Abstract
Background:
The role of non-polymeric phenolic (NP) and polymeric tannin (PT) constituents in the antioxidant and antibacterial properties of six brands of green, black, and herbal teas of Camellia sinensis were investigated.
Materials and Methods:
Total phenolic content (TPC) and ascorbic acid equivalent antioxidant capacity (AEAC) were assessed using the Folin-Ciocalteu and 2,2-diphenyl-1-picrylhydrazyl (DPPH) assays, respectively. Minimum inhibitory dose (MID) against Gram-positive Micrococcus luteus, Staphylococcus aureus, and Bacillus cereus, and Gram-negative. Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa was assessed using the disc-diffusion method. Teas were extracted with hot water successively three times for one hour each time. The extracts were fractionated using Sephadex LH-20 column chromatography to obtain the NP and PT constituents.
Results:
Extraction yields ranged from 12 to 23%. Yields of NP fractions (70–81%) were much higher than those of PT fractions (1–11%), suggesting that the former are the major tea components. Ranking of antioxidant properties of extracts was green tea>black tea>herbal tea. For all six teas, antioxidant properties of PT fractions were significantly higher than extracts and NP fractions. Extracts and fractions of all six teas showed no activity against the three Gram-negative bacteria. Green teas inhibited all three Gram-positive bacteria with S. aureus being the least susceptible. Black and herbal teas inhibited the growth of M. luteus and B. cereus, but not S. aureus. The most potent were the PT fractions of Boh Cameron Highlands and Ho Yan Hor with MID of 0.01 and 0.03 mg/disc against M. luteus.
Conclusion:
Results suggested that NP constituents are major contributors to the antioxidant and antibacterial properties of teas of C. sinensis. Although PT constituents have stronger antioxidant and antibacterial properties, they constitute only a minor component of the teas.
Keywords: Extracts, fractions, minimum inhibitory dose, non-polymeric phenolics, polymeric tannins
INTRODUCTION
Produced from young leaves of Camellia sinensis L. (Kuntz), tea is one of the most popular beverages worldwide. It is cultivated in more than 30 countries worldwide, and of the total amount of tea produced and consumed in the world, 78% is black, 20% is green, and 2% is oolong.[1,2] Black tea is consumed primarily in western countries and in south Asian countries such as India and Sri Lanka, whereas green and oolong teas are consumed mainly in east Asian countries such as China, Japan, and Taiwan.
Teas of C. sinensis undergo different manufacturing processes. Green tea is produced by steaming (Japan) or panning (China) to prevent catechin oxidation by polyphenol oxidase.[3] With no fermentation, green tea leaves retain their green color and almost all of their original polyphenol content. Oolong tea is semi-fermented while black tea is fully fermented.[4–6] The different processes of manufacturing give the various teas their characteristic colors and flavors. Oolong tea has an excellent characteristic combining the freshness of green tea and the fragrance of black tea.[6]
Green tea contains mainly flavanols or catechins of epigallocatechin gallate (EGCG), epigallocatechin (EGC), epicatechin gallate (ECG), and epicatechin (EC).[3,7] In black tea, the major polyphenols are thearubigins and theaflavins.[3,8] The major theaflavins of black tea are theaflavin, theaflavin 3-gallate, theaflavin 3’-gallate, and theaflavin 3,3’-gallate.[9,10] Theaflavins are orange-red compounds responsible for the astringent taste and coppery color of black tea.[11,12] Although thearubigins are most abundant in black tea, their chemical nature and structure are largely unknown. They are water-soluble, acidic, and often rust-brown with structures ranging from dimeric and trimeric to tetrameric, and with molecular weights of 700–2000.[13]
Tea polyphenols are well-known for their antioxidant properties. Green tea has greater antioxidant potential than oolong and black teas.[14–18] Studies have shown that the strong antioxidant properties of green tea are attributed to catechins of EGCG and EGC.[19–22] The three adjacent hydroxyl groups on the B-ring of EGCG, GCG, EGC, and GC are more effective in scavenging free radicals than the two adjacent OH groups of ECG, CG, and EC.[7] Black tea is also known to have potent antioxidant properties which are manifested by its ability to scavenge free radicals, inhibit lipid peroxidation, and chelate metal ions.[9,23] Although green tea has higher total phenolic content (TPC), free radical scavenging activity, and ferric reducing power, its ferrous ion-chelating ability is poorer than black tea.[17,18]
Tea polyphenols are also known for their antibacterial activity. In general, antibacterial activity decreases when the extent of tea fermentation is increased, implying stronger activity in green tea than black tea.[24,25] Green tea catechins, particularly EGCG and ECG, have antibacterial activity against both Gram-positive and Gram-negative bacteria.[26–28] Green tea can prevent tooth decay by inhibiting oral bacteria.[29] The antibacterial activity of black tea has also been reported.[24–26]
Although much work has been done on the antioxidant and antibacterial properties on teas of C. sinensis, it remains unclear whether these bioactivities are attributed to non-polymeric phenolic (NP) or to polymeric tannin (PT) constituents. This prompted us to compare the antioxidant and antibacterial properties of extracts, NP fractions, and PT fractions of different brands of green, black, and herbal teas of C. sinensis.
MATERIALS AND METHODS
Tea samples
Camellia sinensis green teas (Sea Dyke, Ito En, and Boh), and black teas (Boh Cameron Highlands and Boh Bukit Cheeding) were purchased from the supermarket. Herbal tea of Ho Yan Hor was purchased from a Chinese drug store. The tea samples were stored in a cool dry place before analysis. Brief descriptions of each of the teas are given in Table 1.
Table 1.
Brief descriptions of the studied teas

Extraction
Tea samples were extracted using the hot water method.[18] Teas (25 g) were extracted with 250 ml of hot water three times, with continuous swirling at 120 rpm in an orbital shaker, for 1 h each time. The boiling water was allowed to cool throughout the extraction process to mimic tea brewing. After filtration under suction through Whatman No. 1 filter paper, the residues were re-extracted again with 250 ml of hot water. The water in the extracts was removed using a freeze dryer. Dried extracts were kept at -20°C in a freezer for further analysis.
Fractionation
Tannins were fractionated using Sephadex LH-20 column chromatography.[30–32] The crude extract (1 g) was suspended in 10 ml of water and applied onto a chromatographic column (40 × 3 cm) packed with Sephadex LH-20 (GE Health, Sweden) and equilibrated with 100% (v/v) methanol. The column was washed with 200 ml of 100% methanol (v/v) and with 200 ml of 70% acetone (v/v) to obtain the NP and PT constituents, respectively. The fractions were then dried using a rotary evaporator at 50°C.
Folin-Ciocalteu assay
TPC of extracts was determined using the Folin-Ciocalteu method.[33,34] Samples (300 μl, in triplicate) were introduced test tubes wrapped in aluminum foil followed by addition of 1.5 ml of FC reagent (10 times dilution) and 1.2 ml of sodium carbonate solution (7.5% w/v). The tubes were allowed to stand in the dark for 30 min before absorbance was measured at 765 nm. TPC was expressed as gallic acid equivalent (GAE) in mg/g of sample. The calibration equation for gallic acid was y = 0.0111υ + 0.0148 (R2 = 0.9998).
DPPH radical scavenging assay
Antioxidant activity was measured using the DPPH radical scavenging assay.[34,35] Different dilutions of the extracts (1 ml) were added to 2 ml of DPPH (5.9 mg/100 ml methanol) in test tubes wrapped in aluminium foil. Absorbance (A) was measured at 517 nm after 30 min incubation in the dark. All measurements were made with distilled water as blank. The scavenging ability (%) of the samples was calculated as (Acontrol – Asample)/Acontrol × 100) and calculated as IC50, the concentration of sample needed scavenge DPPH free radicals by 50%. IC50 was expressed as ascorbic acid equivalent antioxidant capacity (AEAC) using the equation: AEAC (mg AA/g sample) = IC50 (AA)/IC50 (sample) × 105 . The IC50 of AA used for calculation of AEAC was 0.00387 mg/ml.
Disc-diffusion method
Antibacterial activity of extracts and fractions of green, black, and herbal teas were tested against Gram-positive Micrococcus luteus, Staphylococcus aureus, and Bacillus cereus, and against Gram-negative Escherichia coli, Salmonella typhi, and Pseudomonas aeruginosa. Antibacterial activity was measured using the disc-diffusion method.[32,36] Inoculums (100 μl) were spread evenly onto 20 ml Mueller-Hinton agar set in 90-mm Petri dishes using a sterile cotton swab. Sterilized paper discs (6-mm diameter) were impregnated with plant samples (2 mg per disc) using a micropipette and firmly placed onto the inoculated agar ensuring even distribution to avoid overlapping of zones. Streptomycin susceptibility discs (10 μg) were used as positive controls. After incubation overnight at 37°C, the minimum inhibitory dose (MID) or lowest concentration of extract or fraction in mg/disc required to show a zone of inhibition was recorded.[32,37]
Statistical analysis
All experiments were done in triplicate (n = 3) and results were expressed as means ± standard deviation (SD). Results were analyzed using the Turkey Honestly Significant Difference (HSD) one-way analysis of variance (ANOVA) software developed by Vassar College, New York State, USA. The significant difference was based on P < 0.05.
RESULTS AND DISCUSSION
Extraction and fractionation
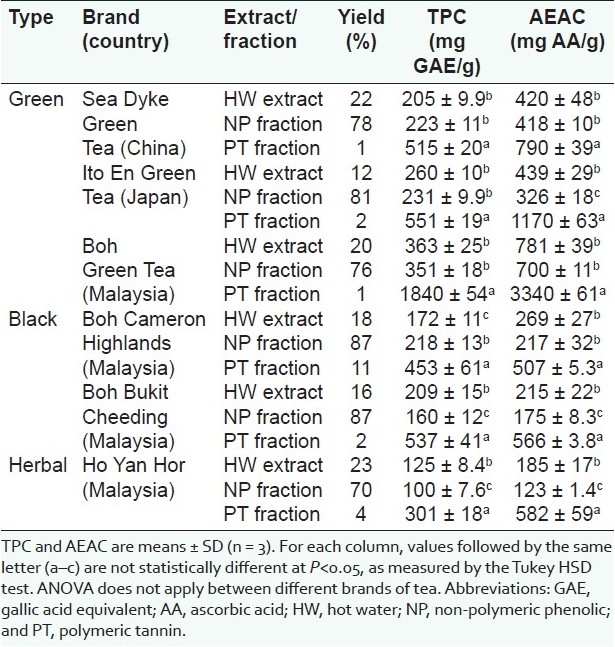
Extraction and fractionation yields of the green, black, and herbal teas are shown in Table 2. Yields of extracts ranged from 12% (Ito En Green Tea) to 23% (Ho Yan Hor). Fractionation yields of NP constituents ranged from 70% (Ho Yan Hor) to 87% (Boh Cameron Highland and Boh Bukit Cheeding). Yields of PT constituents were very low in green teas (1–2%), and low in black teas (2–11%) and herbal tea (4%). Results suggested that NP constituents are the major tea components. In a related study on the antioxidant and antibacterial properties of leaves, rhizomes, and inflorescences of three ginger species, yields of NP fractions (66–92%) were also much higher than those of PT fractions (0.5–10%).[32]
Table 2.
Percentage yield, total phenolic content (TPC), and ascorbic acid equivalent antioxidant capacity (AEAC) of extracts and fractions of Camellia sinensis teas
Hot water was used for extraction since it is the traditional way of brewing tea and previous studies have shown it to be an efficient way of extracting tea. In the extraction of green tea, the yield of hot water extracts was significantly higher than methanol and ethyl acetate extracts.[21,38] Similarly, reported that the yield of green tea extract obtained by the hot water extraction (42%) was higher than methanol (41%) and ethyl acetate (25%) extractions. For herbal teas, hot water extraction of microwave-dried tea yielded TPC and AEAC values which were significantly higher than methanol extraction.[39] Studies have shown that water temperature is an important factor when extracting tea. Significantly higher yields of hot water than cold water extraction of green tea and stronger radical scavenging activity of oolong tea extracted with hot water of increasing temperature have been reported.[40,41] For green, oolong, and black teas, extraction with water at 100 o C for 3 min yielded higher total flavanol content than extraction with water at 60° and 80°C.[22] Commercial green, oolong, and black teas, boiled gently in water for one hour, yielded 15, 17, and 18% of extracts, respectively.[16] It has been reported that higher temperatures reduce the polarity of water, thus increasing its extraction efficiency and capability to dissolve less polar compounds.[42] Raising the temperature of water also reduces its surface tension and viscosity, which increases the diffusion rate and the rate of mass transfer during extraction.
From the literature, data on the content of tannins for green and black teas are somewhat variable and lower than results of this study. In general, the tannin content in black tea is much higher than in green tea. In the processing of black tea, some 75% of the catechins are converted to thearubigins, while another 10% account for the formation of theaflavins and 15% would remain unchanged.[43]
Antioxidant properties
Antioxidant properties of hot water extracts of green, black, and herbal teas are shown in Table 2. For green teas, TPC and AEAC ranged from 205 mg GAE/g extract and 420 mg AA/g extract in Sea Dyke to 363 mg GAE/g extract and 781 mg AA/g extract in Boh, respectively. For black teas, values were 172 mg GAE/g extract and 269 mg AA/g extract for Boh Cameron Highlands, and 209 mg GAE/g extract and 215 mg AA/g extract for Boh Bukit Cheeding, respectively. Values of Ho Yan Hor herbal tea were 125 mg GAE/g extract and 185 mg AA/g extract, respectively.
Ranking based on TPC of extracts was Boh Green Tea > Ito En Green Tea > Sea Dyke Green Tea ~ Boh Bukit Cheeding > Boh Cameron Highlands > Ho Yan Hor. Ranking based on AEAC of extracts was Boh Green Tea > Ito En Green Tea ~ Sea Dyke Green Tea > Boh Cameron Highlands > Boh Bukit Cheeding > Ho Yan Hor. In general, the antioxidant properties of green tea extracts were stronger than those of black and herbal teas.
Antioxidant properties of fractions of green, black, and herbal teas are shown in Table 2. For all six teas, TPC and AEAC of PT constituents were significantly higher than those of NP constituents. PT constituents in Boh Green Tea yielded the highest values of 1840 mg GAE/g fraction and 3340 mg AA/g fraction, respectively.
Ranking based on TPC of PT fractions was Boh Green Tea > Ito En Green Tea ~ Boh Bukit Cheeding > Sea Dyke Green Tea > Boh Cameron Highlands > Ho Yan Hor. Ranking based on AEAC of PT fractions was Boh Green Tea > Ito En Green Tea > Sea Dyke Green Tea > Ho Yan Hor ~ Boh Bukit Cheeding > Boh Cameron Highlands.
Many studies have shown that green tea has stronger antioxidant properties than black tea.[14–18] The main chemical constituents of green tea are catechins of EGCG, EGC, ECG, and EC.[3,5,7] The potent antioxidant activities of catechins in green tea are due to their three adjacent hydroxyl (OH) groups on the B-ring as in EGCG, GCG, EGC, and GC which are more effective in scavenging free radicals than the two adjacent OH groups as in ECG, CG, and EC.[7] The content of EGCG and EGC in green tea is much higher than in black tea.[25] Although green tea has higher TPC, free radical scavenging activity, and ferric reducing power, its ferrous ion-chelating ability is poorer than black tea.[17,18]
The strong antioxidant properties of black tea have been attributed to its chemical components of thearubigins, phenolic acids, catechins, and theaflavins. Theaflavins which impart color, brightness, and astringency to black tea infusion possess potent antioxidant properties.[9,11] Theaflavin 3,3’-gallate has been shown to have higher antioxidant activity than EGCG which is the strongest antioxidant among the green tea catechins.[44,45] The antioxidant properties of theaflavins have been attributed to their gallic acid moiety.[46,47] Theaflavins have more OH groups than catechins since theaflavins are dimers of catechins.[9]
This study showed stronger antioxidant properties in PT than in NP constituents. Tannins in the form of thearubigins, with molecular weights of 1000–40,000 Da, are major constituents of black tea.[3,13] The strong antioxidant properties of tannins are due to the large number of phenolic hydroxyl groups and high degree of hydroxylation of aromatic rings.[48] Tannins have been reported to be 15–30 times more effective in quenching peroxyl radicals than simple phenols.[49] Hydrolyzable tannins having galloyl groups exhibited stronger antioxidant effects than flavonoids.[50] An increase of galloyl groups, molecular weight and ortho-hydroxyl structure can enhance their antioxidant activity.[51] Although tannins may not be absorbed by human due to their big molecular size, they could still exert their antioxidant activity within the digestive tract, and protect lipids, proteins, and carbohydrate from oxidative damage during digestion.[52] They can also serve as food preservatives, and as cosmetics and other skin care products.[53,54]
Antibacterial properties
Antibacterial properties of hot water extracts of green, black, and herbal teas are shown in Table 3. All extracts showed inhibitory effects on Gram-positive but not on Gram-negative bacteria. For green teas, extracts of Ito En and Boh showed strong antibacterial activity with MID of 0.06 mg/disc against M. luteus and B. cereus. Among the three Gram-positive bacteria, S. aureus was the least susceptible with Sea Dyke, Ito En, and Boh having MID of 2.0, 2.0, and 1.0 mg/disc, respectively. For black teas, extracts of Boh Cameron Highlands and Boh Bukit Cheeding inhibited M. luteus and B. cereus with MID of 0.13 and 0.5 mg/disc, respectively. Interestingly, extracts of black teas showed no inhibitory effect on the growth of S. aureus. Extract of Ho Yan Hor herbal tea inhibited M. luteus and B. cereus (MID of 2.0 mg/disc) with no inhibitory activity against S. aureus.
Table 3.
Antibacterial activity of extracts and fractions of Camellia sinensis teas using the disc-diffusion method
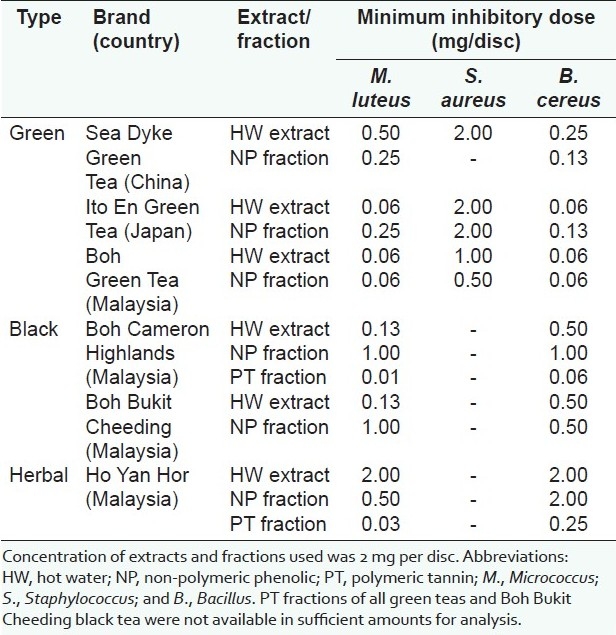
Antibacterial properties of fractions of green, black, and herbal teas are shown in Table 3. All fractions showed inhibitory effects on Gram-positive but not Gram-negative bacteria. For green teas, the NP fraction of Boh green tea displayed the strongest antibacterial activity with MID of 0.06 mg/disc against M. luteus and B. cereus. Good antibacterial activity of NP fractions of Sea Dyke and Ito En green teas was observed with MID of 0.13 and 0.25 mg/disc, respectively. Again, S. aureus appeared to be the least sensitive to the NP fractions of all three green teas. PT constituents fractionated from green teas were insufficient for testing their antibacterial activity. For black teas, the PT fraction of Boh Cameron Highlands strongly inhibited M. luteus and B. cereus with MID of 0.01 and 0.06 mg/disc, respectively. Similar to extracts, fractions of black teas displayed no antibacterial activity against S. aureus. The NP and PT fractions of Ho Yan Hor herbal tea inhibited M. luteus and B. cereus with no activity against S. aureus.
In this study, all extracts and fractions of green, black, and herbal teas showed no antibacterial activity against Gram-negative E. coli, S. typhi, and P. aeruginosa. The inhibition of tea extracts against P. aeruginosa and E. coli has been reported,[25,26] although an earlier study has explicitly reported that tea extracts are not effective against P. aeruginosa and E. coli.[27] The disparity in findings could be due to different strains of bacteria used, and to the different concentrations and types of extracts investigated. Gram-negative bacteria are less susceptible to antibiotics as their outer membrane of lipoproteins and lipopolysaccharides is able to regulate the access of antibacterial agents into the underlying structures.[54]
This study showed that green tea extracts inhibited the growth of Gram-positive M. luteus, S. aureus, and B. cereus, with M. luteus being be most sensitive. Similar findings have been reported earlier.[25,26] Several studies have shown that catechins from green and black teas, particularly EGCG and ECG, inhibited the growth of many bacterialspecies.[28] Contrary to findings from this study, earlier studies have reported that black teas inhibited the growth of S. aureus.[26,55] Extracts of green tea have been reported to be more effective in inhibiting bacterial growth than black tea.[24] In general, antibacterial activity decreased when the extent of tea fermentation increased.[25,27]
In this study, the potent antibacterial activity of PT constituents of black and herbal teas against M. luteus and B. cereus has been demonstrated. The strong and broad spectrum antibacterial properties of tannins have been well documented.[56–59] Tannins affect bacterial growth via several mechanisms such as inhibition of extracellular microbial enzymes, deprivation of the substrates required for microbial growth or direct action on microbial metabolism through inhibition of oxidative phosphorylation.[60]
CONCLUSION
The role of NP and PT constituents in the antioxidant and antibacterial properties of six brands of green, black, and herbal C. sinensis teas were investigated. Extraction yields ranged from 12 to 23%. Much higher fractionation yields of NP constituents (70–81%) than PT constituents (1–11%) suggested that the former are the major tea components. In general, the antioxidant properties of green tea extracts were stronger than those of black and herbal teas. For all six teas, antioxidant properties of PT fractions were significantly higher than crude extracts and NP fractions. Extracts and fractions showed no activity against Gram-negative E. coli, S. typhi, and P. aeruginosa. Green teas inhibited all three Gram-positive bacteria with S. aureus being the least susceptible. Black and herbal teas inhibited the growth of M. luteus and B. cereus, but not S. aureus. The most potent was the PT fractions of Boh Cameron Highlands and Ho Yan Hor with MID of 0.01 and 0.03 mg/disc against M. luteus. Results suggested that NP constituents are major contributors to the antioxidant and antibacterial properties of green, black, and herbal teas. Being non-polymeric, they can be absorbed by the gastrointestinal tract and are therefore orally active. Although PT constituents have stronger antioxidant and antibacterial properties, they constitute only a minor component of the teas.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Graham HN. Green tea composition, consumption, and polyphenol chemistry. Prev Med. 1992;21:334–50. doi: 10.1016/0091-7435(92)90041-f. [DOI] [PubMed] [Google Scholar]
- 2.Muktar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698–702. doi: 10.1093/ajcn/71.6.1698S. [DOI] [PubMed] [Google Scholar]
- 3.Graham HN. Tea. In: Frederick JF, editor. Wiley Encyclopedia of Food Science and Technology. 2nd ed. New Jersey: John Wiley and Sons; 1999. pp. 1–4. [Google Scholar]
- 4.Wheeler DA, Wheeler WJ. The medicinal chemistry of tea. Drug Dev Res. 2004;61:45–65. [Google Scholar]
- 5.Chopade VV, Phatak AA, Upaganlawar AB, Tankar AA. Green tea (Camellia sinensis): Chemistry, traditional, medicinal uses and its pharmacological activities: A review. Phcog Rev. 2008;2:157–62. [Google Scholar]
- 6.Wan X, Li D, Zhang Z. Green tea and black tea manufacturing and consumption. In: Ho CT, Lin JK, Shahidi F, editors. Tea and tea products: Chemistry and health-promoting properties. United States: CRC Press; 2008. pp. 1–8. [Google Scholar]
- 7.Sharma A, Wang R, Zhou W. Functional foods from green tea. In: Shahidi F, editor. Functional foods of the east. United States: CRC Press; 2011. pp. 173–95. [Google Scholar]
- 8.Lee KW, Lee HJ, Lee CY. Antioxidant activity of black tea vs.green tea. J Nutr. 2002;132:785. doi: 10.1093/jn/132.4.785. [DOI] [PubMed] [Google Scholar]
- 9.Łuczaj W, Skrzydlewska E. Antioxidative properties of black tea. Prev Med. 2005;40:910–8. doi: 10.1016/j.ypmed.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Dufresne CJ, Farnworth ER. A review of latest research findings on the health promotion properties of tea. J Nutr Biochem. 2001;12:404–21. doi: 10.1016/s0955-2863(01)00155-3. [DOI] [PubMed] [Google Scholar]
- 11.Ngure FM, Wanyoko JK, Mahungu SM, Shitandi AA. Catechins depletion patterns in relation to theaflavin and thearubigins formation. Food Chem. 2009;115:8–14. [Google Scholar]
- 12.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds - nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–117. [Google Scholar]
- 13.Haslam E. Thoughts on thearubigins. Phytochemistry. 2003;64:61–73. doi: 10.1016/s0031-9422(03)00355-8. [DOI] [PubMed] [Google Scholar]
- 14.Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 15.von Gadow A, Joubert E, Hansmann CF. Comparison of the antioxidant activity of rooibos tea (Aspalathus linearis) with green, oolong, and black tea. Food Chem. 1997;60:73–7. [Google Scholar]
- 16.Yokozawa T, Dong E, Nakagawa T, Kashiwagi H, Nakagawa H, Takeuchi S, et al. In vitro and in vivo studies on the radical scavenging activity of tea. J Agric Food Chem. 1998;46:2143–50. [Google Scholar]
- 17.Chan EW, Lim YY, Chew YL. Antioxidant activity of Camellia sinensis leaves and tea from a lowland plantation in Malaysia. Food Chem. 2007;102:1214–22. [Google Scholar]
- 18.Chan EW, Lim YY, Chong KL, Tan JBL, Wong SK. Antioxidant properties of tropical and temperate herbal teas. J Food Compos Anal. 2010;23:185–9. [Google Scholar]
- 19.Nanjo F, Goto K, Seto R, Suzuki M, Sakai M, Hara Y. Scavenging effects of tea catechins and their derivatives on 1,1-diphenyl-2-picrylhydrazyl radical. Free Radical Biol Med. 1996;21:895–902. doi: 10.1016/0891-5849(96)00237-7. [DOI] [PubMed] [Google Scholar]
- 20.Kondo K, Kurihara M, Miyata N, Suzuki T, Toyoda M. Scavenging mechanisms of (–)-epigallocatechin gallate and (–)-epicatechin gallate on peroxyl radicals and formation of superoxide during the inhibitory action. Free Radical Biol Med. 1999;27:855–63. doi: 10.1016/s0891-5849(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 21.Farhoosh R, Golmovahhed GA, Khodaparast MH. Antioxidant activity of various extracts of old tea leaves and black tea wastes (Camellia sinensis L.) Food Chem. 2007;100:231–6. [Google Scholar]
- 22.Horžić D, Komes D, Belscak A, Ganic KK, Ivekovic D, Karlovic D. The composition of polyphenols and methylxanthines in teas and herbal infusions. Food Chem. 2009;115:441–8. [Google Scholar]
- 23.Wiseman SA, Balentine DA, Frei B. Antioxidants in tea. Crit Rev Food Sci Nutr. 1997;37:705–18. doi: 10.1080/10408399709527798. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari RP, Bharti SK, Kaur HD, Dikshit RP, Hoondal GS. Synergistic antimicrobial activity of tea and antibiotics. Indian J Med Res. 2005;122:80–4. [PubMed] [Google Scholar]
- 25.Almajano MP, Carbó R, Jiménez JA, Gordon MH. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008;108:55–63. [Google Scholar]
- 26.Bancirova M. Comparison of the antioxidant capacity and the anti-microbial activity of black and green tea. Food Res Int. 2010;43:1379–82. [Google Scholar]
- 27.Toda M, Okubo S, Hiyoshi R, Shimamura T. The bactericidal activity of tea and coffee. Lett Appl Microbiol. 1989;8:123–5. [Google Scholar]
- 28.Hamilton-Miller JMT. Antimicrobial properties of tea (Camellia sinensis L.) Antimicrob Agents Chemother. 1995;39:2375–7. doi: 10.1128/aac.39.11.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Provan GJ, Helliwell K. Tea flavonoids: Their functions, utilisation and analysis. Trends Food Sci Technol. 2000;11:152–60. [Google Scholar]
- 30.Hagerman AE, Butler LG. Condensed tannin purification and characterization of tannin-associated proteins. J Agric Food Chem. 1980;28:947–52. doi: 10.1021/jf60231a011. [DOI] [PubMed] [Google Scholar]
- 31.Karamac M. Antioxidant activity of tannin fractions isolated from buckwheat seeds and groats. J Am Oil Chem Soc. 2010;87:559–66. [Google Scholar]
- 32.Chan EW, Ng VP, Tan VV, Low YY. Antioxidant and antibacterial properties of Alpinia galanga, Curcuma longa, and Etlingera elatior (Zingiberaceae) Phcog J. 2011;3:54–61. [Google Scholar]
- 33.Kähkönen MP, Hopia AI, Vuorela HJ, Rauha JP, Pihlaja K, Kujala TS, et al. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999;47:3954–62. doi: 10.1021/jf990146l. [DOI] [PubMed] [Google Scholar]
- 34.Wong SK, Lim YY, Abdullah NR, Nordin FJ. Antiproliferative and phytochemical analyses of leaf extracts of ten Apocynaceae species. Phcog Res. 2011;3:100–6. doi: 10.4103/0974-8490.81957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miliauskas G, Venskutonis PR, van Beek TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004;85:231–7. [Google Scholar]
- 36.Chung PY, Chung LY, Ngeow YF, Goh SH, Imiyabir Z. Antimicrobial activities of Malaysian plant species. Pharm Biol. 2004;42:292–300. [Google Scholar]
- 37.Mackeen MM, Ali AM, El-Sharkawy SH, Manap MY, Salleh KM, Lajis NH, et al. Antimicrobial and cytotoxic properties of some Malaysian traditional vegetables (ulam) Pharm Biol. 1997;35:174–8. [Google Scholar]
- 38.Nkubana A, He Q. A comparative study of antioxidant activity between black tea from Rwandan highlands with green and oolong teas from China. Int J Food Safety Nutr Public Health. 2008;1:159–66. [Google Scholar]
- 39.Chan EW, Lim YY. Antioxidant activity of Thunbergia laurifolia tea. J Trop Forest Sci. 2006;18:130–6. [Google Scholar]
- 40.Lin SD, Liu EH, Mau JL. Effect of different brewing methods on antioxidant properties of steaming green tea. LWT - Food Sci Technol. 2008;41:1616–23. [Google Scholar]
- 41.Su X, Duan J, Jiang Y, Shi J, Kakuda Y. Effects of soaking conditions on the antioxidant potentials of oolong tea. J Food Compos Anal. 2006;19:348–53. [Google Scholar]
- 42.Hassas-Roudsari M, Chang PR, Pegg RB, Tyler RT. Antioxidant capacity of bioactives extracted from canola meal by subcritical water, ethanolic and hot water extraction. Food Chem. 2009;114:717–726. [Google Scholar]
- 43.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds – nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–117. [Google Scholar]
- 44.Engelhardt UH, Lakenbrink C, Pokorny O. Proanthocyanidins, bisflavanols, and hydrolyzable tannins in green and black teas. In: Shahidi F, Weerasinghe DK, editors. Nutraceutical Beverages: Chemistry, Nutrition, and Health Effects. ACS Symposium Series. Washington DC: American Chemical Society; 2003. pp. 254–64. [Google Scholar]
- 45.Leung LK, Su YL, Chen RY, Zhang ZS, Huang Y, Chen ZY. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248–51. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- 46.Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of theaflavins and their gallate esters- radical scavengers or metal chelators? FEBS Lett. 1996;392:40–4. doi: 10.1016/0014-5793(96)00780-6. [DOI] [PubMed] [Google Scholar]
- 47.Shivaki M, Hava Y, Osawa T, Kumon H, Nakayama T, Kawakishi S. Antioxidative and antimutagenic effects of theaflavins from black tea. Mutat Res. 1994;323:29–34. doi: 10.1016/0165-7992(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 48.Koleckar V, Kubikova K, Rehakova Z, Kuca K, Jun D, Jahodar L, et al. Condensed and hydrolysable tannins as antioxidants influencing the health. Mini-Rev Med Chem. 2008;8:436–47. doi: 10.2174/138955708784223486. [DOI] [PubMed] [Google Scholar]
- 49.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–92. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 50.Yoshida T, Mori K, Htanao T, Okumura T, Uehara I, Komagoe K, et al. Studies on inhibition mechanism of autoxidation by tannins and flavonoids, V: Radical scavenging effects of tannins and related polyphenols on 1,1-diphenyl-2-picrylhydrazyl radical. Chem Pharm Bull. 1989;37:1919–21. [Google Scholar]
- 51.Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol. 1998;56:213–22. doi: 10.1016/s0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]
- 52.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism and nutritional significance. Nutr Rev. 1998;56:317–33. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 53.Roedig-Penman A, Gordon MH. Antioxidant properties of catechins and green tea extracts in model food emulsions. J Agric Food Chem. 1997;45:4267–70. [Google Scholar]
- 54.Unten L, Koketsu M, Kim M. Anti-discoloring activity of green tea polyphenols on β-carotene. J Agric Food Chem. 1997;45:2009–12. [Google Scholar]
- 55.Chopra I, Greenwood D. Antibacterial agents: Basis of action. In: Battista J, editor. Encyclopedia of Life Sciences. Hoboken, New Jersey: Wiley; 2001. pp. 1–8. [Google Scholar]
- 56.Turkmen N, Velioglu YS, Sari F, Polat G. Effect of extraction conditions on measured total polyphenol contents and antioxidant and antibacterial activities of black tea. Molecules. 2007;12:484–96. doi: 10.3390/12030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amarowicz R, Dykes GA, Pegg RB. Antibacterial activity of tannin constituents from Phaseolus vulgaris, Fagoypyrum esculentum, Corylus avellana, and Juglans nigra. Fitoterapia. 2008;79:217–9. doi: 10.1016/j.fitote.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 58.Min BR, Pinchak WE, Merkel R, Walker S, Tomita G, Anderson RC. Comparative antimicrobial activity of tannin extracts from perennial plants on mastitis pathogens. Sci Res Essay. 2008;3:66–73. [Google Scholar]
- 59.Doss A, Mohammed MH, Dhanabalan R. Antibacterial activity of tannins from the leaves of Solanum trilobatum Linn. Indian J Sci Technol. 2009;2:41–3. [Google Scholar]
- 60.Scalbert A. Antimicrobial properties of tannins. Phytochemistry. 1991;30:3875–83. [Google Scholar]


