Abstract
Aim:
The aim of our study was to do an agreement analysis of two different laboratory methods used to measure electrolytes i.e., between the ISE based Beckman Coulter Synchron CX9 PRO Biochemistry analyzer and RAL's Ion3 Flame Photometer (Técnica para el Laboratorio, Barcelona, Spain), in serum samples.
Materials and Methods:
This cross sectional study was done over a period of three months from September’09 through December’09 on routine biochemistry samples. A total of 6492 samples were received for routine biochemistry analysis from those 630 blood samples were randomly processed for this study. Two ml of sample was taken in a plain gel tube (LABTECH Disposables, Ahmedabad, India), centrifuged and further processed using both systems within one hour of the sampling to obtain the Na and K concentrations in the samples. The bias and variability of differences in measured values were analyzed according to Bland and Altman method.
Results:
Flame photometry method has drawbacks such as low throughput, requires manual operation, is a time consuming procedure. Ion selective electrodes technique is a more universal method for the high throughput determination of electrolytes in physiological samples; Beckman Coulter Synchron CX9 PRO is an example of such a system. The mean difference between the two methods (standard minus test) and 95% limits of agreement for sodium in serum was -7.8±17.3 (-42.2 to 26.6) and in urine was -22±41 (-104 to 60). Similarly, the mean difference between the two methods for potassium values in serum was found to be -0.25±0.75 (-1.75 to 1.25) and in urine was -5.3±38.9 (-83.1 to 72.5). With 95% confidence interval, the value of sodium and potassium as determined by both the methods lie between the upper and lower limit showing 95% limits of agreement.
Conclusion:
Good degree of agreement was seen on comparing the two methods for measuring the electrolytes; the use of Synchron CX9 in place of Flame photometer for electrolyte analysis in serum and urine is justified or use the two interchangeably.
Keywords: Electrolyte measurement, flame photometry, ion selective electrodes method
INTRODUCTION
Flame photometry[1] is one of the oldest direct potentiometric methods commonly used to measure the concentrations of sodium, potassium, and lithium in serum and urine samples. It is a routinely used reference method, working on emission photometric principle. Nevertheless, this method has drawbacks such as low throughput, requires manual operation, as well as time consuming procedure. No standardization methods are available to date for this technique.
The regular clinical need for the availability of these electrolytes with both patients presenting to emergency departments as well as with inpatients has paved way for the replacement of these techniques by newer ones. One such method is the electrolyte measurement by ion selective electrodes (ISE).[2] ISE are an important class of chemical sensors that has found widespread use today in a number of routine applications. A key driving force for their development was their implication in automated clinical analyzers for the high throughput determination of electrolytes in physiological samples. Over the last several years, numerous instruments have been introduced into the clinical market for testing various critical analyte; all of these systems claim to improve patient care by their simple, rapid, and fully automated user interface. Comparison of any new measurement technique with an established one is often needed to see whether they agree sufficiently for the new to replace the old. Beckman Coulter Synchron CX9 PRO[3] is an example of such a system.
The aim of our study was to do an agreement analysis of two different laboratory methods used to measure electrolytes i.e., between the conventional flame photometer and the automated ion selective electrode method in serum and urine samples.
We planned to measure the electrolyte concentrations (total sodium and ionized potassium) in serum and urine samples using both RAL Ion3 SP Flame Photometer and Beckman Coulter Synchron CX9 PRO electrolyte analyzer and performed an agreement analysis between the two measurements.
MATERIALS AND METHODS
This cross sectional study was done on routine biochemistry samples over a period of three months from September’10 through December’10, after receiving an approval from the institutional ethics committee.
Consecutive patients presenting to the outpatient department were included in the study. A total of 6492 samples were received for routine biochemistry analysis in our laboratory; from those, 630 blood samples were randomly processed for this study and 100 urine samples were also randomly selected for analysis. The samples were analyzed within one hour of collection on both systems. Trauma patients from all age groups and both genders with any severity of injury were included. Samples which we strongly- lipemic, strongly hyperbilirubinemic and hemolysed were excluded from the study. Also the routine samples which were < 2 ml were not processed for the purpose of this study.
Sample collection and processing
From the routine biochemistry samples that were received at our laboratory, two ml of sample was taken in a plain gel tube (LABTECH Disposables, Ahemdabad, India) and centrifuged for five minutes at 10,000 rpm. The supernatant serum was collected from the blood samples for electrolyte measurement using both the analyzers. Whereas for the urine samples, concentrate was collected after similar centrifugation, and further, processed using both Synchron CX9 (Beckman Coulter) biochemistry analyzer and RAL Ion SP3 Flame photometer respectively; at the same time to obtain the electrolytes namely, sodium (Na) and potassium (K) concentrations in the samples. The samples were processed by two different laboratory personals independently to avoid bias.
The Beckman Coulter analyzer was used as per the manufacturer's instructions and proprietary Synchron buffer and reagents were utilized for analysis. The ISE electrolyte buffer and ISE electrolyte reference maintain a constant ion activity on the electrodes.
Flame photometry is a subset of emission photometry. The flame provides energy to the elements leading to the excitement of their valence electrons. These unstable electrons emit energy as photons of a particular wavelength which is characteristic of that element. This intensity of light is directly proportional to the number of photons being emitted, which in turn is directly proportional to the number of atoms in the solution. RAL's Ion3 Flame Photometer (Técnica para el Laboratorio) was designed to measure Na, K, Li ion concentration in serum and Na, K ion concentrations in urine. There is included an automated diluter that permits to prepare in an easy and precise way the samples and calibrations needed for the analysis of Na, K ion. The principle of the instrument has been described in detail by Barnes et al.[4] The solution to be analyzed is discharged through an atomizer in a fine mist into a chamber when it is drawn into a flame. An optical system produces light by the combustion of the elements in the vaporized solution conducted through appropriate filters to impinge upon a photoelectric cell which activates a galvanometer. Under proper operative conditions, the concentration of sodium or potassium in the solution can be estimated from the reading of the galvanometer. The instrument is equipped with an amplifier which permits the analysis of solutions with greatly varying concentrations of sodium and potassium.[1]
This instrument uses Lithiumas an internal standard (STD) reference in order to obtain a greater accuracy and enable a precise compensation of changes in the flame itself when used to determinate Na and K in serum and urine samples. When measuring the Lithiumtion concentration in serum, Potassium is used as the internal STD reference.
Beckman Coulter works on the principle, wherein, the ion selective electrodes (ISE) measure the activity of ions in water which is directly proportional to their concentration. The instrument claims to have advantages such as improved operator safety, short turnaround time, unaffected by color or turbidity.
An ISE constitutes of a thin membrane across which only the selected ion can be transported from a higher to a lower concentration via selective binding with sites within the membrane creating a potential difference. The sample is diluted with a high ionic strength ISE electrolyte buffer, minimizing the variation in the activity coefficients of the analyte to be measured. A potential is generated at the surface of ISE when the diluted sample passes through the flow cell. The magnitude of potential change is proportional to the concentration of sodium or potassium .
The potassium and sodium concentrations of the sample can then be determined from these potentials using the Nernst equation.
ISE based biochemistry analyzers use various types of electrodes for estimation of different analyte, such as glass electrode, solid-state electrode, liquid-based electrode, or compound electrode. In the Synchron CX9 biochemistry analyzer by Beckman Coulter, a glass electrode surface is used to estimate sodium concentration and the validamycin electrode surface is used to estimate potassium concentration. The short response time of Synchron CX9 makes it an ideal instrument for high volume and rapid analysis in routine pathology; the instrument has a linear response of over four to six orders of magnitude, there is no consumption of analytes, non-contaminating. Precision of Synchron CX9 biochemistry analyzer is rarely above 1%; limitations for this instrument are that, the electrodes can be corrupted by proteins or other organic solutes, they are fragile and have limited shelf life and respond to the activity of uncomplexed ion. Interference by other ions can hinder the results. Considering standard deviation of difference in Na values by the two methods as 25, to estimate this difference with 20% precision and 95% confidence, 600 serum samples were required. We have randomly selected 630 blood samples.
Data analysis
Data was recorded in a proforma and managed on an excel sheet. The bias and variability of differences in the Na and K values estimated by the two methods were analyzed according to Bland and Altman method.[5] The difference (test minus standard method) between the two measurements was calculated and plotted against the mean of the two measurements. Then the 95% limits of agreement were calculated as mean difference ±2 SD where ‘SD’ is the standard deviation of the differences in paired measurements. STATA 11.0 statistical software (Stata Corp, TX, USA) was used for data analysis.
RESULTS
A total of 630 blood samples and 100 urine samples were collected from patients with median age 35 years (5-87) with 75% (571) male and 25% females (129).
Scatter plots were obtained on plotting the difference between the ion selective electrode method (test method) and flame photometer (standard method).
Interference by endogenous and exogenous substances with assays for clinical analytes is a common problem in laboratory medicine.[6] Thirty serum samples were excluded due to hemolysis and lipemia; hemolysis has a direct effect on several tests. The cellular components of erythrocytes may in fact raise the analyte concentration as a contaminant. Hemolysis increases the concentration of potassium by 3 mmol/l for each 1 g/l of hemoglobin. Lipemia is the result of circulating chylomicrons which are large lipid particles. They cause interference by turbidity or light scattering and volume displacement. However, methods are not affected by lipemia when the determination is made in the aqueous fraction, such as methods involving ion selective electrodes.[6] To determine agreement between the ion selective method and flame photometer for serum samples, difference between the ion selective electrode method (test method) and flame photometer (standard method) for serum values were calculated (standard minus test). The Mean±S.D. of Na and K in serum and urine samples using both the methods is given in Table 1. The mean difference for Na concentrations in serum was found to be -7.89, standard deviation was 17.28, and similarly for serum K concentrations the mean difference was -0.25, standard deviation was 0.75. These values were plotted against the mean of the two measurements using Bland and Altman method.[5]
Table 1.
Mean Na, K values using Flame photometer and Synchron CX9 Biochemistry analyser
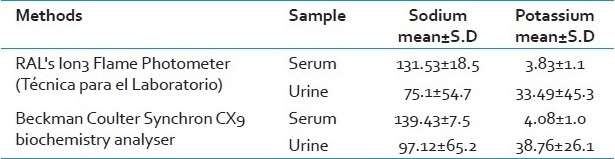
The 95% limits of agreement for sodium values in serum were found to be from -42.2 to 26.6 [Figure 1a]. The value of sodium as determined by both the methods (Flame Photometer and Synchron CX9) lie between the upper and lower limit showing 95% limits of agreement. Similarly, 95% limits of agreement for potassium values in serum were found to be from -1.75 to 1.25 [Figure 1b]. The value of serum potassium as determined by both Flame Photometer and Synchron CX9 lie between the upper limit and lower limit showing 95% limits of agreement. There is only a 5% chance that the values may lay beyond the limits of agreement.
Figure 1.
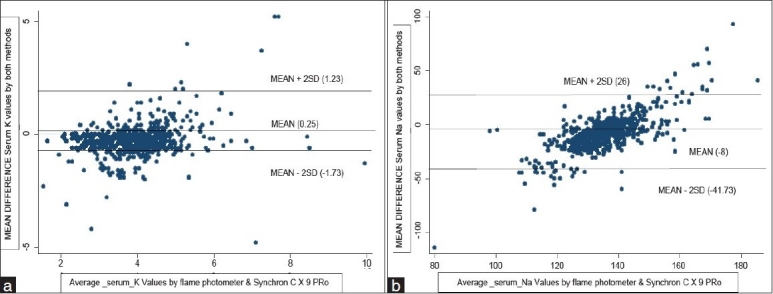
(a) Mean difference serum potassium (K) vs average serum potassium (K) values by Flame Photometer and Synchron Cx9 PRO (Bland and Altman Plot) 95% limits of agreement = mean difference ± 2 SD [1.23, -1.73] (b) Mean difference serum sodium (Na) vs average serum sodium (Na) values by Flame Photometer and Synchron Cx9 PRO (Bland and Altman Plot) 95% limits of agreement =mean difference ± 2 SD [26, -41.73]
The calculated mean of the differences between the two methods for sodium measurement in urine samples was -22.02 and standard deviation was 41.01 plotting the Bland and Altman plot the 95% limits of agreement for sodium measurement in urine samples were from -104 to 60 [Figure 2a]. For urine potassium values, the calculated mean of the differences between the two methods was -5.27 and the calculated standard deviation was 38.91. The 95% limits of agreement were found to be from -83.1 to 72.5. The value of urine potassium as determined by both Flame Photometer and Synchron CX9 lie between the upper limit and lower limit showing 95% limits of agreement [Figure 2b]. Since the two methods do not differ enough to cause problems in clinical interpretation, the use of Synchron CX9 in place of Flame photometer for electrolyte analysis in serum and urine is justified or use the two interchangeably[7]
Figure 2.
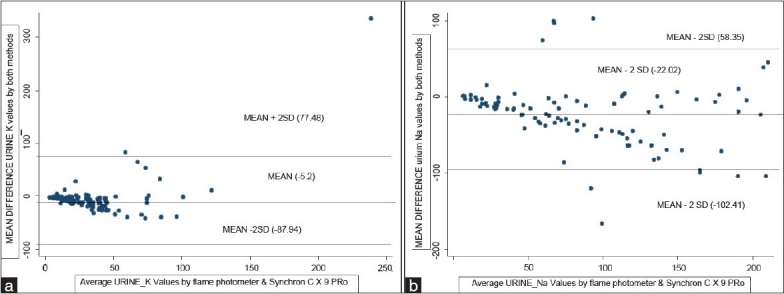
(a) Mean difference urine potassium (K) vs average urine potassium (K) values by Flame Photometer and Synchron Cx9 PRO (Bland and Altman Plot) 95% limits of agreement = mean difference ± 2 SD [77.48, -87.94] (b) Mean difference urine sodium (Na) vs average urine sodium (Na) values by Flame Photometer and Synchron Cx9 PRO (Bland and Altman Plot) 95% limits of agreement = mean difference ± 2 SD [58.35, -102.41]
DISCUSSION
The great clinical importance and common ordering of electrolyte studies in patients warrants the use of rapid, simple, and automated methods. Selective ion electrode is certainly a step towards simplicity and the automated Synchron CX9 PRO chemistry analyzer by Beckman Coulter is one such instrument. It was introduced in our laboratory to decrease the consumption of time engaged in the electrolyte analysis and to give rapid results in emergency cases.
The data obtained in the present study was evaluated by applying Bland and Altman agreement analysis.[6] The 95% limits of agreement estimated by mean difference ±1.96 (standard deviation) of the differences provided an interval within which 95% of differences between measurements by the two methods were found to lie. With 95% of confidence interval, the values of both Na and K determined by both the methods (Flame Photometer and Synchron CX9) lie between the upper limit and lower limit showing 95%limits of agreement; there is a 5% chance where the values may lie beyond the limits of agreement.
In 1983, a Workshop on Direct Potentiometric Measurements in Blood was held in Gaithersburg, MD, under the co sponsorship of The National Committee for Clinical Laboratory Standards (NCCLS) and the National Bureau of Standards (NBS) [now National Institute of Standards and Technology (NIST)],to examine the factors that affect these measurements and to determine what decisions and data were necessary to establish standardization.[5] Guidelines were established as regards to the methods of standardization of these equipments and are strongly supported by the Area Committee on Clinical Chemistry of NCCLS and its subcommittee on electrolytes.[8]
Similar studies have been done to compare and standardize the ion selective technique based instruments with the traditional flame photometric method. In a study by Preusse et al,[9] the two equipments, namely ORION SPACE-STAT (SS-30) and the TECHNICON STAT/ION, were used to investigate quality control of Na and K determinations in test sera (n=8) and in the plasma of 100 patients. The flame photometer IL 543 was used as a reference apparatus. The ion-selective electrode instruments, SS-30 and STAT/ION, gave results very similar to those of flame photometry.
The differences observed were small, within the clinical tolerance interval and without much clinical significance, thus the two methods are equivalent or comparable.
Though some authors showed slight variations in the readings between the ion selective method and the flame photometric method, they strongly recommended this automated method for routine chemistry laboratory setting.
Worth[10] reappraised the working principle for the measurement of sodium and potassium by flame photometry and ion-selective electrode, and advocated all clinical chemistry laboratories having access to a direct-reading ion-selective electrode for sodium estimation.
Spectrophotometer, coulometer, and ion-selective electrode (ISE) technology in a correlation study,[11] were assessed for their precision, actual time required for analysis, correlation, and the total cost per test. The study revealed that flame photometer would be suitable equipment for small scale hospitals, and that for medium and large scale hospitals, ion selective electrode technique would suffice.
In a study by Mikolaenko et al,[12] the newly available Beckman Coulter LX20 (Brea, CA) was analyzed with the standard reference methods. The results were obtained for linearity within- and between-day precision, correlation, interference, and serum vs plasma studies were satisfactory with most assays demonstrating within-day coefficients of variation < 2% and between-day coefficients of variation < 5%. The linearity for all assays were acceptable over the range tested and correlation results to be adequate. The major difference in serum vs plasma studies was potassium. Hence, the authors concluded that the Beckman Coulter LX20 demonstrates good performance capabilities making it suitable for a medium- to high-volume laboratory.
In a center like ours with a huge sample load, a fully automated, high throughput instrument is mandatory especially to meet the emergency need of the patients. Though similar studies have been done previously in other countries, this study was carried out to see how compatible Beckman coulter Synchron CX9 PRO is in our hospital. This ISE method based equipment has a high throughput and efficiency to provide rapid results of other biochemistry tests (sugar, liver function tests, renal function tests, lipid profile, magnesium, calcium, phosphorous, cardiac enzymes) besides the electrolyte measurement using the same sample in a single run in a shorter period of time even though the cost per test is higher than the flame photometric method.
The correlation with the ISE based automated electrolyte analyzers have also been shown previously by various authors in other automated analyzers for serum and plasma samples. In this study, urine samples were also used for the method comparison to see if similar results are observed.
CONCLUSION
Good degree of agreement is seen on comparing the ion selective electrode method and the conventional Flame photometer for measuring the electrolytes, namely sodium and potassium, for serum samples and also for the electrolytes measurement in urine samples.
Ion selective electrode (Synchron CX9 PRO) equipment take a shorter analytic time, high throughput (larger volume of the test) compared to the semi automated Flame photometric method.
A good degree of agreement of results was seen on comparing the two methods, since they do not differ enough to cause problems in clinical interpretation; the use of Synchron CX9 in place of Flame photometer for electrolyte analysis in serum and urine is justified or use the two interchangeably.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Hald PM. The flame photometer for the measurement of sodium and potassium in biological materials. J Biol Chem. 1946;167:499–510. [PubMed] [Google Scholar]
- 2.Burnett RW, Covington AK, Fogh-Andersen N, Külpmann WR, Lewenstam A, Maas AH, et al. Recommendations for measurement of and conventions for reporting sodium and potassium by ion-selective electrodes in undiluted serum, plasma or whole blood. International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). IFCC Scientific Division Working Group on Selective Electrodes. Clin Chem Lab Med. 2000;38:1065–71. doi: 10.1515/CCLM.2000.159. [DOI] [PubMed] [Google Scholar]
- 3.USA: Beckman Coulter Inc; 2003. User manual Beckman coulter synchron CX9 PRO. [Google Scholar]
- 4.Barnes RB, Richardson D, Berry JW, Hood RL. Flame photometry: a rapid analytical procedure. Industr Engin Chem (Anal. Ed) 1945;17:60. [Google Scholar]
- 5.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 6.Kroll MH, Elin RJ. Interference with clinical laboratory analyses. Clin Chem. 1994;40:1996–2005. [PubMed] [Google Scholar]
- 7.Kang JA, Song KE, Lee WK, Choi SM, Kim YS. Comparitive analysis of management and test results among three different electrolyte-anlayzing instruments. Korean J Clin Pathol. 1993;13:51–8. [Google Scholar]
- 8.Koch WF, editor. Vilanova, Pk: NCCLS; 1983. May 18-20, Proceeding of the workshop on direct potentiometric measurements in blood, held at NIST, Gaithersburg, MD. 1985. [Google Scholar]
- 9.Villanova, PA: National Committee for Clinical Laboratory Standards; 1989. NCCLS document C29-P. Standardization of sodium and potassium ionselective electrode systems to the flame photometric reference method. [Google Scholar]
- 10.Preusse CJ, Fuchs C. Comparison of ion-selective electrodes and flame photometry for the determination of serum Na+ and K+ for clinical purposes. J Clin Chem Clin Biochem. 1979;17:639–45. [PubMed] [Google Scholar]
- 11.Worth HG. A comparison of the measurement of sodium and potassium by flame photometry and ion-selective electrode. Ann Clin Biochem. 1985;22:343–50. doi: 10.1177/000456328502200402. [DOI] [PubMed] [Google Scholar]
- 12.Mikolaenko I, Benson E, Konrad RJ, Chaffin C, Robinson CA, Hardy RW. Evaluation of the Beckman Coulter LX20 Clinical Chemistry Analyzer. Lab Med. 2000;31:387–93. [Google Scholar]


