Abstract
Objective
To test if estrogen promotes carcinogenesis in vitro and in a genetic mouse model of ovarian cancer and whether its effects can be inhibited by a novel selective estrogen receptor modulator (SERM), bazedoxifene.
Methods
Bazedoxifene was synthesized and it was confirmed that the drug abrogated the uterine stimulatory effect of 17β-estradiol in mice. To determine if hormones alter tumorigenesis in vivo LSL-K-rasG12D/+PtenloxP/loxP mice were treated with vehicle control, 17β-estradiol or bazedoxifene. Hormone receptor status of a cell line established from LSL-K-rasG12D/+PtenloxP/loxP mouse ovarian tumors was characterized using western blotting and immunohistochemistry. The cell line was treated with hormones and invasion assays were performed using Boyden chambers and proliferation was assessed using MTT assays.
Results
In vitro 17β-estradiol increased both the invasion and proliferation of ovarian cancer cells and bazedoxifene reversed these effects. However, in the genetic mouse model neither treatment with 17β-estradiol nor bazedoxifene changed mean tumor burden when compared to treatment with placebo. The mice in all treatment groups had similar tumor incidence, metastatic nodules and ascites.
Conclusion
While 17β-estradiol increases the invasion and proliferation of ovarian cancer cells, these effects do not translate into increased tumor burden in a genetic mouse model of endometrioid ovarian cancer. Likewise, while the SERM reversed the detrimental effects of estrogen in vitro, there was no change in tumor burden in mice treated with bazedoxifene. These findings demonstrate the complex interplay between hormones and ovarian carcinogenesis.
Keywords: ovarian cancer, 17β-estradiol, selective estrogen receptor modulators, genetic mouse model, hormone replacement therapy
Introduction
In an attempt to reduce the high mortality from ovarian cancer, considerable effort has been invested in improving screening for the early detection of the disease. Unfortunately, screening strategies such as cancer antigen 125 (CA-125) and transvaginal ultrasound have not been shown to decrease ovarian cancer (OvCa) mortality [1]. Ovarian cancer continues to be the most fatal gynecologic cancer, with an estimated 5-year survival of only 50% [2]. Epithelial OvCa is thought to arise either from the ovarian surface epithelium or the fimbrial epithelium of the fallopian tube [3, 4], both environments that may be uniquely susceptible to the effects of sex-steroid hormones. In fact, there is robust epidemiologic evidence that use of oral contraception reduces the risk of OvCa by up to 29% after five years of use [5]. In contrast to the protection conveyed by oral contraceptives, epidemiologic studies indicate that use of postmenopausal hormonal therapy increases the risk of OvCa [6-11]. This risk appears to be attributable to the estrogen component, since use of estrogen only hormonal therapy triples the risk of OvCa, while there is no elevated risk among users of estrogen plus progestin hormonal therapy [11]. In vitro data also suggests that estrogen promotes ovarian carcinogenesis. 17β-estradiol increases growth of several OvCa cell lines [12] and enhances OvCa cell migration and invasion [13-15]. There are limited in vivo studies of estrogen and ovarian carcinogenesis, but there have been reports of increased tumor growth [13, 16] and decreased survival in mice treated with 17β-estradiol [17].
Given the detrimental effects of estrogen in OvCa, it is plausible that drugs that act as antagonists at the estrogen receptor could protect against OvCa. Selective estrogen receptor modulators (SERMs) may prove to be promising drugs in this arena. For example, clinical trials have shown that tamoxifen has a small but favorable effect on recurrent ovarian cancer [18, 19]. Bazedoxifene is a third generation SERM that is known to have an estrogen agonist effect on bone and lipid metabolism, but is an estrogen antagonist in the breast and endometrium [20]. Bazedoxifene is being developed for prevention and treatment of postmenopausal osteoporosis and has undergone phase III clinical trials for this indication. One study has shown that bazedoxifene antagonizes estrogen mediated increases in breast cancer cell proliferation and gene expression [21]. However, it is unknown whether or not bazedoxifene will counteract the detrimental effects of estrogen in OvCa.
Given the limitations of both early detection and the treatment of advanced disease, it is important to develop strategies for OvCa prevention. Towards this end understanding the effects of hormones on ovarian carcinogenesis may provide insights into preventive approaches. Both epidemiologic and in vitro studies have demonstrated a detrimental effect of estrogen in OvCa, indicating that the hormonal milieu may play an important role in carcinogenesis. However, there have been few in vivo studies exploring the effects of estrogen or SERMs on ovarian carcinogenesis. Recent evidence indicates that chemopreventive approaches are not isolated to inhibition of tumor initiation, but also that slowing the progression of low-volume, clinically undetectable disease may be equally important [22]. In this study to further understand the role of reproductive hormones in OvCa prevention, we tested if estrogen increased tumor burden in a genetic mouse model of OvCa. In addition, we asked how estrogen increased tumor burden and whether a novel third generation SERM, bazedoxifene, decreased tumor burden.
Materials and methods
Synthesis of Bazedoxifene
Bazedoxifene was synthesized as previously described [23] following the steps outlined in (Supplemental Fig 1). All chemicals were obtained from Sigma Aldrich and used as received. 1H NMR spectra were recorded on a Bruker Biospin 400MHz spectrometer. Mass spectra were obtained with an Agilent 1100 LC/MSD SL quadruple mass spectrometer equipped with an APCI ionization source. 1H NMR and LC/MSD readings were obtained following each synthesis step (data available upon request). Briefly, 4′-benzyloxy-2-bromopropiophenone (2) was synthesized by dissolving 4′-benzyloxy-propiophenone in dichloromethane (DCM), to which Br2 was added. 4′-benzyloxy-2-bromopropiophenone (2) and 4-benzyloxyaniline hydrochloride were then dissolved in DMF to synthesize 2-Phenyl-3-methyl-1H-indole (3). To synthesize 2-[4-(Hydroxymethyl)phenoxy]acetic acid ethyl ester (5) 4-(hydroxymethyl)phenol in DMF, ethyl iodoacetate and K2CO3 were mixed. 2-[4-(Hydroxymethyl)phenoxy]acetic acid ethyl ester (5) and triethylamine were then dissolved in DCM and methanesulfonyl chloride to form 2-[4-(Chloromethyl)phenoxy]acetic acid ethyl ester (6). 2-Phenyl-3-methyl-1H-indole (3) dissolved in DMF was added to 2-[4-(Chloromethyl)phenoxy]acetic acid ethyl ester (6) forming {4-[5-Benzyloxy-2-(4-benzyloxy-phenyl)-3-methyl-indol-1-ylmethyl]-phenoxy}-acetic acid ethyl ester (7). This was then dissolved in THF to synthesize 2-{4-[5-Benzylxoy-2- (4-benzyloxy-phenyl)-3-methyl-indol-1-ylmethyl]-phenoxy}-ethanol (8). Benzylxoy-2-(4-benzyloxy-phenyl)-3-methyl-indol-1-ylmethyl]-phenoxy}-ethanol (8) was dissolved in THF, CBr4 and PPh3 were added to form 5-Benzylxoy-2-(4-benzyloxy-phenyl)-1-[4-(2-bromo- ethoxy)-benzyl]-3-methyl-1H-indol (9). To form 5-Benzylxoy-2-phenyl-3-methyl-1-[4-(2-azepan-1-yl-ethoxy)-benzyl]-1H-indol; 5-Benzylxoy-2-(4-benzyloxy-phenyl)-1-[4-(2-bromo-ethoxy)-benzyl]-3-methyl-1H-indol (9) was dissolved in THF and hexamethylenimine was added. As a final step, 2-(4-hydroxy-phenyl)-3-methyl-1-[4-(2-Azepan-1-yl-ethoxy)-benzyl]- 1H-indol-5-ol (10) was synthesized by dispersing 10% Pd/C in ethyl acetate, adding 5-Benzylxoy-2-phenyl-3-methyl-1-[4-(2-azepan-1-yl-ethoxy)-benzyl]-1H-indol in THF/EtOH and stirring under H2 atmosphere overnight.
Animals
LSL-K-rasG12D/+PtenloxP/loxP mice were obtained from the Massachusetts Institute of Technology, Boston, MA [24]. After weaning, the genotypes of the female mice were determined by PCR. Virgin female mice were housed in a modified barrier facility with ad libitum access to food and water. A temperature of 25°C and light cycle of 12 hours on/off was maintained. Compounds were prepared in a vehicle of 2% ethanol, 10% Cremophor EL (Sigma-Aldrich), and 88% 1x PBS at defined concentrations, so that the treatment volume was 0.01ml/g body weight. At 5 weeks of age mice were treated 6 days per week with subcutaneous 17β-estradiol (5μg/kg/day), bazedoxifene (5 mg/kg/day), or vehicle. These hormone doses were based on previously published reports both by ourselves and others [25, 26]. The mice were treated for 6 weeks before OvCa was initiated and treatment was continued until the end of the study. OvCa was initiated by intrabursal injection of AdCre virus obtained from the University of Iowa Gene Transfer Vector Core. For the injection mice were sedated, the right ovary was exposed and the ovarian bursa injected with AdCre (2.5 × 107 plaque-forming units [p.f.u]). The left ovary was not injected and served as an internal control. Mice were sacrificed 9 weeks after the injection of the virus. At the time of sacrifice the primary tumor was excised, weighed, measured and the number of metastatic nodules and volume of ascites were recorded. All tissue was fixed in 10% formalin, embedded in paraffin, and stained with hematoxylin and eosin (H&E). All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Chicago.
Uterotrophic Assay
A standard uterotrophic assay was completed as previously described [27]. Briefly, 18 to 21 day old mice were treated daily for 4 days with vehicle control (n=6), 17β-estradiol (10 μg/kg/day, n=9), bazedoxifene (5 mg/kg/day, n=8) or both hormones (n=9). Twenty-four hours after the final treatment the mice were sacrificed and the uterus was weighed and expressed as percent of total body weight.
Reagents and cell lines
The K-ras/Pten mouse OvCa cell line was established by us from ovarian tumors produced by the genetic model as previously reported by our lab [28]. For all in vitro assays the K-ras/Pten mouse OvCa cell line was treated with 17β-estradiol (1×10−8 M), bazedoxifene (1×10−9 M) or vehicle control (0.5% ETOH). The IOSE 7576 cell line was kindly shared by Dr. Nelly Auersperg (University of British Columbia, Canada). The ERα (MC-20) antibody was from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) and the ERβ antibody was from BioGenex Laboratories Inc. (San Ramon, CA). The antibodies detecting phosphorylated AKT, total and phosphorylated ERK and STAT3 were from Cell Signaling Technologies (Berverly, MA). The β-actin antibody and 17β-estradiol were obtained from Sigma-Aldrich (St Louis, MO).
Genotyping for the K-Ras lox-stop-lox cassette and Pten
DNA was extracted from paraffin embedded tumor samples and from K-ras/Pten cells using a DNA extraction kit (Dneasy Tissue Kit; Qiagen, Hilden, Germany) and genotyped for the K-Ras lox-stop-lox cassette. Primer sequences are available upon request. DNA extracted from a tail of a LSL-K-rasG12D/+PtenloxP/loxP mouse was used as positive control and nuclease free water as negative control.
Quantitative Real-Time RT-PCR
The K-ras/Pten mouse OvCa cells were cultured in phenol red-free media with charcoal stripped FBS. RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) and was transcribed into cDNA using high capacity cDNA kit (Applied Biosystems). Quantitative real-time RT-PCR was performed using the Applied Biosystems 7500 Real Time PCR system (Applied Biosystems, Forest City, CA) with TaqMan gene expression assays for ESR1 (Mm01191130_m1) and mouse GAPDH as internal control (Applied Biosystems, Forest City, CA). Relative levels of mRNA expression were calculated using the 2−ΔΔCT method [29]. The cDNA was also subjected to PCR using the mouse estrogen and progesterone receptor primers as previously described [13]. Amplified PCR products were analyzed by electrophoresis on 1% agarose gel with Tris-borate-EDTA buffer.
Western Blot Analysis
Cells were serum starved in phenol red free media for 24 hours and treated with 17β-estradiol, Bazedoxifene or vehicle control. The cells were then lysed in ice-cold radioimmunoprecipitation assay buffer. An equal amount (30μg) of cell extract was separated by 4-20% SDS-PAGE, transferred to a nitrocellulose membrane and blocked with 5% nonfat milk or bovine serum albumin. The membrane was incubated with respective antibodies overnight at 4°C. The blots were incubated with horseradish peroxidase-conjugated secondary antibody and visualized with enhanced chemoluminescence reagents.
MTT Assay
Proliferation of K-ras/Pten mouse OvCa cells was measured using an MTT assay as previously described [30]. The cells were plated in quadruplicate into 96-well plates and treated with vehicle control, 17β-estradiol and/or bazedoxifene. Treatment was continued for 72 hours. Four hours before the desired time point, 20 μl of MTT solution (5mg/ml in PBS) was added and followed by 200 μl of DMSO. The absorbance was measured using a plate reader (Synergy HT; Bio-Tek, Winoosky, VT) at 560 nm excitation and 670 nm emission. The effect of treatment was calculated as a percentage of control cell growth obtained from vehicle treated cells grown in the same 96-well plates. Each experiment was conducted in triplicate.
Invasion Assay
Fifty thousand K-ras/Pten mouse OvCa cells were treated with hormones for 24 hours and then plated in serum free, phenol red free media on each well of a 24-well transwell plate (8-μm pore size) pre-coated with 0.02 mg/ml of collagen type I. The bottom of the transwell was filled with 700 μl of full growth media. After incubation at 37°C overnight, membranes were fixed in 4% paraformaldehyde, stained with Giemsa and dried. The number of invading cells was quantified in five fields using an Axiovert 100 microscope (Zeiss, Göttingen, Germany). Each experiment was done in triplicate [31].
Immunostaining
Mouse tumors were formalin fixed, paraffin-embedded, sectioned and mounted on slides. Slides were deparaffinized in xylene and hydrated with alcohol. The slides were then placed in 3% H2O2/methanol blocking solution followed by antigen unmasking. Incubation with the antibody against ERα was done with a 1:160 dilution and ERβ was done with a 1:50 dilution. The slides were stained using the EnVision avidin-biotin-free detection system and counterstained with hematoxylin. Mouse endometrium served as a positive control for ERα and mouse ovarian follicles served as positive control for ERβ. Negative controls were prepared by omitting the primary antibody. The Hematoxylin and Eosin (H&E) and the immunohistochemistry staining were evaluated by two pathologists (IG, TK) who were unaware of the treatment groups. Quantification of ER immunostaining was performed using the Automated Cellular Imaging System (ACIS, Clarient, San Juan Capistrano, CA) by setting color-specific thresholds to determine brown (positive) and blue (negative) nuclei within 12 representative regions per slide and by calculating the ratio of positively stained nuclei to all nuclei, expressed as a region score [32]. The highest and lowest region scores for each slide were excluded and the average region score was taken of the remaining 10 regions.
Statistical analysis
Data was analyzed by an unpaired, two-tailed Student’s t-test of significance, assuming equal variance of the test and the control populations. For non-parametric data a Kruskal-Wallis rank test was completed to compare the medians for all groups. Data are presented as mean ± standard deviations. A p < 0.05 level was considered significant. All data analysis was performed with STATA 10 (StataCorp, College Station, TX).
Results
Synthesis of Bazedoxifene
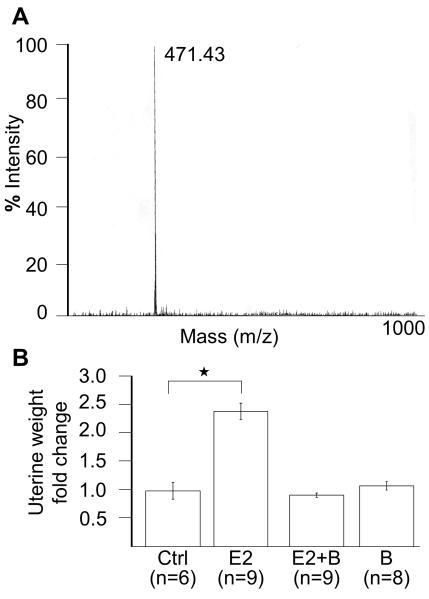
There is a reasonable rationale to use SERMs as prevention for OvCa, therefore, we decided to test bazedoxifene as a preventive agent. To facilitate a more unbiased, industry independent approach to this question we synthesized bazedoxifene ourselves. Briefly, bazedoxifene was synthesized in a step-wise fashion beginning with 4′-benzyloxy-2-bromopropiophenone as previously published and described in detail in the material and methods section [23]. 1H NMR and LC/MSD readings were obtained following synthesis (Fig. 1A).
Fig. 1.
Synthesis of bazedoxifene and physiologic effect.
(A) Mass spectrometry of synthesized bazedoxifene. (B) Uterotrophic assays. Eighteen day old mice were treated with hormones for 4 days and sacrificed. The uterus was weighed and weight changes expressed as percent of total body weight normalized to untreated uterus. Groups: Ctrl= vehicle control (n=6), E2= 17β-estradiol (n=9), E2+B= 17β-estradiol plus bazedoxifene (n=9), B= bazedoxifene alone (n=8). Columns, fold-change in uterus weight compared to placebo. *, p=0.004.
To confirm a physiologic effect of hormones prior to beginning the OvCa experiments, an uterotrophic assay was performed in a subset of mice. This assay is considered as the standard to confirm the effect of hormones in rodents [27]. In this experiment mice were treated with 17β-estradiol and/or bazedoxifene for 4 days and then the uterine weight was determined. It is expected that the estrogen stimulation of the endometrium would cause increase in uterine weight. Indeed, after treatment with 17β-estradiol mice had significantly increased uterine weights when compared to control treated mice. Importantly, the addition of bazedoxifene to 17β-estradiol resulted in uterine weights equal to the control group (p =0.004, Fig. 1B), confirming that the bazedoxifene had the expected estrogen antagonist effect.
Neither 17β-estradiol nor bazedoxifene alters tumor burden in genetic mouse model
17β-estradiol, bazedoxifene, or vehicle control was administered to the mice for 6 weeks followed by OvCa initiation by injection of AdCre virus into the right ovarian bursa (study schedule, Fig. 2A). The treatment was continued until the animals were sacrificed 9 weeks after initiation of cancer. The mean tumor weight was not significantly different in mice treated with 17β-estradiol or bazedoxifene compared to vehicle control (control: 1.2g ±1.2, 17β-estradiol: 0.91g±1.2, bazedoxifene: 0.87g ±0.73, p=0.93). Furthermore, mice in all the treatment groups had a similar number of metastatic nodules, volume of ascites, tumor incidence and rate of premature deaths (Fig. 2B). Gross dissection findings were similar among the three treatment groups with enlarged right ovaries containing both cystic and solid components; the left ovary was not injected with AdCre and served as a normal control (Fig. 2C). The histological appearance of the tumors were similar in all treatment groups (Fig. 2D). There have been reports that 17β-estradiol induces preneoplastic changes and epithelial mesenchymal transition (EMT) in the normal ovaries of mice [14, 17]. We assess for preneoplastic changes by completing a histological evaluation of all the normal left ovaries. However, the ovarian surface epithelium (OSE) of the left ovaries was found to be comparable among the different treatment groups. To evaluate for EMT the left ovaries were stained for E-cadherin by immunohistochemistry. Similar levels of E-cadherin were noted in the 17β-estradiol, bazedoxifene and control groups (data not shown).
Fig. 2.
Hormonal treatment does not alter in vivo tumorigenesis.
(A) Treatment schedule with hormones in the LSL-K-rasG12D/+PtenloxP/loxP genetic mouse model of ovarian cancer. (B) At the end of study mice were sacrificed and tumor burden was quantified. P values were calculated with Kruskal-Wallis or Fisher’s exact test. (C) Representative gross dissection for each treatment group. The left ovary was not injected with AdCre virus and served as an internal control. Yellow outline, ovarian tumors. Star, normal ovary, Dashed outline, uterus. R, right. L, left. (D) H&E stain of representative ovarian tumor from each treatment group. Original magnification, 100x. Inset normal ovary, 200x magnification.
Hormonal treatment alters expression of estrogen receptors in tumors
To characterize the effect of the treatments on estrogen receptor expression levels in the tumors, multiple tumors from each treatment group were stained for estrogen receptor α (ERα) and estrogen receptor β (ERβ) using immunohistochemistry. Analysis of the stains showed that ERα expression was significantly lower in tumors from mice treated with bazedoxifene compared to the control (p=0.003, Fig. 3A). In contrast, ERβ expression was significantly lower in the tumors from mice treated with 17β-estradiol compared mice treated with bazedoxifene (p=0.03, Fig. 3B).
Fig. 3.
Estrogen receptor α and β expression in ovarian tumors.
Immunohistochemistry. Tumors were stained for estrogen receptor α (A) and β (B) expression using a mouse antibody. Representative tumor samples from each treatment group were stained and quantification was performed using an Automated Cellular Imaging System. A region score was calculated as the ratio of positively stained nuclei to all nuclei. Ten representative regions were analyzed per slide. Original magnification, 200x and 400x. Scale bar = 50 μm. Columns, mean region score; bars, SD; *, P<0.5. Groups: Ctrl= vehicle control, E2= 17β-estradiol, B= bazedoxifene
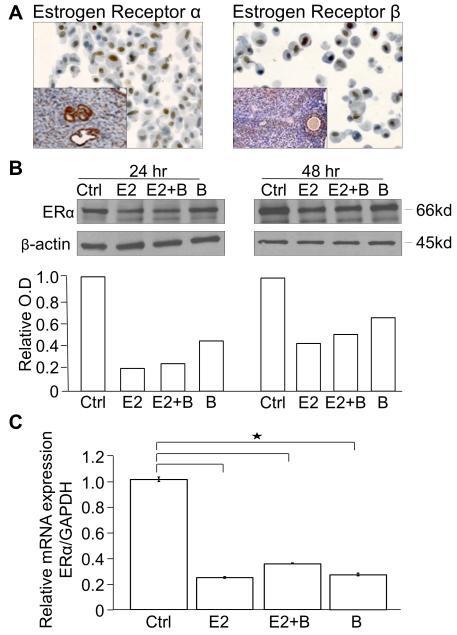
To understand why the hormone treatments did not alter tumor burden in the mouse model, in vitro studies were performed using the Kras/Pten mouse OvCa cell line which was established from tumors produced by LSL-K-rasG12D/+PtenloxP/loxP mice (Supplemental Fig. 2A) [28]. The hormone receptor status in the cell line was evaluated using RT-PCR which demonstrated expression of ERα, ERβ and progesterone receptors (Supplemental Fig. 2B). The genotype of the cell line was also determined and found to be the same as the tumors produced in the mouse model (Supplemental Fig 3). Cell blocks of the Kras/Pten mouse OvCa cell line were made and immunohistochemistry showed nuclear staining for both ERα and ERβ (Fig. 4A). The cell line was then treated with 17β-estradiol and/or bazedoxifene for 24 or 48 hours and western blots were performed for ERα. The results show that there was a decrease in ERα protein levels with 17β-estradiol and bazedoxifene treatment at 24 and 48 hours (Fig. 4B). To further evaluate ERα expression, quantitative real-time RT-PCR was performed which also showed decreased ERα mRNA levels with 24 hours of 17β-estradiol and/or bazedoxifene treatment (Fig. 4C). Given that the cell line expressed the estrogen receptors and was responsive to hormonal treatment we proceeded with in vitro studies evaluating the effect of hormones on important aspects of ovarian carcinogenesis.
Fig. 4.
K-ras/Pten mouse OvCa cell line hormone receptor characterization.
(A) The K-ras/Pten mouse OvCa cell line expresses ERα and ERβ. Cell blocks were made from the K-ras/Pten mouse OvCa cell line and stained by immunohistochemistry for ERα and ERβ. Inset positive control = mouse uterus (ERα) and mouse ovarian follicle (ERβ). (B) The K-ras/Pten mouse OvCa cell line was treated for 24 or 48 hours with hormones in charcoal stripped phenol red free complete media, followed by western blots for ERα and β-actin. Protein expression was quantitated and normalized to β-actin using NIH Image J software. Columns, fold-change in ERα optical density (OD) compared to placebo. (C) The K-ras/Pten cell line was treated with the indicated hormones for 24 hours then total RNA was extracted and the relative expression of ERα normalized to glyceraldehyde-3-phosphate dehydrogenase was measured using TaqMan quantitative real-time RT-PCR.
Groups: Ctrl= vehicle control, E2= 17β-estradiol, E2+ B= 17β-estradiol plus bazedoxifene, B= bazedoxifene alone
17β-estradiol increases invasion and proliferation of ovarian cancer cells
To understand the functional effect of hormones on carcinogenesis, the Kras/Pten mouse OvCa cell line was treated with 17β-estradiol and/or bazedoxifene and invasion and proliferation assays were performed. 17β-estradiol significantly increased the invasion of OvCa cells, while the combination of 17β-estradiol and bazedoxifene or bazedoxifene alone had no significant effect (mean number of invaded cells per field= control: 134±15, 17β-estradiol: 253±20, 17β-estradiol plus bazedoxifene: 133±12, bazedoxifene alone: 105±17, p=0.005, Fig. 5A). 17β-estradiol also increased the proliferation of the cancer cells by up to 76% compared to the placebo, while bazedoxifene alone did not alter the rate of proliferation (p=0.006, Fig. 5B).
Fig. 5.
17β-estradiol increases invasion and proliferation of OvCa cells.
(A) Invasion assay. The K-ras/Pten mouse OvCa cell line was treated for 24 hours with control, 17β-estradiol and/or bazedoxifene and the invading cells on the underside of the filter were enumerated using an inverted microscope. Columns, mean number of invaded cells from a representative experiment. *, P<0.05. (B) Proliferation assay. The K-ras/Pten mouse OvCa cell line was plated in quadruplicate into 96-well plates and treated with vehicle control, 17β-estradiol and/or bazedoxifene for 72 hours. The MTT proliferation assay was then performed. Experiments were performed four times. Columns, percent proliferation relative to control group*, P<0.05. (C) Western blotting. The K-ras/Pten mouse OvCa cell line or an immortalized human ovarian surface epithelium cell line, IOSE 7576, were serum- starved for 24 hours and the treated with the indicated hormones for 24 hours followed by immunoblotting for phosphorylated and total ERK and STAT3 proteins.
Groups: Ctrl= vehicle control, E2= 17β-estradiol, E2+ B= 17β-estradiol plus bazedoxifene, B= bazedoxifene alone
17β-estradiol had previously been shown to alter important cancer cell signaling pathways including increasing MAPK and STAT3 activity [13, 33, 34]. Therefore the effect of 17β-estradiol and bazedoxifene on these signaling pathways was tested in both the Kras/Pten mouse OvCa cell line and an immortalized human ovarian surface epithelial line (IOSE 7576). In the IOSE cell line, treatment with 17β-estradiol decreased phosphorylation of ERK, an important member of the MAPK family, while bazedoxifene had no effect. In the Kras/Pten cancer cells there was no change in ERK and STAT3 phosphorylation with either treatment (Fig. 5C). It has also been reported that estrogen receptor signaling can activate AKT [15, 35]. Therefore, we evaluated whether there was a change in phosphorylation of AKT with hormonal treatment. In our model we did not see increased phosphorylation of AKT with hormonal treatments in either the mouse tumors or the K-ras/Pten mouse OvCa cell line (Supplemental Fig 4).
Discussion
We undertook this study to test if hormonal treatment would alter carcinogenesis in a genetic mouse model. Contrary to our expectations, we found that 17β-estradiol did not increase tumor burden in LSL-K-rasG12D/+PtenloxP/loxP mice. In addition, bazedoxifene, a third generation SERM, also did not decrease tumor burden in the model. The results show that tumor weight, ascites volume, and the number of metastasis and tumor incidence were similar in all treatment groups (Fig. 2).
In addition the present study addressed whether hormones affect key OvCa cell functions. The main finding was that 17β-estradiol treatment increases proliferation of OvCa cells by 72% and invasion by 88% compared to control. Meanwhile, bazedoxifene returned both the level of proliferation and invasion to that of controls. The in vitro findings reported here add to the findings of others indicating that estrogen promotes OvCa cell growth [12], as well as migration and invasion [13-15]. More importantly, this is the first time a novel SERM has been shown to reverse the detrimental effects of 17β-estradiol in the context of OvCa. Although we were unable to demonstrate an impact of hormonal treatment on tumor burden, we thought it was important to further characterize the effect of 17β-estradiol and bazedoxifene in the tumors. There is evidence that levels of ERα and ERβ expression are important in OvCa. For example, ERβ is highly expressed in normal OSE, while ERα is the main form expressed in malignant tumors [36-38]. In addition, decreases in ERβ levels correlate with tumor progression [39]. In our study tumors from mice treated with bazedoxifene had a statistically significant decrease in ERα and an increase in ERβ expression. This may indicate that bazedoxifene was having a protective role at an estrogen receptor level through reducing ERα and increasing ERβ; although, this did not result in decreased tumor burden in this model.
The apparent discrepancies between our findings, which showed no effect of hormones on tumor burden in mice, and those of earlier studies which reported that 17β-estradiol increased tumor burden, may be due to the different mouse models used. Earlier studies utilized xenograft mouse models of OvCa in which human OvCa cell lines are injected into immunocompromized mice, an approach that may miss important steps in carcinogenesis. To overcome the deficits of xenograft models, genetic mouse models of OvCa have been developed in which cancer develops from the normal OSE [17, 24, 40, 41]. Why did bazedoxifene fail to affect tumor development despite the compelling rationale underlying the use of SERMs to prevent OvCa? Tumor burden may not have been altered because, like all genetic mouse models of OvCa, the LSL-K-rasG12D/+PtenloxP/loxP model is predicated on powerful genetic alteration in the OSE. It is possible that in the setting of such potent drivers of carcinogenesis the effects of hormones could not be captured. Although hormones may not prevent cancer initiation in a model driven by activation of Kras and deletion of Pten, we felt that it was biologically plausible that hormones might affect the progression of disease and therefore increase or decrease final tumor burden compared to placebo treatment. Consistent with this approach, in a parallel study we found that in this model treatment with foretinib, a multi-kinase inhibitor of c-Met and VEGFR-2 prevented the progression of primary tumors to invasive adenocarcinoma [42]. One final difference between the model we report here and those used in other studies is that the model we used develops the endometrioid subtype of epithelial OvCa. The most common subtype of OvCa is papillary serous, and perhaps the effect of 17β-estradiol and bazedoxifene would be more pronounced in that setting. Most of the genetic models of OvCa develop the endometrioid subtype. Therefore, despite the advantages of the new genetic mouse models of OvCa, there continues to be significant limitations. Still, our data suggest that bazedoxifene is not useful in the prevention of endometrioid OvCa using this model.
In conclusion, this study adds to the existing body of knowledge demonstrating that 17β-estradiol increases proliferation and invasion OvCa cells in vitro. However, these in vitro effects did not translate into increased tumor burden or changes in the normal ovary in the genetic mouse model. We also provide, to our knowledge, the first report that, as in breast cancer, a novel SERM is able to reverse the negative effects of estrogen on OvCa in vitro. Clinically, understanding the effects of estrogen and SERMs on ovarian carcinogenesis is of vital importance as women and their physicians consider options for post-menopausal hormone therapy and osteoporosis treatment. The hypothesis of hormonal carcinogenesis would suggest that exogenous hormones will increase cellular proliferation, leading to an accumulation of genetic errors and ultimately malignant transformation [43]. While this phenomenon has been well characterized in other hormonally responsive tissues, such as breast epithelium, it has not been well developed in OvCa. In fact, there appears to be a hormonal paradox at play in the ovary, with oral contraception providing protection but postmenopausal hormone therapy increasing the risk of OvCa. Further studies will be needed to clarify the complex relationship between the hormonal milieu and ovarian carcinogenesis.
Supplementary Material
Fig S1. Synthesis steps for bazedoxifene.
1H NMR spectra were recorded on a Bruker Biospin 400MHz spectrometer. Mass spectra were obtained with an Agilent 1100 LC/MSD SL quadruple mass spectrometer equipped with an APCI ionization source. 1H NMR and LC/MSD readings were obtained following each of the outlined synthesis steps. Reagents and conditions: (a) Br2, DCM; (b) 4-benzyloxyaniline hydrochloride, Et3N, DMF; (c) K2CO3, R-bromoethyl acetate; (d) SOCl2, THF; (e) NaH, DMF; (f) LiAlH4, THF; (g) TPP, CBr4, THF; (h) hexamethylenimine, THF; (i) H2, Pd/C, EtOH/THF.
Fig S2. Hormone expression in the K-ras/Pten mouse OvCa cell line (A) Phase contrast photograph of the cell line established from mouse ovarian tumors. Original magnification, 400x. (B) Real time PCR for ERα, ERβ and progesterone receptor, in triplicate.
Total RNA was extracted from the K-ras/Pten mouse OvCa cell line and mouse pituitary which is known to express all three receptor types, transcribed into cDNA and subjected to PCR using mouse hormone receptor primers. ERα= estrogen receptor alpha, ERβ= estrogen receptor beta, PR= progesterone receptor, +Ctrl= positive control, mouse pituitary.
Fig S3. Genotype of the K-ras/Pten mouse OvCa cell line
Genotyping for the K-Ras lox-stop-lox cassette and Pten were performed on the K-ras/Pten mouse ovarian cancer cell line and on tumors generated by the LSL-K-rasG12D/+PtenloxP/loxP mouse model. The positive control demonstrated amplification of both wild type K-ras at 500 bps (lane 1), the K-ras lox-stop-lox cassette at 550 bps (lane 2) and Pten at 1200 bps (lane 3). The K-ras/Pten cell line and tumor samples had no evidence either the K-ras lox-stop-lox cassette (lane 2) or Pten (lane 3). (+) Control: Positive control is DNA extracted from a tail of a LSL-K-rasG12D/+PtenloxP/loxP mouse. (−) Control: Negative control is nuclease free water.
Fig S4. Hormonal treatment does not increase phosphorylation of AKT
(A) The K-ras/Pten mouse OvCa cell line was treated for 24 or 48 hours with hormones in charcoal stripped phenol red free complete media followed by western blots for phospho-AKT and β-actin. (B) Tumor lysates were collected from mice treated with Control or hormones and equal amount of lysates (30 μg) was resolved by 10% SDS-PAGE and immunoblotted with phospho-AKT antibody. The membranes were stripped and rehybridized with antibodies detecting β-actin. The level of protein expression was quantitated using NIH image J software and phospho-AKT was normalized to β-actin. Columns, mean of two experiments, fold-change in phospho-AKT optical density (OD) compared to placebo. ; bars, SD; Groups: Ctrl= vehicle control, E2= 17β-estradiol, B= bazedoxifene
Acknowledgements
We thank Gail Isenberg for critically reviewing the manuscript. Funding: This work was supported by grants from the Reproductive Scientist Development Program (NIH 2K12HD00849-22) and the Cancer Research Foundation (to I.L.R). Ernst Lengyel holds a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund and is supported by grants from the Ovarian Cancer Research Fund (Liz Tilberis Scholars Program), and the National Cancer Institute (RO1 CA111882).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement Dr. Greene is a consultant for Pfizer and receives sponsored research funding from the company to study molecular mechanisms of estrogen receptor action in the presence of SERM and estrogen mixtures.
Reference List
- [1].Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- [2].Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- [3].Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177(3):1053–64. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Folkins AK, Jarboe EA, Roh MH, Crum CP. Precursors to pelvic serous carcinoma and their clinical implications. Gynecol Oncol. 2009;113(3):391–6. doi: 10.1016/j.ygyno.2009.01.013. [DOI] [PubMed] [Google Scholar]
- [5].Collaborative Group on Epidemiological Studies of Ovarian C. Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: Collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet. 2008;371(9609):303–14. doi: 10.1016/S0140-6736(08)60167-1. [DOI] [PubMed] [Google Scholar]
- [6].Greiser CM, Greiser EM, Doren M. Menopausal hormone therapy and risk of ovarian cancer: Systematic review and meta-analysis. Hum Reprod Update. 2007;13(5):453–63. doi: 10.1093/humupd/dmm012. [DOI] [PubMed] [Google Scholar]
- [7].Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. A prospective study of postmenopausal hormone use and ovarian cancer risk. Br J Cancer. 2007;96(1):151–6. doi: 10.1038/sj.bjc.6603527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Beral V, Bull D, Green J, Reeves G. Ovarian cancer and hormone replacement therapy in the Million Women Study. Lancet. 2007;369(9574):1703–10. doi: 10.1016/S0140-6736(07)60534-0. [DOI] [PubMed] [Google Scholar]
- [9].Morch LS, Lokkegaard E, Andreasen AH, Kruger-Kjaer S, Lidegaard O. Hormone therapy and ovarian cancer. JAMA. 2009;302(3):298–305. doi: 10.1001/jama.2009.1052. [DOI] [PubMed] [Google Scholar]
- [10].Pearce CL, Chung K, Pike MC, Wu AH. Increased ovarian cancer risk associated with menopausal estrogen therapy is reduced by adding a progestin. Cancer. 2009;115(3):531–9. doi: 10.1002/cncr.23956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hildebrand JS, Gapstur SM, Feigelson HS, Teras LR, Thun MJ, Patel AV. Postmenopausal hormone use and incident ovarian cancer: Associations differ by regimen. Int J Cancer. 2010;127:2928–35. doi: 10.1002/ijc.25515. [DOI] [PubMed] [Google Scholar]
- [12].Syed V, Ulinski G, Mok SC, Yiu GK, Ho SM. Expression of gonadotropin receptor and growth responses to key reproductive hormones in normal and malignant human ovarian surface epithelial cells. Cancer Res. 2001;61(18):6768–76. [PubMed] [Google Scholar]
- [13].Armaiz-Pena GN, Mangala LS, Spannuth WA, Lin YG, Jennings NB, Nick AM, et al. Estrous cycle modulates ovarian carcinoma growth. Clin Cancer Res. 2009;15(9):2971–8. doi: 10.1158/1078-0432.CCR-08-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park SH, Cheung LW, Wong AS, Leung PC. Estrogen regulates Snail and Slug in the down-regulation of E-cadherin and induces metastatic potential of ovarian cancer cells through estrogen receptor alpha. Mol Endocrinol. 2008;22(9):2085–98. doi: 10.1210/me.2007-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hua K, Feng W, Cao Q, Zhou X, Lu X, Feng Y. Estrogen and progestin regulate metastasis through the PI3K/AKT pathway in human ovarian cancer. Int J Oncol. 2008;33(5):959–67. [PubMed] [Google Scholar]
- [16].Spillman MA, Manning NG, Dye WW, Sartorius CA, Post MD, Harrell JC, et al. Tissue-specific pathways for estrogen regulation of ovarian cancer growth and metastasis. Cancer Res. 2010;70(21):8927–36. doi: 10.1158/0008-5472.CAN-10-1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Laviolette LA, Garson K, Macdonald EA, Senterman MK, Courville K, Crane CA, et al. 17{beta}-Estradiol Accelerates Tumor Onset and Decreases Survival in a Transgenic Mouse Model of Ovarian Cancer. Endocrinology. 2010;151(3):929–38. doi: 10.1210/en.2009-0602. [DOI] [PubMed] [Google Scholar]
- [18].Hatch KD, Beecham JB, Blessing JA, Creasman WT. Responsiveness of patients with advanced ovarian carcinoma to tamoxifen. A Gynecologic Oncology Group study of second-line therapy in 105 patients. Cancer. 1991;68(2):269–71. doi: 10.1002/1097-0142(19910715)68:2<269::aid-cncr2820680209>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- [19].Hurteau JA, Brady MF, Darcy KM, McGuire WP, Edmonds P, Pearl ML, et al. Randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent-only epithelial ovarian, fallopian tube or primary peritoneal carcinoma after a complete response to first-line platinum/taxane chemotherapy with an evaluation of serum vascular endothelial growth factor (VEGF): A Gynecologic Oncology Group Study. Gynecol Oncol. 2010;119:444–50. doi: 10.1016/j.ygyno.2010.08.002. [DOI] [PubMed] [Google Scholar]
- [20].Kung AW, Chu EY, Xu L. Bazedoxifene: a new selective estrogen receptor modulator for the treatment of postmenopausal osteoporosis. Expert Opin Pharmacother. 2009;10(8):1377–85. doi: 10.1517/14656560902980228. [DOI] [PubMed] [Google Scholar]
- [21].Chang KC, Wang Y, Bodine PV, Nagpal S, Komm BS. Gene expression profiling studies of three SERMs and their conjugated estrogen combinations in human breast cancer cells: Insights into the unique antagonistic effects of bazedoxifene on conjugated estrogens. J Steroid Biochem Mol Biol. 2009;118:117–24. doi: 10.1016/j.jsbmb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- [22].Lippman SM, Heymach JV. The convergent development of molecular-targeted drugs for cancer treatment and prevention. Clin Cancer Res. 2007;13(14):4035–41. doi: 10.1158/1078-0432.CCR-07-0063. [DOI] [PubMed] [Google Scholar]
- [23].Miller CP, Collini MD, Tran BD, Harris HA, Kharode YP, Marzolf JT, et al. Design, synthesis, and preclinical characterization of novel, highly selective indole estrogens. J Med Chem. 2001;44(11):1654–7. doi: 10.1021/jm010086m. [DOI] [PubMed] [Google Scholar]
- [24].Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11(1):63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- [25].Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149(6):2970–9. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Komm BS, Kharode YP, Bodine PV, Harris HA, Miller CP, Lyttle CR. Bazedoxifene acetate: a selective estrogen receptor modulator with improved selectivity. Endocrinology. 2005;146(9):3999–4008. doi: 10.1210/en.2005-0030. [DOI] [PubMed] [Google Scholar]
- [27].Reel JR, Lamb JC, IV, Neal BH. Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam Appl Toxicol. 1996;34(2):288–305. doi: 10.1006/faat.1996.0198. [DOI] [PubMed] [Google Scholar]
- [28].Romero IL, Gordon IO, Jagadeeswaran S, Mui KL, Lee WS, Dinulescu DM, et al. Effects of oral contraceptives or a gonadotropin-releasing hormone agonist on ovarian carcinogenesis in genetically engineered mice. Cancer Prev Res (Phila Pa) 2009;2(9):792–9. doi: 10.1158/1940-6207.CAPR-08-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLoS One. 2010;5(4):e10202. doi: 10.1371/journal.pone.0010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lengyel E, Ried S, Heiss MM, Jager C, Schmitt M, Allgayer H. Ras regulation of urokinase-type plasminogen activator. Methods Enzymol. 2001;333:105–16. doi: 10.1016/s0076-6879(01)33049-5. [DOI] [PubMed] [Google Scholar]
- [32].Sawada K, Radjabi AR, Shinomiya N, Kistner E, Kenny H, Becker AR, et al. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67(4):1670–9. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- [33].Syed V, Ulinski G, Mok SC, Ho SM. Reproductive hormone-induced, STAT3-mediated interleukin 6 action in normal and malignant human ovarian surface epithelial cells. J Natl Cancer Inst. 2002;94(8):617–29. doi: 10.1093/jnci/94.8.617. [DOI] [PubMed] [Google Scholar]
- [34].Suga S, Kato K, Ohgami T, Yamayoshi A, Adachi S, Asanoma K, et al. An inhibitory effect on cell proliferation by blockage of the MAPK/estrogen receptor/MDM2 signal pathway in gynecologic cancer. Gynecol Oncol. 2007;105(2):341–50. doi: 10.1016/j.ygyno.2006.12.030. [DOI] [PubMed] [Google Scholar]
- [35].La RP, Pesiri V, Marino M, Acconcia F. 17beta-Estradiol-induced cell proliferation requires estrogen receptor (ER) alpha monoubiquitination. Cell Signal. 2011;23(7):1128–35. doi: 10.1016/j.cellsig.2011.02.006. [DOI] [PubMed] [Google Scholar]
- [36].Brandenberger AW, Tee MK, Jaffe RB. Estrogen receptor alpha (ER-alpha) and beta (ER-beta) mRNAs in normal ovary, ovarian serous cystadenocarcinoma and ovarian cancer cell lines: down-regulation of ER-beta in neoplastic tissues. J Clin Endocrinol Metab. 1998;83(3):1025–8. doi: 10.1210/jcem.83.3.4788. [DOI] [PubMed] [Google Scholar]
- [37].Lau KM, Mok SC, Ho SM. Expression of human estrogen receptor-alpha and -beta, progesterone receptor, and androgen receptor mRNA in normal and malignant ovarian epithelial cells. Proc Natl Acad Sci U S A. 1999;96(10):5722–7. doi: 10.1073/pnas.96.10.5722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pujol P, Rey JM, Nirde P, Roger P, Gastaldi M, Laffargue F, et al. Differential expression of estrogen receptor-alpha and -beta messenger RNAs as a potential marker of ovarian carcinogenesis. Cancer Res. 1998;58(23):5367–73. [PubMed] [Google Scholar]
- [39].Rutherford T, Brown WD, Sapi E, Aschkenazi S, Munoz A, Mor G. Absence of estrogen receptor-beta expression in metastatic ovarian cancer. Obstet Gynecol. 2000;96(3):417–21. doi: 10.1016/s0029-7844(00)00917-0. [DOI] [PubMed] [Google Scholar]
- [40].Flesken-Nikitin A, Choi KC, Eng JP, Shmidt EN, Nikitin AY. Induction of carcinogenesis by concurrent inactivation of p53 and Rb1 in the mouse ovarian surface epithelium. Cancer Res. 2003;63(13):3459–63. [PubMed] [Google Scholar]
- [41].Wu R, Hendrix-Lucas N, Kuick R, Zhai Y, Schwartz DR, Akyol A, et al. Mouse model of human ovarian endometrioid adenocarcinoma based on somatic defects in the Wnt/beta-catenin and PI3K/Pten signaling pathways. Cancer Cell. 2007;11(4):331–3. doi: 10.1016/j.ccr.2007.02.016. [DOI] [PubMed] [Google Scholar]
- [42].Zillhardt M, Park SM, Romero IL, Sawada K, Montag AG, Krausz T, et al. Foretinib (GSK1363089), an orally available multi-kinase inhibitor of c-Met and VEGFR-2, blocks proliferation, induces anoikis, and impairs ovarian cancer metastasis. Clin Cancer Res. 2011;17(12):4042–51. doi: 10.1158/1078-0432.CCR-10-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Henderson BE, Feigelson HS. Hormonal carcinogenesis. Carcinogenesis. 2000;21(3):427–33. doi: 10.1093/carcin/21.3.427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Synthesis steps for bazedoxifene.
1H NMR spectra were recorded on a Bruker Biospin 400MHz spectrometer. Mass spectra were obtained with an Agilent 1100 LC/MSD SL quadruple mass spectrometer equipped with an APCI ionization source. 1H NMR and LC/MSD readings were obtained following each of the outlined synthesis steps. Reagents and conditions: (a) Br2, DCM; (b) 4-benzyloxyaniline hydrochloride, Et3N, DMF; (c) K2CO3, R-bromoethyl acetate; (d) SOCl2, THF; (e) NaH, DMF; (f) LiAlH4, THF; (g) TPP, CBr4, THF; (h) hexamethylenimine, THF; (i) H2, Pd/C, EtOH/THF.
Fig S2. Hormone expression in the K-ras/Pten mouse OvCa cell line (A) Phase contrast photograph of the cell line established from mouse ovarian tumors. Original magnification, 400x. (B) Real time PCR for ERα, ERβ and progesterone receptor, in triplicate.
Total RNA was extracted from the K-ras/Pten mouse OvCa cell line and mouse pituitary which is known to express all three receptor types, transcribed into cDNA and subjected to PCR using mouse hormone receptor primers. ERα= estrogen receptor alpha, ERβ= estrogen receptor beta, PR= progesterone receptor, +Ctrl= positive control, mouse pituitary.
Fig S3. Genotype of the K-ras/Pten mouse OvCa cell line
Genotyping for the K-Ras lox-stop-lox cassette and Pten were performed on the K-ras/Pten mouse ovarian cancer cell line and on tumors generated by the LSL-K-rasG12D/+PtenloxP/loxP mouse model. The positive control demonstrated amplification of both wild type K-ras at 500 bps (lane 1), the K-ras lox-stop-lox cassette at 550 bps (lane 2) and Pten at 1200 bps (lane 3). The K-ras/Pten cell line and tumor samples had no evidence either the K-ras lox-stop-lox cassette (lane 2) or Pten (lane 3). (+) Control: Positive control is DNA extracted from a tail of a LSL-K-rasG12D/+PtenloxP/loxP mouse. (−) Control: Negative control is nuclease free water.
Fig S4. Hormonal treatment does not increase phosphorylation of AKT
(A) The K-ras/Pten mouse OvCa cell line was treated for 24 or 48 hours with hormones in charcoal stripped phenol red free complete media followed by western blots for phospho-AKT and β-actin. (B) Tumor lysates were collected from mice treated with Control or hormones and equal amount of lysates (30 μg) was resolved by 10% SDS-PAGE and immunoblotted with phospho-AKT antibody. The membranes were stripped and rehybridized with antibodies detecting β-actin. The level of protein expression was quantitated using NIH image J software and phospho-AKT was normalized to β-actin. Columns, mean of two experiments, fold-change in phospho-AKT optical density (OD) compared to placebo. ; bars, SD; Groups: Ctrl= vehicle control, E2= 17β-estradiol, B= bazedoxifene