Abstract
The reaction of p-bromoanilino acetohydrazide(II) with aromatic aldehydes in alcohol yielded 2-[4-bromo aniline] N-substituted benzylidine hydrazides (IIIa-IIIj), which in presence of yellow mercuric oxide and iodine in DMF, yielded corresponding 4-bromo[(N-5-substituted 1,3,4 oxadiazole-2 –yl)methyl]aniline (IVa-IVj). Structures of the compounds synthesized were confirmed by IR, 1HNMR and MASS spectroscopic analysis. The newly synthesized compounds were screened for antibacterial, antifungal and anti-inflammatory activities. Some of the compounds showed remarkable antibacterial, antifungal and anti-inflammatory activities.
Keywords: 1,3,4-Oxadiazoles; antibacterial activity; antifungal activity; anti-inflammatory activity
INTRODUCTION
Oxadiazoles are five-membered heterocyclic compounds with two nitrogen and one oxygen atoms. They are synthesized by ring condensation and rearrangement reactions. Some of the recent studies have shown that 1,3,4-oxadiazoles and its derivatives were reported to possess antimicrobial,[1] anti-inflammatory,[2] antibacterial,[3] anticancer,[4] antifungal,[5] tuberculostatic[6] and analgesic[7] activities. Moreover the amino compounds are very commonly used as antimicrobial and germicidal drug. By considering all the above factors, it was thought to synthesize some substituted oxadiazoles derivatives derived from p-bromo aniline moiety. The compounds synthesized were screened for anti-inflammatory, antibacterial and antifungal activities.
MATERIALS AND METHODS
All the melting points were determined by open capillary method and were uncorrected. The final products were purified by recrystallization. The IR spectra were recorded by PERKIN ELMER FT-IR Spectrophotometer using a thin film supported on KBr pellets. 1HNMR spectra were obtained in DMSO and chemical shift values are reported as values in ppm relative to TMS (δ = 0) as internal standard. Mass spectra were recorded on Jeol SX 102/Da-600 mass spectrometer.
Synthesis of ethyl-2-(4-bromophenylamino) acetate (I)
A mixture of p-bromo aniline (13.9 g., 0.1 mol), ethylchloroacetate(12.25 ml, 0.1 mol) and anhydrous potassium carbonate (19.5 g, 0.15 mol) in dry acetone was refluxed on a water bath for 24 hours at 70°C. The resultant reaction mixture was cooled and filtered. From the filtrate, excess of acetone was removed by distillation. The resultant solid was recrystallized from ethanol mp:150-152°C;
IR(KBr) cm-1: 3374 (NH), 1720(C = O), 1600(C = C) 2851 (CH),599 (C--Br).
1H nmR(δ ppm): 7.16- 7.36 (4H, m,Ar),7.89 (1H,s,NH), 4.2-4.1 (2H,q,CH2), 3.85 (2H, s, CH2), 1.28-1.24 (2H,t,CH3).
Synthesis of 2-(4-bromophenylamino) acetohydrazide (II)
A mixture of (I) (12.9 g.,0.05mol) and hydrazine hydrate (2.4 ml.,99%, 0.075mol) in ethanol (100 ml) was refluxed on a water bath for 6 hours. From the reaction mixture, excess of ethanol was removed by distillation. On cooling the resultant mixture, white needle like crystals of p-bromo anilino aceto hydrazide began to separate. It was collected, filtered and recrystallized from ethanol mp:184-186°C;
IR(KBr) cm–1:3374(NH str), 2902 (C-H), 1640 (C = O), 600(C--Br).1HNMR(δ ppm): 9.5(1H,s,CONH), 8.5 (1H,s,NH), 6.4-7.1 (4H,m,Ar),4.2-4.15 (2H,s,NH2), 3.84 (2H,s,CH2).
Synthesis of 2-(4-bromophenylamino)-N’- (substituted benzylidene) acetohydrazide(IIIa-IIIj)
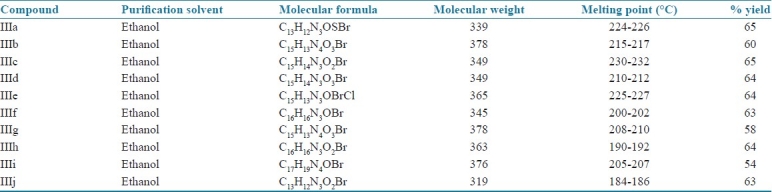
A mixture of (II) (0.01 mol) and different aromatic or heterocyclic aldehyde (0.01 mol) in ethanol was refluxed in presence of few drops of glacial acetic acid in a round bottom flask on a water bath for 6 hours. The reaction mixture was cooled and the solid thus separated was filtered, washed with ice cold water and recrystallized from ethanol. Table 1 enlists various substituents and Table 2 summarizes physical data of the above compounds.
Table 1.
Various substituents in titled compounds (IVa-IVj)
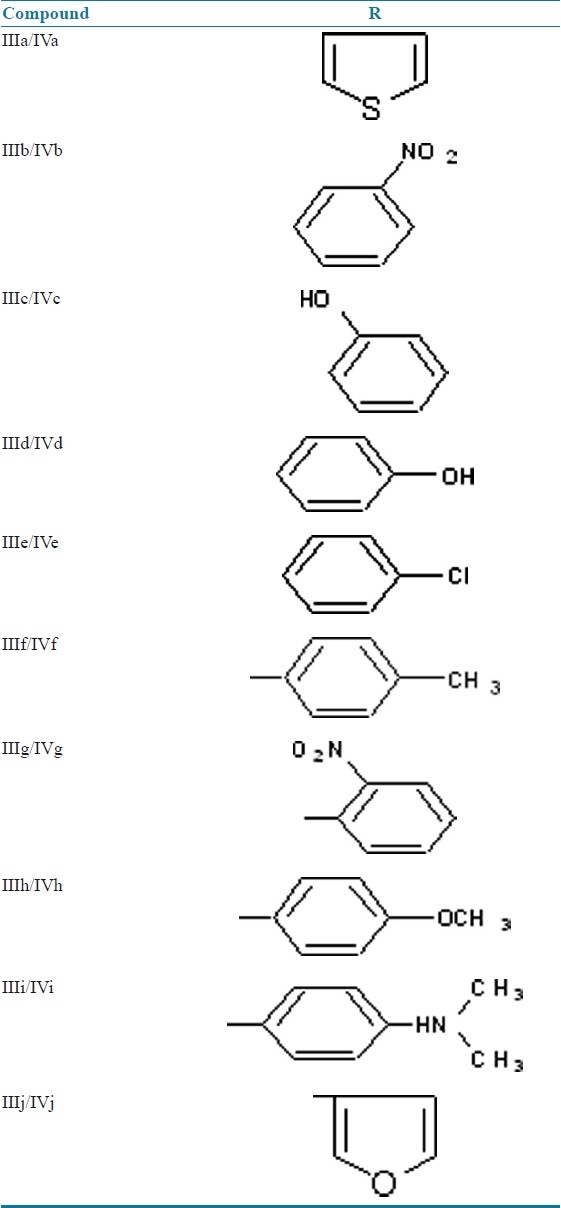
Table 2.
Physical data of compounds (IIIa-IIIj)
Synthesis of 4-bromo-N-((5-(substitutedphenyl)-1,3,4-oxadiazol-2-yl)methyl)aniline:(IVa-IVj)
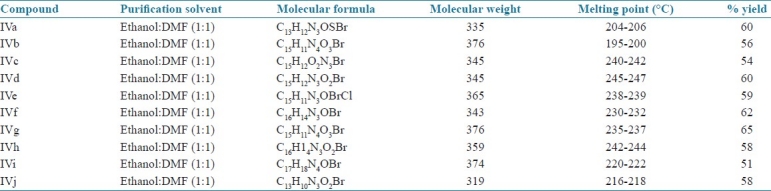
A solution of Schiff base (IIIa-IIIj) (3.78 g.,0.01 mol(III b) in DMF (40 ml) was stirred in presence of yellow mercuric oxide (3 g) and iodine (1.5 g) at room temperature for 48 hr under anhydrous conditions. The reaction mixture was filtered and poured onto crushed ice and stirred well. The solid thus separated out was washed with water and recrystallized from DMF::ethanol (1:1). Table 3 summarizes the physical data of these compounds.
Table 3.
Physical data of compound (IVa-IVj)
IIId: 2-(4-Bromophenylamino)-N’-(4-hydroxybenzylidene) acetohydrazide
IR (KBr) cm-1: 3374OH str,3292(NH str), 3111 (C-H str in CH2), 1668,(C = O) str in amide,1601(C = C str), 502 (C--Br str).1HNMR(δppm)9.82(1H,s,OH),9.78(1H,s,CONH),8.33(1H,s,NH),6.84-7.9(9H,mAr and N=CH),3.8-3.9(2H,s,CH2).
IVd:4-(5-((4-Bromophenylamino)methyl)-13,4-oxadiazol-2-yl)phenol
IR (KBr) cm–1: 3398(NH)3269, (OH),1602(C=N),1589(C=C),1169 (C-O-C), 577 (C-Br).1H nmR(δ ppm):
10.36(1H,s,OH), 8.9(1H,s,NH),6.8--8.1(8H,m, Ar--H), 2.59 (2H,s,CH2) Mass in m/z: Molecular ion peak was observed at m/z 345.
IV g.4-Bromo-N-((5-(2-nitrophenyl)-1,3,4-oxadiazol-2-yl)methyl)aniline
IR (KBr) cm-1: 3359(NH str), 1615(C=N str),1579(C=C str), 1158(C-O-C str),531(C-Br str)1 H nmR(δ ppm):
8.37(1H, s, NH), 6.7--7.8(8H, m, Ar-H), 2.33(2H, s, CH2) Mass in m/z: Molecular ion peak was observed at m/z 376.
IV h.4-Bromo-N-((5-(4-methoxy)--1,3,4-oxadiazol-2-yl)methyl)aniline
IR (KBr) cm–1: 3289(NH str), 1600(C=N str),1596(C=C str), 1132(C-O-C str),562(C-Br str)1 H nmR(δ ppm):
9.12(1H, s, NH),7.1-7.9(8H, m, Ar--H), 3.86 (3H,s, OCH3), 2.56(2H, s, CH2) Mass in m/z: Molecular ion peak was observed at m/z 359.
RESULTS AND DISCUSSION
The main aim of this work was to synthesize various substituted 4-bromo [(N-5-substituted [1,3,4 -oxadiazole-2 –yl) methyl] aniline derivatives (Scheme 1). Initially N-substituted benzylidines (IIIa-IIIj) were synthesized by reacting p-bromoaceto hydrazide (II) with substituted aromatic aldehyde in ethanol. The titled compounds (IVa-IVj) were obtained by cyclization of N-substituted benzylidine hydrazide in presence of iodine and mercuric oxide in DMF.
Scheme 1.
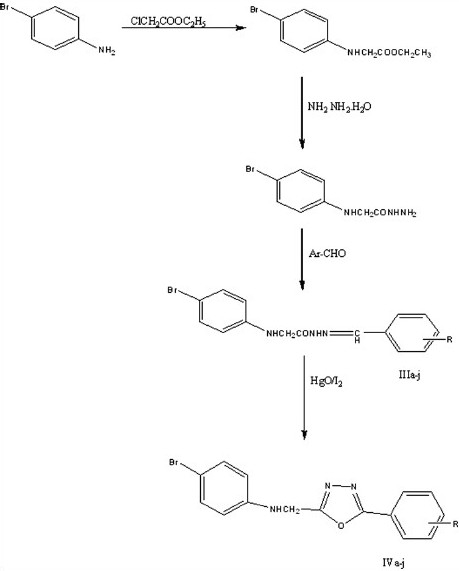
(III a-j) 2-(4-bromophenylamino)-N’- (substituted benzylidene) acetohydrazide (IVa-j) 4-bromo-N-((5-(substituted phenyl)-1,3,4-oxadiazol-2-yl)methyl)aniline
All the synthesized compounds resulted in good yields with 50-65%. The formation of title compounds (IVa-IVj) is indicated by the disappearance of peak due to CH = N of the intermediate material Schiff base in IR and 1H-NMR spectrum as given above. The IR of these compounds showed the presence of peaks due to (C=N and C-O-C) of the oxadiazole ring in all the compounds (IVa-IVj). The mass spectra of the title compounds are in confirmative with the assigned structure. The mass spectrum of these compounds showed molecular ion peaks corresponding to their molecular formula [Table 3].
Among the newly synthesized compounds IVb, IVc, IVd and IVg showed good antifungal activity. The electron withdrawing groups like chlorine and electron releasing groups like methoxy and methyl (IVe, IVf, IVh) showed better antibacterial activity. Compounds like IVa, IVd, IVf, IVg and IVj showed moderate anti-inflammatory activity.
Biological evaluation
Antibacterial and antifungal activity
All the synthesized compounds were screened for their antibacterial activity[8] against Staphylococcus aureus (MTCC 96), Bacillus subtilis, (MTCC 441), Pseudomonas aeruginosa (MTCC 1688) and Escherichia coli (MTCC 443) [strain no. indicating the source of pure culture for all species used] using amoxicillin as standard drug. All the synthesized compound were also screened for their antifungal activity against Candida albicans (MTCC 227) and Aspergillus niger (MTCC 282) using ketoconazole as standard drug. Compounds with substituents like – OH, – NO2, [IVb, IVc, IVd, IVg] showed moderate antifungal activity. Compounds with substituents like p-OCH3, p-Cl, p-CH3, [IVe, IVf, IVh] showed better antibacterial activity. Data of antimicrobial activity is summarized in Table 4.
Table 4.
Antimicrobial activity of titled compounds (IVa-IVj)

Anti-inflammatory activity by Carrageenan-induced rat hind paw edema method

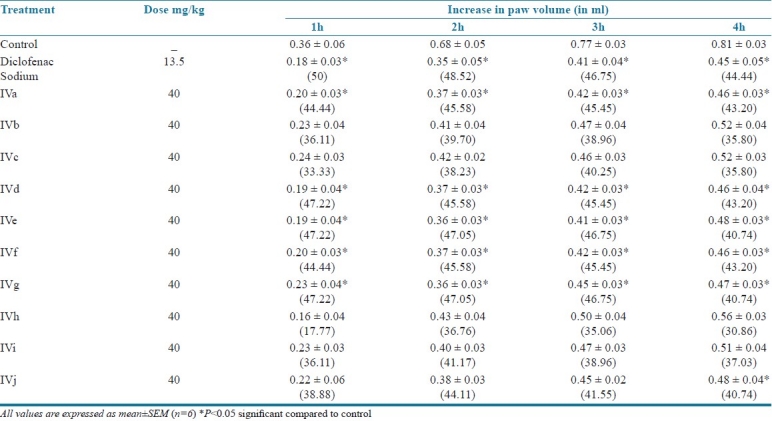
Animals were divided into 12 different groups contained six in each group. After 30 minutes of administration of standard and test compounds an inflammatory edema was induced in the left hind paw by injection of 0.1 ml of 1% of carrageenan solution in the plantar tissue of the paw of all the animals. The initial paw volume was measured plethysmographically (IITC digital plethysmograph, IITC-520) within 30 second of the injection. The relative increase in paw volume was measured in control, standard and test groups at 1, 2, 3 and 4-hour intervals after carrageenan injection.[9]
The percentage inhibition of edema volume was calculated by using the formula
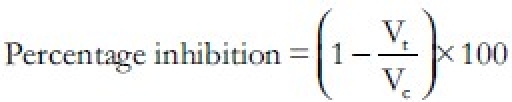
Where Vt and VC are the relative change in the edema volume of paw after the administration of the test and control, respectively. Data of anti-inflammatory activity is summarized in Table 5.
Table 5.
Anti-inflammatory activity of titled compounds (IVa-IVj)
Statistical analysis
All the values were expressed as mean ± SEM using One-way ANOVA followed by Dunnet's —t test.
CONCLUSION
The 1,3,4 oxadiazole derivatives reported showed good antimicrobial and anti-inflammatory activities. Derivatives IVb, IVc, IVd, IVg showed moderate antifungal activity. Compounds IVe, IVf, IVh, respectively, showed better antibacterial activity, whereas all the compounds showed good anti-inflammatory activity.
ACKNOWLEDGMENT
The authors are thankful to Nitte University, Deralakatte, Mangalore, India, for providing necessary facilities and also to SAIF-Punjab University, CDRI Lucknow for providing spectral data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ingole SP, Mohane SR, Berad BN. Synthesis and antimicrobial activity of 2-alkyl –aryl -5-(Pyrid-4-yl)-1,3,4-oxadiazole. Asian J Chem. 2007;19:2683–6. [Google Scholar]
- 2.Amir M, Javed SA, Kumar H. Synthesis of some 1,3,4oxadiazole derivatives as potential anti-inflammatory agents. Indian J Chem. 2007;46B:1014–9. [Google Scholar]
- 3.Siddiqui N, Khan SA, Bhat MA. Synthesis and antibacterial activity of coumarin incorporated 1,3,4oxadiazoles. Indian J Het Chem. 2005;14:271–2. [Google Scholar]
- 4.Shivarama Holla B, Poojary KN, Subrahmanya Bhat K, Ashok M, Poojary B. Synthesis and anticancer activity studies on some 2-Chloro-1, 4-bis-(-substituted-1, 3, 4-oxadiazole-2-ylmethyl enoxy) phenylene derivatives. Indian J Chem. 2005;44B:1669–73. [Google Scholar]
- 5.Mishra A, Singh DV, Mishra RM. Synthesis, antifungal activity of new1,3,4oxadiazole(3,2-b)-s-triazine-5-ones and their thiones analogues. Indian J Het Chem. 2005;14:289–92. [Google Scholar]
- 6.Franski R. Biological activities of the compounds bearing 1,3,4-oxa(thia) diazole ring. Asian J Chem. 2005;17:2063–75. [Google Scholar]
- 7.Reddy VM, Reddy PS, Reddy PC, Ratmanc V. Cyclisation by dehydrosulfuration of 1-(2-amino benzoyl) -4-aryl-3 thiosemicarbazides. Formation of 1,3,4benzotriazepinones, 1,3,4 thiadiazoles and 1,3,4 oxadiazoles. Indian J Het Chem. 1997;7:17–20. [Google Scholar]
- 8.Satyanarayana D, George S, Subrahmanyam EVS, Kalluraya B. Studied the biological activity of 5-pyridyl- 3-arylaminomethyl-1, 3, 4-oxadiazol-2 thiones. Indian J Het Chem. 1997;11:189–92. [Google Scholar]
- 9.Winter CA, Risely EA, Nuss EV. Carrageenin induced oedema in hint paw of the rats as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]


