns in ICUs of Australia and New Zealand revealed that a total of 656 antibiotics were “empirically” prescribed by 84 intensivists during the 4-month study period and the main areas for noncompliance with guidelines were provision for broader cover for resistant infections.[11] Biswal et al[12] reported that nearly 62% patients in a tertiary care ICU in northern India received antibiotics, while Shrikala et al[13] reported that 64% of ICU patients received empiric antibiotics. Data from other countries report 60%–75% rates of antibiotic prescription in the ICU[14,15] and studies from Europe report an average antibiotic use of 58%–61%.[7,16] However, these studies have reported data of overall antibiotic usage and not specifically of antibiotic usage at admission, which is probably why our figures are much higher than the others. In a relatively older study on antibiotic usage patterns, of the 220 patients receiving antibiotics for an infection in a Danish ICU, 87% were treated on day 1, but only 34% on day 11[17] However, an Indian study noted that in 32.03% of the patients, who were on empirical antibiotics initially, the microbiological sensitivity patterns were contrary to the treatment given. Despite this, 42.42% continued to receive the same treatment.[13] In our study, although 95% of patients were prescribed some antibiotic, the number of antibiotics prescribed per patient (2.09 per prescription) was similar to that described in other studies.[15] In our audit, most patients (70%) received 2 or less antibiotics. Hariharan et al reported that 60% of the patients studied in a Caribbean ICU received two antimicrobials.[18] Data from the western ICUs indicates that fewer antimicrobials are prescribed in western ICUs. A study in a Danish university hospital ICU reported that the majority of their patients were on one antibiotic,[16] whereas in a German surgical ICU, 36.7% of cases were treated with only one antibiotic agent, 14.1% were given a combination of 2, and 7.2% were given a combination of ≥3 antibiotic agents.[19]
Antibiotics choices made during the first hour of treatment in severe sepsis have been recognized as an important predictor of clinical outcomes. Capp et al retrospectively reviewed the data on 1400 patients admitted to the ICU and noted that effective antibiotic therapy was prescribed in 82% of the patients.[20] They found that utilizing set guidelines for community and health care-associated infections was 100% sensitive in selecting patients who had infections caused by the more resistant organisms. In our ICU, the most frequently prescribed antibiotics were 3rd generation cephalosporins followed by metronidazole. The DDD/100 bed-days for the 5 most frequently prescribed antibiotics were found to be 18.48 (3rd generation cephalosporins), 14.65 (metronidazole), 15.97 (levofloxacin), 16.47 (meropenem), and 13.42 (ceftriaxone). Biswal et al reported that the most frequently prescribed antibiotic at ICU admission was metronidazole followed by cefotaxime, amoxycillin/clavulinic acid, cefipime, and ciprofloxacin.[12] In another Indian study, the most commonly prescribed antibiotics at admission were cefaperazone/sulbactum or piperacillin/tazobactam.[13] In a study from Nepal, the utilization of penicillins, fluoroquinolones, 2nd-generation cephalosporins and 3rd-generation cephalosporins were 55.1, 5.34, 0.82, and 13.74 DDD/100 bed-days, respectively.[9]
On analysis of the factors contributing to an increased antibiotic prescription at admission in the ICU, it was found that patients who eventually had a poor outcome had been prescribed a significantly higher number of antibiotics. On subdividing patients on the basis of their APACHE II scores, the majority of patients (101 patients) had APACHE II scores between 15 and 30. In this group, the number of antibiotics prescribed correlated significantly with higher mortality. Patients at either extreme of APACHE II scores did not show this trend, probably because they were either too well or too sick for the number of antibiotics to be a factor predicting mortality. Montravers et al studied the decision-making process of antibiotic therapy in 41 French medical and surgical ICUs.[21] They found that 61% of the patients received new antibiotic therapy, which were generally prescribed by senior physicians and during out-of-hours periods. Of the new antibiotics ordered, 22% were judged to be inappropriate and they concluded that the predictive factors of mortality in these patients were absence of set protocols for empiric antibiotic therapy, age > 60 years, high SAPS II scores, and rapidly fatal underlying disease. In our study, the age, gender (males), patients with higher APACHE II scores, and patients from medical wards had higher number of antibiotics prescribed, although this was not a statistically significant difference. The average number of drugs prescribed was significantly more in patients with higher APACHE II scores. The cost analysis of the antibiotics and the total drugs prescribed at admission revealed that patients were prescribed drugs and antibiotics worth nearly Rs 2725 per patient and Rs 1995 per patient, respectively, and antibiotic costs accounted for 71% of the total drug expenditure. Biswal, et al have reported that patients spent about Rs 19,725 on total drug costs and antibiotics contributed to 51.3% of the total drug expenditure.[12] Shankar, et al from Nepal report an average expenditure of Rs 1958.53 ± 1267.8 on the drugs prescribed in ICU.[9] Data from the western literature report ICU drug costs per patient-day ranging from $208 to $312.[3] In a study of costs incurred due to antibiotic usage in bloodstream infections in 310 patients in an ICU in Belgium, the mean overall daily antimicrobial cost was €114.25, with higher costs in patients with nosocomial infections.[22] In a Turkish university hospital, the mean daily antibiotic cost was $89.64, with higher costs for patients with nosocomial infections and meropenem was the most expensive drug for treatment in this group.[23] In our study too, meropenem was the most expensive drug ordered (accounting for 34.7% of the total antibiotic costs). On univariate analysis, age was the most significant factor influencing the costs of total drugs as well as of antibiotics prescribed in our study population. Medical patients and those with higher APACHE II scores spent more on antibiotics as well as on total drugs prescribed at admission, although this was not a statistically significant association.
The strengths of our study were that it was conducted prospectively, and we studied the interrelation of the number and cost of antibiotics prescribed with various parameters, such as severity of illness, age, diagnosis, gender, LOS, and mortality. To date, there are limited studies on drug utilization and expenditure due to antibiotic usage, especially from Indian adult ICUs. These data are relevant as our health care system is largely based on individual paying capacity with limited insurance or government funding to critically ill patients. Hence, data on drug costs, especially antibiotic costs are particularly useful in formulating policies on health care systems and expenditure in India. Such data also serve as a reference for comparison among various Indian ICUs. The limitations of our study were that only the drug prescriptions at admission were audited, and hence this may not reflect drug usage patterns as a whole in the ICU. Also, the subsequent reduction or escalation of antibiotics, which were started empirically could not be studied. The utilization of intravenous fluids, vasoactive drugs, sedatives, insulin, and equipments used, such as tubes, catheters, and so on, was not studied. Thus, our data underestimates the cost of total drugs used at admission in the ICU.
The results of our study highlight several areas that need improvement. Most importantly, there is a need to formulate strict antibiotic restriction policy and implement protocols for antibiotic usage in order to streamline the judicious use of these drugs. Studies have revealed that the implementation of rational antibiotic policies have had a significant positive economic benefit and improved quality of care delivered to patients.[24,25] Thursky et al studied the use of a computerized decision support tool, which reduced their total antibiotic utilization by 10.55% and increased the number of switches to narrow spectrum antibiotics.[25] Implementation of local antibiotic management programs, infectious disease specialist consultation, and restricted authorization to prescribe antibiotics have all been reported to result in marked reductions in antibiotic consumption.[26] Siddiqui et al, reporting from a developing country, found that creation of a restriction program using an infectious disease specialist helped reduce the antibiotic prescriptions of broad spectrum antibiotics by 34%.[27] The use of diagnosis-related groups (DRG)-based health care systems have also been suggested in order to reduce costs of antibiotic therapy. Here, it is possible to identify subsets of infections and the patient records that have a potentially negative DRG-result, that is, the costs are higher than the reimbursement. This can help to find out if a different therapeutic approach, for example, by different choices in initial (empirical) antibiotic treatment may cause other outcomes leading to a better balance of clinical and economical outcomes in patients with severe infections.[28]
Antibiotic policies need to be formulated by national, state level, and individual hospital bodies. Here, it is important to incorporate local data on the hospital microbiological flora along with epidemiologic data to formulate guidelines for empirical antibiotic usage and de-escalation therapies so as to aid clinicians to make appropriate antibiotic choices for their patients. Other considerations to remember while making antibiotic choices are, recognizing colonizations from true infections in patients admitted into the ICU and use of biomarkers, such as C-reactive protein, erythrocyte sedimentation rates, serum procalcitonin, and so on, to help in differentiating sepsis from systemic inflammatory response syndromes. Use of antibiotic rotation policies in the ICU, regular audits, and feedback reviews are also useful tools to check the use of irrational antibiotic therapy in the ICUs. Education of the prescriber is the cornerstone of any successful antibiotic stewardship program and teaching of guidelines and clinical pathways will aid in improving antimicrobial prescribing behavior to a large extent.
Conclusion
In conclusion, our study reveals that antibiotics continue to be widely prescribed in critically ill patients and form a significant proportion of the total drugs consumed in the ICU. At admission into the ICU, the elderly and sicker patients are prescribed more antibiotics, especially antibiotics that are more expensive. The high utilization rates and costs of antibiotics prescribed at admission in the ICU are a matter of great concern and need to be urgently addressed by the use of guidelines, protocols, educational initiatives, surveillance, and antibiotic restriction policies at all levels of health care.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Esposito S, Leone S. Antimicrobial treatment for intensive care unit (ICU) infections including the role of the infectious diseases specialist. Int J Antimicrob Agents. 2007;29:494–500. doi: 10.1016/j.ijantimicag.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Lockhart SR, Abramson MA, Beekman SE, Gallagher G, Riedel SR, Diekma DJ, et al. Antimicrobial resistance among gram-negative bacilli as causes of infections in intensive care unit patients in the United States between 1993 and 2004. J Clin Microbiol. 2007;45:3352–9. doi: 10.1128/JCM.01284-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber RJ, Kane SL, Oriolo VA, Saul M, Skledar SJ, Dasta JF. Impact of intensive care drug costs: A descriptive analysis, with recommendations for optimizing ICU pharmacotherapy. Crit Care Med. 2003;31:17–24. doi: 10.1097/00003246-200301001-00003. [DOI] [PubMed] [Google Scholar]
- 4.Paterson DL, Rogers BA. How Soon Is Now? The urgent need for randomized, controlled trials evaluating treatment of multidrug-resistant bacterial infection. Clin Infect Dis. 2010;51:1245–7. doi: 10.1086/657243. [DOI] [PubMed] [Google Scholar]
- 5.ATC index with DDDs. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2002. WHO Collaborating Centre for Drug Statistics Methodology. [Google Scholar]
- 6.Tavallaee M, Fahimi F, Kiani S. Drug-use patterns in an intensive care unit of a hospital in Iran: An observational prospective study. Int J Pharm Pract. 2010;18:370–6. doi: 10.1111/j.2042-7174.2010.00065.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergmans DCJJ, Bontena MJM, Gaillard CA, van Tiel FH, van der Geesta S, de Leeuwa PW, et al. Indications for antibiotic use in ICU patients: A one-year prospective surveillance. J Antimicrob Chemother. 1997;39:527–35. doi: 10.1093/jac/39.4.527. [DOI] [PubMed] [Google Scholar]
- 8.Stratton CW, 4th, Ratner H, Johnston PE, Schaffner W. Focused microbiological surveillance by specific hospital unit: Practical application and clinical utility. Clin Ther. 1993;15(Suppl A):12–20. [PubMed] [Google Scholar]
- 9.Shankar PR, Partha P, Dubey AK, Mishra P, Deshpande VY. Intensive care unit drug utilization in a teaching hospital in Nepal. Kathmandu Univ Med J (KUMJ) 2005;3:130–7. [PubMed] [Google Scholar]
- 10.Smythe MA, Melendy S, Jahns B, Dmuchowski C. An exploratory analysis of medication utilization in a medical intensive care unit. Crit Care Med. 1993;21:1319–23. doi: 10.1097/00003246-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Dulhunty JM, Webb SA, Paterson DL, Bellomo R, Myburgh J, Roberts JA, et al. A survey of antibiotic prescribing practices in Australian and New Zealand intensive care units. Crit Care Resusc. 2010;12:162–70. [PubMed] [Google Scholar]
- 12.Biswal S, Mishra P, Malhotra S, Puri GD, Pandhi P. Drug Utilization Pattern in the Intensive Care Unit of a Tertiary Care Hospital. J Clin Pharmacol. 2006;46:945–51. doi: 10.1177/0091270006289845. [DOI] [PubMed] [Google Scholar]
- 13.Shrikala B, Kranthi K, Nafisa A prospective study on evaluation of antibiotic prescription practices in an intensive care unit of a tertiary care hospital. J Clin Diag Res. 2010;4:3387–91. [Google Scholar]
- 14.Erbay A, Bodur H, Akinci E, Colpan A. Evaluation of antibiotic use in intensive care units of a tertiary care hospital in Turkey. J Hosp Infect. 2005;59:53–61. doi: 10.1016/j.jhin.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Hanssens Y, Ismaeil BB, Kamha AA, Elshafie SS, Adheir FS, Saleh TM, et al. Antibiotic prescription pattern in a medical intensive care unit in Qatar. Saudi Med J. 2005;26:1269–76. [PubMed] [Google Scholar]
- 16.Hartmann B, Junger A, Brammen D, Röhrig R, Klasen J, Quinzio L, et al. Review of antibiotic drug use in a surgical ICU: management with a patient data management system for additional outcome analysis in patients staying more than 24 hours. Clin Ther. 2004;26:915–24. doi: 10.1016/s0149-2918(04)90135-x. [DOI] [PubMed] [Google Scholar]
- 17.Røder BL, Nielsen SL, Magnussen P, Engquist A, Frimodt-Møller N. Antibiotic usage in an intensive care unit in a Danish university hospital. J Antimicrob Chemother. 1993;32:633–42. doi: 10.1093/jac/32.4.633. [DOI] [PubMed] [Google Scholar]
- 18.Hariharan S, Pillai G, McIntosh D, Bhanji Z, Culmer L, Harper-McIntosh K. Prescribing patterns and utilization of antimicrobial drugs in a tertiary care teaching hospital of a Caribbean developing country. Fundam Clin Pharmacol. 2009;23:609–15. doi: 10.1111/j.1472-8206.2009.00713.x. [DOI] [PubMed] [Google Scholar]
- 19.Meyer E, Jonas D, Schwab F, Rueden H, Gastmeier P, Daschner FD. Design of a surveillance system of antibiotic use and bacterial resistance in German intensive care units (SARI) Infection. 2003;31:208–15. doi: 10.1007/s15010-003-3201-7. [DOI] [PubMed] [Google Scholar]
- 20.Capp R, Chang Y, Brown DF. Effective Antibiotic Treatment Prescribed by Emergency Physicians in Patients Admitted to the Intensive Care Unit with Severe Sepsis or Septic Shock: Where is the Gap? J Emerg Med. 2011 doi: 10.1016/j.jemermed.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Montravers P, Dupont H, Gauzit R, Veber B, Bedos JP, Lepape A, et al. Strategies of initiation and streamlining of antibiotic therapy in 41 French intensive care units. Crit Care. 2011;15:R17. doi: 10.1186/cc9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vandijck DM, Depaemelaere M, Labeau SO, Depuydt PO, Annemans L, Buyle FM, et al. Links Daily cost of antimicrobial therapy in patients with Intensive Care Unit-acquired, laboratory-confirmed bloodstream infection. Int J Antimicrob Agents. 2008;31:161–5. doi: 10.1016/j.ijantimicag.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 23.Inan D, Saba R, Gunseren F, Ongut G, Turhan O, Yalcin AN, et al. Daily antibiotic cost of nosocomial infections in a Turkish university hospital. BMC Infect Dis. 2005;5:5. doi: 10.1186/1471-2334-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanc P, Von Elm BE, Geissler A, Granier I, Boussuges A, Durand Gasselin J. Economic impact of a rational use of antibiotics in intensive care. Intensive Care Med. 1999;25:1407–12. doi: 10.1007/s001340051089. [DOI] [PubMed] [Google Scholar]
- 25.Thursky KA, Buising KL, Bak N, Macgregor L, Street AC, Macintyre CR, et al. Reduction of broad-spectrum antibiotic use with computerized decision support in an intensive care unit. Int J Qual Health Care. 2006;18:224–31. doi: 10.1093/intqhc/mzi095. [DOI] [PubMed] [Google Scholar]
- 26.Peto Z, Benko R, Matuz M, Csullog E, Molnar A, Hajdu E. Results of a local antibiotic management program on antibiotic use in a tertiary intensive care unit in Hungary. Infection. 2008;36:560–4. doi: 10.1007/s15010-008-7377-8. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui S, Hussein K, Manasia R, Samad A, Salahuddin N, Zafar A, et al. Impact of antibiotic restriction on broad spectrum antibiotic usage in the ICU of a developing country. J Pak Med Assoc. 2007;57:484–7. [PubMed] [Google Scholar]
- 28.Wilke MH, Grube R. Pharmaco-economic evaluation of antibiotic therapy strategies in DRG-based healthcare systems - a new approach. Eur J Med Res. 2010;15:564–70. doi: 10.1186/2047-783X-15-12-564. [DOI] [PMC free article] [PubMed] [Google Scholar]


