Abstract
Hydroalcoholic extract of Pycnocycla spinosa has spasmolytic effect in vitro and antidiarrhoeal action in vivo. The aim of this research was to separate fractions of total hydroalcoholic extract of P. spinosa guided by their spasmolytic activity. Aerial parts of P. spinosa were extracted with ethanol. The concentrated extract was subjected to column chromatography and thin layer chromatography. Initially four fractions were obtained (F1, F2, F3, and F4) and their spasmolytic activities were determined on ileum contraction induced by KCl (80 mM). The more active fraction was subjected to further isolation and tested to find its most active components. The active component was phytochemically characterized using phytochemical methods including ultraviolet and infrared spectroscopy. Hydroalcoholic extract of P. spinosa (10-320 μg/ml) in a concentration dependent manner inhibited ileum contraction with the IC50 value of 47 ± 8.1 μg/ml (mean ± S.E.M., n=6). Fraction F2 was the most potent inhibitor of ileum contraction (IC50= 3.4 ± 0.33 μg/ml). From five sub-fractions separated from fraction F2 (F2a, F2b, F2c, F2d, and F2e, respectively), F2c was a more active component with the IC50 value of 2.6 ± 0.27 μg/ml. The primary results of target fraction (F2c) showed sugar moiety in its structure or in one of its components. In this research we have isolated pharmacological active fraction which is most likely responsible for antispasmodic action of P. spinosa hydroalcoholic extract.
Keywords: Pycnocycla spinosa, Extract, Ileum, Bioactivity, Anti-spasmodic
INTRODUCTION
Pycnocycla spinosa Decne. ex Boiss., var. spinosa (Fam. Umbelliferae) is a wild plant growing in many parts of Iran(1,2). The essential oil has reported to have spasmolytic activity on ileum(3). The hydroalcoholic extract is also a potent relaxant of ileum and inhibits rat ileum contractions induced by acetylcholine (ACh) , serotonine (5-HT), (IC50=13 ± 2.5 μg/ml) and KCl (IC50=40 ± 7.3 μg/ml) (3–6). Hydroalcoholic extract of P. spinosa inhibits rat ileum at lower concentrations than those inhibit rat bladder or uterus contractions(7,8) which may indicate that P. spinosa extract has a relative selective inhibitory action on the ileum. In addition, P. spinosa extract has shown to have a dose dependent antidiarrhoeal action with doses of 250 μg/kg, 500 μg/kg and 1 mg/kg in mice(3,9). Comparative studies have been shown that the antidiarrhoeal activity of hydroalcoholic extract of P. spinosa extract and its effect on small intestinal transit of charcoal meal are relatively similar to loperamide and it is more potent than dicyclomine(9).
Other studies have shown that intravenous injection of P. spinosa extracts at doses that inhibit diarrhea only had a transient effect on blood pressure and heart rate(10). General assessment of drug tolerance and behavioral response also indicated that antidiarrhoeal doses of P. spinosa extract had no significant effect on CNS activity, although at higher doses it caused sedation and reduced motor activity. Therefore, P. spinosa extract has relatively potent antispasmodic effect and prevents diarrhea in mice. The lethal dose test study shows that it has a relatively good margin of safety (LD50=140 mg/kg)(11).
P. spinosa aerial extract is rich in chemical substances and contains alkaloids, flavonoids, tannins, and saponine like substances(6,12,13). The antispasmodic action of the extract is mainly due to flavonoid and alkaloid-like components in the extract with alkaloid fraction being the most bioactive components(6). Several fractions were isolated from the alkaloid rich fractions and all tested for pharmacological activity on the ileum(12). Although most of these fractions had a relaxant effect on isolated ileum, however, the productivity during isolation stages sharply reduced(6,12). Therefore, in this research we have used thin layer and column chromatography separation techniques as an alternative method to isolate the active components for further pharmacological studies and development.
MATERIALS AND METHODS
Plant material
Leaves and branches of Pycnocycla spinosa Decne. ex Boiss were collected from Isfahan University Campus (Isfahan, Iran), in June 2006 . A voucher specimen (access number A24) was deposited in the herbarium of Dept. Pharmacognosy, Isfahan School of Pharmacy, Isfahan, Iran.
Extractions and isolations
Dry, powdered aerial parts (450 g) of P. spinosa were defatted with light petroleum ether and extracted with 2500 ml of ethanol (Nasr, Iran) using perculation(14). The solution was evaporated to dryness, and the residue was suspended in methanol, coated on silica gel, and applied on top of a 40 × 4.1 cm silica gel column. Column chromatography was performed on Matrix silica gel 100A (particle size 70-230 μm). The column was eluted with light petroleum-EtOAc gradiantly (2 L), collecting 20 ml fractions. The fractions were pooled according to thin layer chromatography (TLC) profiles, evaporated, and repeatedly purified by normal-phase preparative TLC (PTLC) using chloroformethyl acetate (5:1). TLC separations were performed on precoated silica gel 60 F254 plates. Visualisation of the separated bands was carried out under UV light (365 nm). The new fractions namely F2a, F2b, F2c, F2d, F2e were detected as 5 separate bands which were scraped off and eluted with methanol in order to evaluate and compare their antispasmodic activities.
Solvents and materials
The following material and solvents were used for separation procedure: glass column (Jahad, Iran), silica gel (Merck, Germany), TLC plate (Merck, Germany), sulfuric acid (Sigma, UK). All solvents used for extraction and isolation procedures were obtained from Merck (Germany), unless stated otherwise.
UV and IR analysis
The solution of each fraction was separated by TLC and scanned using a Secomam S-1000 UV spectophotometer (France) and a Rayleigh WQF-510 IR spectophotometer (China). The UV scanning was performed between 200 and 350 nm. The major UV and IR absorption peaks were compared with known spectrophotometer patterns.
Experimental procedure
Male Wistar rats, 200-250 g, bred and kept at room temperature in School of Pharmacy animal house were killed by a blow on the head, followed by exsanguination. A piece of ileum was cut and placed in Tyrode's solution at room temperature. The ileum was trimmed and sectioned into 2-3 cm long tissues. Then the tissue was suspended via a thread in an organ bath (Harvard Apparatus, England) in Tyrode's solution at 37°C with the following composition (mM): NaCl, 136.9; KCl, 2.68; CaCl2, 1.8; MgCl2, 1.05; NaHCO3, 11.9; NaH2PO4, 0.42 and glucose 5.55 and continuously gassed with O2. From a resting tension of 1g, isotonic contractions, elicited by KCl (80 mM) were recorded using a Harvard transducer and displayed on a Harvard Universal Oscillograph pen recorder device (Harvard Apparatus, England).
Initially effects of the extract/fractions were studied on two tissues. The extract/fractions were directly added into the bath in a cumulative manner. Each concentration of extract/fractions remained in contact with the tissue for at least 10 min before its effect was evaluated. After initial screening for selected fractions the pharmacological investigation was completed on six different tissues. When appropriate, experiments were conducted in parallel with time matched control tissues.
Measurements and statistical analysis
Contractions were measured as area under the contraction records from pre-contraction baseline 5 min before addition of the next extract concentration and expressed as percentage of initial values before addition of the extracts. Mean and standard error of mean (S.E.M.) values were calculated for each group of results and inter-group comparisons were made with one way ANOVA and further compared with their time matched controls using Student's t test. Differences considered statistically significant for P<0.05.
Drugs and solutions
The solvent in the hydroalcoholic extracts was evaporated in the rotary at 50°C. The solidified extract was then weight out and prepared as 10 mg/ml stock solution in 70% ethanol and further diluted to 1 mg/ml in Tyrode's solution. Dried factions F2, F3 and F4 were prepared in 70% ethanol as 10 mg/ml stock solution and concentrations of 1 mg/ml and 100 μg/ml were prepared in Tyrode's solution. Other fractions were prepared as 1 mg/ml in 70% ethanol and further ten folds serial dilutions were prepared in Tyrode's solution. KCl was made up in distilled water as 2 M stock solution.
RESULTS
Characterization of fractions
In the column chromatography isolation procedure four fractions were obtained based on their TLC profiles which were called F1, F2, F3, and F4. F2 showed more antispasmodic activity and therefore it was selected for further bioactivity guided isolation.
In the PTLC isolation procedure fraction F2 was further separated into 5 subfractions according to their Rfvalues, namely F2a (Rf 0.22, pale yellow), F2b (Rf=0.4, cream), F2c (Rf0.54, pale green), F2d (Rf=0.64, green), and F2e (Rf0.8, blue), respectively. F2c was more active fraction and seemed to be a single component containing a sugar moiety in its structure as confirmed with Mulish reagent, UV (Max. abs. 229, 242, 258 nm) and IR (3350 cm-1, 2950 cm-1) patterns. The aglycon part of the structure needs more chemical analysis in order to confirm its chemical structure.
Bioactivity testing
Addition of KCl (80 mM) in to the bath caused a rapid contraction in the rat ileum which was maintained as long as KCl remained in the bath. Total extracts of P. spinosa (10 μg/ml to 160 μg/ml) inhibited contraction induced by KCl (IC50=47 ± 8.1 μg/ml) in a concentration dependent manner and at concentration of 160 μg/ml abolished the contractile response (Fig. 1).
Fig. 1.
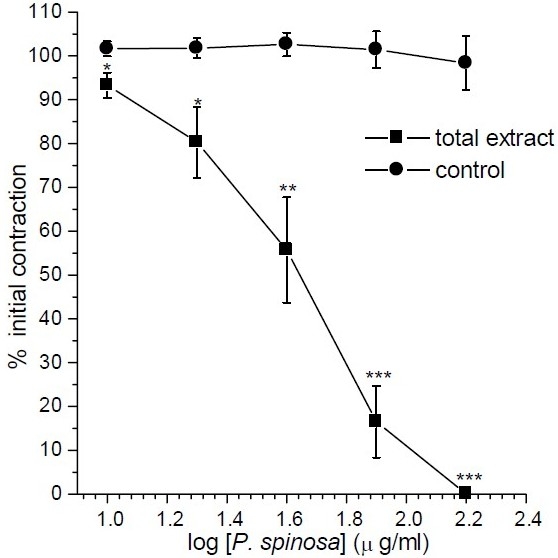
Effect of Pycnocycla spinosa total extract on tonic contractions developed in rat isolated ileum by KCl (80mM). Time-matched control tissues treated with vehicle in equivalent volume. Results are given as mean and vertical bars indicate S.E.M. (n=6). Stars show statistically significant differences between the test and control groups at corresponding points. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test).
From the initial fractions, fraction F1 mainly contained the solvent so it was discarded. Fraction F4 lacked any significant bioactive substances (IC50>320 μg/ml) and was not further persuade. Fraction F2 (IC50= 3.4 ± 0.33 μg/ml) and F3 (IC50= 6.0 ± 2 μg/ml) were the most bioactive fractions. As the F2 fraction was more potent (Fig. 2) and contained more abundant substance on gram weight basis, it was subjected to further isolation. From fraction F2, five sub-fractions were isolated and their spasmolytic activities were determined on ileum contraction induced by KCl. The active components were F2a (IC50=13.0 ± 3.0 μg/ml), F2b (IC50<1μg/ml), F2c (IC50=2.6 ± 0.27 μg/ml) and F2d (IC50=14.0 ± 2.0 μg/ml), respectively. Fraction F2e was less active than the original fraction. Although, F2b was found to be the most pharmacologically active component, however, its yield was not enough for persuading further investigation. Therefore, further studies were performed on fraction F2c. The relaxant effect of fraction F2c was very similar to that of its original F2 fraction (Fig. 3).
Fig. 2.
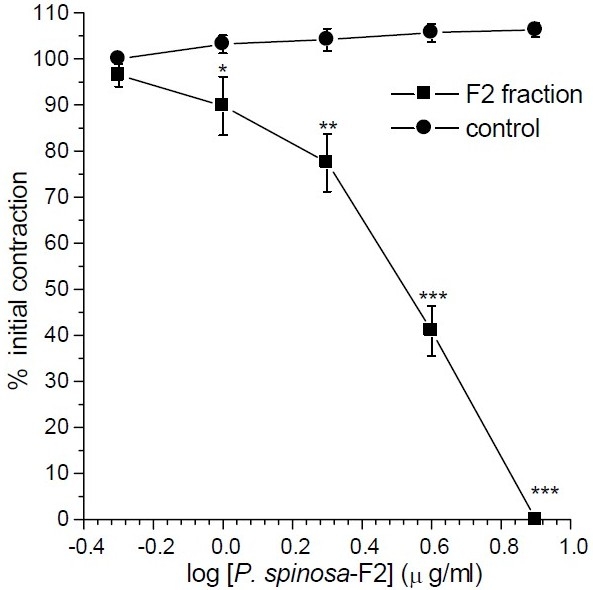
Effect of fraction F2 of Pycnocycla spinosatotal extracts on tonic contractions developed in rat isolated ileum by KCl (80mM). Time-matched control tissues treated with vehicle in equivalent volume. Results are given as mean and vertical bars indicate S.E.M. (n=6). Stars show statistically significant differences between the test and control groups at corresponding points. *P<0.05, **P<0.01, ***P<0.001 (Student's t-test).
Fig. 3.
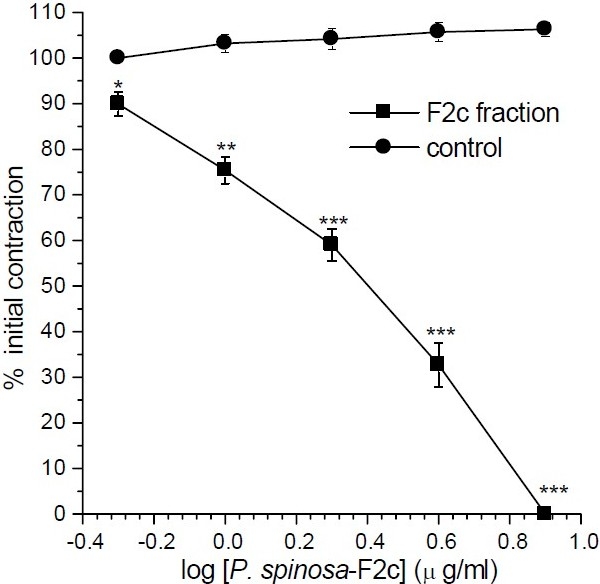
Effect of sub-fraction F2c of Pycnocycla spinosaextracts on tonic contractions developed in rat isolated ileum by KCl (80mM). Time-matched control tissues treated with vehicle in equivalent volume. Results are given as mean and vertical bars indicate S.E.M. (n=6). Stars show statistically significant differences between the test and control groups at corresponding points. *P<0.05 **P<0.01, ***P<0.001 (Student's t-test).
DISCUSSION
P. spinosa extract is a relaxant of isolated ileum and in the current study we have shown that it's inhibitory effect on rat ileum starts with about 10 μg/ml extract in the bath and at 160 μg/ml bath concentration totally removes the contractile response to KCl. These results are in consistence with previous reported results(3,6,7,12). In addition to a relative good potency of the extract(9), the relative high extract yield (12-14%) makes P. spinosa extract a suitable remedy for the treatment of diarrhea and an antispasmodic herbal medicine(9). However, the hydroalcoholic extract of P. spinosa is a mixture of unknown substances with different pharmacological activities. Therefore, it is essential that the active substances that are relaxant of ileum smooth muscle be identified. Administration of active components rather than the total extract has two main advantages. Firstly, the relative purity is increased and it is more feasible to give a standard dose. Secondly, administration of inactive substances is avoided. Although the inactive substances might be a weak relaxant of smooth muscle, but they may have other unknown pharmacological actions which might contribute to unwanted effects of the extract which are not yet known. Therefore, when an extract is being used as medicine it is essential to avoid giving unnecessary substances to the patients.
The hydroalcoholic extract usually contains a mixture of polar and non polar substances including the essential oils. However, for pharmacological studies we have used dried extract and it has been shown that there is no traceable essential oil in dried extract of P. spinosa(3). Thus, only solid components are responsible for relaxant effect of P. spinosa extract on rat ileum. The main objective of the present study was to isolate the active components of the P. spinosa extract and to compare it with the total extract. The main problem is that we have no idea about the chemical properties of the components exist in the extract. Therefore, we have used a bioassay technique based on the bioactivity properties of the separated components of the P. spinosa extract on contracted rat ileum in vitro and compared with the parent extract. In the initial phase of separation, four fractions were obtained of which fractions F2 and F3 mostly contained the active substances. More interestingly the relative potency was substantially increased and fraction F2 was at least 10 times more potent than the total extract (IC50 ratio=13.8). The fraction F3 was slightly less active than fraction F2 but again it was far more active than the total extract (IC50 ratio=7.8). Fraction F4 was even less active than the total extract. Therefore, it was concluded that the active substance(s) are mainly concentrated in fractions F2 and F3. The differences between potency of fractions F2 and F3 is an indication that there are different components, or as these fractions separated by column chromatography, it might contain different concentration of the same components.
As the fraction F2 was the most active fraction, it was further separated into five sub-fractions, all of which were relaxant of rat ileum. Fraction F2c was almost equipotent to the original fraction F2, while relaxant potency of fraction F2b was relatively improved. Fraction F2a and F2d were slightly less active than the original fraction F2. The activity of fraction F2e was substantially reduced although it still was more active than the total extract. Differences in relaxant potency of these sub-fractions again confirm the presence of a numbers of active components in the total extract which are responsible for its spasmolytic activity. Therefore, it seems that more than one component are responsible for antispasmodic effect of P. spinosa extract.
CONCLUSION
In conclusion, in this research we have adapted a suitable isolation technique for separation and identification of active components of P. spinosa hydroalcoholic extract using bioassay technique. Structural identification of most active components is recommended for further development of P. spinosa extract as a herbal remedy.
ACKNOWLEDGMENT
We would like to thanks the research department of Isfahan University of Medical Sciences, Isfahan, I.R.Iran, for financially supported this project.
REFERENCES
- 1.Mozaffarian V. A dictionary of Iranian plant names. Tehran: Farhang Moaser; 1996. pp. 443–444. [Google Scholar]
- 2.Jalili A, Jamzad Z. Red data book of Iran, A preliminary survey of endemic, rare and endangered plant species in Iran. Tehran: Research Institute of Forests and Rangelands; 1999. pp. 689–690. [Google Scholar]
- 3.Sadraei H, Asghari G, Naddafi A. Relaxant effect of essential oil and hydro-alcoholic extract of Pycnocycla spinosa Decne. exBoiss. on ileum contractions. Phytother Res. 2003;17:645–649. doi: 10.1002/ptr.1217. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadi L, Mirza M. Volatile constituents of the essential oil of Pycnocyla spinosa Decne ex. Boiss from Iran. J Essent Oil Res. 1998;10:197–198. [Google Scholar]
- 5.Asgahri G, Hoshfar G, Mahmoudi Z. Seasonal variation of mono- and sesquiterpenes in the essential oil of Pycnocyla spinosa Decne. exBoiss. Iranian J Pharm Res. 2002;1:61–63. [Google Scholar]
- 6.Sadraei H, Asghari G, Hekmatti AA. Antispasmodic effect of three fractions of hydroalcoholic extract of Pycnocycla spinosa. >J Ethnopharmacol. 2003;86:187–190. doi: 10.1016/s0378-8741(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 7.Sadraei H, Asghari G, Andisha M. Antispasmodic effect of Pycnocycla spinosa seed and aerial part extracts on rat ileum and uterus smooth muscle contractions. Daru. 2008;13:160–163. [Google Scholar]
- 8.Sadraei H, Asghari G, Arabzadah A. Effect of hydroalcoholic extract of Pycnocycla spinosa on rat isolated bladder contraction. Iranian J Pharm Res. 2004;4:237–241. [Google Scholar]
- 9.Sadraei H, Asghari G, Shams M. Antidiarrhoeal action of hydroalcoholic extract of Pycnocycla spinosa in comparison with loperamide and dicyclomine. Iranian J Pharm Res. 2011 (in press) [PMC free article] [PubMed] [Google Scholar]
- 10.Sadraei H, Asghari G, Hajhashemi V, Nezami M. Evalutaion of cardiovascular effect of Pycnocycla spinosa Decne. exBoiss., var. spinosa extracts in anaensthetized rat. Daru. 2008;14:11–14. [Google Scholar]
- 11.Sadraei H, Asghari G, Zeinodin M. Evaluation of systemic effect of Pycnocycla spinosa extract in mice using Irwin test. Res Pharm Sci. 2006;1:22–29. [Google Scholar]
- 12.Sadraei H, Asghari G, Khazael M. Relaxant effect of four fractions separated from alkaloid extract of Pycnocycla spinosa on rat isolated ileum. Res Pharm Sci. 2008;3:79–86. [Google Scholar]
- 13.Shokoohinia Y. Phytochemical analysis of Pycnocycla spinosa Decne. Ex. Boiss. PharmD [Thesis], Isfahan Isfahan University of Medical Sciences. 2007 [Google Scholar]
- 14.Samuelsson G. Drugs of natural origin. 4th ed. Stock-holm: Swedish Pharmaceutical Press; 1999. pp. 48–49. [Google Scholar]


