Abstract
Introduction:
Although most general anaesthesia procedures are performed without any complications, volatile agents may have adverse effects on various living systems. This study aims to compare the antioxidant effects of isoflurane and N-acetylcysteine (NAC) on liver function.
Methods:
Forty-one patients in the ASA I-II risk groups, who were scheduled to undergo gynaecologic laparoscopy, were randomly divided into two groups: The placebo (group P, n=21) and the NAC group (group N, n=20). In both groups, anaesthesia was maintained with 1–2% isoflurane in 50% Oxygen–50% N2O at 6 l/min, also administered by inhalation. Venous blood samples were obtained before anaesthesia induction, and then in the postoperative 1st hour and at the 24th hour. The samples were centrifuged and serum levels of glutathione S-transferase (GST), malondialdehyde (MDA), aspartate amino transferase (AST), alanine amino transferase (ALT), lactate dehydrogenase (LDH), gamma glutamyltranspeptidase (GGT), prothrombin time (PT), activated partial thromboplastin time (aPTT) and international normalised ratio were determined.
Results:
GST levels were significantly higher in group N than in group P in the postoperative 1st hour. Postoperative values of GST in the two groups were higher when compared to preoperative values (P<0.05). When postoperative levels were compared with preoperative levels, the postoperative MDA levels of group N were significantly higher (P<0.05). Levels of AST, ALT, GGT and LDH in both groups revealed significant decreases at the postoperative 1st hour and postoperative 24th hour compared to preoperative values (P<0.05, P<0.001). PT values were significantly higher in both groups in the postoperative 1st hour and 24th hour (P<0.05, P<0.001), although there were no differences in aPTT levels.
Conclusion:
Our results showed that liver functions were well preserved with administration of NAC during anaesthesia with isoflurane. Isoflurane with NAC has lesser effect on liver function tests compared to isoflurane alone.
Keywords: Isoflurane, liver function tests, N-acetylcysteine
INTRODUCTION
Volatile anaesthetics have exhibited various organ and system toxicities since they have became operational.[1] Of particular importance is the occurrence of fatal toxicities in the liver where these anaesthetics are actually metabolised. Like all the other branches of medicine, anaesthesiology recently made great improvements, but the search for the ideal anaesthetic agent is still ongoing.
There are reports of postoperative hepatitis after halothane anaesthesia with a wide spectrum, ranging from mildly elevated bilirubin levels to fatal fulminant hepatic necrosis.[2] Isoflurane is metabolised very little (0.17%) and is presented as safer than halothane.[3] There are also reports of hepatitis related to isoflurane anaesthesia; but these occurrences are not as severe as in halothane cases.[4,5] There is a general consensus on the toxicity of volatile anaesthetics. These agents are not toxic themselves, but their by-products or end-products of metabolism are toxic.[6] Isoflurane is halogenated ether and an isomer of enflurane. Its anaesthetic induction is faster because it is nearly insoluble in blood. Only 0.17% of isoflurane is metabolised in the liver; the amount of its metabolites [fluoride and trifluoroacetic acid (TFA)] is not sufficient to cause nephrotoxicity.[7] TFA is a reactive metabolite that has been shown to be the main reason for hepatotoxicity and also cross-sensitivity to other halogenated agents. TFA binds to hepatocyte proteins and acts like a “hapten.” These antigens are then attacked by patient's antibodies.[8]
The toxicity of volatile anaesthetics can be seen after biodegradation. Biodegradation causes lipid peroxidation and depletion of antioxidants like glutathione.[7] N-acetylcysteine (NAC), a precursor of glutathione synthesis, can exert important antioxidant cytoprotective effects and anti-inflammatory effects. It is also used in cases of paracetamol intoxication and chronic obstructive pulmonary diseases, inflammatory arthritic diseases, adult respiratory distress syndrome, and prevention of human immunodeficiency virus (HIV) expression.[9–12]
Free radicals increase the formation of lipid peroxides in the cell membrane, and thus participate in many pathological processes such as ischaemia, tissue anoxia, and diabetes. During surgery with general anaesthesia, changes in tissue and organ perfusion and the degree of oxygenation can affect oxidative stress, defined as the equilibrium between oxidising factors (lipid peroxidation) and antioxidising factors (mainly the glutathione system). Glutathione S-transferase (GST) and malondialdehyde (MDA) levels in the tissue samples are assessed to be indicators of lipid peroxidation.[13,14]
The aim of the present study is to investigate the effects of widely used volatile agent, isoflurane, and an antioxidant, NAC, on liver functions.
METHODS
This study was approved by Institutional Review Board and written informed consent was obtained from all the patients. In this prospective, double-blind, randomised, comparative study, a total of 41 patients, aged between 33 and 65 years, of ASA I-II, undergoing elective gynaecologic laparoscopic surgery (hysterectomy, myomectomy and ovarian cysts) were randomised into two groups, group P (n=21, placebo) and group N (n=20, NAC), and they were divided randomly by drawing a sealed envelope.
Patients who had severe cardiovascular, pulmonary, renal, hepatic, endocrine, neuropsychiatric diseases, history of using coumadin recently, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), vitamins, corticosteroids, immune depressants or any drug interfering with metabolic functions, any history of drug, alcohol, or cigarette abuse, a history of hepatitis B or C, inflammatory bowel disease, malabsorption, infection with/without fever, or open abdominal surgery in 5 years were excluded.
Physical examination was performed the day before the scheduled surgery. No premedication was given. Age, sex and weight were recorded. On the operation table, we inserted a peripheral intravenous (i.v.) line with 20-gauge cannula on dorsum of the hand and started 0.9% saline infusion. All the patients were monitored using a Siemens SC 6002 monitor before induction with standard 5-lead electrocardiogram (ECG) (lead II and V5 particularly), automatic cuffed non-invasive blood pressure, and pulse oxymetry with a finger probe. Basal values of heart rate (HR), systolic arterial pressure (SAP), diastolic arterial pressure (DAP), mean arterial pressure (MAP), and peripheral oxygen saturation (SpO2) were measured and recorded. Group N received 150 mg/kg NAC in 250 ml 0.9% saline and group P received only 250 ml 0.9% saline before induction.
All the patients were preoxygenated with 100% O2. For induction, thiopental 3–6 mg/kg and vecuronium 0.1 mg/kg were given. Patients were intubated and connected to the ventilator after 3 minutes. All patients received 50% Oxygen–50% N2O with 1–2% isoflurane in fresh gas flow at 6 l/minute. Tidal volume was 6–8 ml/kg; frequency was 10–12/minute.
HR, SAP, DAP, MAP and SpO2 values were recorded at pre-induction and post-induction, and after intubation at the 1st, 30th, 60th, 90th and 120th minute.
The presence of light reflex, pupillary dilatation, autonomic responses (sweating, tears), somatic responses (movement, knitting the eyebrow, eye opening, swallowing), and more than 20% increase in basal SAP, DAP, HR values were all accepted as superficial anaesthesia. In these superficial anaesthesia cases, isoflurane inhalation was increased up to 0.5–3% and anaesthesia was maintained. Isoflurane inhalation was gradually decreased when basal SAP, DAP, and HR values decreased more than 20%. When necessary, 5 mg ephedrine was administered in order to increase BP. When HR decreased below 50 beats/minute, we accepted this HR as bradycardia and administered 0.5 mg atropine sulphate i.v. Group N received a 12.5 mg/kg/hour NAC i.v. infusion throughout the operation, whereas group P received saline infusion at the same amount.
In order to evaluate the liver functions; serum aspartate amino transferase (AST), alanine amino transferase (ALT), lactate dehydrogenase (LDH), gamma glutamyltranspeptidase (GGT), prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalised ratio (INR), GST and MDA levels were monitored as the main variables from peripheral venous blood at preoperative, postoperative 1st hour and the postoperative 24th hour. AST, ALT, LDH, GGT were measured with a Roche Modular spectrophotometric autoanalyser. PT, PTT, and INR were measured with STA-Compact and STA-R devices using Stago kits.
Perioperative adverse or side effects like arrhythmias, nausea, vomiting, flushing, hypotension, cough, and urticaria were all recorded.
Statistical analysis
To evaluate all these data, the SPSS 13.0 program was used. Parameters with normal distribution were evaluated with the Student's t-test and parameters without normal distribution were evaluated with the Mann–Whitney U-test. In-group and inter-group comparisons were done with analysis of variance (two-way ANOVA).
RESULTS
There were no statistical differences according to demographic data (age, sex, weight).
There were no statistical differences between groups in AST, ALT, GGT and LDH levels at preoperative, postoperative 1st hour or postoperative 24th hour (P>0.05). Analysis of AST, ALT, GGT and LDH in both groups revealed significant decreases at postoperative 1st hour and postoperative 24th hour versus preoperative values (P<0.05, P<0.001).
PT values revealed significant increases at the postoperative 1st hour and postoperative 24th hour compared to preoperative values in both groups (P<0.05, P<0.001) [Table 1]. In both groups, there were no statistical differences in PTT compared to preoperative, postoperative 1st hour and postoperative 24th hour values (P>0.05) [Table 1]. INR values in both groups revealed highly significant increases at the postoperative 1st hour and postoperative 24th hour compared to preoperative values (P<0.001) [Table 1].
Table 1.
Biochemical parameters and values according to the time
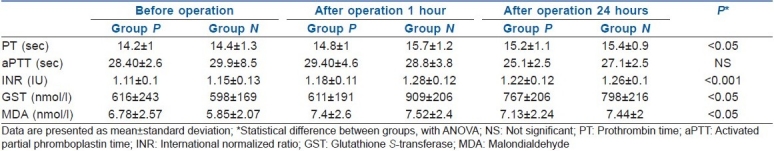
In both in-group and inter-group analyses of GST, group N revealed significant increases compared to group P at the postoperative 1st hour. Postoperative 1st and 24th hour values were higher when compared to preoperative values (P<0.05).
On comparison of MDA values of groups, it was found that in Group P, MDA revealed no significant changes at the postoperative 1st hour and postoperative 24th hour compared to preoperative values (P>0.05). Group N revealed significant increases in MDA at the postoperative 1st hour and postoperative 24th hour compared to preoperative values (P<0.05).
There were no statistical differences in SAP, MAP, DAP, HR and SpO2 values at the preoperative, postoperative 1st hour and postoperative 24th hour (P>0.05). On in-group analysis of SAP, MAP, DAP, HR and SpO2 values, some statistical differences were found, but none of these were clinically significant.
Regarding the side effects, both groups had one patient with nausea. Group N revealed more drug-related issues, such as 11 patients with hypotension during induction and 9 patients with flushing during NAC infusion. In cases of hypotension, we administered ephedrine (an alpha agonist) and decreased the isoflurane concentration to less than 0.5%. Also, in group N, two patients had cough and reported a metallic taste, and two patients had urticaria, for which we administered pheniramine 20 mg i.v.
DISCUSSION
The liver is the most affected organ in cases of anaesthetic agent toxicity, since it is the site where inhalational agents are metabolised. In this study where isoflurane was used, no adverse effect was found on the liver.
Routine laboratory tests are not indicated unless there is no abnormal finding in history or physical examination of asymptomatic, otherwise healthy cases. There is no routine need for liver function tests preoperatively. Preoperative liver function tests should be based on any history of hepatic injury and physical examination findings. In these cases, AST and ALT levels are measured primarily.[15,16]
In a study of sevoflurane for intermediate-duration non-hepatic surgeries, ALT levels increased non-significantly whereas AST levels remained unchanged.[17] Repeated sevoflurane anaesthesia caused relatively increased ALT, AST, LDH levels in another study, where sevoflurane was administered to monkeys for 3 hours a day for 3 days in a total of 8 weeks period.[18] Hepatic enzymes returned to normal within 4–8 weeks. There were no signs of histopathological hepatic damage. Thus, they concluded that sevoflurane has no significant effect on healthy individuals. Postoperative ALT and AST levels were found non-significantly reduced in a study conducted on 50 children and infants using desflurane 2–3%.[19] They concluded that our up-to-date information on volatile anaesthetics could not explain this decrease. In the present study, serum AST, ALT, GGT, LDH levels also decreased in both the groups, which is similar to results of the above-mentioned studies.[17–19]
The basic reactions of liver drug metabolism are biphasic in nature. Liver p450 monooxidases and oxidase systems carry out Phase I reactions which are biotransformations.[20] This enzyme system is the basal metabolic pathway for sevoflurane, isoflurane, enflurane and desflurane. Only sevoflurane needs the Phase II reactions for excretion of metabolic end products.[21]
After sevoflurane and isoflurane anaesthesia, liver function enzymes were evaluated in a study. Both sevoflurane and isoflurane groups revealed that there was no significant increase during the first 24 hours. Isoflurane group revealed peak increase on the 7th day. However, this increase was not statistically significant in terms of AST, ALT, GGT, and LDH levels. Also, this presented study evaluated coagulation parameters like PT, PTT, and INR.[22] All the coagulation factors except Factor VIII are synthesised in the liver.[23,24] Both groups revealed significant increases in PT values at postoperative 1st hour and postoperative 24th hour compared to preoperative values, whereas PTT values remained within normal ranges. In our study, all our patients had preoperatively normal PT, PTT, and INR values, but this issue might be problematic for patients who have coagulation defects.
In animals anaesthetised with halothane, it was shown that cardiac tissue MDA concentrations were increased.[25] If vitamin E was given together with halothane, cardiac tissue MDA concentrations decreased. This decrease in vitamin E might be explained by protection of cardiac tissues against free radicals and prevention of lipid peroxidation. Vitamin E is a powerful antioxidant and might scavenge some radicals by attracting unpaired single electrons towards it.
The present study shows that preoperative NAC infusion significantly increased serum GSH levels. Increased postoperative GST level in isoflurane+saline group (P) might indicate indirect exposure to oxidative stress. Serum MDA concentrations increased in both groups. NAC, as an antioxidant, was not able to decrease serum MDA levels. We think that this failure might be due to a lesser concentration (<1%) of isoflurane used because of the haemodynamic instability in the NAC infused group N.
Oxidative stress occurs as a result of an imbalance between free oxygen radicals and natural endogenous antioxidant systems. NAC is the most used exogenous antioxidant against this oxidative tissue damage. The antioxidant effect of NAC is because of the direct effects itself or secondary effects by increasing glutathione production. The direct effect is the reaction with hydroxyl radicals, thereby inactivating them. NAC also prevents glutathione depletion and increases hepatic glutathione levels. In an animal acute renal failure model, glutathione has been shown to prevent ischaemia-reperfusion injury.[26] Further, a study was conducted on 83 chronic renal failure patients undergoing computerised tomography using non-ionic contrast material. They showed that NAC significantly decreases contrast-induced nephropathy in high-risk patients.[27]
All inhalational agents decrease hepatic blood flow in a dose-dependent manner. Depending on the chosen agent, this decrease of hepatic blood flow is about 20–25% during anaesthesia and surgery. The important factors of this decrease might be the higher sympathetic tonus or pressure on the vena caval system due to increased intrathoracic pressure during controlled ventilation. In the end, decreased hepatic flow occurs together with hepatic hypoxia.[28,29] The effects of isoflurane and sevoflurane anaesthesia on total (portal vein+hepatic artery) hepatic blood flow were investigated. Sevoflurane partially decreases portal blood flow but increases hepatic arterial flow; therefore, it has the same effect as isoflurane on total hepatic blood flow.[30]
In a hypoxic environment, the hepatotoxicity of anaesthetic volatile agents increases because the energy needs of tissues and cells are not sufficiently met.[31,32] Especially in hypoxic environments, halothane has been shown to cause more histopathological injury than does isoflurane in studies conducted on rat livers.[33,34] In the present study, we eliminated the risk of a hypoxic liver by holding the MAP over 80 mmHg. NAC was administered to 11 patients with hypotension from group N; therefore, we had to decrease isoflurane to less than 0.5% MAC and administer ephedrine in order to achieve an MAP over 80 mmHg.
CONCLUSION
We conducted this randomised controlled study in order to show the effects of perioperative NAC infusion on liver during general anaesthesia maintained by isoflurane, the halogenated volatile agent used the most today. Under isoflurane anaesthesia, preoperative NAC caused increased levels of glutathione and MDA; however, liver function tests showed no significant difference. We conclude that more extensive and prolonged studies are needed to investigate the effect of NAC in patients undergoing general anaesthesia with halogenated volatile agents.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Kharasch ED. Adverse drug reactions with halogenated anesthetics. Clin Pharmacol Ther. 2008;84:158–62. doi: 10.1038/clpt.2008.97. [DOI] [PubMed] [Google Scholar]
- 2.Reichle FM, Conzen PF. Halogenated inhalational anaesthetics. Best Pract Res Clin Anaesthesiol. 2003;17:29–46. doi: 10.1053/bean.2002.0265. [DOI] [PubMed] [Google Scholar]
- 3.Bedirli N, Ofluoglu E, Kerem M, Utebey G, Alper M, Yilmazer D, et al. Hepatic energy metabolism and the differential protective effects of sevoflurane and isoflurane anesthesia in a rat hepatic ischemia-reperfusion injury model. Anesth Analg. 2008;106:830–7. doi: 10.1213/ane.0b013e3181616fc9. [DOI] [PubMed] [Google Scholar]
- 4.Wark HJ. Hepatic failure after cardiopulmonary bypass is unlikely to be isoflurane hepatitis. Anesthesiology. 2002;97:1323–4. doi: 10.1097/00000542-200211000-00046. [DOI] [PubMed] [Google Scholar]
- 5.Ihtiyar E, Algin C, Haciolu A, Isiksoy S. Fatal isoflurane hepatotoxicity without re-exposure. Indian J Gastroenterol. 2006;25:41–2. [PubMed] [Google Scholar]
- 6.Lu CC, Tsai CS, Hu OY, Chen RM, Chen TL, Ho ST. Pharmacokinetics of isoflurane in human blood. Pharmacology. 2008;81:344–9. doi: 10.1159/000122960. [DOI] [PubMed] [Google Scholar]
- 7.Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth Analg. 2009;109:1506–10. doi: 10.1213/ANE.0b013e3181b6709a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Njoku DB, Shrestha S, Soloway R, Duray PR, Tsokos M, Abu-Asab MS, et al. Subcellular localization of trifluoroacetylated liver proteins in association with hepatitis following isoflurane. Anesthesiology. 2002;96:757–61. doi: 10.1097/00000542-200203000-00036. [DOI] [PubMed] [Google Scholar]
- 9.Picker O, Beck C, Pannen B. Liver protection in the perioperative setting. Best Pract Res Clin Anaesthesiol. 2008;22:209–24. doi: 10.1016/j.bpa.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Hynninen MS, Niemi TT, Pöyhiä R, Raininko EI, Salmenperä MT, Lepäntalo MJ, et al. N-acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: A randomized, double-blind, placebo-controlled trial. Anesth Analg. 2006;102:1638–45. doi: 10.1213/01.ANE.0000219590.79796.66. [DOI] [PubMed] [Google Scholar]
- 11.Naughton F, Wijeysundera D, Karkouti K, Tait G, Beattie WS. N-acetylcysteine to reduce renal failure after cardiac surgery: A systematic review and meta-analysis. Can J Anaesth. 2008;55:827–35. doi: 10.1007/BF03034054. [DOI] [PubMed] [Google Scholar]
- 12.Oliveira DM, Gomes ES, Mussivand T, Fiorelli AI, Gomes OM. Effects of n-acetylcysteine on ischemic preconditioning: Study in isolated rat hearts. Rev Bras Cir Cardiovasc. 2009;24:23–30. doi: 10.1590/s0102-76382009000100006. [DOI] [PubMed] [Google Scholar]
- 13.Warholm M, Guthenberg C, von Bahr C, Mannervik B. Glutathione transferases from human liver. Methods Enzymol. 1985;113:499–504. doi: 10.1016/s0076-6879(85)13065-x. [DOI] [PubMed] [Google Scholar]
- 14.Ceylan BG, Yilmaz F, Eroglu F, Yavuz L, Gulmen S, Vural H. Oxidant and antioxidant activities of different anesthetic techniques.Propofol versus desflurane. Saudi Med J. 2009;30:371–6. [PubMed] [Google Scholar]
- 15.Fischer SP, Bader AM, Sweitzer BJ. Preoperative Evaluation. In: Miller RD, editor. Miller's Anesthesia. 6th ed. Philadelphia: Elsevier Churchill Livingstone; 2005. pp. 928–98. Chapter 25. [Google Scholar]
- 16.Toker A, Girgin NK, Turker G, Kutlay O. [Are preoperative routine laboratory tests necessary in minor and moderate surgical procedures?] Dicle Med J. 2008;35:120–7. [Google Scholar]
- 17.Nishiyama T, Yokoyama T, Hanaoka K. Effects of sevoflurane and isoflurane anesthesia on arterial ketone body ratio and liver function. Acta Anaesthesiol Scand. 1999;43:347–51. doi: 10.1034/j.1399-6576.1999.430318.x. [DOI] [PubMed] [Google Scholar]
- 18.Soma LR, Tierney WJ, Hogan GK, Satoh N. The Effects of multiple administrationsevoflurane to Cynomolgus Monkeys: Clinical pathologic, hematologic and pathologic study. AnesthAnalg. 1995;81:347–51. doi: 10.1097/00000539-199508000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Wissing H, Kuhn I. The Effect of desflurane on liver function markers in infants and children. Acta Anaesth Scand. 2000;44:1149–57. doi: 10.1034/j.1399-6576.2000.440920.x. [DOI] [PubMed] [Google Scholar]
- 20.Kharasch ED, Thummel KE. Identification of cytocrome P450 2E1 as the predominant enzyme catalysing human liver microzomal desfluorinasation of sevoflurane, isoflurane and metoxyflurane. Anesthesiology. 1993;79:795–79. doi: 10.1097/00000542-199310000-00023. [DOI] [PubMed] [Google Scholar]
- 21.Frink EJ. The hepatic effects of sevoflurane. Anesth Analg. 1995;81:46–52. doi: 10.1097/00000539-199512001-00007. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama T, Yokoyama T, Hanaoka K. Liver function after sevoflurane or isoflurane anaesthesia in neurosurgical patients. Can J Anaesth. 1998;45:753–6. doi: 10.1007/BF03012146. [DOI] [PubMed] [Google Scholar]
- 23.De Gasperi A, Corti A, Mazza E, Prosperi M, Amici O, Bettinelli L. Acute liver failure: Managing coagulopathy and the bleeding diathesis. Transplant Proc. 2009;41:1256–9. doi: 10.1016/j.transproceed.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka KA, Key NS, Levy JH. Blood coagulation: Hemostasisand thrombin regulation. Anesth Analg. 2009;108:1433–46. doi: 10.1213/ane.0b013e31819bcc9c. [DOI] [PubMed] [Google Scholar]
- 25.Beaussier M, Deriaz H, Abdelahim Z, Aissa F, Lienhart A. Comparative effects of desflurane and isoflurane on recovery after long lasting anaesthesia. Can J Anaesth. 1998;45:429–34. doi: 10.1007/BF03012578. [DOI] [PubMed] [Google Scholar]
- 26.Itoh Y, Yano T, Sendo T, Oishi R. Clinical and experimental evidence for prevention of acute renal failure induced by radiographic contrast media. J Pharmacol Sci. 2005;97:473–88. doi: 10.1254/jphs.crj05002x. [DOI] [PubMed] [Google Scholar]
- 27.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 28.Frink EJ, Jr, Morgan SE, Coetzee A, Conzen PF, Brown BR., Jr The effects ofsevoflurane, halothane, enflurane and isoflurane on hepatic blood flow oxygenisation in chronically. Anesth Analg. 1992;76:85–91. doi: 10.1097/00000542-199201000-00013. [DOI] [PubMed] [Google Scholar]
- 29.Conzen PF, Vollmar B, Habazettl H, Frink EJ, Peter K, Messmer K. Systemic and regional hemodynamic of isoflurane and sevoflurane in rats. AnesthAnalg. 1992;74:79. doi: 10.1213/00000539-199201000-00014. [DOI] [PubMed] [Google Scholar]
- 30.Bernard JM, Doursout MF, Wouters P, Hartley CJ, Merin RG, Chelly JE. Effects of sevoflurane and isoflurane on hepatic circulation in the chronically instrumented dog. Anesthesiology. 1992;77:541–5. doi: 10.1097/00000542-199209000-00021. [DOI] [PubMed] [Google Scholar]
- 31.Pohorecki R, Howard BJ, Matsushita M, Stemmer PM, Becker GL, Landers DF. Isoflurane isomers differ in preservation of ATP in anoxic rat hepatocytes. J Pharmacol Exp Ther. 1994;268:625–8. [PubMed] [Google Scholar]
- 32.Howard BJ, Pohorecki R, Becker GL, Landers DF. Energy status in anoxic rat hepatocytes: Effects of isoflurane, solution composition, and hypothermia. Liver Transpl Surg. 1995;1:220–4. doi: 10.1002/lt.500010404. [DOI] [PubMed] [Google Scholar]
- 33.Eger EI, Johnson BH, Strum DP, Ferell LD. Studies of the toxicity of I–653, halothane and isoflurane in enzyme induced hypoxic rats. AnesthAnalg. 1987;66:1227–9. [PubMed] [Google Scholar]
- 34.Gelman S, Riverman V, Fowler KC, Bishop SP, Bradley EL. The effect of halothane, isoflurane and blood loss on hepatotoxicity and hepatic oxygen availability in phenobarbital pre treated hypoxic rats. AnesthAnalg. 1984;63:965–72. [PubMed] [Google Scholar]


