Abstract
Quinazolinones are interesting molecules with a wide range of biological activities. We prepared a number of quinazolinone derivatives by the condensation of 5-bromo- or 5-nitro-substituted anthranilic acids with chloro-acyl chlorides. Anthranilic acid derivatives were treated with either 3-chloro-propionyl chloride or 4-chloro-butyryl chloride to yield the corresponding N-acyl-anthranilic acids. The resultants were reacted with acetic anhydride to afford the benzoxazinone intermediates, which upon condensation with elected amines in either DMF or ethanol gave the corresponding tricyclic 4(3H)-quinazolinone derivatives. It was found that reactions in DMF produced higher yields.
Keywords: Anthranilic acid, Benzoxazinone, Tricyclic quinazolinone
INTRODUCTION
4(3H)-Quinazolinone ring backbone have been incorporated in several important heterocyclic compounds(1). Some of those have been prepared for their antibacterial, antifungal(2–4), anti inflammatory(5) and anticancer(6) properties. Literature surveys revealed that the syntheses of quinazolinones were achieved by the use of anthranilic acid(7–9), 2-aminobenzamide(7,10) and 2-aminobenzonitril or their derivatives(7).
Quinazolinone based natural products demanding more structurally complex precursors have been constructed indirectly via thioamid formation, oxidation of dehydro-quinazolinone and azawitting condensation(11,12). Most of these procedures have significant drawbacks such as long reaction times, harsh reaction condition, difficult work-up and use of environmentally toxic reagents or media(11). In contrast to the hitherto described methods, herein, in a simple and direct method, we report the reactions of substituted anthranilic acids with 3-chloro propionyl chloride or 4-chloro butyryl chloride for the preparation of the tricyclic quinazolinone target compounds.
MATERIALS AND METHODS
Instrumentation
Melting points were determined in open capillaries using electrothermal 9200 melting point apparatus and are uncorrected. IR (KBr discs) was recorded with a WQF -510 FT-IR-spectrophotometer. 1H-NMR spectra were recorded on Bruker 400 or 80 MHz spectrometers using TMS as an internal standard and either DMSO-d6 or CDCl3 as solvents. Mass spectra were recorded on Shimadzu Mass spectrometer. All chemical were purchased from Merck Company.
Preparation of compounds
Various synthetic procedures, as described throughout the text where appropriate, were used to prepare target compounds. Synthetic routes for the preparation of the target compounds are shown in Schemes 1–4. The target compounds were then purified by column chromatography and/or preparative thin layer chromatography and their structures were confirmed by 1HNMR, mass spectrometer and FT-IR spectrophotometer.
Scheme 1.
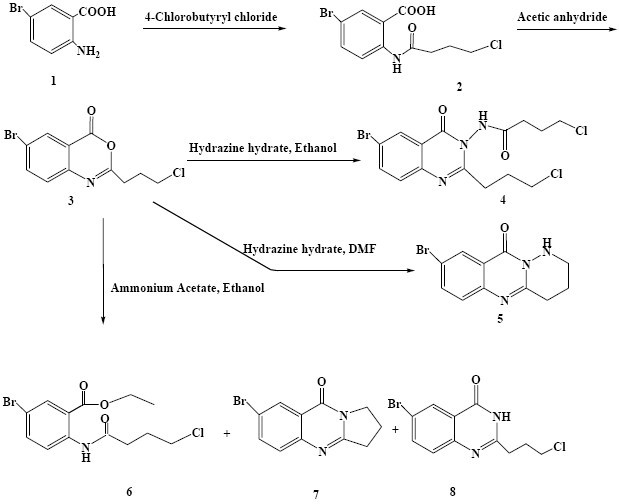
Synthesis of the target compounds 4-8
Scheme 4.
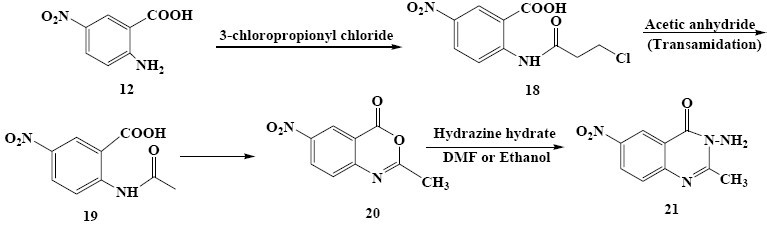
Synthesis of compound 21
RESULTS
Details of preparation procedures and chemistry of synthesized compounds
5-Bromo-2-(4-chlorobutanamido) benzoic acid (2)
4-Chlorobutyrylchloride (0.075 mol, 9 ml) was added drop wise to a solution of (0.069 mol, 15 g) 5-bromo anthranilic acid (1) in dimethyl formamide (35 ml) and stirred at room temperature for 3 h. The mixture was poured into water. The precipitate was collected by filtration, washed with water, and dried under reduced pressure to give 2 as a white solid (79% yield). (Scheme 1), m.p.: 152.5-154.4° C. MS (m/z) 320.4 (M+), 322 (M+2) for (C11H11BrCl NO3) ; M.W. 320.4. IR νmax, 3323(N-H), 3060 (C-H Ar), 2966 (C-H), 1676, 1700 (C=O), 1600 (C=C Ar), 791 (C-Cl) cm-1. 1HNMR δH(80 MHz; CDCl3), 11.2 (1H, s, NH), 8.5 (1H, d, J=8.8 Hz, H-3 Ar), 8.1 (1H, s, H-6 Ar), 7.7 (1H, d, J=8.8 Hz, H-4 Ar), 3.7 (2H, t, J=8.4Hz, NH-CO-CH2-CH2-CH2-Cl), 2.6 (2H, t, J=8.4 Hz, NH-CO-CH2-CH2-CH2-Cl), 2.1 (2H, qui, J=8.4 Hz, NH-CO-CH2-CH2-CH2-Cl).
6-Bromo-2-(3-chloropropyl)-4H-benzo[d] [1,3] oxazin-4-one (3)
Compound 2 (0.018 mol, 6 g) was dissolved in acetic anhydride (180 ml) and heated for 1 h with vigorous stirring. The solvent was removed by distillation under reduced pressure to give 3 as a light yellow solid (62% yield). (Scheme 1), m.p.: 114.7-115° C. 1HNMR δH (80MHz; CDCl3), 8.2 (1H, s, H-5 Ar), 7.8 (1H, d, J=6.9 Hz, H-8 Ar), 7.4 (1H, d, J=6.9 Hz, H-7 Ar), 3.7 (2H, t, J=5.2 Hz, N=C-CH2-CH2-CH2-Cl), 2.8 (2H, t, J=5.2 Hz, N=C-CH2-CH2-CH2-Cl),2.3 (2H, qui, J=5.2 Hz, N=C-CH2-CH2-CH2-Cl).
N-(6-Bromo-2-(3-chloropropyl)-4-oxoquinazolin- 3(4H)-yl)-4-chloro-butanamide (4) and 8-bromo-1,2,3,4-tetrahydropyridazino[6, 1-b] quinazolin-10-one(5)
Benzoxazinone (3) (0.11 mol, 3.51 g) was treated with excess amounts of hydrazine hydrate in ethanol or DMF under reflux condition for 2 h. The obtained product by either procedure was purified by column chromatography on silica gel using CHCl3-MeOH (49:1) as eluent to afford 4 and 5 (Scheme 1).
(4): White niddle crystals (25% yield), m.p.: 208.8-209.1° C, MS (m/z, %): 421 (M+,16), 317 (100), 253 (10) for (C15H16BrCl2N3O2); M.W. 421.12 , IR νmax, 3263 (N-H), 3122-3076 (C-H , Ar), 2926- 2854 (C-H), 1691, 1680 (C=O), 1620(C=N) cm-1. 1HNMR δH(400MHz; CDCl3), 10.9 (1H, s, NH), 8.7 (1H, d, J=9.2 Hz, H-8 Ar), 8.0 (1H, S, H-5 Ar), 7.6 (1H, d, J=9.2 Hz, H-7 Ar), 3.7 (2H, t, J=6 Hz, N=C-CH2-CH2-CH2-Cl), 3.6 (2H, t, J=6.4 Hz, -N-NH-CO-CH2-CH2-CH2-Cl), 3.1 (2H, t, J=7.6 Hz, N=C-CH2-CH2-CH2-Cl), 2.7 (2H, t, J=7.2Hz, -N-NH-CO-CH2-CH2-CH2-Cl), 2.3 (2H, qui, J=6.8 Hz, N=C-CH2-CH2-CH2-Cl), 2.2 (2H, qui, J=6.4Hz, -N-NH-CO-CH2-CH2-CH2-Cl). (5): White crystals (50% yield), m.p.: 203.7-204.8°C, MS (m/z, %): 280 (M+,100), 251 (25), 224 (7.5) for (C11H10Br N3O); M.W. 280. IR νmax, 3251 (N-H), 2954 (C-H), 1664 (C=O), 1606 (C=C, Ar) cm-1; 1HNMR δH(400MHz; CDCl3), 8.4 (1H, s, H-9 Ar), 7.8 (1H, d, J=8 Hz, H-7 Ar), 7.7 (1H, d, J=8 Hz, H-6 Ar), 7.1 (1H, s, N-H), 3.3 (2H, t, J=7.2 Hz, N=C-CH2-CH2-CH2), 3.2 (2H, t, J=7.2 Hz, N=C-CH2-CH2-CH2), 2.2 (2H, qui, J=7.2 Hz, N=C-CH2-CH2-CH2).
Ethyl -5- bromo -2- (4-chlorobutanamido) benzoate (6), 7-bromo-2, 3-dihydropyrrolo 2,1-b] quinazolin-9(1H)-one (7) and 6-bromo-2-(3-chloropropyl) quinazolin-4(3H)-one (8)
Benzoxazinone (3) (0.11 mol, 3.51 g) was treated with excess amounts of ammonium acetate in ethanol or DMF and refluxed for 2 h. The residues from both reactions were purified by column chromato-graphy on silica gel using an eluent of CHCl3-MeOH 19:1 to obtain 6, 7 and 8 from the ethanolic solution and 7, 8 from DMF (Scheme 1).
(6): White crystals (24% yield), m.p.: 79.5-80.8° C. MS (m/z, %): 348 (M+, 26), 243 (100), 215(10), 197(40) for (C13H15 BrCl NO3); M.W. 348. IR νmax, 3321 (N-H), 2925-2852 (C-H), 1678, 1725 (C=O), 1597 (C=C, Ar) cm-1. 1HNMR δH (400 MHz ; CDCl3), 11.2 (1H, s, NH), 8.6 (1H, d, J=9.2 Hz, H-3 Ar), 8.1 (1H, d, J=2.4 Hz, H-6 Ar), 7.6 (1H, dd, J=8.8 Hz, J= 2.4 Hz, H-4 Ar), 4.4 (2H, qua, J=7.2 Hz, COO-C H2-CH3), 3.6 (2H, t, J=6.4Hz, NH-CO-CH2-CH2-CH2-Cl), 2.6 (2H, t, J=6.8 Hz, NH-CO-C H2-CH2-CH2-Cl), 2.2 (2H, qui, J=6.8 Hz, NH-CO-CH2-CH2-CH2-Cl), 1.4 (3H, t, J=7.2 Hz, COO-CH2-CH3).
(7): Light yellow (24% yield from ethanol, 31% yield from DMF), m.p.:187.8-189.5° C. MS (m/z, %): 265 (M+, 100), 238 (6.6), 184 (25) for (C11H9 Br N2O); M.W. 265. IR νmax, 2925-2852 (C-H), 1685 (C=O), 1616 (C=C Ar), 1259(C-N) cm-1. 1HNMR δH (400MHz; CDCl3), 8.4 (1H, d, J=3.8 Hz, H-8 Ar), 7.8 (1H, dd, J=8.8 Hz J=4 Hz H-6 Ar), 7.5 (1H, d, J=8.4 Hz, H-5 Ar), 4.2 (2H, t, J=7.2, N=C-CH2-CH2-CH2), 3.1 (2H, t, J=8 Hz N=C-CH2-CH2-CH2), 2.3 (2H, qui, J=7.2 Hz N=C-CH2-CH2-CH2).
(8): White crystals (50% yield from ethanol, 63% yield from DMF), m.p.: 251-252° C. MS (m/z, %): 265 (100), 236 (62), 210 (20) for (C11H10BrClN2 HCl); M.W. 301.5. IR νmax, 3076 (C-H Ar), 3026-2962 (C-H), 1645 (C=O), 1022-1074 (Br-Ar) cm-1. 1HNMR δH (400MHz; DMSO), 8.1 (1H, d, J=2 Hz, H-5 Ar), 7.9 (1H, dd, J=9 Hz J=2Hz H-7 Ar), 7.5 (1H, d, J=8.5 Hz, H-8 Ar), 4.2 (2H, t, J=7.5 Hz, N=C-CH2-CH2-CH2-Cl), 3.0 (2H, t, J=8 Hz, N=C-C H2-CH2-CH2-Cl), 2.2 (2H, qui, J=7.5 Hz, N=C-CH2-C H2-CH2-Cl).
5-Bromo-2-(3-chloropropanamido)benzoic acid (9)
3-Chloropropionyl chloride (0.075 mol, 7.21 ml) and 5-bromoanthranilic acid (0.069 mol, 15 g) were reacted according to the procedure explained for 2 to give 9 as a light yellow solid (65% yield). (Scheme 2), m.p.: 184.7-185.9°C. MS (m/z) 306.5 (M+), 308.8 (M+2) for (C10H9BrCl NO3), M.W. 306.5. IR νmax, 3319 (N-H), 3060 (C-H Ar), 2974 (C-H), 1678, 1700 (C=O), 1599 (C=C Ar), 789 (C-Cl) cm-1. 1HNMR δH (80 MHz;DMSO), 11.2 (1H, s, NH), 8.5 (1H, d. J=8.4 Hz, H-3 Ar), 8.1(1H, d, J=4.2 Hz, H-6 Ar), 7.7 (1H, dd, J=8.5 Hz, J=4.2 Hz, H-4 Ar), 3.9 (2H, t, J=8 Hz, NH-CO-CH2-C H2-Cl), 2.9 (2H, t, J=8 Hz, NH-CO-CH2-CH2-Cl).
Scheme 2.
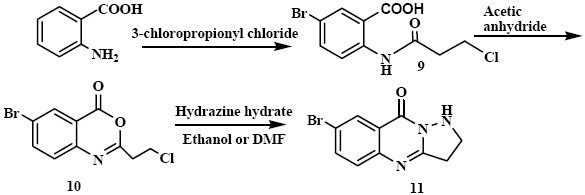
Synthesis of the target compound 11
6-Bromo-2-(2-chloroethyl)-4H-benzo[d] [1,3] oxazin-4-one (10)
Compound 9 (0.019 mol, 6 g) was dissolved in acetic anhydride (15 ml) and heated for 1 h with vigorous stirring. The solvent was re-moved by distillation under reduced pressure. The obtained unstable yellow residue was used for the next step without purification (Scheme 2).
7-Bromo-2, 3-dihydropyrazolo [5,1-b] quinazolin-9(1H)-one (11)
Compound 10 (0.012 mol, 3.5 g) and exces of hydrazine hydrate were refluxed in DMF or Ethanol for 3 h. The reaction mixtures under both conditions were purified by column chromatography on silica gel using an eluent of CHCl3-MeOH (49:1) to give 11 as light yellow crystals (68% yield from DMF, 37% yield from ethanol), (Scheme 2) m.p.: 212.5-213°C, MS (m/z, %): 266 (M+, 100), 253 (4), 238 (16.6), 210 (20), 197 (13) for (C10H8 BrN3O); M.W. 266, IR νmax, 3228 (N-H), 2914(C-H), 1658 (C=O), 1622(C=C, Ar) cm-1. 1HNMR δH (400MHz ; CDCl3), 8.4 (1H, s, H-8 Ar), 7.8 (1H, d, J=8 Hz, H-6 Ar), 7.5 (1H, d, J=8 Hz, H-5 Ar), 5.8 (1H, br s, NH), 3.7 (2H, t, J=7.6 Hz, N=C-CH2-CH2), 3.4 (2H, t, J=7.6 Hz, N=C-CH2-CH2).
2-(4-Chlorobutanamido)-5-nitrobenzoic acid (13)
4-Chlorobutyrylchloride (0.059 mol, 6.6 ml) was added dropwise to a solution of (0.054 mol, 10g) 5-nitroanthranilic acid (12) in DMF (27 ml). The mixture was poured into water and stirred for 1 h. The precipitated product was collected by filtration, washed with cold water, and dried under reduced pressure to give 13 as a light yellow solid (77% yield). (Scheme 3) m.p.: 150.5-151.2° C. MS (m/z) 286.5(M+), 288 (M+2) for (C11H11ClN2O5); M.W. 286.5 IR: νmax, 3124 (N-H), 2970 (C-H), 1703, 1630 (C=O), 1576, 1352 (NO2) cm-1. 1HNMR δH(400 MHz; DMSO): 13.0 (1H, bs,
Scheme 3.
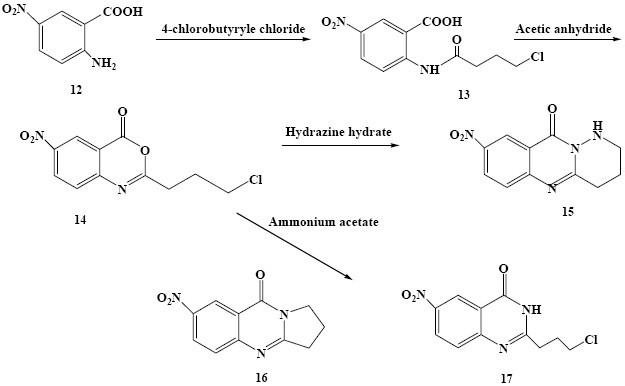
Synthesis of the target compounds 15-17
2-(4-Chlorobutanamido)-5-nitrobenzoic acid (13)
4-Chlorobutyrylchloride (0.059 mol, 6.6 ml) was added dropwise to a solution of (0.054 mol, 10g) 5-nitroanthranilic acid (12) in DMF (27 ml). The mixture was poured into water and stirred for 1 h. The precipitated product was collected by filtration, washed with cold water, and dried under reduced pressure to give 13 as a light yellow solid (77% yield). (Scheme 3) m.p.: 150.5-151.2° C. MS (m/z) 286.5(M+), 288 (M+2) for (C11H11ClN2O5); M.W. 286.5 IR: νmax, 3124 (N-H), 2970 (C-H), 1703, 1630 (C=O), 1576, 1352 (NO2) cm-1. 1HNMR δH(400 MHz; DMSO): 13.0 (1H, bs, COOH), 11.5 (1H, s, NH), 8.6 (2H, m, H-6, H-3 Ar), 8.4 (1H, d, J=6.4 Hz, H-4 Ar), 3.7 (2H, m, NH-CO-CH2-CH2-CH2-Cl), 2.6(2H, m, NH-CO-CH2-CH2-CH2-Cl), 2.0 (2H, m, NH-CO-CH2-CH2-CH2-Cl).
2-(3-Chloropropyl)-6-nitro-4H-benzo[d] [1,3] oxazin-4-one (14)
Compound 13 (0.013 mol, 4 g) was dissolved in acetic anhydride (12 ml) and heated for 1 h with vigorous stirring. The solvent was removed by distillation under reduced pre-ssure. The obtained unstable residue was used for the next step without purification (Scheme 3).
8- Nitro -1,2,3,4- tetrahydropyridazino [6,1-b] quinazolin-10-one (15)
To a solution of 14 (0.012 mol, 3.48 g) in ethanol or DMF was added excess of hydrazine hydrate and heated at reflux temperature for 2 h. Colored suspensions, orange (in ethanol) and red (in DMF) were filtrated off and washed with iso-propanol to provide 15 as pure light brown solid (50% yield from DMF, 25% yield from ethanol). (Scheme 3), m.p.: 251-252°C. MS (m/z, %): 246 (M+, 100), 219 (16) for (C11H10N4O3); M.W. 246, IR νmax, 3251 (N-H), 3032-2879 (C-H), 1666 (C=O), 1608 (C=C Ar), (1595, 1342 NO2) cm-1. 1HNMR δH (400MHz; CDCl3), 9.1 (1H, d, J=2.8 Hz, H-9 Ar) , 8.5 (1H, dd, J=9.2 Hz, J=2.8 Hz, H-7 Ar), 7.7 (1H, d, J=9.2 Hz, H-6 Ar), 7.1(1H, s, NH), 3.3 (2H, t, J=6.8 Hz, N=C-CH2-CH2-C H2), 3.1 (2H, t, J=7.2 Hz, N=C-CH2-CH2-CH2), 2.2 (2H, qui, J=7.2 Hz, N=C-CH2-CH2-CH2).
7-Nitro-2,3-dihydropyrrolo [2, 1-b] quinazolin-9(1H)-one (16) and 6-nitro 2-(3-chloropropyl)- quinazolin-4(3H)-one (17)
The benzoxazinone (14) (0.012 mol, 3.48 g) was reacted with excess of ammonium acetate in ethanol or DMF to give yellow suspensions which was filtrated off and washed with iso-propanol. The yellowish precipitated products from both solvents were fractionated by column chromatography on silica gel using an eluent of CHCl3-MeOH (49:1) to give 16 and 17 (Scheme 3).
(16): Light yellow, (35.7% yield from DMF, 30% yield from ethanol), m.p.: 190.8-191.3° C. MS (m/z, %): 231 (M+, 100), 201 (38), 185 (21), 173(16) for (C11H9N3O3); M.W. 231, IR νmax, 3105-3076 (C-H, Ar), 2970 (C-H), 1693 (C=O), 1606 (C=C, Ar), 1570, 1388 (NO2) cm-1. 1HNMR δH (400MHz; CDCl3), 9.0 (1H, d, J=2.4 Hz, H-8 Ar), 8.4 (1H, dd, J=8.8 Hz, J=2.4 Hz, H-6 Ar), 7.7 (1H, d, J=8.8 Hz, H-5 Ar), 4.2 (2H, t, J=7.6 Hz, N=C-CH2-CH2-C H2), 3.2 (2H, t, J=8 Hz N=C-CH2-CH2-CH2), 2.3 (2H, qui, J=7.6 Hz, N=C-CH2-CH2-CH2).
(17): Light yellow, (33% yield from DMF, 24% yield from ethanol), m.p.: 228.9-229.5° C. MS (m/z, %): 267(M+, 31), 253(8), 222 (8), 231(100), 185 (23) for (C11H10ClN3O3); M.W. 267.5, IR νmax, 3093 (C-H, Ar), 3041 (C-H), 1645 (C=O), 1612 (C=C Ar), 1520, 1338 (NO2) cm-1. 1HNMR δH (400MHz; DMSO), 8.7 (1H, s, H-5 Ar), 8.5 (1H, d, J=8.4 Hz, H-7 Ar), 7.7 (1H, d, J=8.8Hz, H-8 Ar), 4.3 (2H, t, J=7.6 Hz, N=C-CH2-CH2-CH2-Cl), 3.0 (2H, t, J=8Hz, N=C-C H2-CH2-CH2-Cl), 2.2 (2H, qui, J=7.6 Hz, N=C-CH2-CH2-CH2-Cl).
2(3-chloropropanamido)- 5-nitrobenzoic acid (18)
3-Chloropropionyl chloride (0.059 mol, 5.6 ml) was added dropwise to a solution of (0.054 mol, 10 g) 5-nitro anthranilic acid (12) in DMF (25 ml). The mixture was poured into water and stirred for 1 h. The precipitated product was collected by filtration, washed with cold water, and dried under reduced pressure to give 18 as a yellow solid (70% yield). Since the product was precipitated in reaction and was pure enough further recrystallizaion was not required (Scheme 4).
3-Amino-2-methyl-6-nitroquinazolin-4(3H)-one (21)
Compound 18 (0.02 mole, 6 g) was treated with acetic anhydride to give the corresponding benzoxazinone (20). The resulting benzoxazinone was refluxed in ethanol in the presence of excess hydrazine hydrate for 2 h. “This reaction was repeated in DMF with the same reaction condition”. The red solution was cooled to give a precipitate, which was fractionated by column chromatography on silica gel using an eluent of CHCl3-MeOH (49:1) to give 21 as orange solid (25% yield from DMF, 18% yield from ethanol), m.p.: 197.7-198.8° C. MS (m/z, %): 220 (M+, 100), 191 (37.5) for (C9H8N4O3); M.W. 220, IR νmax, 3419 (N-H), 2962-2856 (C-H), 1558, 1340 (NO2) cm-1. 1HNMR δH (400MHz; CDCl3), 9.1 (1H, d, J=5 Hz, H-5 Ar), 8.5 (1H, dd, J=8.8 Hz, J=5 Hz, H-7 Ar), 7.7 (1H, d, J=8.5 Hz, H-8 Ar), 4.9 (2H, s, NH2), 2.7 (3H, s, CH3).
Formation of unexpected product as 21 could be explained by trans amidation of 18 to 19 (was not isolated) by means of direct effect of acetic anhydride. Further steps were carried out as the usual procedure for preparation of benzoxazinon (20) (was not isolated) and quinazolinon (21) (Scheme 4).
DISCUSSION
In the first step to produce quinazolinone derivatives, 5-bromoanthranilic acid (1) was treated with 4-chloro-butyryl chloride to yield amide 2. Treatment of 2 with acetic anhydride afforded the benzoxazinone (3) through dehydrative cyclization mechanism(8,13). The benzoxazinone (3) was then reacted with hydrazine hydrate in ethanol or DMF to give 4 and 5, respectively. Interestingly, the products obtained from the third step of the reactions were found to be mainly depended on the nature of the solvent used. The reaction of 3 with excess of hydrazine hydrate in ethanol yielded 4, while refluxing a mixture of 3 with excess of hydrazine hydrate in DMF gave rise to a cyclic quinazolinone product (Scheme 1, compound 5).
In ethanol, hydrazine hydrate acted as a nucleophile and attacked the carbonyl group of the benzoxazinone molecule which resulted in the ring opening to afford the intermediate compound A. Subsequently the lone pair electron of nitrogen of the intermediate compound A attacked to the carbonyl group of the side chain to form an intermediate, which upon its dehydration produced compound B. Then, NH2 functionality in compound B attacked to 2 to afford 4 and 5-bromo anthranilic acid as shown in Scheme 5.
Scheme 5.
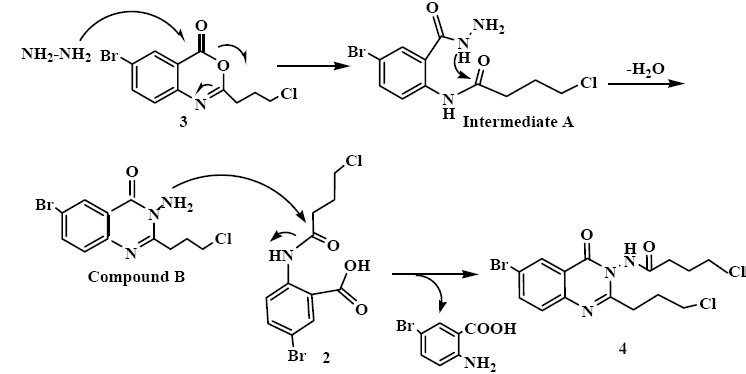
Proposed mechanism for the synthesis of compound 4
In DMF solvent hydrazine hydrate acted as a nucleophile and reacted with the carbonyl group of cyclic ester 3. As shown in Scheme 6, nucleophilic attacks of individual carbohydrazine nitrogens to the carbonyl group of the amide and methylene chloride functionality resulted in the production of 5. In a similar manner, the benzoxazinone (3) was treated with excess amounts of ammonium acetate in ethanol or DMF. The residues from either reaction were purified by column chromatography to obtain 6, 7 and 8 (from ethanol) and 7 and 8 (from DMF) (Scheme 1).
Scheme 6.
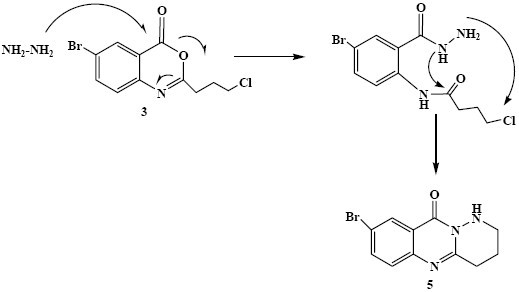
Proposed mechanism for the synthesis of compound 5
Similarly, we synthesized benzoxazinone (10) using 3-chloro propionyl chloride. The benzoxazinone (10) and excess hydrazine-hydrate were refluxed in DMF or ethanol for 3 h. The reaction mixtures from both solvents were purified by column chromatography to obtain 11 (Scheme 2). Reaction in DMF was cleaner compared with the one in ethanol. Compound 11 which is a five-membered ring analogue of 5 was produced by a similar mechanism explained for the synthesis of 5.
Next, as shown in Scheme 3, 5-nitroanthranilic acid (12) was used as the starting material. 4-Chlorobutyryl chlorid was added to a solution of 5-nitroanthranilic acid in DMF to produce 13 which was subsequently cyclized to the benzoxazinone intermediate 14 by heating with acetic anhydride. Then, to a solution of 14 in ethanol or DMF excess hydrazine hydrate was added. Orange ethanolic and reddish DMF suspensions were filtrated off and washed with isopropanol to provide 15 as a pure product. In a similar reaction, the benzoxazinone (14) was reacted with excess ammonium acetate in ethanol or DMF to give yellow suspensions which was filtrated off and washed with isopropanol. The yellowish precipitated products in both solvents were fractionated by column chromatography to give 16 and 17 (Scheme 3).
Finally, 5-nitroanthranilic acid (12) was reacted with 3-chlopropionyl chloride (Scheme 4) to obtain 18, which treated with acetic anhydride to give the corresponding benzoxazinone (20) via intermediate (19).
Benzoxazinone (20) was then refluxed in ethanol or DMF with excess of hydrazine hydrate to give 21 (Scheme 4). Reaction of 18 with acetic anhydride did not follow the usual manner to give the corresponding benzoxazinone; instead, transamidation of (18) with the acetic anhydride gave 19 which upon its ring closure afforded intermediate (20).
CONCLUSION
In conclusion, application of chloro acyl chloride instead of acyl chloride in quinazolinone synthetic procedures resulted in a second ring closure. This second ring closure was achieved when an intramolecular nucleophilic attack to the end methylen chloride group was possible. Formation of highly stable five- or six-membered ring may be counted as a good reason for this ring closure. Generally, reaction in DMF resulted in more clean reactions with higher yields compared with that of ethanol. Initial screening of the target compounds indicated that some of them had considerable anti-bacterial, antifungal and cytotoxic activities.
ACKNOWLEDGMENT
This work was financially supported by research council of Isfahan University of Medical Sciences.
REFERENCES
- 1.Khosropour AR, Mohammadpoor-Baltork I, Ghorbankhani H. Bi (TFA) 3-[nbp] Fecl4: A new, efficient and reusable promoter system for the synthesis of 4(3H)-quinazolinone derivatives. Tetrahedron Lett. 2006;47:3561–3564. [Google Scholar]
- 2.Patel JA, Mistry BD, Desai KR. Synthesis and antimicrobial activity of newer quinazolinone. Eur J Chem. 2006;3:97–102. [Google Scholar]
- 3.Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D. Synthesis an antimicrobial activities of some novel substituted 2-imidazolyl–N-(4-oxo-quinazolin-3(4H)-yl) acetamides. Chem Pharm Bull. 2007;55:1615–1619. doi: 10.1248/cpb.55.1615. [DOI] [PubMed] [Google Scholar]
- 4.Jantova S, Stankovsky S, Spirkova k. In vitro antibacterial activity of ten series of substituted quinazolines. Biologia Bratislava. 2004;59:741–752. [Google Scholar]
- 5.Laddha SS, Wadod Kar SG, Meghal SK. Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1. Synthesis, characterization and anti-inflammatory, antimicrobial activity of 6,8-disubstituted 2-phenyl-3-[substituted- benzothiazol-2-yl]-4(3H)-quinazolinone. Arkivoc. 2006:1–20. [Google Scholar]
- 6.Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT. Synthesis and in vitro antitumor activity of 4(3H)-quinazolinone derivatives with dithiocar-bamate side chains. Bioorg Med Chem Lett. 2005;15:1915–1917. doi: 10.1016/j.bmcl.2005.01.083. [DOI] [PubMed] [Google Scholar]
- 7.Van zyl EF. A survey of reported synthesis of methaqualone and some positional and structural isomers. Forensic Sci Int. 2001;122:142–149. doi: 10.1016/s0379-0738(01)00484-4. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Ryder H, Pretswell I, Depledge P, Milton J, Hancox TC, et al. Studies on quinazolinones as dual inhibitors of Pgp and MRP1 in multidrug resistance. Bioorg Med Chem Lett. 2002;12:571–574. doi: 10.1016/s0960-894x(01)00804-6. [DOI] [PubMed] [Google Scholar]
- 9.Jatav V, Mishra P, Kashaw S, Stables JP. Synthesis and CNS depressant activity of some novel 3-[5-substituted 1, 3, 4-thiadiazol-2-yl] -2- styryl quinazoline-4(3H)-ones. Eur J Med Chem. 2008;43:135–141. doi: 10.1016/j.ejmech.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Deb roy A, Subramanian A, Mukhopadhyay B, Roy R. A one- pot synthesis of novel sugar derived 5,6-dihydro-quinazolino [4,3-b] quinazolin-8-one :an entry towards highly fuctionalized sugar-heterocyclic hybrids. Tetrahedron Lett. 2006;47:6857–6860. [Google Scholar]
- 11.Kshirsagar UA, Mhaske SB, Argade NP. Hexamethyldisilazane-iodine induced intramolecular dehydrative cyclization of diamides: a general access to natural and unnatural quinazolinones. Tetrahedron Lett. 2007;48:3243–3246. [Google Scholar]
- 12.Eguchi S. Recent progress in the synthesis of heterocyclic natural products by the Staudinger/ intramolecular aza-witting reaction. Arkivoc. 2005:98–119. [Google Scholar]
- 13.Eissa AMF, El-metwally AM, El-hashash MA, El-gohary AMF. Synthesis and biological evaluation of some new 2-propyl-4(3H)-quinazolinone derivatives as anti-bacterial. J korean chem Soc. 2008;52:328–337. [Google Scholar]


