Abstract
Background:
Discovery of new plant species with antioxidant properties is a priority of many research teams. Most of the species included in this study are unstudied for antioxidant properties, but they are taxonomically related to reference plants with well-documented antioxidant activity.
Materials and Methods:
Free radical scavenging activity of plant extracts was evaluated using a 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. An aluminum chloride colorimetric method was used for flavonoid determination. The amount of phenolic compounds in the extracts was estimated by using the Folin–Ciocalteu reagent.
Results:
As a result of screening, it was found that the significant antioxidant properties possess several unstudied until now plant species (Veronica bellidioides L., V. kellereri Deg. et Urm, V. vindobonensis (M. Fisher) M. Fisher, V. beccabunga L., V. rhodopaea L., V. austriaca (Velen.) Degen., Clinopodium vulgare L., Stachysrecta L., Clematis vitalba L., and Xeranthemum annum L.). The antioxidant potential of the new species is comparable to that of reference medicinal plants.
Conclusions:
The existing data presented here provide new information for antioxidant potential of plant species that have not been traditionally used as medicinal plants.
Keywords: Asteraceae, DPPH, flavonoids, phenols, Salvia, Veronica
INTRODUCTION
Recently, there is an increasing interest toward plant extracts as a potential source of naturally occurring new antioxidants. The evaluation of antioxidant properties of plant extracts has been extensively performed by 1,1-diphenyl-2-picrylhydrazyl (DPPH) assays. This is a quick, reliable, and reproducible method.[1–4] It has been observed that phenols and flavonoids contribute significantly to the antioxidant capacity of plant extracts.[5–7] That is why many research groups investigated the connection between the antioxidant capacity and polyphenol content of plants.[7–10] In most cases, the surveys have been related to traditionally used medicinal plants.[6,11] However, there are a lot of species taxonomically related to medicinal plants that have not been evaluated for their antioxidant capacity.
Previously, we have performed phytochemical studies on the genus Veronica and Salvia.[12,13] We have identified flavonoid compounds from these species. However, we have not studied their biological activity.
This study deals with estimation of antioxidant potential, total phenolic content, and total flavonoid content of extracts from 38 plant species, the most of which are unstudied for antioxidant properties, with a aim to discover potential extracts as newly sources of antioxidant activity.
MATERIALS AND METHODS
Plant material
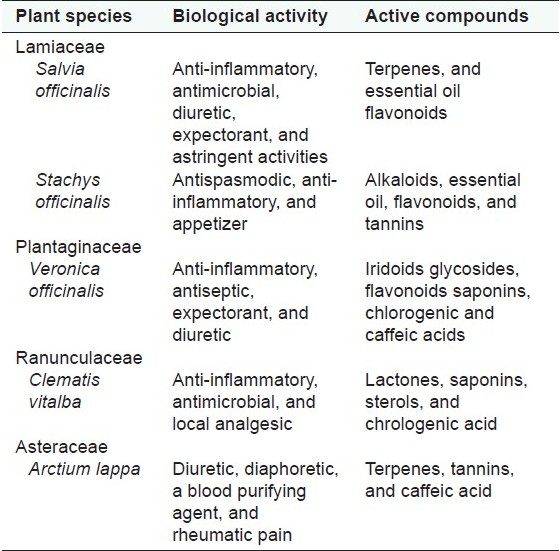
The plants used for this study were collected from natural habitats in Bulgaria. Voucher specimens were deposited at the Herbarium of the Institute of Biodiversity and Ecosystem Research, Sofia (SOM). Data about pharmacological activity and main group biologically active compounds of reference medicinal plants are presented in Table 1.
Table 1.
Pharmacological properties and main group components of reference medicinal plants[14]
Preparation of extracts
Two grams of air-dried ground plant material was extracted with 80% methanol three times. The combined MeOH extracts were evaporated under vacuum to give crude MeOH extracts that were subject to subsequent analysis.
DPPH radical scavenging activity
The DPPH radical scavenging method was used for the determination of the antioxidant capacity of the extracts.[2] Different concentrations of the plant extract (10, 20, 50, 100, 200, and 300 μg/ml, in methanol) were added at an equal volume (2.5 ml) to methanol solution of DPPH (0.3 mM, 1 ml). After 30 min at room temperature, the Ab values were measured at 517 nm on a spectrophotometer (Jenway 6320D) and converted into the percentage antioxidant activity using the following equation: DPPH antiradical scavenging capacity (%) = [1 – (Abof sample – Abof blank)/Abof control] × 100. Methanol (1.0 ml) plus plant extract solution (2.5 ml) was used as a blank, while DPPH solution plus methanol was used as a control. The IC50 values were calculated by the sigmoid non-linear regression model using plots, where the abscissa represented the concentration of tested plant extracts and the ordinate the average percent of scavenging capacity (Software Prizm 3.00). IC50 values denote the concentration of the sample required to scavenge 50% of DPPH radicals.
Flavonoid content
The content of flavonoids was determined by a pharmacopeia method (1989) using rutin as a reference compound.[7] One milliliter of the plant extract in methanol (10 g/l) was mixed with 1 ml aluminum trichloride in ethanol (20 g/l) and diluted with ethanol to 25 ml. The absorption at 415 nm was noted after 40 min at room temperature. Blank samples were prepared from 1 ml plant extract and one drop of acetic acid, and diluted to 25 ml. The absorption of rutin solutions was measured under the same conditions. Standard rutin solutions were prepared from 0.05 g rutin. All determinations were carried out in duplicate. The amount of flavonoids in plant extracts in rutin equivalents (RE) was calculated by the following formula: X = (A × m0 × 10)/(A0 × m), where: X is the flavonoid content, mg/g plant extract in RE; A is the absorption of plant extract solution; A0 is the absorption of standard rutin solution; m is the weight of plant extract, g; and m0 is the weight of rutin in the solution, g.
Phenol content
The amount of total phenolics in the extracts was determined by the Folin-Ciocalteu procedure, using gallic acid as the standard.[4,15] Distilled water (2 ml) was combined with 0.5 ml of sample, 200 μl of Folin–Ciocalteu's reagent, and 2 ml of Na2CO3 (6%). After incubation at room temperature for 40 min, the absorbance of the mixture was measured at 765 nm against a blank without the sample. Quantification was done on the basis of the standard curve of gallic acid. Results were expressed as milligrams of gallic acid equivalents (GAE) per gram of extracts.
Statistical analysis
All of the experiments were carried out in triplicate. The content of total phenols and flavonoids was presented as mean ± SD. The correlation coefficient was calculated using MS Excel software (CORREL statistical function).
RESULTS AND DISCUSSION
Methanol extracts of 38 species listed in Table 2 were examined for their antiradical potential using a DPPH assay. Most of species included in this study are unstudied for antioxidant properties, but they are taxonomically related to reference plants (Salvia officinalis, Salvia tomentosa, Stachys officinalis, and Veronica officinalis) with well-documented antioxidant activity. Among the plants screened, the extracts of Salvia scabiosifolia, Veronica chamaedrys, Clinopodium vulgare, Veronica vindobonensis, Salvia ringens, Veronica bellidioides, Tanacetum macrophyllum, Veronica persica, Veronica rhodopaea, Stachys recta, Clematis vitalba, and Vetronica beccabunga are the most active and their IC50 values are 17.72, 21.65, 22.33, 24.48, 25.71, 31.52, 37.85, 37.85, 39.21, 39.79, 41.17, 46.73, and 47.85 μg/ml, respectively. Other plant extracts of Salvia pratensis, S. nemorosa, Veronica kellereri, V. austriaca, and Xeranthemum annum also possessed significant activity and their IC50 values were between 50 and 100 μg/ml. The extracts of Veronica polita, V. teucrium, and V. hederifolia have shown IC50 values below 200 μg/ml. Little antioxidant activity (>200 μg/ml) was observed for 15 extracts. These results show that free radical scavenging effects (IC50 values) of part of newly studied species were similar to those of the reference plants: Salvia officinalis (IC50 = 35.72), Salvia tomentosa (IC50 = 11.32), and Veronica officinalis (IC50 = 17.59) which defines them as perspective objects for further research.
Table 2.
Antiradical activity, total flavonoids, and phenols of methanol extracts of the studied plant species
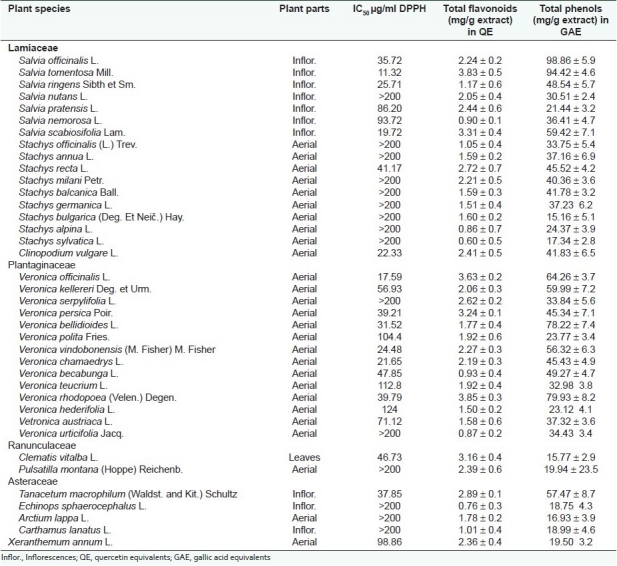
The content of phenolic compounds in the studied methanol extracts expressed in GAE, varied between 9.91 and 98.86 mg/g. The highest amounts were found in the extracts of Salvia officinalis and Salvia tomentosa. A high content of phenolic compounds also exhibited the extracts of Veronica bellidioides, V. rhodopaea, V. officinalis, V. kellereri, Salvia scabiosifolia, and Tanacetum macrophilum.
The level of flavonoids, expressed in quercetin equivalents in mg/g of plant extract, varied from 0.6 to 3.8 mg/g. The highest amounts were found for the extracts of Salvia tomentosa, S. scabiosifolia, Veronica officinalis, V. persica, Tanacetum macrophyllum, and Clematis vitalba.
Although in some cases the extracts with strong antiradical activity are abundant in flavonoids or phenolic compounds, statistically significant correlation between these indicators is not established. The correlation coefficient (R) between a DPPH assay and data of flavonoid content of the studied extracts was only 0.286 while between free radical scavenging activity and total phenolic compounds was 0.560. The received result that the antioxidant properties of the studied extracts are correlated more strongly with the content of phenolics than that of flavonoids is in agreement with finding by other authors.[5,7,16]
According to the received results, it was concluded that 16 plant extracts (Veronica bellidioides, V. persica, V. rhodopaea, V. vindobonensis, V. chasmaedrys, V. beccabunga, V. kellereri, V. austriaca, Salvia scabiosifolia, S. ringens, S. pratensis, Clinopodium vulgare, Tanacetum macrophyllum, Stachys recta, Clematis vitalba, and Xeranthemum annum) of 38 tested have potent antioxidant activity, achieved by scavenging abilities observed against DPPH. The existing data give new information for the antioxidant potential and polyphenol content of plant species that have not been traditionally used as medicinal plants.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Cimpoiu C. Analysis of some natural antioxidants by thin-layer chromatography and high performance thin-layer chromatography. J Liq Chromatogr Relat Technol. 2006;29:1125–42. [Google Scholar]
- 2.Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, et al. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002;163:1161–8. [Google Scholar]
- 3.Kukić J, Petrović S, Niketić M. Antioxidant activity of four endemic Stachys taxa. Biol Pharm Bull. 2006;29:725–9. doi: 10.1248/bpb.29.725. [DOI] [PubMed] [Google Scholar]
- 4.Matkowski A, Piotrowska M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia. 2006;77:346–53. doi: 10.1016/j.fitote.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 5.0Katalinic V, Milos M, Kulisic T, Jukic M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006;94:550–7. [Google Scholar]
- 6.Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from Bulgarian herbs. Phytother Res. 2006;20:961–5. doi: 10.1002/ptr.1985. [DOI] [PubMed] [Google Scholar]
- 7.Miliauskas G, Venskutonisa PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 8.Bajpai M, Pande A, Tewari SK, Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr. 2005;56:287–91. doi: 10.1080/09637480500146606. [DOI] [PubMed] [Google Scholar]
- 9.Kim K-H, Tsao R, Yang R, Cui S.W. Phenolic acid profiles and antioxidant activities of wheat bran extracts and the effect of hydrolysis conditions. Food Chem. 2006;95:466–73. [Google Scholar]
- 10.Wojdylo A, Oszmiañski J, Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–9. [Google Scholar]
- 11.Ivanova D, Gerova D, Chervenkov T, Yankova T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol. 2005;96:145–50. doi: 10.1016/j.jep.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Nikolova M, Renée JG, Genova E, Porter EA. Exudate flavonoids from Bulgarian species of Salvia. Biochem Syst Ecol. 2006;34:360–4. [Google Scholar]
- 13.Nikolova M, Taskova R, Peev D. Exudate flavonoid aglycones of Veronica: ecological and systematic implications. Biochem Syst Ecol. 2005;33:1258–68. [Google Scholar]
- 14.Nikolov S, editor. Sofia: Publishing House Trud; 2007. Specialized encyclopedia of the medicinal plants in Bulgaria. [Google Scholar]
- 15.Nićiforović N, Mihailović V, Masković P, Solujić S, Stojković A, Muratspahić DP. Antioxidant activity of selected plant species: Potential new sources of natural antioxidants. Food Chem Toxicol. 2010;48:3125–30. doi: 10.1016/j.fct.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Moussa AM, Emam AM, Diab YM, Mahmoud ME, Mahmoud AS. Evaluation of antioxidant potential of 124 Egyptian plants with emphasis on the action of Punica granatum leaf extract on rats. Int Food Res J. 2011;18:535–42. [Google Scholar]


