Abstract
The present study was designed to evaluate the cardioprotective effects of methanolic extract of Litsea deccanensis (MELD) against isoproterenol-induced myocardial infarction in rats by studying cardiac markers, lipid peroxidation, lipid profile, and histological changes. Male Wistar rats were treated orally with MELD (100 and 200 mg/kg) daily for a period of 21 days. After 21 days of pretreatment, isoproterenol (100 mg/kg) was injected subcutaneously to rats at an interval of 24 h for 2 days to induce myocardial infarction. Isoproterenol-induced rats showed significant (P < 0.05) increase in the levels of serum creatine kinase, lactate dehydrogenase, thiobarbituric acid reactive substances, and lipid hydro peroxides. The serum lipid levels were altered in the isoproterenol-induced myocardial infarcted rats. The histopathological findings of the myocardial tissue evidenced myocardial damage in isoproterenol-induced rats. The oral pretreatment with MELD restored the pathological alterations in the isoproterenol-induced myocardial infarcted rats. The MELD pretreatment significantly reduced the levels of biochemical markers, lipid peroxidation and regulated the lipid profile of the antioxidant system in the isoproterenol-induced rats. An inhibited myocardial necrosis was evidenced by the histopathological findings in MELD pretreated isoproterenol-induced rats. Our study shows that oral pretreatment with MELD prevents isoproterenol-induced oxidative stress in myocardial infarction. The presence of phenolic acid and flavonoid contents were confirmed by preliminary phytochemical tests. The reducing power and free radical scavenging activities of the MELD may be the possible reason for it pharmacological actions.
Keywords: Antioxidant, infarct size, lipid metabolism, lipid peroxidation
INTRODUCTION
Recently, there has been a growing interest in establishing the therapeutic potentials of natural products against various diseases. Herbal medicine has been prescribed in many countries over centuries for treating various diseases, including infectious and malignant diseases.[1] The use of plant products for medicinal purposes seems to be more natural, less expensive and without side effects. The therapeutic activities of plants are due to the presence of various active compounds like vitamins, flavonoids and polyphenols. The consumption of plant foods, such as antioxidant supplements or antioxidant-containing foods may be used to protect against various diseases, including cancer, cardio and cerebrovascular diseases.[2] Litsea is one of the most diverse genera of evergreen trees or shrubs belong to family Lauraceae. Plants belong to Litsea genus exhibit a variety of biological activities, including antimicrobial, hypothermic and anti-tumor activities.[3] It has been reported the aphrodisiac activity of ethanolic extract of the bark of Litsea chinensis.[4] The methanolic extract of Litsea cubeba and its fractions showed remarkable antioxidant activity in comparison with vitamin E and ascorbic acid.[5] The leaves of Litsea decanensis are used as folk medicine for chest pain by tribal people in Andhra Pradesh state, India. Previous study from our laboratory explained the presence of phytochemical constituents present in Litsea decanensis and confirmed the presence of phenolic compounds.[6] The GC--MS analysis and in vitro antioxidant activity of Litsea decanensis stimulated us to study the pharmacological efficacy of this plant against cardiovascular diseases.
Cardiovascular disease (CVD) is a major global health problem, reaching epidemic proportions in the Indian subcontinent.[7] The majority of cardiovascular events such as myocardial infarction (MI) arise from individuals with unpretentious elevation of many etiological factors.[8] Myocardial infarction (MI) is one of the leading causes of morbidity and mortality worldwide. MI occurs due to imbalance between myocardial blood supply and demand resulting in development of ischemia followed by necrosis.[9] Reactive oxygen species (ROS) play an important role in oxidative stress and related myocardial damage. ROS-induced lipid peroxidation influence cardiac cell injury and affect the membrane integrity of cardio myocyte. Hyperlipidemia and hypertriglyceridemia have also been among the major reasons for the pathogenesis of MI. The current knowledge on the pathophysiology of MI stimulated the therapeutic intervention to reduce the risk of MI.
Isoproterenol [1-(3,4-dihydroxyphenyl)-2-isopropyl amino ethanol hydrochloride] (ISO) is a synthetic catecholamine and β-adrenergic agonist. The excess amount of ISO produces free radicals through its metabolites which are responsible for oxidative stress, and cardiac damage. The rat model of ISO-induced MI serves as a standard model to estimate the effect of cardio protective drugs in preclinical study and show many metabolic and morphologic alterations in the heart tissue of the experimental animals similar to those observed in human MI.[10]
MATERIALS AND METHODS
Plant collection and extraction
Litsea decanensis was collected during the month of January from the forest regions of Chittoor district in Andhra Pradesh. The plant material was authenticated by Dr. Madava Chetty, Asst. Professor, Department of Botany, Sri Venkateshwara University, Tirupathi. A voucher specimen has been deposited in the Department of Pharmacognosy, Jayamukhi College of Pharmacy (Herbarium No.: 2-2010/Ph/JCP). The leaves were manually separated and dried at room temperature for 72 h then ground to a granulated powder using a grinder. The powdered leaves were extracted in a soxhlet extractor with petroleum ether (60°C for 8 h) and defatted leaves powder was re-extracted in a soxhlet apparatus for 72 h with methanol at 60°C. The methanolic extract of Litsea deccanensis (MELD) was allowed to dry and powdered.
Chemicals
Gallic acid and Isoproterenol were purchased from Sigma Chemical Co., St. Louis, MO, USA, Ascorbic acid, Folin-Ciocalteu reagent, 2,3,5-triphenyltetrazolium chloride, and rutin were purchased from Merck chemicals, India. All the other chemicals used were of the analytical grade.
Determination of total phenolic content
The content of total phenolic compound in MELD was determined by the method of Folin-Ciocalteu, 1927.[11] All determinations were performed in triplicate. Total content of phenolic compounds of MELD in Gallic acid equivalents (GAE) was calculated by the following: C = cV/m, where C is the total content of phenolic compounds, mg/g MELD, in GAE; c is the concentration of gallic acid established from the calibration curve, mg/ml; V is the volume of MELD, ml; and m is the weight of MELD, g.
Total flavonoid content
The content of flavonoids was determined according to colorimetric method as described by Zou et al.[12] In brief, 0.5 ml of sample solution (MELD) was mixed with 2 ml of distilled water and subsequently with 0.15 ml of 5% NaNO2 solution. After 6 min of incubation, 0.15 ml of 10% AlCl3 solution was added and then allowed to stand for 6 min, followed by adding 2 ml of 4% NaOH solution to the mixture. Immediately water was added to the sample to bring for another 15 min. The mixture absorbance was determined at wavelength 510 nm. The total flavonoid content was expressed in milligrams of rutin equivalents per gram of MELD. The amount of flavonoids in MELD in rutin equivalents (RE) was calculated by following formula: X = (Amo10)/Aom, where X is flavonoid content, mg/g MELD in RE; A is the absorption of MELD, mo is the weight of rutin in the solution, g; Ao is the absorption of standard rutin solution; m is the weight of MELD in g.
Determination of free radical scavenging activity by 2,2-diphenyl-1-picryl-hydrazyl
The free radical scavenging activities of various concentrations of MELD were measured in vitro by diphenyl-1-picryl-hydrazyl (DPPH) free radical scavenging assay.[13] The percentage scavenging of the sample was calculated according to the equation: %Scavenging = (AC-AS/AC) ×100, where, AC is the absorbance of control, AS is the absorbance of sample, ascorbic acid, gallic acid, and used as control.
Reducing power assay
The total reducing power of MELD was determined according to method Oyaizu, 1986.[14] Higher absorbance of the reaction mixture indicated greater reducing power.
Experimental animals
All the experiments were carried out with male albino Wistar rats (Rattus norvegicus) weighing 180--200 g, purchased from Mahaveer Enterprises, Hyderabad, India. They were housed in polypropylene cages (47 cm × 34 cm × 20 cm) lined with husk, renewed every 24 h under a 12 h light/dark cycle at 22 ± 5 °C with 50% humidity. The animals were allowed to adapt to the new housing environment for 10 days before the experiment. They were provided with standard food pellets and water. All animal experiments were performed according to the guidelines of Committee for Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India, approved by Institutional Animal Ethical Committee of Jayamukhi College of Pharmacy (Approval no: 17, date 12-06-2010).
Acute toxicity studies
For acute toxicity studies, rats were divided into six groups of three animals in each group. MELD was dissolved in an appropriate volume of normal saline was administered orally at doses of 100, 200, 500, 1000, and 2000 mg/kg body weight/day for a period of 30 days. Same volume of normal saline without MELD was given to control group of animals. Morphological, behavioral, and toxic symptoms of the animals were also observed for 24, 48, and 72 h and the animals were weighed biweekly for the whole treatment period for delayed toxicity. MELD showed no lethal effect up to dose of 2000 mg/kg weight, indicating that LD50 if any should be higher than this dose. MELD was found to be safe at all the doses. Hence, 1/10th and 1/20th of the maximum safe dose corresponding to 200 and 100 mg/kg orally were selected as high and low dose, respectively.
Induction of myocardial infarction
ISO was dissolved in normal saline and injected to rats (100 mg/kg) at an interval of 24 h for 2 days to induce experimental myocardial infarction.[15] Animals were sacrificed 48 h after the first dose of ISO.
Experimental design
In our study, a total of 48 rats were used. They were divided into six groups of eight rats each. Two rats from each group were used for histological studies, i.e., 2,3,5-triphenyl tetrazolium chloride (TTC) staining technique and histopathological analysis. Remaining six animals in each group were used for biochemical analysis. Group I: normal control rats were given 2 ml of saline orally by gastric incubation daily for a period of 21 days; Group II: normal rats were treated with MELD (100 mg/kg) in 2 ml of saline orally by gastric incubation daily for a period of 21 days; Group III: normal rats were treated with MELD (200 mg/kg) in 2 ml of saline orally by gastric incubation daily for a period of 21 days, Group IV: rats were subcutaneously injected with ISO (100 mg/kg) in 2 ml of saline once a day for 2 days (on 22nd and 23rd day); Group V: rats were pretreated with MELD (100 mg/kg) in 2 ml of saline orally by gastric incubation daily for a period of 21 days and then subcutaneously injected with ISO (100 mg/kg) once a day for 2 days (on 22nd and 23rd day); Group VI: rats were pretreated with MELD (200 mg/kg) in 2 ml of saline orally by gastric incubation daily for a period of 21 days and then subcutaneously injected with ISO (100 mg/kg) once a day for 2 days (on 22nd and 23rd day). Twenty four hours after the second dose of ISO, rats of all the groups were anesthetized and sacrificed by cervical decapitation and blood was collected in two tubes, i.e., one with anticoagulant (ethylene diamine tetra acetic acid) for plasma separation, and another without anticoagulant for serum separation. Both the plasma and serum were separated from each sample and used for the biochemical analysis.
Biochemical parameters
The activities of creatine kinase (CK), criatine kinase-MB fraction (CK-MB) and lactate dehyrogenase (LDH) in the serum were determined by commercial diagnostic kits purchased from Accurex, Mumbai, India. The instructions from the manufacturer of the kits were followed. Thiobarbituric acid reactive substances (TBARS) in the plasma were measured by the method of Yagi.[16] Lipid hydroperoxides (LOOH) in the plasma was estimated by the method of Jiang et al.[17]
Lipid profile
Total cholesterol, high density lipoproteins (HDL) cholesterol, and triglycerides (TG) in the serum were measured by standard diagnostic kits (Accurex, Mumbai, India) according to the instruction given by the manufacturers. Very low density lipoproteins (VLDL) and low density lipoprotein (LDL) in the serum were calculated as per Friedewald et al.[18] The level of free fatty acids (FFA) in the serum was estimated by the method of Falholt et al.,[19] and the level of phospholipids (PL) in the serum were estimated by the method of Zilversmit and Davis.[20] The activity of 3-hydroxy-3 methyl glutryl CoA (HMG CoA) reductase in the liver tissue homogenate was assayed by the method of Rao and Ramakrishnan.[21] Superoxide dismutase (SOD) activity in the plasma was assayed by the method of Kakkar et al.[22] The activity of catalase in the plasma was assayed by the method of Sinha.[23]
Determination of myocardial infarction size by direct staining method
Myocardial infarction size was measured in the cardiac tissue by the method of Ojha et al.[24] The slices were photographed after TTC staining.
Histopathology
Myocardial tissue after removal was immediately fixed in 10% buffered neutral formalin solution. After fixation was complete, tissues were embedded in paraffin and serial sections were cut. Each section was stained with hematoxylin and eosin. The sections were examined under light microscope (45×) and photomicrographs were taken.
Statistical analysis
The results are expressed as mean ± S.D. from six rats in each group. Statistical comparison between group by one-way analysis of variance (ANOVA) and a group by group comparison was performed by Duncan's multiple range test (DMRT). Differences between the groups were considered statistically at P < 0.05.
RESULTS
Total phenolic and total flavonoid analysis
The total phenolic content and flavonoid content of MELD were measured in our study and found to be a notable concentration 0.2 mg/ml Gallic Acid equivalent and 2.26 mg/g of MELD in Rutin equivalents, respectively.
Reducing power assay
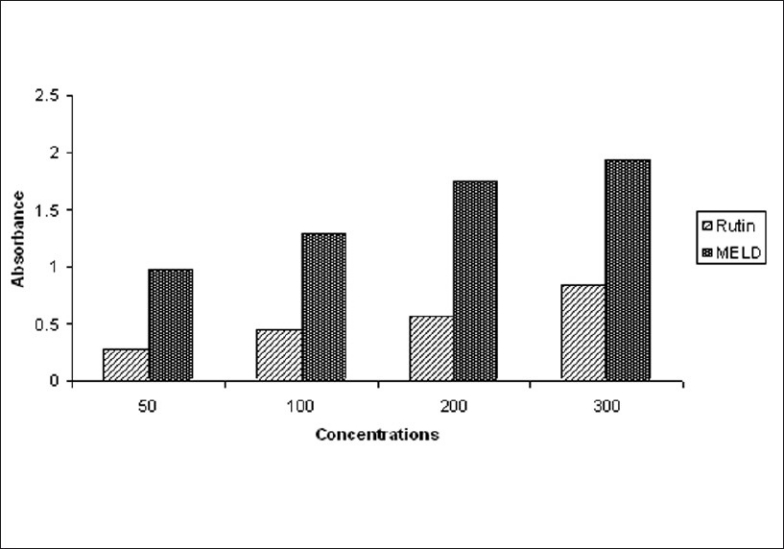
The reductive capabilities of MELD compared to rutin as shown in the Figure 1. The reducing power of MELD was very potent and the reducing power was increased with increase in the concentration of MELD. The MELD could reduce the most Fe3+ ions, which had a lesser reductive activity than the standard of rutin.
Figure 1.
Reducing power of MELD
Free radical scavenging assay
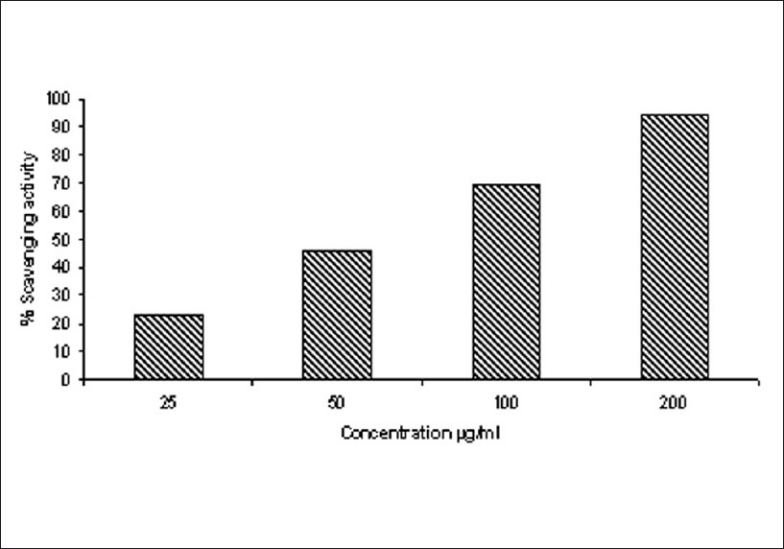
DPPH (1,1-diphenyl-2-picryl-hydrazil) is a stable free radical that accepts an electron or hydrogen radical to become a stable diamagnetic molecule.[25] Antioxidant activity of MELD studied by DPPH radical scavenging activity and the percentage scavenging activity increased with increase in the concentration of MELD [Figure 2].
Figure 2.
Scavenging activity of MELD against DPPH free radicals
Effect of MELD on levels of cardiac markers
The activities of serum CK, CK-MB, and LDH in normal and experimental rats were studied. The ISO-induced rats showed significant (P < 0.05) increase in the activities of CK, CK-MB, and LDH in the serum, when compared to normal control rats. Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days reduced the levels of these enzymes in the serum, when compared to ISO-induced myocardial infarcted rats [Figure 3].
Figure 3.
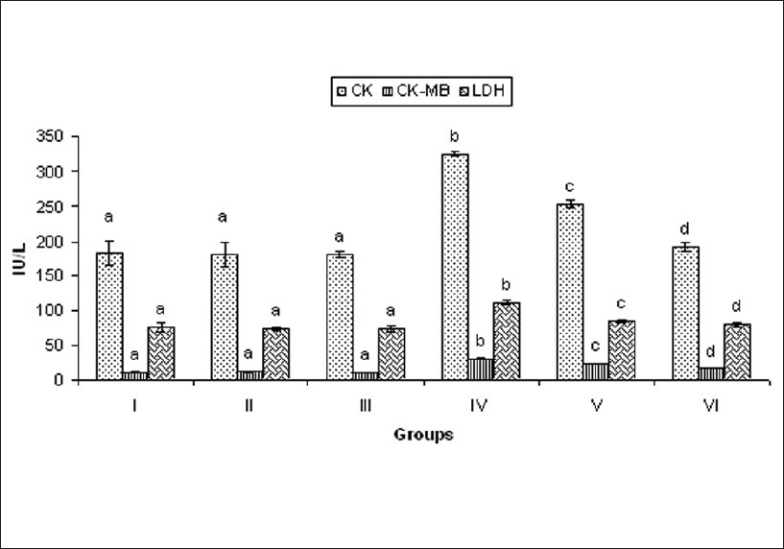
Effect of MELD on cardiac markers. Values are expressed as mean ± standard deviation from six animals in each group. Significant (P < 0.05) compared to normal
Effect of MELD on levels of TBARS and LOOH in heart
ISO-induced rats showed significant (P < 0.05) increase in the levels of plasma TBARS and LOOH, when compared to normal control rats. Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days inhibited the levels of these enzymes in the plasma, when compared to ISO alone treated rats [Figure 4].
Figure 4.
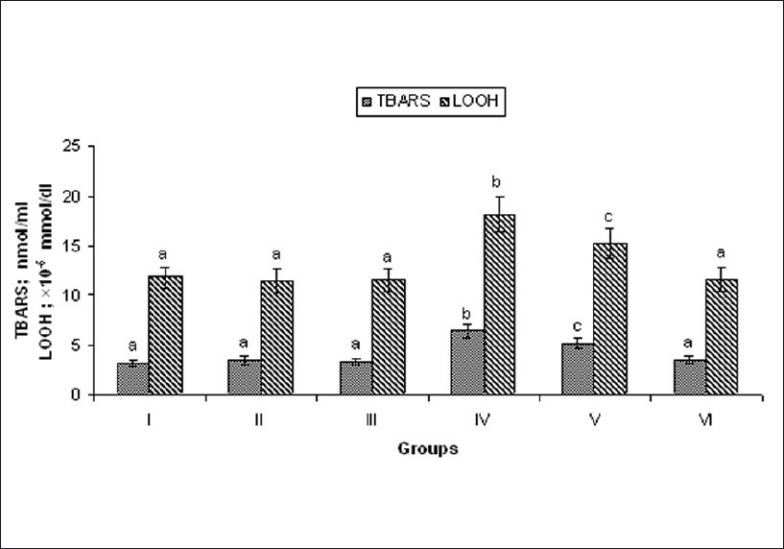
Effect of MELD on levels of lipid peroxidation products. Values are expressed as mean ± standard deviation from six animals in each group. Significant (P < 0.05) compared to normal
Effect of MELD on levels of lipid profile
ISO-induced rats showed significant (P < 0.05) increase in the levels of total cholesterol, LDL, and VLDL. The decreased levels of HDL in the plasma were also observed. Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days significantly reduced total cholesterol, LDL, VLDL, and increased HDL [Figure 5].
Figure 5.
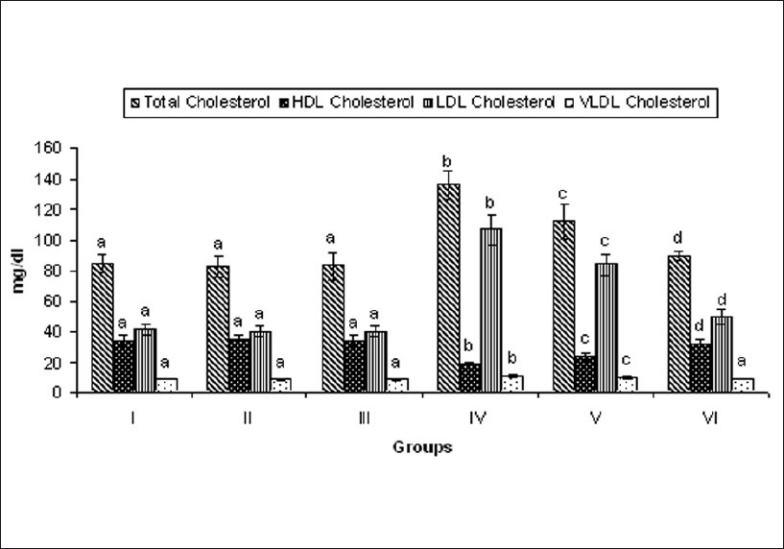
Effect of MELD on levels of lipids and lipoproteins. Values are expressed as mean ± standard deviation from six animals in each group. Significant (P < 0.05) compared to normal
Estimation of MELD on levels of TG, FFAs, PLs
Rats induced with ISO showed significant (P < 0.05) increase in the levels of TG, FFA, and PL in the serum, when compared to normal control rats. Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days normalized the activities of these enzymes in the serum, when compared to ISO alone treated rats [Figure 6].
Figure 6.
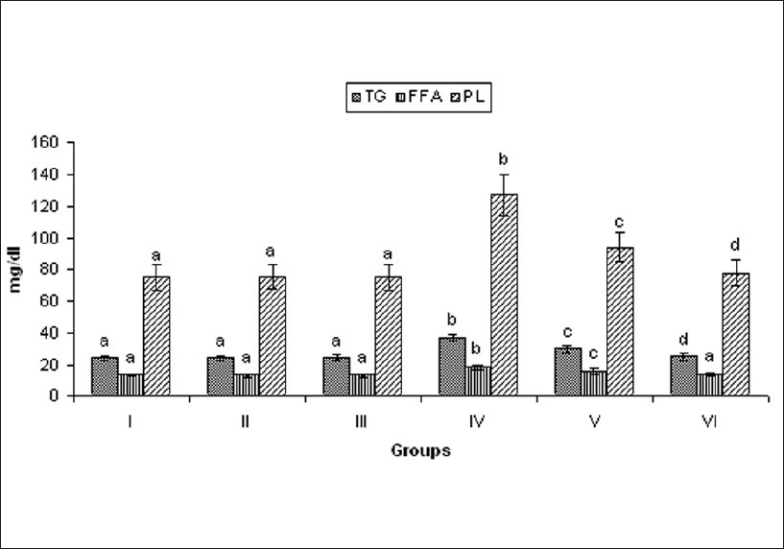
Effect of MELD on levels of TG, FFA, and PL. Values are expressed as mean ± standard deviation from six animals in each group. Significant (P < 0.05) compared to normal
Effect of MELD on HMG CoA reductase activity
ISO-induced rats showed significant (P < 0.05) increase in the activity of HMG CoA reductase in the liver tissue homogenate compared to normal control rats. Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days reduced the activities of these enzymes in the serum compared to ISO-induced rat [Figure 7].
Figure 7.
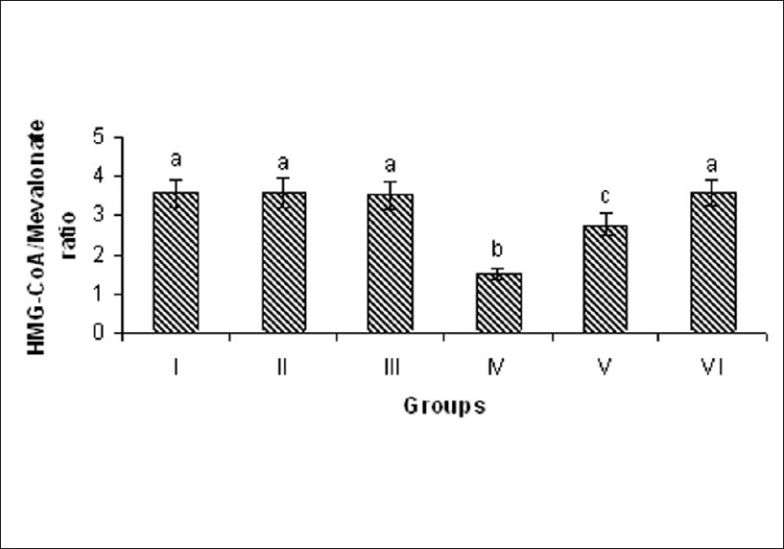
Effect of MELD on the activity of HMG-CoA in the liver. Values are expressed as mean ± standard deviation from six animals in each group. Significant (P < 0.05) compared to normal
Effect of MELD on antioxidant enzymes (SOD, catalase)
ISO-induced rats showed significant (P < 0.05) decrease in the activities of SOD, catalase in the plasma compared to normal control rats. This result reflects the diminished antioxidant enzymes. Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days significantly increase the activities of these enzymes in the plasma, when compared to ISO alone treated rats [Figure 8].
Figure 8.
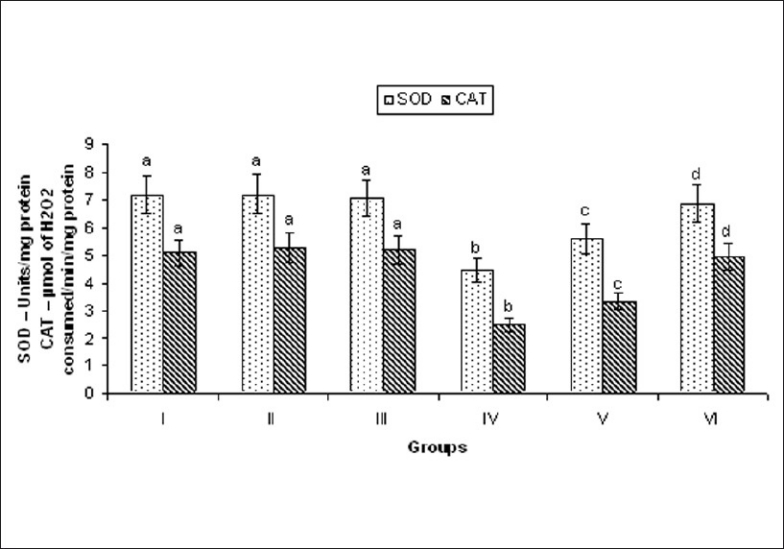
Effect of MELD on the activities of SOD and CAT. Values are expressed as mean ± standard deviation from six animals in each group. Significant (P < 0.05) compared to normal
For all the biochemical parameters studied, pretreatment with MELD (100 mg/kg and 200 mg/kg) daily for a period of 21 days to the normal control rats did not show any significant effect.
Quantification of myocardial infarct size
Detection of myocardial infarct size by direct staining method using 2,3,5- triphenyltetrazolium chloride (TTC) dye, which forms a red formazan precipitate with dehydrogenase of the viable myocardial tissue shows normal viable tissue with red color/dark spot. ISO-induced rats clearly showed the infarct region by pale yellow color/bright spot. The oral pretreatment with MELD (100 mg/kg, 200 mg/kg) markedly reduced the infarcted size in ISO-induced rats [Figure 9].
Figure 9.
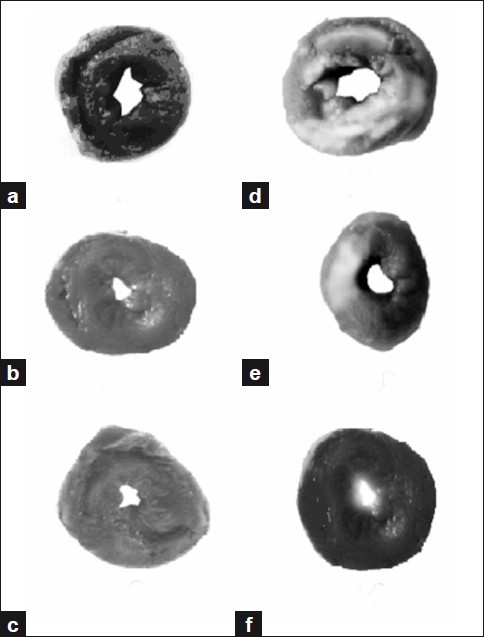
Myocardial infarct size quantification assay (a) Heart slice showing viable cells in normal control group. (b) Heart slice of MELD (100 mg/kg) treated rats showing no significant change from the normal control group. (c) Heart slice of MELD (200 mg/kg) treated rats also showed no significant change. (d) Heart slice of ISO-induced showed increased infarct size and indicating non-viable cells. (e) Heart slice of MELD (100 mg/kg) pretreated and ISO-induced rat showing less infarct size than ISO-induced group. (f) Heart slice of MELD (200 mg/kg) pretreated and ISO-induced rat showing further reduced infarct size, when compared to (d) and (e).
Histopathology
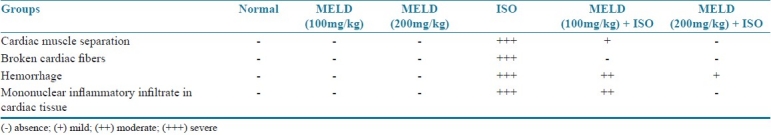
The ISO-induced rats showed the evidence of myocardial necrosis, tissue damage, infiltration of inflammatory cells and hemorrhage. The rats received MELD (100 mg/kg and 200 mg/kg) as oral pretreatment showed decreased necrosis, less tissue damage and reduced infiltration of inflammatory cells, when compared to ISO-induced myocardial infarcted rats [Figure 10]. The quantification of histological damage was mentioned in Table 1.
Figure 10.
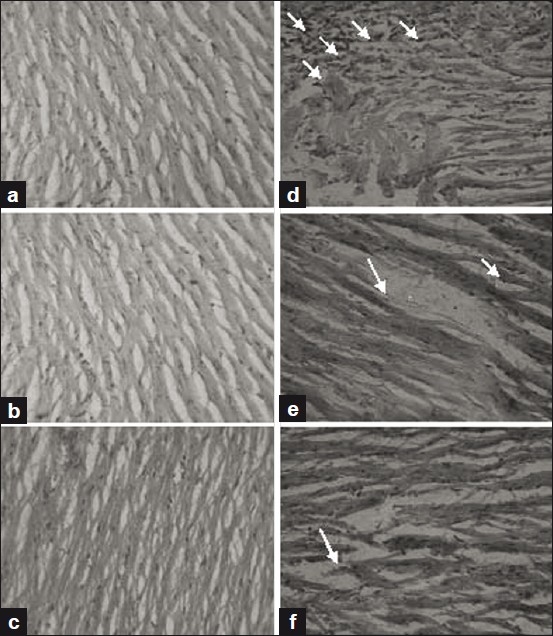
Histopathology of the heart tissue of normal and experimental groups of rats. (a) Section of heart tissue from normal control rat showing normal architecture of heart tissue. (b) Section of heart tissue of MELD (100 mg/kg) treated rats showing no significant change from the normal architecture. (c) Section of heart tissue of MELD (200 mg/kg) treated rats also showing nearly normal architecture. (d) Section of heart tissue of ISO-induced rats showed necrotic damage, infiltration of neutrophils and altered structure of myofiber. (e) Section of heart tissue of MELD (100 mg/kg) pretreated and ISO-induced rat showing mild focal degenerative changes in the cardiac muscle fiber. (f) Section of heart tissue of MELD (200 mg/kg) pretreated and ISO-induced rat showing almost normal architecture.
Table 1.
Quantitative evaluation of histopathological study
DISCUSSION
The phytochemical analysis of MELD confirmed the presence of phenolic contents and particularly flavonoids. High phenolic compounds may cause the anti-oxidative activities of this plant.[26] The beneficial effect of polyphenols is associated with a multitude of biological activities, including antioxidant and free radical-scavenging properties, anti-platelet aggregation and inhibition of vascular smooth muscle cell proliferation.[27] Our study suggested that the presence of phenolic and flavonoid content and its free radical scavenging action of MELD.
Isoproterenol is a potent, nonselective β-receptor agonist. ISO metabolized by Catechol-O-methyl transferase, and excessive dose can produce cardiac ischemia, sympathetic hyper activation and leads to myocardial tissue damage. The metabolic products of ISO are responsible for the production of free radical and myocardial damage in ISO-induced rats. The myocardium is having abundance of enzymes and these enzymes serve as diagnostic marker for cardiovascular diseases. When myocardial cells are damaged, the cardiac membrane integrity will be affected and released these enzymes into the systemic circulation. The significant increase in the levels of cardiac markers in ISO-induced rats is an indication of the severity of necrotic damage in the myocardium induced by ISO. Oral pretreatment with MELD (100 mg/kg, 200 mg/kg) significantly (P < 0.05) reduced the levels of cardiac markers in the serum of ISO-induced rats, and it is indicating the membrane stabilizing effect of MELD.
Lipid peroxidation is an important pathogenic event that damage myocardial membrane. Increased lipid peroxidation is thought to be a consequence of oxidative stress which occurs when the dynamic balance between pro-oxidant and antioxidant mechanism is impaired.[28] The degree of lipid peroxidation in the normal and experimental was estimated by measuring TBARS, LOOH in normal and experimental rats. ISO-induced rats showed increased levels of lipid peroxidation products (TBARS and LOOH) in the plasma. Our results are in consistent with previous reports.[29] MELD pretreatment (100 mg/kg, 200 mg/kg) decreased the levels of TBARS, LOOH in ISO-induced rats. This anti-lipid peroxidation effect of MELD may be responsible for its membrane stabilizing effect.
Cardiovascular diseases are clearly associated with Dyslipidemia and dyslipoproteinemia.[30,31] An altered lipid metabolism can alter the cardiac function by changing the properties of cardiac cell membrane and these changes may contribute to the cell death. We observed the increased levels of total cholesterol, LDL and VLDL and decreased level of HDL in the serum of ISO-induced rats. These changes in lipid levels might be due to enhanced lipid biosynthesis by cardiac cyclic adenosine monophosphate. High levels of LDL cholesterol show a positive correlation with myocardial infarction, whereas HDL cholesterol has a negative correlation.[32] The oral administration of MELD reduced the levels of LDL and increases the levels of HDL in ISO-induced rats. These results show its positive impact against dyslipidemia. Clinical trails support lipid lowering therapy to minimize the risk of cardiovascular diseases. Thus, MELD pretreatment can be considered to reduce the risk of cardiovascular diseases.
Hypertriglyceridemia is one of the major pathological conditions related to MI. We observed an increased level of TG in the serum of ISO-induced rats and it might be due to decreased activity of lipoprotein lipase, and decreased uptake of TG from the circulation. A significant increase in free fatty acid and phospholipids in the serum of ISO-induced rats were also observed. The breakdown of membrane phospholipids, by the free radicals produced by ISO metabolites, the increased peroxidation of membrane phospholipids by releases free fatty acids by the action of phospholipase A2 are the reasons for increased phospholipids.[33] Oral pretreatment with MELD (100 mg/kg and 200 mg/kg) significantly (P < 0.05) decreased the levels of phospholipids and free fatty acids in the serum of ISO-induced rats. The effect of MELD in the reduced levels of FFA and PL reflected its protective action against PL rich myocardial membrane. The findings are supporting its beneficial effect on lipid metabolism.
Most of drugs acting on dyslipidemia are the HMG CoA reductase inhibitors. HMG CoA reductase catalysis the conversion of HMG CoA to mevalonate and the inhibition of this conversion is the rate limiting step in cholesterol biosynthesis. Our results indicated that the pretreatment with MELD showed beneficial effect on dyslipidemia. Phytochemicals those showed beneficial effects on dyslipidemia have inhibited HMG CoA reductase in the animal models.[34,35] We hypothesized that the inhibiting effect of MELD on dyslipidemia in the ISO-induced rats may be due to its inhibiting character on HMG CoA reductase. To confirm this action we studied the ability of MELD on HMG CoA/mevalonate ratio. HMG CoA reductase plays a major role in the regulation of cholesterol metabolism and a rate limiting enzyme in the pathway of cholesterol biosynthesis.[29] The significant increased in the activity of HMG CoA reductase in the liver of ISO-induced rats was observed by decrease ratio between HMG CoA and mevalonate. An increased HMG CoA reductase activity leads to excessive biosynthesis of cholesterol and accumulation of cholesterol in the cardiovascular system which may lead to atherosclerosis, myocardial necrosis and infarction. Pretreatment with MELD (100 mg/kg, 200 mg/kg) significantly reduced the activity of HMG CoA reductase in ISO-induced rats and indicated by significantly increased ratio of HMG CoA and mevalonate. This might be one of the reasons for the beneficial effect of MELD on lipid and lipoprotein metabolism.
The supramaximal dose of ISO produce free radicals and generation of ROS affect the balance between cellular antioxidant defense and free radicals. Antiperoxidative enzymes such as SOD and catalase are the first line cellular defense antioxidant enzymes against oxidative stress. We observed decrease in the activities of SOD and catalase in ISO-induced rats. The enhanced lipid peroxidation and increased generation of ROS such as superoxides and hydrogen peroxides. These free radicals utilized the endogenous antioxidant enzymes SOD and catalase and make the myocardium more susceptible for further free radical attack. Oral pretreatment with MELD (100 mg/kg, 200 mg/kg) increased the levels of SOD, catalase in the heart tissue and demonstrate its antioxidant effect.
TTC is a redox indicator commonly used in biochemical experiments to differentiate between metabolically active and inactive cells and tissues. The presence of electron donor NADH favors oxidation of TTC into 1,3,5-triphenylformazan TPF. In the cell death, the NADH decreases and prevents the oxidation of TTC to TPF.[36] The presence of NADH and dehydrogenase enzymes (i.e., succinate dehydrogenase and lactate dehydrogenates) will convert the TTC in to TPF which shows red color spots in viable myocardial tissue. In the present study we observed an increased infarct size which is indicated by a clear bright/yellow color spot in the heart slices of ISO-induced rats. The utilization of NADH by the free radicals produced by ISO and leakage of dehydrogenase enzymes from the damaged myocardial cells are the possible mechanisms that might prevented the conversion of TTC in to TPF. This may be the reason for the colorless bright spot in ISO-induced rat heart and reflects the presence of non viable cells. This finding was also supported by the increased levels of LDH in the serum of ISO-induced rats, where LDH might release out from the myocardium. Oral pretreatment with MELD (100 mg/kg, 200 mg/kg) markedly reduce the infarcted size in ISO-induced rats. The antioxidant and membrane stabilizing activity of MELD could be the possible reason for reduction in the myocardial infarct size.
To further confirm the beneficial effect of MELD, histopathological study was performed on normal and experimental animals. The results showed a better insight of cardio protective effect of MELD. The normal heart showed no change in the architecture of myocardial tissue. The rats pretreated with MELD (100 mg/kg, 200 mg/kg) also showed the similar architecture as normal heart. The ISO-induced rats showed the evidence of myocardial necrosis, tissue damage, infiltration of inflammatory cells and hemorrhage. The rats received MELD (100 mg/kg) as oral pretreatment showed decreased necrosis, less tissue damage and reduced infiltration of inflammatory cells. The rats pretreated with MELD (200 mg/kg) further reduced the pathological changes and brings back the cellular architecture almost similar to the normal.
The reducing power and free radical scavenging activity of MELD were studied to confirm the mechanism action of MELD in vitro showed better reducing power than the standard compound rutin. The in vitro study results confirmed the possible antioxidant activity by MELD.
In conclusion, our results of the present study indicate that the oral pretreatment with MELD reduced the cardiac marker levels in the serum of ISO-induced rats. MELD also showed anti lipid peroxidative effect and regulated the altered lipid metabolism in the ISO-induced rats. The improvement in the activities of SOD and catalase in the ISO-induced rats showed the antioxidant potentials of MELD. The histological study by infarct size quantification assay and histo-architectural analysis of the myocardium confirmed the cardioprotective effect of MELD at various doses. Our in vitro analysis of antioxidant activity against DPPH free radicals confirmed the scavenging effect of MELD. This could be the possible mechanism of MELD against the ISO-induced pathological events of MI in rats.
ACKNOWLEDGMENTS
The authors P. Bharath Kumar and M. Mari Kannan are grateful to T.V.R.N. Reddy, Joint Secretary, Jayamukhi Institutions for his constant support and encouragement during the research work; Dr. S. Vasudeva Murthy, Principal, Jayamukhi College of Pharmacy, for his valuable suggestions to improve the plan of research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–14. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ozen T, Turkeku I. Antioxidant activities of Sarcodon imbricatum wildly grown in the black sea region of Turkey. Pharmacogn Mag. 2010;6:89–97. doi: 10.4103/0973-1296.62892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao-dong Y, Li-juan Y, Shu Y, Jing-feng Z, Hong-bin Z, Liang Li. Flavonoids from Litsea chingpingensis. Chem Nat Comp. 2008;44:642–3. [Google Scholar]
- 4.Ageel AM, Islam MW, Ginawi OT, Al-Yahya MA. Evaluation of the aphrodisiac activity of Litsea chinensis (Lauraceae) and Orchis malculata (Orchidaceae) extracts in rats. Phytother Res. 2000;8:103–5. [Google Scholar]
- 5.Hwang HK, Choi EM, Lee JH. Antioxidant activity of Litsea cubeba. Fitoterapia. 2005;76:684–6. doi: 10.1016/j.fitote.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Bharath Kumar P, Mari Kannan M, Lavanya B, Suthakaran R, Darlin Quine S. GC-MS analysis of methanolic extract of Litsea decanensis gamble and its free radical scavenging activity. J Pharm Res. 2011;4:100–3. [Google Scholar]
- 7.Banerjee A. Coronary artery disease and its problems in management. J Indian Med Assoc. 2001;9:474–5. [PubMed] [Google Scholar]
- 8.Goenka S, Prabhakaran D, Ajay VS, Reddy KS. Preventing cardiovascular disease in India -Translating evidence to action. Curr Sci. 2009;97:367–77. [Google Scholar]
- 9.De Bono DP, Boon NA. Diseases of the cardiovascular system. In: Edwards CR, Boucheir IA, editors. Davidson's principles and practice of medicine. Hong Kong: Churchill Livingstone; 2002. pp. 249–340. [Google Scholar]
- 10.Nirmala C, Anand A, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol Cell Biochem. 1996;159:85–93. doi: 10.1007/BF00420910. [DOI] [PubMed] [Google Scholar]
- 11.Folin O, Ciocalteu V. On tyrosine and tryptophane determination in proteins. J Biol Chem. 1927;27:627–50. [Google Scholar]
- 12.Zou Y, Lu Y, Wei D. Antioxidant activity of a flavonoid rich extract of Hypericum perforatm L. in vitro. J Agric Food Chem. 2004;52:5032–9. doi: 10.1021/jf049571r. [DOI] [PubMed] [Google Scholar]
- 13.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–200. [Google Scholar]
- 14.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr. 1986;44:307–15. [Google Scholar]
- 15.Punithavathi VR, Prince PS. Combined effect of Quercetin and alpha tocopherol on lipids and glycoprotein components in isoproterenol induced wistar rats. Chem Biol Interact. 2009;181:322–7. doi: 10.1016/j.cbi.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Yagi K. Lipid peroxides and human diseases. Chem Phys Lipids. 1987;45:337–51. doi: 10.1016/0009-3084(87)90071-5. [DOI] [PubMed] [Google Scholar]
- 17.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–9. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Falholt K, Lund B, Falholt W. An easy colorimetric micromethod for routine determination of free fatty acids in plasma. Clin Chim Acta. 1973;46:105–11. doi: 10.1016/0009-8981(73)90016-8. [DOI] [PubMed] [Google Scholar]
- 20.Zilversmith DB, Davis AK. Micro determination of plasma phospholipids by trichloroacetic acid precipitation. J Lab Clin Med. 1950;35:155–60. [PubMed] [Google Scholar]
- 21.Rao AV, Ramakrishnan S. Indirect assessment of HMG CoA reductase (NADPH) activity in liver tissue. Clin Chem. 1975;21:1523–5. [PubMed] [Google Scholar]
- 22.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–2. [PubMed] [Google Scholar]
- 23.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 24.Ojha N, Roy S, Radtke J, Simonetti O, Gnyawali S, Zweier JL. Characterization of the structural and functional changes in the myocardium following focal ischemia–reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;294:H2435–43. doi: 10.1152/ajpheart.01190.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhujbal SS, Kewatkar SM, More LS, Patil MJ. Antioxidant effects of roots of Clerodendrum serratum Linn. Pharmacogn Res. 2009;1:294–8. [Google Scholar]
- 26.Ebrahimzadeh MA, Ehsanifar S, Eslami B. Sambucus ebulus elburensis fruits: A good source for antioxidants. Pharmacogn Res. 2009;5:213–8. [Google Scholar]
- 27.Ramchoun M, Harnafi H, Alem C, Benlyas M, Elrhaffari L, Amrani S. Study on antioxidant and hypolipidemic effects of polyphenol-rich extracts from Thymus vulgaris and Lavendula multifida. Pharmacogn Res. 2009;1:106–12. [Google Scholar]
- 28.Kumari SS, Menon VP. Changes in levels of lipid peroxides and activities of superoxide dismutase and catalase in isoproterenol induced myocardial infarction in rats. Indian J Exp Biol. 1987;25:419–23. [PubMed] [Google Scholar]
- 29.Prince PS, Sathya B. Pretreatment with quercetin ameliorates lipids, lipoproteins and marker enzymes of lipid metabolism in isoproterenol treated cardiotoxic male Wistar rats. Eur J Pharmacol. 2010;635:142–8. doi: 10.1016/j.ejphar.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 30.Fuster V, Gotto AM., Jr Risk reduction. Circulation. 2000;102:IV94–102. doi: 10.1161/01.cir.102.suppl_4.iv-94. [DOI] [PubMed] [Google Scholar]
- 31.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: The Framingham study. Clin Chem. 2004;50:1189–200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 32.Buring JE, O’Connor GT, Goldhaber SZ, Rosner B, Herbert PN, Blum CB, et al. Decreased HDL-2 and HDL-3 cholesterol, APO A-11 and increased risk of myocardial infarction. Circulation. 1992;85:22–9. doi: 10.1161/01.cir.85.1.22. [DOI] [PubMed] [Google Scholar]
- 33.Nalbone G, Grynberg A, Chevalier A, Leonardi J, Termine E, Lafont H. Phospholipase A activity of cultured rat ventricular myocyte is affected by the nature of cellular polyunsaturated fatty acids. Lipids. 1990;25:301–6. doi: 10.1007/BF02544337. [DOI] [PubMed] [Google Scholar]
- 34.Devika PT, Stanely Mainzen Prince P. Protective effect of (–) epigallocatechin gallate (EGCG) on lipid peroxide metabolism in isoproterenol induced myocardial infarction in male Wistar rats: A histopathological study. Biomed Pharmacother. 2008;62:701–8. doi: 10.1016/j.biopha.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Devipriya N, Sudheer AR, Vishwanathan P, Menon VP. Modulatory potential of ellagic acid, a natural plant polyphenol on altered lipid profile and lipid peroxidation status during alcohol-induced toxicity: A pathohistological study. J Biochem Mol Toxicol. 2008;22:101–12. doi: 10.1002/jbt.20226. [DOI] [PubMed] [Google Scholar]
- 36.Csonka C, Kupai K, Kocsis GF, Novák G, Fekete V, Bencsik P, et al. Measurement of myocardial infarct size in preclinical studies. J Pharmacol Toxicol. 2010;61:163–70. doi: 10.1016/j.vascn.2010.02.014. [DOI] [PubMed] [Google Scholar]


