Abstract
Drug–drug interactions (DDIs) are defined as two or more drugs interacting in such a manner that the effectiveness or toxicity of one or more drugs is altered. DDI in patients receiving multidrug therapy is a major concern. The aim of the present study was to assess the incidence and risk factors of DDIs in patients admitted in cardiology unit of a teaching hospital. A prospective, observational study was carried out for a period of 3 months (April–July 2009). During the study period, a total of 600 prescriptions were analyzed and it was found that 88 patients had at least one DDI. The percentage of DDIs was higher in females compared to males (56.82% vs. 43.18%). DDIs were observed more in the age group of 60 years and above (57.96). Patients with more than 10 prescribed drugs developed DDIs more frequently [58 (65.91%)]. Heparin [55 (62.25%)] and aspirin [42 (47.72%)] were the most common drugs responsible for DDIs. Bleeding was the commonest clinical consequence [76 (86.63%)] found in this study population. On assessment of severity of DDIs, majority of the cases were classified as moderate in severity (61.36%). Aging, female gender and increase in concurrent medications were found to be associated with increased DDIs. Patients having these risk factors can be actively monitored during their stay in the cardiology department to identify DDIs.
Keywords: Cardiology, drug–drug interactions, hospitalized patients
INTRODUCTION
Drug therapy is growing more complex, thus making appropriate decision on drug therapy increasingly challenging. Drug interactions are most important in this context and proper handling of drug–drug interactions (DDIs) may prevent harmful events.[1] DDIs are defined thus: “two or more drugs interacting in such a manner that the effectiveness or toxicity of one or more drugs is altered”.[2–5] DDI in patients receiving multidrug therapy is a major concern. Such interactions may lead to an increased risk of hospitalization and higher health care costs.[6] The incidence of actual occurrence of drug interactions has been reported to be much smaller, ranging from 0 to 1.3%.[7,8] Some studies have found that up to 11% of patients experience symptoms associated with DDIs and that DDIs are responsible for up to 2.8% of hospital admissions.[9,10] Research has also shown that DDIs are associated with increased health care use.[11] According to a recently published study, 1% of all hospital admissions are caused by DDIs, and 0.05% emergency department visits, 0.6% of the hospital admissions and 0.1% of re-hospitalizations are caused by adverse drug reactions (ADRs) due to DDIs.[12,13]
The incidence of cardiovascular diseases (CVDs) has increased in recent decades. It has been estimated that CVDs are the most common cause of death in India and as a result cardiovascular drugs have moved to the second place among all drug classes prescribed in the country.[14]
Patients with cardiovascular diseases are particularly vulnerable to DDIs due to their advanced age, polypharmacy and the influence of heart disease on drug metabolism. The DDI potential for a particular cardiovascular drug varies with the individual, the disease being treated, and the extent of exposure to other drugs.[15]
Potential for drug interaction is higher with cardiac drugs[16] and there are reports on potential DDIs in cardiology department from India.[17] There are no studies reporting actual incidence of DDIs in the Indian setting. Hence, the present study was designed to assess the incidence and pattern of DDIs in hospitalized cardiac patients in a tertiary care hospital, with the assessment of reaction characteristics, outcome and causality.
MATERIALS AND METHODS
Study design, population and data collection
A prospective observational study was carried out for a period of 3 months (April–July 2009) in a tertiary care teaching hospital. Ethical approval was obtained from Institutional ethics committee prior to initiation of the study (UEC/19/2009). Patients who were admitted consecutively to cardiology unit were included in the study. Prescriptions with two or more drugs prescribed during the hospitalization were only selected for the study. The study population comprised all patients aged 18 years or older admitted to the hospital and had a length of stay greater than 24 hours. Patients referred to the cardiology unit for evaluation, patients visiting on outpatient basis and patients who died during hospital stay were excluded from the study. Demographic information (age and gender), number of drugs taken, length of hospital stay, main diagnosis (ICD-10) and the number of additional diagnoses and laboratory investigations made were obtained from the clinical records.
All the prescriptions of the study population were screened for DDIs by using computerized DDI database system.[18] For determining the ADRs, both the medications added and as well as discontinued were considered. All drugs were classified as per Anatomical Therapeutic Chemical Classification (ATC code, level one).[19] Certain demographic characteristics were studied to find out the predictors of DDIs, such as patient characteristics [gender, age (more than 18 years old), concurrent morbidities and length of stay], drug characteristic (number of drugs) and laboratory investigations [International Normalized Ratio (INR), bleeding time, serum creatinine and serum potassium level].
The patient's medical records were screened for the presence of ADRs and the numbers of DDIs were calculated from the total number of DDIs. To classify the causality of the hospital admission to the drug, the Naranjo algorithm was used. The Naranjo algorithm or Naranjo Scale is a questionnaire designed by Naranjo et al., for determining the likelihood of whether an ADR is actually due to the drug rather than the result of other factors. Probability is assigned via a score termed definite, probable, possible or doubtful. Values obtained from this algorithm are sometimes used in peer reviews to verify the validity of author's conclusions regarding ADRs.[20] The interactions observed were classified into mild, moderate and severe according to severity and undesirable effects. The data on severity was obtained from the DDI data of the drug database.[18]
Statistical analysis
Frequencies with percentage were used to summarize sex, diagnosis, number of drugs dispensed, frequency of DDIs, drugs involved in the DDIs and severity of DDIs. Mean with 95% confidence interval was used to summarize age and length of stay. Chi-square test was used to find the association between sex, number of drugs and DDIs. Spearman's correlation was used to find the correlation between numbers of drugs, length of stay with DDIs. A “P” value of <0.05 was considered statistically significant. All analyses were performed using SPSS v. 15.
RESULTS
Patients, drug characteristics and DDIs
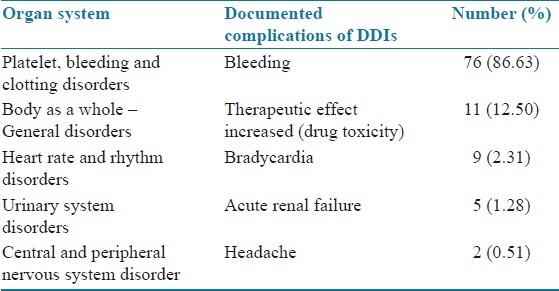
A total of 600 prescriptions were analyzed during the study period and it was found that 88 patients were confirmed with minimum of one DDI (14.66%). A significant proportion of patients with DDIs was females numbering 50 (56.82%), followed by males [38 (43.18%)]. The age group of more than 60 years had 51 (57.96%) DDIs, and was followed by other age groups. Patients who had taken more than 10 drugs developed higher number of DDIs [58 (65.91%)]. Patient characteristics and statistical significance of the results are summarized in Table 1. The patients who stayed for 5–10 days developed DDIs more frequently than other groups [48 (54.55%)]. On an average, each patient had 3 coded diagnosis in which anterior wall myocardial infarction was the most common condition [16 (18.18%)], followed by inferior wall myocardial infarction [14 (15.91%)], atrial fibrillation [9 (10.23%)], hypertension [4 (04.54%] and others [45 (51.14%)]. The most common drug classes involved in ADRs were the anti-platelets [67 (76.13%)] and anticoagulant drugs [64 (72.72%)]. Heparin [55 (62.25%)] and aspirin [42 (47.72%)] were the most common drugs responsible for DDIs. Clinically important DDIs among the prescribed drugs are summarized in Table 2. Bleeding was the commonest clinical consequence in 76 (86.63%) cases. Clinical consequences of DDIs are summarized in Table 3. The data evaluated for the specific systems affected by DDIs are summarized in Table 4.
Table 1.
Patient characteristics
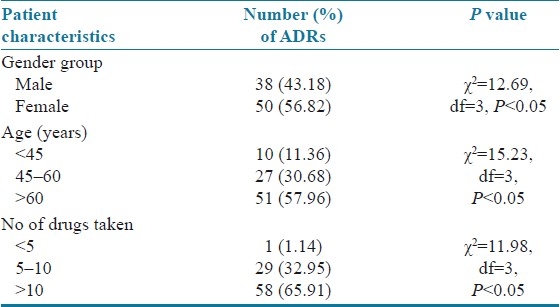
Table 2.
Clinically important DDIs among the prescribed drugs
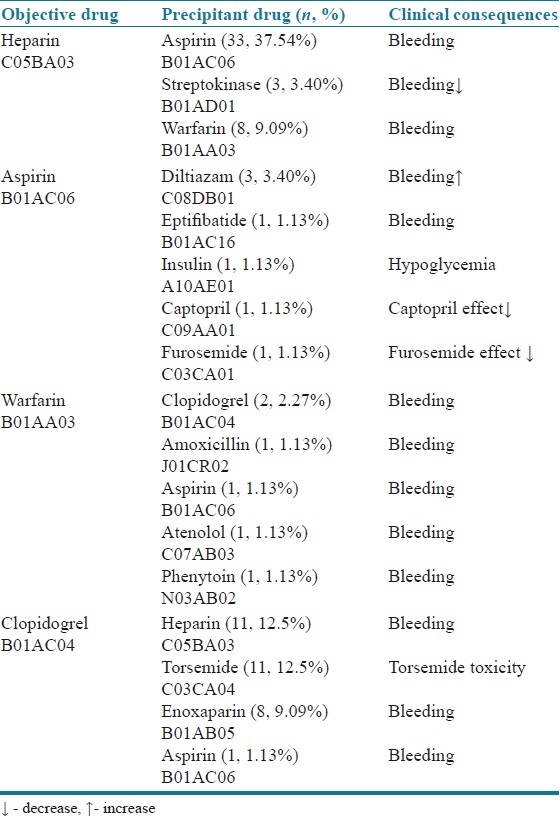
Table 3.
Clinical consequences of DDIs

Table 4.
Organ systems affected by the DDIs
Causality and severity of DDIs
All of the DDIs were assessed to have the “probable” causality by using Naranjo algorithm. The interacting drugs were withdrawn in 70 cases (79.54%) and dose was altered in 12 cases (13.63%). Sixty (68.18%) patients improved after withdrawal of interacting drugs. Rechallenge was performed in 65 patients and recurrence of symptoms occurred in 8 patients. Upon causality assessment, majority of the DDI reports were rated as probable [46 (52.27%)] followed by possible rating for 24 (27.27%) reports. These DDIs were assessed for severity in which 54 cases (61.36%) were classified as moderate followed by 33 severe cases (37.50%) and 1 mild case (1.14%).
DISCUSSION
This study revealed the overall incidence of clinically important DDIs in cardiology department to be 14.66%. The incidence compared with another study published from the same setting on potential DDIs which reported an incidence of about 30%. This study focused on the incidence of actual DDIs compared to the reported study on potential DDIs which was about the possible DDIs which may arise out of the given combination.[17] Findings of the present study showed that the patterns of incidence of DDIs are positively associated with patients’ age, gender, number of drugs prescribed and length of hospital stay. A higher rate of DDI was present in women and patients who were more than 60 years of age. This corresponds to results of other studies reporting that DDIs are common in elderly people who are on multiple drug regimens.[6,21,22]
The results showed that higher rate of DDIs seen in elderly patients was due to the increased number of medications prescribed to this population. These results are in accordance with the observation of reported studies.[23] Positive association was observed between the number of drugs prescribed and length of stay with DDIs.
In fact, some of these drug combinations are used for therapeutic benefit in clinical practice and others are introduced internationally despite the increased risk of DDIs. Some of the most common drug classes involved in DDIs were anti-platelets (76.13%) and anticoagulants (72.72%). Among these drug classes, heparin and aspirin (37.54%), clopidogrel and heparin (12.5%), clopidogrel and torsemide (12.5%) and heparin and warfarin (9.09%) were the most commonly observed drug pairs resulting in DDIs, and bleeding was the most common clinical consequence observed in the present study. This result correlates with the results of similar studies.[24] In 2006, the NPSA risk assessment of anticoagulation therapy highlighted co-prescribing of nonsteroidal anti-inflammatory drugs (NSAIDs) and other interacting medicines in anticoagulated patients as one of 15 key high-risk prescribing practices.[25] The potential consequences of such prescribing practice are an increase in the risk of bleeding complications, which have been reported to affect between 7 and 26% of warfarinized patients annually. Of these bleeds, 6–15% were reported to be minor, 1–8% were major, and 0.25–4.8% were fatal.[26,27]
Caution must be exercised in comparing the exact rates of each DDI by maintaining the normal range of activated partial thromboplastin time (aPTT) and INR value because even slight increase or decrease in plasma drug concentration can have profound clinical effects. On the other hand, for this same reason, patients using heparin and warfarin are often subject to rigorous monitoring of aPTT or INR and doses might be adjusted according to lab reports.
DDIs were reported from cardiology department. Concurrent use of many drugs and frequent addition of new drugs makes this group of patients vulnerable to DDIs. Despite all this, there is a need to increase the awareness of possible DDIs in all hospital departments, as a sizeable number of DDIs have been recorded in all of them. The age, gender, number of drugs taken and multiple disease states were identified as the risk factors for developing DDIs (P<0.0001). There was an extremely significant linear relationship (r=0.98; P<0.01) between the number of drugs prescribed and the DDIs in patients. Similarly, a significant linear relationship (r=0.96; P<0.0001) was observed between length of stay and DDIs. These results are in accordance with previous reports available in the literature.[28]
Consistent with previous research, it was observed in this study that the use of multiple medications was associated with significantly increased risk of being prescribed potentially harmful drug–drug combinations; in fact, the odds of being prescribed potentially interacting drug more than doubled for each additional medication prescribed, after controlling for other factors.[29,30] With regard to management approach for DDIs, drug withdrawal or dose reduction is usually the first step to be employed for the management of DDIs. In present study, in 70 cases (79.54%) the suspected drug was withdrawn, and in 12 (13.63%) cases the dose of the suspected drug was altered. Drug withdrawals and dose alterations of suspected drugs have been reported in the literature.[25]
On causality assessment of DDIs by using Naranjo algorithm, DDIs were confirmed to have the probable causality. Considering the severity assessment of the reactions, majority of the reactions were categorized as moderate in nature, followed by severe and mild severity, and these findings are different when compared with the reports of spontaneous reporting studies.[21,31]
The usefulness of computerized screening depends on the quality, including proper validation, of data held in the software. Furthermore, updating such systems requires knowledge, judgment and continuous effort by specialists maintaining the drug interaction database.[31]
Limitation of this study is its short duration without any intervention component. Controlled study to evaluate whether good clinical management of DDIs can reduce drug-related morbidity or mortality is needed in the future in this discipline.
CONCLUSION
This study reports the incidence of DDIs in the cardiology department in a hospital from Indian setting. This study also examined patient, drug characteristics, causality and severity of DDIs. This study shows that DDIs are frequent among hospitalized cardiac patients. The factors influencing DDIs are age, gender, number of prescribed drugs and length of hospital stay and cost. Thus, development and implementation of cautionary guidelines and computer-based screening might help to prevent potentially harmful drug interactions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Blix HS, Viktil KK, Moger TA, Reikvam A. Identification of drug interactions in hospitals -computerized screening vs. bedside recording. J Clin Pharma Therap. 2008;33:131–9. doi: 10.1111/j.1365-2710.2007.00893.x. [DOI] [PubMed] [Google Scholar]
- 2.Hartshorn EA. Handbook of drug interactions. Hamilton, IIlinois: Drug Intelligence Publications; 1973. [Google Scholar]
- 3.Peterson JF, Bates DW. Preventable medication errors: Identifying and eliminating serious drug interactions. J Am Pharma Assoc. 2001;41:159–60. doi: 10.1016/s1086-5802(16)31243-8. [DOI] [PubMed] [Google Scholar]
- 4.Ray WA, Murray KT, Meredith S, Narasimhulu SS, Hall K, Stein cm. Oral erythromycin and the risk of sudden death from cardiac causes. N Engl J Med. 2004;351:1089–96. doi: 10.1056/NEJMoa040582. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson AK, Spigset O, Jacobson I, Hagg S. Cerebral haemorrhage induced by warfarin -the influence of drug-drug interactions. Pharmaco Drug Safety. 2007;16:309–15. doi: 10.1002/pds.1291. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton RA, Briceland LL, Andritz MH. Frequency of hospitalization after exposure to known drug-drug interaction in a medical population. Pharmacotherapy. 1998;18:112–20. [PubMed] [Google Scholar]
- 7.Kurfees JF, Dotson RL. Drug interactions in elderly. J Fam Pract. 1987;25:477–88. [PubMed] [Google Scholar]
- 8.Ho YF, Huang SH, Lin HN. Detecting drug-drug interactions in medication profiles of psychiatric inpatients: A two stage approach. J Formosan Med Assoc. 2002;101:294–7. [PubMed] [Google Scholar]
- 9.Grymonpre RE, Mitenko PA, Sitar DS, Aoki FY, Montgomery PR. Drugs associated hospital admissions in older medical patients. J Am Geriatr Soc. 1988;36:1092–8. doi: 10.1111/j.1532-5415.1988.tb04395.x. [DOI] [PubMed] [Google Scholar]
- 10.Jankel CA, Speedie SM. Detecting drug interactions: A review of the literature. DICP. 1990;24:982–9. doi: 10.1177/106002809002401014. [DOI] [PubMed] [Google Scholar]
- 11.Jankel CA, McMillan JA, Martin BC. Effect of drug interactions on outcomes of patients receiving warfarin or theophylline. Am J Hosp Pharm. 1994;51:661–6. [PubMed] [Google Scholar]
- 12.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18820 patients. BMJ. 2004;329:15–29. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker ml, Kallewaard M, Caspers PW, Visser LE, Leufkens HG, Stricker BH. Hospitalization and emergency department visits due to drug-drug interactions: A literature review. Pharmaco Drug Saf. 2007;16:641–51. doi: 10.1002/pds.1351. [DOI] [PubMed] [Google Scholar]
- 14.Disease burden in India. Estimations and causal analysis. [accessed on 2009 Nov 21]. Available from: http://www.whoindia.org/LinkFiles/Commision_on_Macroeconomic_and_Health_Bg_P2Burden_of_Disease_Estimations_and_Casual_analysis.pdf .
- 15.Faulx MD, Francis GS. Adverse drug reactions in patients with cardiovascular disease. Curr Probl Cardiol. 2008;33:703–68. doi: 10.1016/j.cpcardiol.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Nolan PE, Jr, Marcus FI. Cardiovascular drug use in elderly. Am J Geriatr Cardiol. 2000;9:127–9. doi: 10.1111/j.1076-7460.2000.80021.x. [DOI] [PubMed] [Google Scholar]
- 17.Patel VK, Acharya LD, Rajakannan T, Mallayasamy S, Guddttu V, Padmakumar R. Potential drug interactions in patients admitted to cardiology wards of a south Indian teaching hospital. AMJ. 2011;4:9–14. doi: 10.4066/AMJ.2011.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomson micromedex. DRUGDEX® system. [accessed on 2010 Jan 16]. Available from: http:// www.micromedex.com/products/drugdex/
- 19.Anatomical therapeutic chemical classification system. [cited on 2010 Jan 16, accessed on 2010 Jan 16]. Available from: http://www.whocc.no/atc_ddd_index/
- 20.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roborts EA. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1991;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 21.Bjorkman IK, Fastbom J, Schmidt IK, Bernsten CB. Drug-drug interactions in the elderly. Ann Pharmacother. 2002;36:1675–81. doi: 10.1345/aph.1A484. [DOI] [PubMed] [Google Scholar]
- 22.Kohler G, Bode-Boger S, Busse R, Hoopmann M, Welte T, Boger R. Drug-drug interactions in medical patients: Effect of in-hospital treatment and relation to multiple drug use. Int J Clin Pharmacol Ther. 2000;38:504–13. doi: 10.5414/cpp38504. [DOI] [PubMed] [Google Scholar]
- 23.Snaith A, Pugh L, Simpson CR, McLay JS. The potential for interaction between warfarin and co-prescribed medication: A retrospective study in primary care. Am J Cardiovasc Drugs. 2008;8:207–12. doi: 10.2165/00129784-200808030-00007. [DOI] [PubMed] [Google Scholar]
- 24.UK national patient safety agency. Risk assessment of anticoagulants therapy. [cited on 2006 Jan, accessed on 2010 March 9]. Available from: http://www.npsa.nhs.uk/site/media/docouments/1773_anticoagulantreport.pdf .
- 25.Palarerti G, Leali N, Coccheri S. Bleeding complications of oral anticoagulant treatment: An inception cohort, prospective collaborative study (ISCOAST): Italian study on complications of oral anticoagulation therapy. Lancet. 1996;348:423–819. doi: 10.1016/s0140-6736(96)01109-9. [DOI] [PubMed] [Google Scholar]
- 26.Fitzmaurice DA, Blann Ad, Lip Gyh. ABC of antithrombotic therapy. BMJ. 2002;325:828–31. doi: 10.1136/bmj.325.7368.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aparasu R, Baer R, Aparasu A. Clinically important potential drug-drug interactions in outpatient settings. Res Social Adm Pharm. 2007;3:426–37. doi: 10.1016/j.sapharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhan C, Correa-De-Araujo R, Bierman AS, Sangal J. Suboptimal prescribing in elderly out patients: Potentially harmful drug-drug and drug disease combinations. J Am Geriatr Soc. 2005;53:262–7. doi: 10.1111/j.1532-5415.2005.53112.x. [DOI] [PubMed] [Google Scholar]
- 29.Malone DC, Hutchins DS, Haupert H, Hansten P, Duncan B, Van Bergen RC, et al. Assessment of potential drug-drug interactions with a prescription claims database. Am J Heath Syst Pharm. 2005;62:1983–91. doi: 10.2146/ajhp040567. [DOI] [PubMed] [Google Scholar]
- 30.Jose J, Rao P. Pattern of adverse drug reactions notified by spontaneous reporting in an Indian tertiary care teaching hospital. Pharma Res. 2006;54:226–33. doi: 10.1016/j.phrs.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Nightingle PG, Adu D, Richards NT, Peters M. Implementation of rules based computerized bedside prescribing and administration: Intervention study. BMJ. 2000;320:750–3. doi: 10.1136/bmj.320.7237.750. [DOI] [PMC free article] [PubMed] [Google Scholar]


