Abstract
Background:
Thousands of chemical compounds are used in paint products, like pigments, extenders, binders, additives, and solvents (toluene, xylene, ketones, alcohols, esters, and glycol ethers). Paint manufacture workers are potentially exposed to the chemicals present in paint products although the patterns and levels of exposure to individual agents may differ from those of painters. The aim of the present study was to evaluate genome damage induced in peripheral blood lymphocytes and oral mucosa cells of paint industry workers.
Materials and Methods:
Genotoxicity was evaluated using the alkaline Comet assay in blood lymphocytes and oral mucosa cells, and the Micronucleus test in oral mucosa cells. For the micronucleus test in exfoliated buccal cells, no significant difference was detected between the control and paint industry workers.
Results:
The Comet assay in epithelia buccal cells showed that the damage index (DI) and damage frequency (DF) observed in the exposed group were significantly higher relative to the control group (P≤0.05). In the same way, the Comet assay data in peripheral blood leukocytes showed that both analysis parameters (DI and DF) were significantly greater than that for the control group (P≤0.05).
Conclusions:
Chronic occupational exposure to paints may lead to a slightly increased risk of genetic damage among paint industry workers.
Keywords: Comet assay, micronucleus test, oral mucosa cells, paint industry workers, peripheral blood lymphocytes
INTRODUCTION
According to recent studies, occupational exposure to paint may cause an increased risk of several kinds of cancer, including lung, bladder and pancreas cancer,[1] and lymphatic and hematopoietic tumors.[1,2] These findings are consistent with the 1989 report issued by the International Agency for Research on Cancer, which classified painting as an occupationally related cause of cancer and provided further evidence that the risk of certain cancers is increased by exposures in the paint manufacturing process. However, occupational exposure in paint manufacture is not classifiable as to its carcinogenicity.[3]
Thousands of chemical compounds are used in the manufacturing of paint products, like pigments, extenders, binders, additives, and solvents (toluene, xylene, ketones, alcohols, esters, and glycol ethers). Paint manufacture workers are potentially exposed to the chemicals found in paint products although the patterns and levels of exposure to individual agents may differ from those of painters.
The little information available regarding genotoxic effects associated to exposure to paints describes positive and negative results. Higher values of chromosomal aberrations (CAs), sister chromatid exchange (SCE), micronuclei (MN) (in lymphocytes and in oral mucosa cells), and DNA damage detected by the Comet assay in leukocytes are reported for workers exposed to automobile coatings and painters in general.[4,5] In addition, Diaz et al.[6] describe an increase in MN in peripheral lymphocytes and in oral mucosa cells of paint industry workers in Cuba. More recently, in vivo genotoxic studies have demonstrated that dust and fumes of lead-based paints cause chromosomal damage that result in a significant increase in heritable CAs levels in painters.[7] In contrast, Cárdenas-Bustamante et al.[8] investigated the degree of exposure to organic solvents and related genotoxic consequences in paint factory workers using cytogenetic monitoring (MN and Comet assay), finding no statistical differences regarding genetic biomarkers between exposed and non-exposed workers. Cytogenetic analysis of peripheral blood lymphocytes has been accepted as a technique suitable for the biological monitoring of genetic damage in somatic cells since the early 1970s.[9] In the present study, we used the MN test in exfoliated mucosa cells and the single cell gel electrophoresis (SCGE) or the Comet assay because of the advantages these systems afford in the screening of DNA damage caused by environmental mutagens.
MN are acentric chromosome fragments or whole chromosomes delayed during mitotic cellular division, and appear in the cytoplasm of interphase cells as small additional nuclei. The MN test is faster and easier than metaphase analysis and it can be used both in vivo and in vitro in a variety of cells. This assay has also been shown to be a reliable and sensitive biomarker for human biomonitoring.[10] The frequency of MN in human exfoliated cells is considered a useful biomarker of genotoxic effects in populations exposed to genotoxicants, through direct contact with ingested or inhaled compounds.[11]
Briefly, in the Comet assay, which is a simple and sensitive method for studying DNA damage and repair, cells are embedded in agarose on a microscope slide, lysed with detergent and high salt to form the nucleoids containing supercoiled loops of DNA linked to the nuclear matrix. Then, they are electrophoresed in alkaline medium. In cells with increased DNA damage, the results are structures resembling comets, because the DNA migrates from the nucleiod, an event observable by microscopy. The intensity of the comet tail relative to the head reflects the number of DNA breaks. The likely basis for this is that loops containing a break lose their supercoiling and become free to extend towards the anode.[12] Similarly to other genotoxicity tests, the Comet assay is not predictive of individual cancer risk but represents a useful tool to evaluate early and still repairable genotoxic effects due to occupational or environmental exposure.[13]
Considering that, to our knowledge, there is no published cytogenetic data concern paint industry workers in Brazil. Therefore, the objective of this study was to evaluate the genotoxic risk of these workers using the Comet assay in peripheral blood leukocytes and oral mucosa cells and the MN test in oral mucosa cells.
MATERIAL AND METHODS
Study population and sample collected
This study was approved by Brazilian National Ethical Committee on Research (Comissão Nacional de Ética em Pesquisa – CONEP, Protocols 061/2008) and informed written consent was obtained from each individual prior to the start of the study.
The study included 58 male workers of a paint manufacturing company employed in the sectors where they were occupationally exposed to solutions containing organic mixtures. The control group consisted of 30 healthy males with no occupational exposure that worked at the administrative function of the same company.
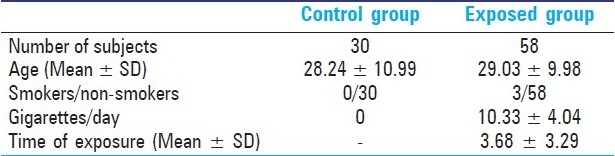
All the individuals examined in the study were required to answer a Portuguese version of a questionnaire from the International Commission for Protection against Environmental Mutagens and Carcinogens[14] and participate in a face-to-face questionnaire which included standard demographic data (age, gender,) as well as questions relating to medical issues (exposure to X-rays, vaccinations, medications), life style (smoking, coffee, alcohol, diet,) and their occupation (number of hours worked per day, time exposed to organic solvents, use of protective measures). Individuals were selected for the two groups (control and paint exposed) in such a manner so as to ensure that except for occupational exposure to organic solvents, there were no marked differences between the members of the groups. Individuals who smoked more than five cigarettes per day for at least 1 year were considered smokers. The characteristics of the two groups are presented in Table 1.
Table 1.
Demographic characteristics of the groups of study
Blood and urine samples were obtained from individuals in the two groups on the same day at the end of a normal shift during the workers’ periodical medical examinations by the nurses from a laboratory (BioLabor, Criciúma, Santa Catarina, Brazil). All blood samples were collected using venipuncture and heparinized vacutainers and processed as quickly as possible to avoid the damage associated with storage. The blood cell samples were transported to the university laboratory at or below 8°C and processed within 5 h of collection.
Hippuric acid analysis
As a biomarker of toluene exposure, 50 mL of urine samples were collected at the end of the working day and analyzed for hippuric acid (HA) using high performance liquid chromatography (HPLC) with UV-VIS detector in a commercial laboratory (Alvaro Laboratory, Cascavel-PR, Brazil).
Analysis of Hematological parameters
The following hematological markers were measured: leukocytes (granulocytes, lymphocytes, and monocytes), erythrocytes, hemoglobin, hematocrit.
All blood tests were analyzed in a laboratory (BioLabor, Criciúma, Santa Catarina, Brazil) according to standard hematological methods.
Genotoxicity tests
Comet assay in peripheral blood leukocytes
The alkaline Comet assay was performed as described by Singh et al.,[15] with the modifications suggested by Tice et al.[16] Samples of 5 μL of whole peripheral blood were embedded in 95 μL of 0.75% low-melting point agarose and added to a microscope slide (two slides per donor) precoated with normal agarose (1.5% buffer solution). When the agarose solidified the slides were placed in lysis buffer (2.5 MNaCl, 100m MEDTA and 10 mM Tris; pH 10.0–10.5) containing freshly added 1% (v/v) Triton X-100 and 10% (v/v) dimethylsulfoxide (DMSO) for a minimum of 1 h and a maximum of 2 weeks. After treatment with lysis buffer, to allow DNA unwinding, slides were incubated in a freshly made alkaline electrophoresis buffer (0.3 M NaOH and 1 mM EDTA; pH > 13) for 20 min in a horizontal electrophoresis tank and the DNA was electrophoresed for 20 min at 25 V (0.90 V/cm) and 300 mA. Every step was carried out under indirect yellow light. After electrophoresis, slides were washed three times in a neutralization buffer (0.4 M Tris; pH 7.5) for 5 min, rinsed three times in distilled water, and left to dry overnight at room temperature. Slides were stained with silver nitrate as previously described by Villela et al.:[17] the slides were fixed for 10 min in trichloroacetic acid 15% w/v, zinc sulfate 5% w/v, glycerol 5% v/v, rinsed three times in distilled water, and dried for 2 h at 37°C. The dry slides were re-hydrated for 5 min in distilled water, and then stained (sodium carbonate 5% w/v, ammonium nitrate 0.1% w/v, silver nitrate 0.1% w/v, tungstosilicic acid 0.25%, formaldehyde 0.15% w/v, freshly prepared in the dark), and constantly shaken for 35 min. The stained slides were rinsed twice with distilled water, and submerged in the stop solution (acetic acid 1%), rinsed again, and immediately coded for analysis in an optical microscope. Images of 100 randomly selected cells were analyzed per individual. Cells were scored visually into five classes, according to tail size and shape (from undamaged – 0, to maximally damaged – 4), and a value (damage index (DI)) was assigned to each Comet according to its class.[17] DI thus ranged from 0 (completely undamaged: 100 cells×0) to 400 (with maximum damage: 100 cells×4.[12] The damage frequency (DF) (%) was calculated based on the percentage of damaged cells (0–100%). International guidelines and recommendations for the Comet assay consider that visual scoring of comets is a well-validated evaluation method. It has a high correlation with computer-based image analysis.[12] Negative controls were processed together with workers’ samples and analyzed by one investigator.
Comet assay in epithelial buccal cells
Buccal mucosa cells were obtained by swabbing the left inner cheek with a cervical brush. The cells were washed with a phosphate buffer solution and centrifuged at 800 rpm for 10 min. Then, 20 μL of the pellet was resuspended in 80 μL of 0.75% low-melting point agarose. The Comet assay was then performed as described above.
MN test in epithelial buccal cells
Exfoliated buccal mucosa cells were collected by swabbing the right inner cheek of the individuals with a moistened wooden tongue depressor and was smeared over clean slides containing two drops of physiological solution. Cells were fixed in a methanol–acetic acid (3:1) solution for 10 min, dried at 50°C in a chamber for 5 min, and stained with Giemsa 5% (phosphate buffer solution, pH 5.8). Then the slides were washed in distilled water and stained with Fast green for 1 min, washed again, and stained with total Giemsa for 1 min. After this, they were washed in distilled water again and dried at room temperature.
The criteria used for MN analysis were those of Tolbert et al.[18] and Titenko-Holland et al.,[19] i.e. for a structure to be considered as a micronucleus it must be: (a) less than one third of the diameter of the main nucleus; (b) be in the same plane of focus as the main nucleus e; (c) have the same color, texture, and refraction as the main nucleus; (d) have a smooth oval or round shape; and (e) be clearly separated from the main nucleus. Only cells that were not smeared, clumped or overlapped, and those who contained intact nuclei were included in the analysis. According to Tolbert et al.[18] and Gomez-Arroyo et al.,[20] exfoliated buccal cells undergo degenerative processes which can produce anomalies that are difficult to distinguish from MN (binucleates, pycnosis, karyorrhexis, and karyolysis). In our study, these were excluded from the micronucleus analysis and all the slides were coded to blind analysis. The MN frequency was estimated based on the number of normal exfoliated buccal cells counted using a bright-field Zeiss microscope at a magnification of 1000×. For each volunteer, 2000 buccal cells (i.e. 1000 from each of the duplicate slides) were scored.
Statistical analysis
The normality of variables was evaluated by the Kolmogorov–Smirnov test; χ2 and t-tests were used to compare the demographic characteristics of study populations. The statistical analysis of differences in HA, MN test, and DNA damage measured by the Comet assay were carried out using the non-parametric Mann–Whitney U-test. Correlations between different variables were determined by the Spearman rank correlation test, when appropriate. The critical level for rejection of the null hypothesis was considered to be a two-tailed P value of 5%. All analyses were performed using GraphPad Prism version 4.00 for Windows, GraphPad Software, San Diego, California, USA, www.graphpad.com.
RESULTS
The main characteristics of the two groups studied are presented in Table 1. No significant differences were observed between the mean age of subjects in the different groups (Student's t-test). Regarding smoking habits, in the exposed group just three subjects smoking an average of 10.33 ± 4.04 cigarettes per day, while in the control group all individuals were non-smokers. The duration of exposure in the exposed group was 3.68 ± 3.29 years, ranging from 0.5 to 12 years. Analysis of questionnaires revealed that all paint industry workers used silicone gloves to prevent skin contact with organic solvents, glasses, and breathing masks. We also observed that the work areas in the company were equipped with ventilation devices.
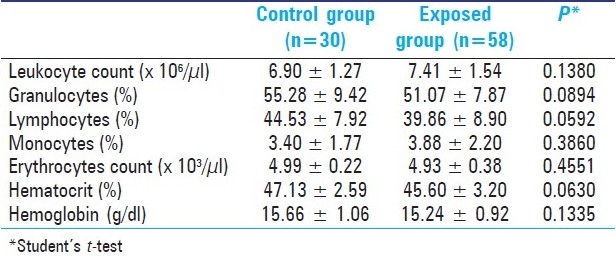
In relation to hematological parameters, Table 2 reports that no significant differences were found between the two groups, and both presented normal hematological values similar to reference values observed in the literature for several other Brazilian populations as described by.[21]
Table 2.
Hematological parameters in study groups (Mean ± SD)
The comparison of the mean values (g/g creatinine) of urine HA level of the control and exposed group is shown in Figure 1. A significant increase in HA levels was observed in the exposed group relative to the controls (P≤0.05, Mann–Whitney U-test).
Figure 1.
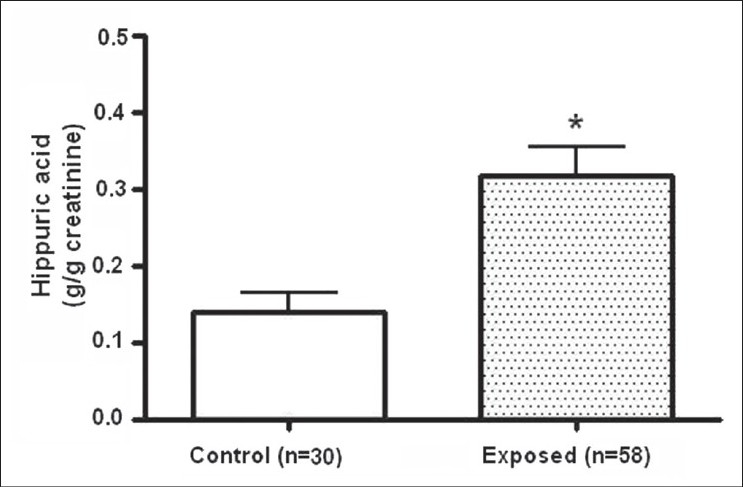
Hippuric acid (HA) concentration (mean ± SEM) in urine of control and exposed group. *Significant difference relative to the control group at P≤0.05 (Mann-Whitney U-test, two-tail)
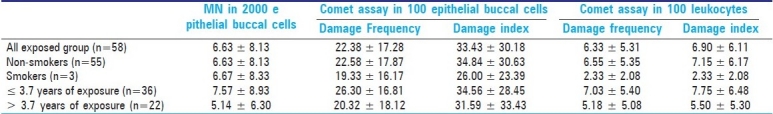
Table 3 shows the data obtained using the three cytogenetic assays for control and exposed groups, and that were analyzed using the Mann-Whitney U- test. For the micronucleus test in buccal exfoliated cells, no significant difference was detected between the control and paint industry workers. The Comet assay in epithelial buccal cells showed that the DI and DF observed in the exposed group were significantly higher relative to the control group (P≤0.05). In the same way, the Comet assay data in peripheral blood leukocytes showed that both analysis parameters of this assay (DI and DF) were significantly greater than that for the control group (P≤0.05). In addition, no significant differences were observed between smokers and non-smokers in the exposed group for all cytogenetic analysis. Also, no significant differences were observed in relation to exposure time for all assays [Table 4].
Table 3.
Mean values (± SD) obtained by MN test and Comet assay in control and exposed group
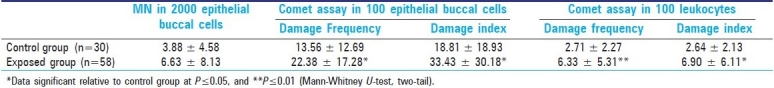
Table 4.
Mean values (± SD) obtained by MN test and Comet assay in the exposed group according to smoking habit and time of exposure
DISCUSSION
Many approaches and techniques have been developed for monitoring human populations exposed to environmental mutagens. The traditional approach has been to use the readily available blood cells (e.g., lymphocytes and red blood cells) as biomarkers to document mutagenic effects.[11] Although long-term diseases are not expected from the affected blood cells, it is generally accepted that the blood cells can be used as sentinel cells types to provide early warning signals for adverse health outcome. It is also suitable to determine whether the biomarker effects observed in blood cells are consistent with those in available target cells. In this paper, we provided a study using two types of cells and two genotoxicity assays to evaluate the occupational exposure of paint industry workers.
Paint industry workers are exposed to complex mixtures of organic solvents, heavy metals such as lead, zinc, chromium, cadmium, and many other compounds with potential mutagenic properties, such as phthalic acid and chlorophenols.[3] Because the main solvent present in the mixtures of organic solvents used in the paint production process is toluene, we analyze the HA concentration in urine of the paint industry workers. Our data indicate a higher mean concentration of this toluene metabolite appearing in the urine of these workers in relation to the control group, and these levels confirmed exposure to toluene among paint industry workers [Figure 1]. However, the HA values observed for all volunteers can be considered low according to NR-7[22] (up to 1.5 g/g creatinine). Nevertheless, similar result was found by Pelclova et al.,[23] in printers exposed to toluene in Poland. The HA in urine in this group of printers were significantly higher than in controls; however, the levels nonetheless remained lower than the Czech limit for occupational settings (i.e., 2.5 g/L of urine). In addition, De Rosa et al.[24] carried out a monitoring of subjects working in a printing company, who were exposed to toluene, using urine samples collected before and after the work shift for the determination of HA. They found many correlations between levels of HA in urine and the environmental samples of toluene collected at the company, concluding that HA is a valid test for evaluating even low exposures to toluene.
Some of the biological effects of exposure to organic solvents are hematological changes.[25] These effects may result in a decreased production of red blood cells, white blood cells, and platelets.[26] However, in contrast to the results of Beving et al.,[27] with painters in Sweden, the values obtained in our study for hematological parameters showed no significant differences between control and exposed group.
The influences of age, sex, and smoking on DNA damage are well-known problems in industrial monitoring.[7,28] However, in this study these factors could be excluded; in both groups, the mean age was similar, all persons were men, and just three workers had smoked habits although in this case the values obtained for cytogenetic tests were not different from control subjects. Similarly, Silva et al.[29] concluded that smoking habits do not represent a significant factor in terms of production of the various types of chromosome aberrations found in their occupational monitoring with car painters.
The results obtained in this study show that there is no exposure-related induction of MN in buccal epithelia cells of workers exposed to solvents in the paint manufacturing industry. Although only a few studies have been conducted with paint workers, data reported using these cells indicate positive results.[6,26,30] The use of the MN test in exfoliated cells has substantially increased, since it is considered a useful biomarker of genotoxic effects in population exposed to genotoxicants through direct contact with ingested or inhaled compounds. It must be recalled that epithelial cells are highly proliferative and are the origin of more than 90% of cancers, for which their use in biomonitoring can be really useful.[11] On the other hand, the Comet assay values for the paint industry workers were significantly higher than the values of the control group both in the peripheral blood and buccal exfoliated cells. Positive results in the Comet assay do not always correspond to positive results in the MN test, especially when the exposure to genotoxic agents is small. The Comet assay usually detects more defects than the MN test.[31] The positive results in the Comet assay and MN tests are due to different mechanisms; the MN test detects injuries that survive at least one mitotic cycle, while the Comet assay identifies reparable injuries or alkali-label sites.[31,32] Consequently, Goethem et al.[31] suggested the use of both the MN test and the Comet assay.
The possibility of cytogenetic damage in various occupations exposed to organic solvents has been discussed in several papers.[6–8,26,29,30,32–35] The increasing use and diversity of solvents raises concern about possible risks in occupational exposure. Several earlier reports suggest harmful effects in subjects occupationally exposed to paint and their components. Madhavi et al.[7] reported that occupational exposure to lead-based paints has been associated with an increase in the frequency of CA in the workers when compared to the controls. A monitoring study was designed by Pinto et al.[26] to determine occupational exposure risk in outdoor painters. Painters showed CA and SCE in lymphocytes and MN in oral epithelia cells greater than in the control group. Diaz et al.[6] analyzed lymphocytes and oral mucosa cell MN in 21 Cuban paint industry workers. Both MN assays showed the same results, i.e. a statistically significant difference between the workers and the control group. Another study reporting CA in lymphocytes from car painters in Brazil showed that there was a significantly higher frequency of aneuploidies and chromosome deletions in the peripheral lymphocytes of car painters than in control subjects.[29]
The MN test and the Comet assay were applied to exfoliated buccal cells in order to evaluate the genotoxic risk associated with occupational exposure of 10 car painters by Martino-Roth et al.[30] Highly significant effects of occupational exposure were found in this study with both the MN test and the Comet assay. On the other hand, Cardenas-Bustamante et al.[8] investigated the degree of exposure to organic solvents and related genotoxic consequences in paint factory workers, determining MN frequency in lymphocytes and DNA damage using the Comet assay. They found no statistical differences regarding genetic biomarkers between exposed and non-exposed workers.
A major problem in interpreting biomonitoring studies is estimating the degree of exposure. Possible abuse or misuse could lead to significant levels of exposure.[36] Until now, many biomonitoring studies have been performed in people from different regions and under a variety of exposure conditions, using several different biomarkers. In this context, it is not surprising that the results obtained by different authors have also shown variability. Also, since workers are frequently exposed to complex mixtures of organic solvents present in paints, it is difficult to attribute the genotoxic damage to any particular chemical or compound. Thus, the DNA damaged observed in our study and in all these listed above should not be attributed only to one compound, but to the cumulative effect of many chemical compounds that are used in paint products.
In the present study, even though paint workers had said that they used adequate personal protection equipment, an increase in HA concentration in urine was observed together with an increase in Comet assay values, both for leukocytes as buccal cells. Organic solvent levels in the samples were apparently low, which is consistent with the absence of mutagenicity and the presence of only genotoxicity in cells. It can be concluded from this study that occupational exposure to paints may lead to a slightly increased risk of genetic damage among paint industry workers. Due to these considerations and the complex multi-composition of paints and solvents, hygienic measures were suggested. A better understanding of variables related to cytogenetic damage would greatly reduce the uncertainty in the carcinogenic risk assessment among paint industry workers.
ACKNOWLEDGMENTS
The authors express their gratitude to all the individuals who volunteered to participate in this study. We especially thank the chairman of the paint manufacturing company and also Marcelo Comim for his valuable help during the sampling.
Footnotes
Source of Support: Programa de Pós-Graduação em Ciências da Saúde - Universidade do Extremo Sul Catarinense (UNESC) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
Conflict of Interest: None declared.
REFERENCES
- 1.Brown LM, Moradi T, Gridley G, Plato N, Dosemeci M, Fraumeni JF., Jr Exposures in the painting trades and paint manufacturing industry and risk of cancer among men and women in Sweden. J Occup Environ Med. 2002;44:258–64. doi: 10.1097/00043764-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Lundberg I, Milatou-Smith R. Mortality and cancer incidence among Swedish paint industry workers with long-term exposure to organic solvents. Scand J Work Environ Health. 1998;24:270–5. doi: 10.5271/sjweh.320. [DOI] [PubMed] [Google Scholar]
- 3.IARC (International Agency for Research on Cancer), Some Organic Solvents, Resin Monomers and Related Compounds, Pigments and Occupational Exposures in Paint Manufacture and Painting, IARC Monographs on the Evaluation of the Carcinogenic Risks of Chemicals to Humans IARC. 1989:47. [PMC free article] [PubMed] [Google Scholar]
- 4.Piña-Calva A, Madrigal-Bujaidar E, Fuentes MV, Neria P, Pérez-Lucio C, Vélez-Zamora NM. Increased frequency of chromosomal aberrations in railroad car painters. Arch Environ Health. 1991;46:335–9. doi: 10.1080/00039896.1991.9934399. [DOI] [PubMed] [Google Scholar]
- 5.Testa A, Festa F, Ranaldi R, Giachelia M, Tirindelli D, De Marco A, et al. A multi-biomarker analysis of DNA damage in automobile painters. Environ Mol Mutagen. 2005;46:182–8. doi: 10.1002/em.20147. [DOI] [PubMed] [Google Scholar]
- 6.Diaz S, Fonseca G, Fernandez I. Analysis of lymphocyte and oral mucosa cell micronuclei in Cuban paint industry workers. Hereditas. 1990;113:77–80. doi: 10.1111/j.1601-5223.1990.tb00700.x. [DOI] [PubMed] [Google Scholar]
- 7.Madhavi D, Devi KR, Sowjanya BL. Increased frequency of chromosomal aberrations in industrial painters exposed to lead-based paints. J Environ Pathol Toxicol Oncol. 2008;27:53–9. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i1.60. [DOI] [PubMed] [Google Scholar]
- 8.Cárdenas-Bustamante O, Varona-Uribe M, Patiño-Florez R. Bogotá paint-industry workers’ exposure to organic solvents and genotoxic effects. Rev Salud Publica. 2007;9:275–88. doi: 10.1590/s0124-00642007000200011. [DOI] [PubMed] [Google Scholar]
- 9.Sram RJ, Rössner P, Smerhovsky Z. Cytogenetic analysis and occupational health in the Czech Republic. Mutat Res. 2004;566:21–48. [PubMed] [Google Scholar]
- 10.Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, et al. Report from the in vitro micronucleus assay working group. Mutat Res. 2003;540:153–63. doi: 10.1016/j.mrgentox.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Salama SA, Serrana M, Au WW. Biomonitoring using accessible human cells for exposure and health risk assessment. Mutat Res. 1999;436:99–112. doi: 10.1016/s1383-5742(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 12.Collins AR, Oscoz AA, Brunborg G, Gaivão I, Giovannelli L, Kruszewski M, et al. The comet assay: Topical issues. Mutagenesis. 2008;23:43–151. doi: 10.1093/mutage/gem051. [DOI] [PubMed] [Google Scholar]
- 13.Møller P, Knudsen LE, Loft S, Wallin H. The comet assay as a rapid test in biomonitoring occupational exposure to DNA-damaging agents and effect of confounding factors. Cancer Epidemiol Biomarkers Prev. 2000;9:1005–15. [PubMed] [Google Scholar]
- 14.Carrano AV, Natarajan AT. Considerations for population monitoring using cytogenetic techniques, International Commission for Protection against Environmental Mutagens and Carcinogens (ICPEMC publication 14) Mutat Res. 1988;204:379–406. doi: 10.1016/0165-1218(88)90036-5. [DOI] [PubMed] [Google Scholar]
- 15.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantification of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 16.Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H. Single-cell gel/Comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen. 2000;35:206–21. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Villela IV, Oliveira IM, Silva J, Henriques JA. DNA damage and repair in haemolymph cells of golden mussel (Limnoperna fortunei) exposed to environmental contaminants. Mutat Res. 2006;605:78–86. doi: 10.1016/j.mrgentox.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: Method development. Mutat Res. 1992;271:69–77. doi: 10.1016/0165-1161(92)90033-i. [DOI] [PubMed] [Google Scholar]
- 19.Titenko-Holland N, Jacob RA, Shang N, Balaraman A, Smith MR. Micronuclei in lymphocytes and exfoliated buccal cells of postmenopausal women with dietary changes in folate. Mutat Res. 1998;417:101–14. doi: 10.1016/s1383-5718(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Arroyo S, Diaz-Sanchez Y, Meneses-Perez MA, Villalobos-Pietrini R, De Leon-Rodriguez J. Cytogenetic biomonitoring in a Mexican floriculture worker group exposed to pesticides. Mutat Res. 2000;466:117–24. doi: 10.1016/s1383-5718(99)00231-4. [DOI] [PubMed] [Google Scholar]
- 21.Karazawa EH, Jamra M. Parâmetros hematológicos normais. Rev Saude Publica. 1989;23:58–66. doi: 10.1590/s0034-89101989000100008. [DOI] [PubMed] [Google Scholar]
- 22.Norma Regulamentadora No7 (NR-7). Segurança e Medicina do Trabalho – NR-7 – Programa de Controle Médico de Saude Ocupacional, Portaria GM/SSSTb n. 24. (DOU December 30, 1994) [Google Scholar]
- 23.Pelclova D, Cerna M, Pastorkova A, Vrbikova V, Prochazka B, Hurychova D, et al. Study of the genotoxicity of toluene. Arch Environ Health. 2000;55:268–73. doi: 10.1080/00039890009603417. [DOI] [PubMed] [Google Scholar]
- 24.De Rosa E, Brugnone F, Bartolucci GB, Perbellini L, Bellomo ML, Gori GP, et al. The validity of urinary metabolites as indicators of low exposures to toluene. Int Arch Occup Environ Health. 1985;56:135–45. doi: 10.1007/BF00379385. [DOI] [PubMed] [Google Scholar]
- 25.Descatha A, Jenabian A, Conso F, Ameille J. Occupational exposures and haematological malignancies: Overview on human recent data. Cancer Causes Control. 2005;16:939–53. doi: 10.1007/s10552-005-2301-3. [DOI] [PubMed] [Google Scholar]
- 26.Pinto D, Ceballos JM, García G, Guzmán P, Del Razo LM, Vera E, et al. Increased cytogenetic damage in outdoor painters. Mutat Res. 2000;467:105–11. doi: 10.1016/s1383-5718(00)00024-3. [DOI] [PubMed] [Google Scholar]
- 27.Beving H, Malmgren R, Petren S, Vesterberg O. Haematological changes in house painters using epoxy paints. Occup Med. 1991;41:102–6. doi: 10.1093/occmed/41.3.102. [DOI] [PubMed] [Google Scholar]
- 28.Faust F, Kassie F, Knasmuller S, Boedecker RH, Mann M, Mersch-Sundermann V. The use of the alkaline comet assay with lymphocytes in human biomonitoring studies. Mutat Res. 2004;566:209–29. doi: 10.1016/j.mrrev.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Silva JM, Santos-Mello R. Chromosomal aberrations in lymphocytes from car painters. Mutat Res. 1996;368:21–5. doi: 10.1016/s0165-1218(96)90036-1. [DOI] [PubMed] [Google Scholar]
- 30.Martino-Roth MG, Viégas J, Roth DM. Occupational genotoxicity risk evaluation through the comet assay and the micronucleus test. Genet Mol Res. 2003;2:410–7. [PubMed] [Google Scholar]
- 31.Goethem FV, Lison D, Kirsch-Volders M. Comparative evaluation of the in vitro micronucleus test and the alkaline single cell gel electrophoresis assay for the detection of DNA damaging agents: Genotoxic effects of cobalt powder, tungsten carbide and cobalt-tungsten carbide. Mutat Res. 1997;392:31–43. doi: 10.1016/s0165-1218(97)00043-8. [DOI] [PubMed] [Google Scholar]
- 32.Vrzoc M, Petras ML. Comparison of alkaline single cell gel (Comet) and peripheral blood micronucleus assays in detecting DNA damage caused by direct and indirect acting mutagens. Mutat Res. 1997;381:31–40. doi: 10.1016/s0027-5107(97)00143-7. [DOI] [PubMed] [Google Scholar]
- 33.Pitarque M, Vaglenov A, Nosko M, Hirvonen A, Norppa H, Creus A, et al. Evaluation of DNA damage by the Comet assay in shoe workers exposed to toluene and other organic solvents. Mutat Res. 1999;441:115–27. doi: 10.1016/s1383-5718(99)00042-x. [DOI] [PubMed] [Google Scholar]
- 34.Çok I, Sarda S, Kadioglu E, Ozcagli E. Assessment of DNA damage in glue sniffers by use of the alkaline comet assay. Mutat Res. 2004;557:131–6. [PubMed] [Google Scholar]
- 35.Heuser VD, Andrade VM, Silva J, Erdtmann B. Comparison of genetic damage in Brazilian footwear-workers exposed to solvent-based or water-based adhesive. Mutat Res. 2005;583:85–94. doi: 10.1016/j.mrgentox.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 36.Bolognesi C. Genotoxicity of pesticides: A review of human biomonitoring studies. Mutat Res. 2003;543:251–72. doi: 10.1016/s1383-5742(03)00015-2. [DOI] [PubMed] [Google Scholar]


