Abstract
To evaluate the safety, efficacy, advantages, and limitations of femtosecond laser-assisted cataract surgery through a review of the literature. A PubMed search was conducted using topic-appropriate keywords to screen and select articles. Initial research has shown appropriate safety and efficacy of femtosecond laser-assisted cataract surgery, with improvements in anterior capsulotomy, phacofragmentation, and corneal incision. Limitations of these studies include small sample size and short-term follow-up. Cost-benefit analysis has not yet been addressed. Preliminary data for femtosecond laser-assisted cataract surgery shows appropriate safety and efficacy, and possible advantage over conventional cataract surgery. Questions to eventually be answered include comparisons of long—term postoperative complication rates—including infection and visual outcomes-and analysis of contraindications and financial feasibility.
Keywords: Capsulorhexis, Capsulotomy, Cataract, Cataract Laser Extraction, Clear Corneal Incision, Femtosecond, Femtosecond Laser-Assisted Cataract Surgery, Femtosecond-Assisted, Fragmentation, Laser, LensAR, LenSx, Optical Coherence Tomography, OptiMedica, Phacoemulsification, Phacofragmentation, Refractive Cataract Surgery
INTRODUCTION
The femtosecond laser (FSL) is useful in ocular surgeries due to its ultrafast pulses in the range of 10-15 seconds and its decreased energy requirements for tissue destruction, allowing for reduced unintended destruction of surrounding tissues.1,2 While FSLs were previously FDA-approved for use in lamellar corneal surgery, the modality was approved in 2010 for cataract surgery. There are three companies-OptiMedica (Santa Clara, CA), LenSx (recently acquired by Alcon, Fort Worth, TX), and LensAR (Winter Park, FL)-which will be discussed in this review. LensAR recently received 501(k) FDA approval for lens fragmentation and anterior capsulotomy. LenSx is now approved for lens fragmentation, anterior capsulotomy, and corneal incisions. OptiMedica is currently seeking FDA approval and is already available outside of the United States. Although there may be other laser platforms, this review will focus on these three companies due to their presence in published literature.
Lasers have been utilized in cataract surgery since the 1970s, when Krasnov reported a laser modality for phacopuncture.3 Subsequently in 1987, Peyman and Katoh focused an Erbium:YAG laser on the lens nucleus, inducing photoablation.4 These efforts were harbingers of laser use in ocular surgery, which eventually led to investigations into FSL-assisted cataract surgery (FLACS). Methods to increase accuracy and precision in cataract surgery are being investigated because as lens implants become more advanced, patient expectations for near-perfect vision are increasing. These premium intraocular lenses (IOLs) also depend more on precise centration for optimal performance.5–7 Accuracy standards for cataract surgery were set in the United Kingdom by Gale et al. in 2006 to reach ±0.50 diopter (D) for 55% of cases, and ±1.00 D for 85% of cases.8 In comparison to these guidelines, Murphy et al. showed that with standard cataract surgery methods, 45% of patients were within the 0.50 D range, and 72% of patients were in the 1.00 D range.9 As cataract surgery is the most common operation in the United States,10 these studies and others suggest there is opportunity for improvement. Although FLACS is a promising surgical modality, there is question of its widespread utility and accessibility. This review will focus on the recent literature regarding laser-assisted cataract surgery.
MATERIALS AND METHODS
A PubMed search in July 2011 was conducted using the following search terms: femtosecond, ultrafast, cataract, assisted, phacoemulsification, phacofragmentation, capsulotomy, capsulorhexis, clear corneal incision, OptiMedica, LensAR, and LenSx. All abstracts were first screened for application to the topic, and those articles selected were then reviewed. The references of the articles selected were also utilized as a resource.
Femtosecond lasers: Mechanism of action
The FSL causes tissue disruption with its near-infrared scanning pulse focused to 3 μm with an accuracy of 1 μm.1 Photodisruption is essentially induced by vaporization of target tissues, which occurs through the following steps: the focused laser energy increases to a level where a plasma is generated; the plasma expands and causes a shock wave, cavitation, and bubble formation; and then the bubble expands and collapses, leading to separation of the tissue.1,2 Because FSLs function nearly at an infrared wavelength, they are not absorbed by optically clear tissues.11 This makes FSL-assisted surgery amenable to anterior chamber targeting at various depths, as the anterior chamber provides an optically clear tissue space.2 The near-infrared wavelength is not absorbed by the cornea, and the waves are known to dissipate approximately 100 μm from the lens tissue target.11
Steps in femtosecond laser-assisted cataract surgery
Although the steps for each laser platform vary, they all require pupillary dilatation and topical anesthesia, followed by applanation of the cornea with a docking system that involves a contact lens with a circumferential suction skirt distributing pressure evenly on the cornea. The docking system minimally distorts anatomy while increasing intraocular pressure (IOP), although reportedly less IOP increase than seen in FSL refractive surgery.2 According to Friedman et al., the OptiMedica platform has a liquid optics interface which increases IOP by 15 mmHg and avoids corneal folds.12 Other platforms have not specified a degree of IOP increase. Once docking is complete, anterior segment imaging is then performed. LenSx and OptiMedica utilize Fourier-domain optical coherence tomography (FD-OCT), while LensAR utilizes Scheimpflug imaging technology. This step is required to find anatomical landmarks for laser pattern mapping. It is also crucial that specific boundaries are mapped, including the iris and the posterior surface of the lens. The posterior surface of the lens must be identified in order to avoid puncture of the posterior capsule. Preprogrammed corneal incisions for temporal wound, paracentesis, and any optional limbal-relaxing incisions (LRIs) can be adjusted at this point to surgeon preference.13 The pattern is then centered and the laser is activated.
Using the OptiMedica and LenSx systems, laser-assisted capsulotomy is performed, followed by lens fragmentation. This sequence is justified because lens fragmentation causes release of gas bubbles, which can distort the anatomy and affect capsulotomy planning.13 If a corneal incision is created, it is the last step before the patient is moved to the operating room. The integrity of the anterior chamber is not disturbed before the patient is sterile, as this initial incision does not penetrate the posterior corneal surface. Once in the sterile field, any partial-thickness corneal incisions are then completed with a microsurgical blade.13 Patients then undergo removal of the anterior capsulotomy, followed by standard phacoemulsification.
Can femtosecond lasers improve cataract surgery?
The vast majority of cataract surgeons use a clear corneal incision (CCI) to access the anterior chamber, although this method has been associated with an increased risk of postoperative endophthalmitis.14 A recent study utilized anterior segment OCT after cataract surgery to show that a majority of eyes had an internally gaping corneal wound and detachment of Descemet's membrane after CCI.15 Other studies have supported this finding,16,17 and it is hypothesized that these wound abnormalities may be one factor which increases the risk of postoperative endophthalmitis. FSLs have been investigated to perform corneal incisions because the laser may allow for more square architecture, which has proven more resistant to leakage.18 Although not a standard indication in cataract surgery, these FSL systems can also create LRIs to treat astigmatism. Since the FSL systems are capable of delivering cuts to precise depths and lengths, these LRIs may be more accurate and standardized compared to manual techniques.
Multiple research studies have shown that manual capsulorhexis-known to be the most technically difficult part of cataract surgery for trainees19 -leads to tears in 0.8% of cases.20 Trivedi et al. demonstrated that smooth, regular edges may offer superior capsular strength and resistance to capsular tears.21 Additionally, the unpredictable diameter observed in manual capsulorhexis can have effects on IOL centration, with subsequent poor refractive outcomes, unpredictable anterior chamber depths, and increased rates of posterior capsular opacification.20,22,23 In fact, Norrby observed that IOL position was the largest contributor to postoperative refractive error in cataract patients.22 It has also been observed that a shift of 1 mm in IOL position-which can stem from an inappropriately sized capsulotomy-can lead to approximately 1.25 D change in refractive error.24 The FSL may be able to deliver a more circular, stronger, precisely planned and executed capsulorhexis, which could afford more control over capsulotomy unpredictability and offer more accurate refractive outcomes.
Ultrasound phacoemulsification carries the risk of corneal injury. Various hypotheses exist as to why this damage is generated, including long duration of ultrasound exposure, damage caused by heat or mechanical manipulation, and injury caused by the fragmenting lens. Using rabbit eyes, Murano et al., studied the effect of ultrasound oscillations in the anterior chamber. The authors observed oxidative stress and cellular necrosis after ultrasound, and concluded that the corneal endothelial cell damage was caused by free radicals.25 Similarly, Shin et al., showed that increasing ultrasound time and energy had a direct relationship to cell injury.26 With consideration to a reduction in ultrasound energy and instrumentation, FSLs may show improved safety and decreased complications. However, controversy still exists as to the amount and clinical relevance of the damage caused by advanced phacoemulsification techniques. When comparing the phaco-chop to the divide-and-conquer technique, Storr-Paulsen et al., found that the two methods caused the same amount of corneal cell loss, and the loss was minimal.27
Current femtosecond laser-assisted cataract surgery studies
While there have been relatively few studies demonstrating the utility of FSL in all steps of cataract surgery, there have been multiple publications detailing FSL use in the separate steps of cataract surgery. We will first review the studies on laser-assisted incision, capsulorhexis, and lens fragmentation separately, followed by studies showing clinical outcomes.
Femtosecond laser-assisted corneal incision
Masket et al., conducted a cadaver eye study in which they showed decreased leakage, added stability, and reproducibility at various IOPs after FSL-guided corneal incision.28 Additionally, Palanker et al., observed they could create an incision using the FSL that formed a one-way, self-sealing, and water-tight valve under normal IOP.13 To date, no authors have published data on the rate of postoperative endophthalmitis with the advent of FSL-guided incision in cataract surgery, and there are no published comparative studies between standard keratome and FSL-guided incisions.
Femtosecond laser-assisted capsulorhexis
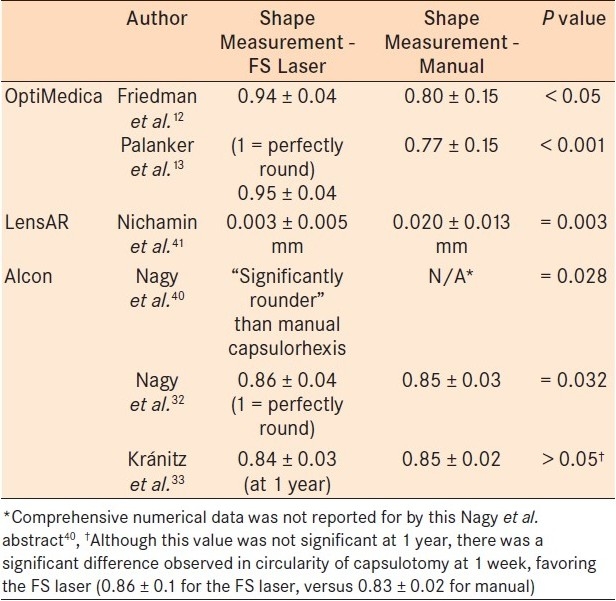
Nagy et al., performed anterior capsulotomies in 54 eyes, comparing the LenSx laser to manual capsulorhexis 1 week after cataract surgery. In the FSL group, the authors observed a higher degree of circularity, fewer patients with incomplete capsulorhexis-IOL overlap (11% of laser patients compared to 28% of manual capsulorhexis patients), and better IOL centration. FSL-guided capsulotomy diameter did not show correlation to pupil diameter, eye size, or curvature of the cornea, assuming the pupil was appropriately dilated. However, manual capsulotomy size was directly correlated with these variables. This suggests that manual capsulotomy is prone to deceptive influence from the pupil size, eye size, and curvature of the cornea, while laser capsulotomy avoids this inappropriate influence.29 Using the OptiMedica FSL platform, two studies similarly demonstrated a statistical advantage for the FSL-assisted capsulotomy in terms of precision, accuracy, and reproducibility in human eyes.12,13
Kránitz et al., studied the LenSx platform and compared manual capsulorhexis to FSL-assisted capsulorhexis with 1 year of follow-up. The authors observed greater capsulorhexis-IOL overlap in the FSL group and greater amounts of horizontal IOL decentration in the manual capsulorhexis group. This study suggested that decentration of the IOL was six times more likely to occur in the setting of manual capsulorhexis. No FSL-assisted capsulorhexis patient experienced > 0.4 mm of horizontal decentration during the study. There was a statistically significant difference between circularity of the capsulotomy at postoperative week one—favoring laser-assisted surgery. However, at 1 year, there was no longer a statistical difference between the two groups. In fact, the manual capsulotomy group showed a nonsignificant trend toward greater circularity compared to the FSL group.30
In regards to capsulotomy strength, Friedman et al. and Nagy et al., showed greater strength in FSL-guided capsulotomies in porcine eyes.12,31 Summaries of all reported capsulorhexis parameter studies allow for direct comparison [Tables 1–3].
Table 1.
Capsulotomy diameter ranges
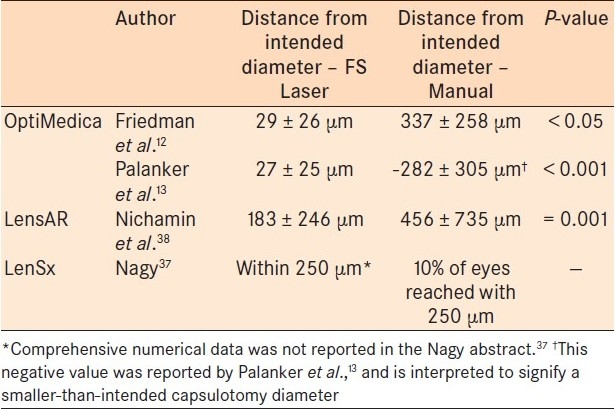
Table 3.
Intraocular lens centration
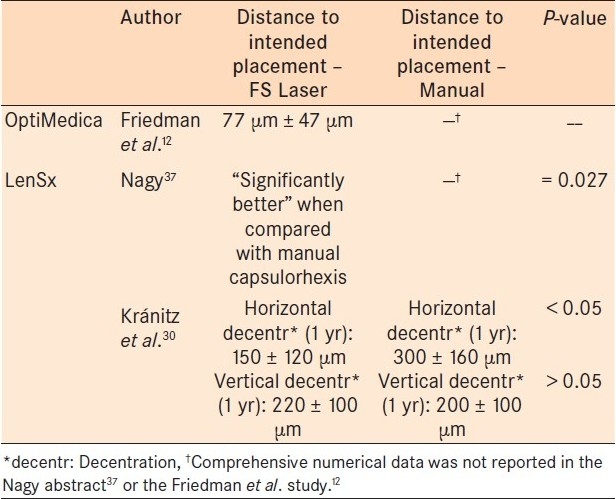
Table 2.
Capsulotomy Shape
Femtosecond laser-assisted phacofragmentation
Preliminary work has shown that FSL systems reduce ultrasound energy necessary for all grades of cataract.32,33 Nagy et al., showed that the FSL reduced phacoemulsification power by 43% and operative time by 51% in a porcine eye study.31 Two studies have compared human eyes receiving FSL-assisted capsulorhexis and phacofragmentation to fellow eyes receiving traditional cataract surgery. Both show easier phacoemulsification in the FSL group.13,33 In one of these studies, Palanker et al. observed a decrease in the perceived hardness of nuclear sclerotic cataract after the laser-assisted procedure, estimated by the surgeon to decrease from grade four to grade two. A 39% average reduction in dispersed energy for phacoemulsification was also observed in the FSL group.13 Furthermore, Uy showed that with grade three or higher cataracts, FSL-assisted lens fragmentation also reduced the amount of energy, suggesting fewer complications for these more difficult cataracts.34
Preliminary clinical outcomes
Upon our most recent literature search, there were limited articles describing outcomes in FLACS, and even fewer comparing outcomes with conventional cataract surgery. In 2009, Nagy et al., studied the LenSx system and reported results for nine human subjects. Three patients had FSL-assisted capsulotomies, three had FSL-assisted phacofragmentation, and three had both FSL-assisted procedures. There were no operative complications. On postoperative day one, the eyes had mild corneal edema and trace cell and flare, which resolved within 1 week. Corneal incision was not made with the FSL. At 1 month postoperatively, all eyes were 20/20, and no eyes had IOP > 21 mmHg at any time.31
Palanker et al., studied 50 patients who underwent FLACS using the OptiMedica platform in one eye, with the fellow eye receiving traditional cataract surgery. Postoperatively, 80% of patients showed small petechial conjunctival hemorrhages and vasodilatation in a ring pattern around the area of the suction contact lens, which resolved by 7-day follow-up. Additionally, 38% of laser patients and 70% of traditional cataract surgery patients experienced corneal edema. Best corrected visual acuity (BCVA) showed a gain of 4.3±3.8 lines in the laser group (n=29) and a gain of 3.5±2.1 lines in the traditional group (n=30), although the difference was not statistically significant. Lastly, the authors also tested 12 rabbit eyes to assess retinal safety using maximal laser settings of 6 μJ and 100 kHz. With fluorescein angiography and fundoscopic imaging at 1 hour, and then at 3 days, they observed no retinal or other damage.13
Although unpublished at this time, there are multiple abstracts which have demonstrated preliminary results for FLACS. Slade presented 50 eyes treated with LenSx FLACS, and showed the corneal incisions self-sealed and were reproducible and architecturally sound. The author showed less induced coma and astigmatism, manipulation, and phacoemulsification time. There was also less variation in lens position. He reported 100% of eyes were 20/30 or better BCVA after one week.35 Edwards et al., performed a fellow eye study with conventional versus LensAR FLACS, which included 60 FSL-treated eyes and 45 conventionally treated eyes. The authors found no significant difference in the outcomes of BCVA, IOP, or corneal thickness between the two groups. No major complications were reported in either group.36
CONCLUSIONS
It is possible that in the coming years, FSLs will revolutionize the way we perform cataract surgery. The method has already shown excellent results for precise and self-sealing corneal incision,13,28,35 highly circular, strong, and accurate capsulorhexis,12,13,29,30,35,37–39 and safer and less technically difficult phacofragmentation and subsequent phacoemulsification.13,32–35
When looking at a technique that may cause such deep-rooted changes in the most common surgical procedure in the United States, it is important to consider questions that must be answered in the coming years. Arguably, the most important questions are: is FLACS safe, and is it effective? Current studies support the safety and efficacy of FLACS,13,31,36 although small patient populations and short-term follow-up limit the ability to adequately assess such safety factors as the frequency of discontinuous FSL-assisted capsulorhexis and associated anterior capsular tears. Additionally, there are still many unanswered questions at this point. For FSL-assisted corneal incision, it is important to research the difference in postoperative endophthalmitis rates after laser-assisted corneal incision. Although this research will be a difficult undertaking requiring very large patient populations, this is a key question because postoperative endophthalmitis is the terminal outcome measure that will ultimately justify FSL use in corneal incisions. Also, the pilot cadaver study by Masket et al., which reported better integrity of FSL-guided incisions—carries the limitation that full-thickness incisions were made using the FSL.28 This is in contrast to current study procedures, which state the incision is partially made by the FSL and then completed with a microsurgical blade in the operating room. Although Palanker et al., demonstrated FSL-guided corneal incisions with great integrity even after completion with a microsurgical blade,13 this subject will require future comparative studies.
Regarding capsulorhexis, we hope to understand if the improved centration outcomes seen with laser-derived capsulotomy will show long-term superiority for visual outcomes and rates of posterior capsular opacification. This is a crucial question because positioning of the IOL is the biggest contributor to visual error after cataract surgery.22 Future studies will need to elucidate if there truly are superior visual outcomes in long-term follow-up of laser-assisted cataract surgery. Also, a smooth curvilinear capsulorhexis is known to prevent intraoperative complications. However, we do not know if the capsule edge strength of a FSL-derived capsulotomy is truly superior, and if this is clinically significant. Authors have stated that a limitation of measuring capsule strength is that is has only been done in porcine samples, and this tissue is known to be thicker and more resistant to tearing than human tissue.12
For phacofragmentation and phacoemulsification, it is not yet known if there is clinical significance to the decreased power seen in laser-assisted phacofragmentation, and if it will lead to fewer intraoperative and postoperative complications. Also, any limitations of FSL use in very high-grade opacifications have not yet been delineated in published studies. Many of these lasers have only recently received FDA approval, and therefore there are few published studies that can answer these questions. Additionally, studies have not been able to achieve large enough patient populations to accurately assess complication rates. The longest study published at this time is limited to 1 year of follow-up. This study focused on centration parameters only, and showed long-term promising results with superior centration data in the FSL group.30 However, it will be important to see what 1-year follow-up shows for patients who have had corneal incision, capsulorhexis, and phacofragmentation with the FSL.
The limitations of FLACS are not well-established at this time. Based on relative contraindications to FSL refractive surgery, we hypothesize similar contraindications may apply to FLACS. Logistically, those patients who have deep set orbits or those with tremors or dementia may do poorly with the initial docking of the lens that requires patient cooperation. Other exclusions may include anterior basement membrane dystrophy, corneal opacities (such as arcus senilis, corneal dystrophies, and trauma- or contact lens-induced scars), ocular surface disease, pannus with encroaching blood vessels, or recurrent epithelial erosion syndrome. Additionally, the level of increase in IOP induced by the docking device has not been adequately quantified in published studies. This may be a significant contraindication for patients with glaucoma, optic neuropathies, or borderline endothelial pathology. Lastly, diabetics may have undiagnosed epithelial disease making them prone to epithelial defects.40 A foreseeable complication could be persistent epithelial defects from the trauma of docking into an optical system, in an otherwise routine cataract surgery
Because FLACS relies on anterior segment imaging for laser pattern mapping, any patients with poor dilation would be poor candidates. Such patients would include those with posterior synechiae, intraoperative floppy iris syndrome suspects, or those on chronic miotic medications. High-quality images for mapping of the posterior lens are critical. More studies are needed to assess if dense posterior subcapsular cataracts, those with vacuoles, anterior subcapsular cataracts, and other types or combinations of cataracts will perform differently with FSL-assisted phacofragmentation. Additionally, having a stable, stationary lens is needed for precise laser mapping and execution. Patients with phacodonesis and zonular dialysis, or even those with risk factors such as pseudoexfoliation syndrome or trauma, may not be ideal candidates.
The final discussion point for FLACS is cost. These laser machines with integrated OCT or Scheimpflug technology will add considerable cost to a currently standard procedure. The final cost -benefit analysis will be more complete once long-term data on the laser systems is available. Because the size of the laser platforms is quite large, many surgery centers may require an extra step of moving a patient from the preoperative laser suite to the operating room. This will also be an extra cost in both time and efficiency
In conclusion, FSL-assisted cataract surgery appears safe and efficacious, and may eventually be proven superior to conventional cataract surgery. The coming years of research will tell how far the reach of laser cataract surgery will extend.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Sugar A. Ultrafast (femtosecond) laser refractive surgery. Curr Opin Ophthalmol. 2002;13:246–9. doi: 10.1097/00055735-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 2.He L, Sheehy K, Culbertson W. Femtosecond laser-assisted cataract surgery. Curr Opin Ophthalmol. 2011;22:43–52. doi: 10.1097/ICU.0b013e3283414f76. [DOI] [PubMed] [Google Scholar]
- 3.Krasnov MM. Laser-phakopuncture in the treatment of soft cataracts. Br J Ophthalmol. 1975;59:96–8. doi: 10.1136/bjo.59.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyman GA, Katoh N. Effects of an erbium: YAG laser on ocular structures. Int Ophthalmol. 1987;10:245–53. doi: 10.1007/BF00155632. [DOI] [PubMed] [Google Scholar]
- 5.Walkow T, Anders N, Pham DT, Wollensak J. Causes of severe decentration and subluxation of intraocular lenses. Graefes Arch Clin Exp Ophthalmol. 1998;236:9–12. doi: 10.1007/s004170050035. [DOI] [PubMed] [Google Scholar]
- 6.Cekic O, Batman C. The relationship between capsulorhexis size and anterior chamber depth relation. Ophthalmic Surg Lasers. 1999;30:185–90. [PubMed] [Google Scholar]
- 7.Wolffsohn JS, Buckhurst PJ. Objective analysis of toric intraocular lens rotation and centration. J Cataract Refract Surg. 2010;36:778–82. doi: 10.1016/j.jcrs.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 8.Gale RP, Saldana M, Johnston RL, Zuberbuhler B, McKibbin M. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye (Lond) 2009;23:149–52. doi: 10.1038/sj.eye.6702954. [DOI] [PubMed] [Google Scholar]
- 9.Murphy C, Tuft SJ, Minassian DC. Refractive error and visual outcome after cataract extraction. J Cataract Refract Surg. 2002;28:62–6. doi: 10.1016/s0886-3350(01)01027-6. [DOI] [PubMed] [Google Scholar]
- 10.Cullen K, Hall M, Golosinskiy A. Ambulatory surgery in the United States, 2006. [Last Accessed on 2011 Jul 7];Natl Health Stat Rep. 2009 28:1–25. Available from: http://www.cdc.gov/nchs/data/nhsr/nhsr011.pdf . [PubMed] [Google Scholar]
- 11.Juhasz T, Loesel F, Kurtz R, Horvath C, Bille J, Mourou G. Corneal refractive surgery with femtosecond lasers. IEEE J Sel Top Quantum Electron. 1999;5:902–10. [Google Scholar]
- 12.Friedman NJ, Palanker DV, Schuele G, Andersen D, Marcellino G, Seibel BS, et al. Femtosecond laser capsulotomy. J Cataract Refract Surg. 2011;37:1189–98. doi: 10.1016/j.jcrs.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Palanker DV, Blumenkranz MS, Andersen D, Wiltberger M, Marcellino G, Gooding P, et al. Femtosecond laser-assisted cataract surgery with integrated optical coherence tomography. Sci Transl Med. 2010;2:58ra85. doi: 10.1126/scitranslmed.3001305. [DOI] [PubMed] [Google Scholar]
- 14.Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: A systematic review of the literature. Arch Ophthalmol. 2005;123:613–20. doi: 10.1001/archopht.123.5.613. [DOI] [PubMed] [Google Scholar]
- 15.Xia Y, Liu X, Luo L, Zeng Y, Cai X, Zeng M, et al. Early changes in clear cornea incision after phacoemulsification: An anterior segment optical coherence tomography study. Acta Ophthalmol. 2009;87:764–8. doi: 10.1111/j.1755-3768.2008.01333.x. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell DP, Jr, Diamond JG, May DR. Surgical wound defects associated with endophthalmitis. Ophthalmic Surg. 1994;25:157–61. [PubMed] [Google Scholar]
- 17.Montan PG, Koranyi G, Setterquist HE, Stridh A, Philipson BT, Wiklund K. Endophthalmitis after cataract surgery: Risk factors relating to technique and events of the operation and patient history: A retrospective case-control study. Ophthalmology. 1998;105:2171–7. doi: 10.1016/S0161-6420(98)91211-8. [DOI] [PubMed] [Google Scholar]
- 18.Ernest PH, Kiessling LA, Lavery KT. Relative strength of cataract incisions in cadaver eyes. J Cataract Refract Surg. 1991;17(Suppl):668–71. doi: 10.1016/s0886-3350(13)80681-5. [DOI] [PubMed] [Google Scholar]
- 19.Dooley IJ, O’Brien PD. Subjective difficulty of each stage of phacoemulsification cataract surgery performed by basic surgical trainees. J Cataract Refract Surg. 2006;32:604–8. doi: 10.1016/j.jcrs.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 20.Dick HB, Pena-Aceves A, Manns M, Krummenauer F. New technology for sizing the continuous curvilinear capsulorhexis: Prospective trial. J Cataract Refract Surg. 2008;34:1136–44. doi: 10.1016/j.jcrs.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 21.Trivedi RH, Wilson ME, Jr, Bartholomew LR. Extensibility and scanning electron microscopy evaluation of 5 pediatric anterior capsulotomy techniques in a porcine model. J Cataract Refract Surg. 2006;32:1206–13. doi: 10.1016/j.jcrs.2005.12.144. [DOI] [PubMed] [Google Scholar]
- 22.Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34:368–76. doi: 10.1016/j.jcrs.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 23.Hollick EJ, Spalton DJ, Meacock WR. The effect of capsulorhexis size on posterior capsular opacification: One-year results of a randomized prospective trial. Am J Ophthalmol. 1999;128:271–9. doi: 10.1016/s0002-9394(99)00157-9. [DOI] [PubMed] [Google Scholar]
- 24.Sanders DR, Higginbotham RW, Opatowsky IE, Confino J. Hyperopic shift in refraction associated with implantation of the single-piece Collamer intraocular lens. J Cataract Refract Surg. 2006;32:2110–2. doi: 10.1016/j.jcrs.2006.07.030. [DOI] [PubMed] [Google Scholar]
- 25.Murano N, Ishizaki M, Sato S, Fukuda Y, Takahashi H. Corneal endothelial cell damage by free radicals associated with ultrasound oscillation. Arch Ophthalmol. 2008;126:816–21. doi: 10.1001/archopht.126.6.816. [DOI] [PubMed] [Google Scholar]
- 26.Shin YJ, Nishi Y, Engler C, Kang J, Hashmi S, Jun AS, et al. The effect of phacoemulsification energy on the redox state of cultured human corneal endothelial cells. Arch Ophthalmol. 2009;127:435–41. doi: 10.1001/archophthalmol.2009.39. [DOI] [PubMed] [Google Scholar]
- 27.Storr-Paulsen A, Norregaard JC, Ahmed S, Storr-Paulsen T, Pedersen TH. Endothelial cell damage after cataract surgery: divide-and-conquer versus phaco-chop technique. J Cataract Refract Surg. 2008;34:996–1000. doi: 10.1016/j.jcrs.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Masket S, Sarayba M, Ignacio T, Fram N. Femtosecond laser-assisted cataract incisions: Architectural stability and reproducibility. J Cataract Refract Surg. 2010;36:1048–9. doi: 10.1016/j.jcrs.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 29.Nagy ZZ, Kranitz K, Takacs AI, Mihaltz K, Kovacs I, Knorz MC. Comparison of intraocular lens decentration parameters after femtosecond and manual capsulotomies. J Refract Surg. 2011;27:1–6. doi: 10.3928/1081597X-20110607-01. [DOI] [PubMed] [Google Scholar]
- 30.Kránitz K, Takacs A, Mihaltz K, Kovacs I, Knorz MC, Nagy ZZ. Femtosecond laser capsulotomy and manual continuous curvilinear capsulorrhexis parameters and their effects on intraocular lens centration. J Refract Surg. 2011:1–6. doi: 10.3928/1081597X-20110623-03. [DOI] [PubMed] [Google Scholar]
- 31.Nagy Z, Takacs A, Filkorn T, Sarayba M. Initial clinical evaluation of an intraocular femtosecond laser in cataract surgery. J Refract Surg. 2009;25:1053–60. doi: 10.3928/1081597X-20091117-04. [DOI] [PubMed] [Google Scholar]
- 32.Fishkind W, Uy H, Tackman R, Kuri J. Boston, Massachusetts: 2010. Apr 9-14, Alternative fragmentation patterns in femtosecond laser cataract surgery [abstract]. In: Program and Abstracts of American Society of Cataract and Refractive Surgeons Symposium on Cataract, IOL and Refractive Surgery. [Google Scholar]
- 33.Koch D, Batlle J, Feliz R, Friedman N, Seibel B. Paris, France: 2010. Sep 4-10, The use of OCT-guided femtosecond laser to facilitate cataract nuclear disassembly and aspiration [abstract]. In: Program and Abstracts of XXVIII Congress of the ESCRS. [Google Scholar]
- 34.Uy HS. Illinois, USA: 2010. Oct 15-16, Femtosecond laser lens fragmentation for higher grade cataracts. In: Program and Abstracts of the Annual meeting of ISRS. [Google Scholar]
- 35.Slade SG. Illinois, USA: 2010. Oct 15-16, First 50 accommodating IOLs with an image-guided femtosecond laser in cataract surgery. In: Program and Abstracts of the Annual Meeting of ISRS. [Google Scholar]
- 36.Edwards KH, Frey RW, Naranjo-Tackman R, Villar Kuri J, Quezada N, Bunch T. Clinical outcomes following laser cataract surgery. Invest Ophthalmol Vis Sci. 2010;51 E-Abstract 5394. [Google Scholar]
- 37.Nagy Z. Paris, France: 2010. Sep 4-8, Comparative analysis of femtolaser-assisted and manual capsulorhexis during phacoemulsification [abstract]. In: Program and Abstracts of XXVIII Congress of the ESCRS. [Google Scholar]
- 38.Nichamin L, Naranjo-Tackman R, VillarKuri J, Fishkind W. Paris, France: 2010. Sep 4-8, Laser capsulotomy with the LensAR laser system (LLS) [abstract]. In: Program and Abstracts of XXVIII Congress of the ESCRS. [Google Scholar]
- 39.Toropygin SG, Krause M, Akkaya A, Riemann I, Seitz B, Mestres P, et al. Experimental femtosecond laser-assisted nanosurgery of anterior lens capsule. Eur J Ophthalmol. 2011;21:237–42. doi: 10.5301/EJO.2010.1445. [DOI] [PubMed] [Google Scholar]
- 40.Halkiadakis I, Belfair N, Gimbel HV. Laser in situ keratomileusis in patients with diabetes. J Cataract Refract Surg. 2005;31:1895–8. doi: 10.1016/j.jcrs.2005.03.075. [DOI] [PubMed] [Google Scholar]


