Abstract
Introduction:
This randomized double blind study was started with an objective of management of spinal anaesthesia-induced hypotension in elective caesarean section by combining two commonly used vasopressors – ephedrine and phenylephrine in half of their usual doses with an expectation of reducing their foetomaternal side effects.
Methods:
One hundred and thirty two patients were randomized into three groups to receive either 100 μg/ml phenylephrine (group-P, n=31) or 3 μg/ml ephedrine (group-E, n=33) or 50 mg phenylephrine plus 1.5 mg ephedrine/ml (group-PE, n=29). Immediately after spinal injection the study solution was started prophylactically in every patient at the rate of 40 ml/h. A predefined algorithm was used to adjust the infusion rate according to the systolic blood pressure (SBP).
Results:
Mean fall of SBP was significantly more in group-E than group-P (P=0.009) and group-PE (P=0.013). This was not significantly different when compared between group-P and group-PE (P=0.9). Episodes of hypotension and tachycardia were more in group-E than the other two groups. Statistically significant tachycardia was seen in Group-E than that in other two groups. Incidence of bradycardia and hypertension did not differ significantly among the groups. Maternal nausea and Apgar score were also comparable in three groups.
Conclusion:
Current study claims that prophylactic phenylephrine 100 mg/ml is a better choice than ephedrine (3 mg/ml) or 50 mcg phenylephrine plus 1.5 mg ephedrine/ml in prevention of spinal anaesthesia-induced hypotension in elective caesarean section. Combination of two drugs in half the usual dose has no added advantage over phenylephrine, but this is better than ephedrine alone.
Keywords: Bradycardia, elective caesarean section, hypotension, spinal anaesthesia, vasopressors
INTRODUCTION
Spinal anaesthesia (SA) is nowadays considered the standard anaesthetic technique for elective caesarean section.[1] However, the chance of hypotension is a major limitation of this technique. The incidence of hypotension is more than 80% without any prophylactic measures.[2,3] This hypotension with or without bradycardia has detrimental effects on both mother and foetus.[4,5] The incidence of hypotension can be lowered by several ways but till date, no single method completely prevents hypotension.[4,5] Contemporary articles emphasize on the arterial rather than venous circulation and project the reduced systemic vascular resistance as the primary factor for the genesis of maternal hypotension.[6–8] Over the last few years, there is a trend to rely more on vasopressors than either crystalloid or colloid alone.[4,6,7]
Different vasopressors are commonly used at present with varying degrees of success.[6] Despite the use of prophylactic intravenous (i.v.) infusion[6] or bolus[7] ephedrine for the last three decades, a good number of failures have also been reported[9] and a rescue phenylephrine bolus dose appears effective when ephedrine alone fails to correct hypotension.[3] Prophylactic phenylephrine infusion significantly lowers the incidence of spinal anaesthesia-induced maternal hypotension[6,10] despite its limitations like bradycardia, hypertension and reduced cardiac output at higher dose.[3,8]
Combining two vasopressors with different mechanisms of action appeared promising as the consumption of individual drug is presumed to be less, thereby minimizing untoward effects of each drug . Different studies assumed different potency ratios of phenylephrine and ephedrine like (80:1),[2] (60:1),[11] (45:1)[12] and (33:1).[13] We followed the 33:1 potency ratio (100 mg of phenylephrine was considered equivalent to 3 mg of ephedrine) for our study to use proportionately more phenylephrine than ephedrine because the literature[9–12] indicates that this would result in better haemodynamic control.
We hypothesized that combining the phenylephrine and ephedrine, at half the usual dosage, would reduce the incidence of predelivery maternal hypotension (primary outcome), along with reduction of drug-related adverse effects.
METHODS
After obtaining institutional ethics committee's approval, 160 nonlaboring women older than 18 years, American Society of Anaesthesiologists (ASA) physical status I or II, weighing more than 50 kg and less than 90 kg, height 145–165 cm, having uncomplicated singleton pregnancy beyond 36 weeks, scheduled to have elective caesarean section under spinal anaesthesia were eligible for this prospective, randomized, double-blind study. Foetal malpresentation, pregnancy-induced hypertension (PIH), hypertension, cardiac disease, renal disease, foetal anomaly, diabetes mellitus and patients on chronic medication were excluded from the study. Written informed consent was obtained in their own language from every patient.
A visit by one of the authors was made to every patient on the day before operation. Haemodynamic variables were noted and patients were advised overnight fasting. Monitors (ECG, NIBP and SpO2) were attached after receiving patients at operation room. Baseline maternal haemodynamic variables were recorded from the mean of three recordings: Last antenatal visit, preanaesthetic check-up on the day before surgery and on the day of operation in ward with uterine displacement. Intravenous preloading was done with 15 ml/kg lactated Ringer's solution over 15 min. SA was administered at L3-L4 or L4-L5 interspace with 25-G Whitacre needle in left lateral position. A dose of 12.5 mg hyperbaric bupivacaine was injected over 10-15 seconds.
Patients were randomly allocated by block randomization method, where one patient had every chance to get allocated in any group by using computer generated random numbers. The sealed opaque envelope technique was used for concealment. Patients were assigned into three groups to receive phenylephrine 100 mcg/ml (group-P) or ephedrine 3 mg/ml (group-E) or combination of phenylephrine 50 mcg plus ephedrine 1.5 mg/ml (group-PE). Either of the vasopressors is supplied to the attending anaesthesiogist in an unlabelled 50 ml syringe, filled up to 40 ml. For group-P, 4 mg phenylephrine was taken with tuberculin syringe up to the mark 4 of that and was mixed with normal saline to make volume 40 ml. For group-E, 120 mg ephedrine (4 one ml ampoule plus 36 ml normal saline) was supplied in 40 ml clear solution. In the combination group, 60 mg ephedrine (2 ml) and 2 mg of phenylephrine were taken to form a volume of 40 ml by adding normal saline.
Patients were placed supine with 15° left-tilt immediately after the spinal injection and a prophylactic vasopressor i.v. infusion was started. Data of vasopressor administration were collected till clamping of umbilical cord. After SA, all patients received i.v. lactated Ringer's solution at the rate of 5 ml/min till cord clamping.
Upper level of sensory block was observed after 5 minutes of spinal injection and every 5 minutes thereafter till 30 minutes by bilateral loss of pinprick discrimination. Surgery was started as soon as upper level of sensory block reached T5. After spinal injection, data [systolic blood pressure (SBP), diastolic blood pressures (DBP), heart rate (HR)] were taken every 1 minute for 10 minutes and every 2 minutes thereafter until cord clamping.
Just after induction of SA, vasopressor infusion started at the rate of 40 ml/h in all patients. A predefined algorithm was used to adjust the infusion rate according to the SBP. This rate was maintained if SBP remained within 90-110% of baseline. The rate of infusion was halved (20 ml/h) if SBP was increased more than 110%. The infusion was stopped if SBP was increased to more than 120% of baseline value and restarted at 40 ml/h if SBP decreased back to between 90% and 110%. The rate was doubled (80 ml/h) if SBP was decreased to between 80% and 90% of baseline.
Hypotension (SBP less than 80% of baseline) was treated with i.v. 6 mg ephedrine, repeated as necessary. Bradycardia (maternal HR less than 50 beats per minute) if associated with hypotension was treated with 0.6 mg i.v. atropine. Bradycardia, if clinically tolerable and not associated with maternal hypotension, was treated by stopping the infusion temporarily. A backup plan was designed anticipating some critical events. These situations allowed the anaesthesiologist to adopt any measure to manage maternal events.
The anaesthesiologist, who prepared and supplied the drug as per randomization number, was not related to data collection, monitoring or conduct of anaesthesia. One senior teacher observed the different study techniques to minimize interpersonal variation. Outcome assessors were unaware of group allocation, patient profile and the study drugs. A coding system was used where each patient was allocated one code number for blinding outcome assessors. Apgar scores at 1 minute and 5 minutes were assessed by a paediatrician, who was unaware of the study.
Presuming the effectiveness of vasopressors over i.v. fluid as 30% and the difference between PE over E as 30% to be clinically relevant, with a power of 80% (β=0.2) at 0.05 level of significance (α=0.05), the sample size was calculated as 44 in each group (n=44). We assessed 160 patients for study eligibility keeping the chance of possible dropouts. All data were entered into an Excel sheet and imported into SPSS version 17.0. Data were presented as mean±SD unless mentioned otherwise. Analyses of mean fall of SBP in each of the three groups were done by an independent sample t-test. Demographic and obstetric data (mean±SD) were compared between three groups by a one-way analysis of variance (ANOVA) test. Primary outcome variables were the incidence and degree of maternal hypotension. Outcome measures were compared by number needed to treat (NNT), proportion and Chi-square tests as required.
RESULTS
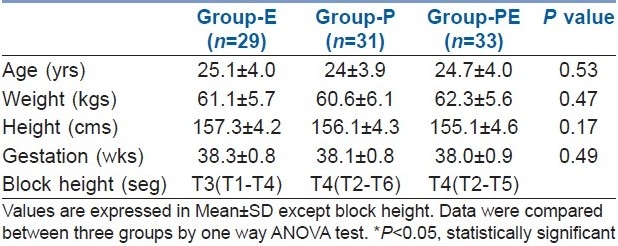
Between August 2007 and July 2008, a total of 160 patients were assessed for eligibility and finally data from 93 patients were available for analysis. After enrolment and randomization, emergency caesarean sections were done in 5 patients, 6 patients and 6 patients in groups-E, P and PE respectively. Instrumental malfunction was noticed in 6, 4 and 3 patients in groups-E, P and PE respectively. Patients changed their minds, wanted GA or refused to participate in 2, 2 and 1 patient(s) in groups-E, P and PE respectively. Haemodynamics changes during operation and noncompliance to backup plan were observed in 2, 1 and 1 patient(s) in groups-E, P and PE respectively. The groups were comparable with respect to age, body weight, height, gestational weeks and block height [Table 1].
Table 1.
Demographic and obstetric data
Repeated measures analysis shows that there were significant changes of SBP among all the groups of patients over different time points. However the incidence of hypotension was significantly more in group-E; not only in the number of patients suffering from hypotension but also in frequencies [Figure 1, Tables 2 and 3].
Figure 1.
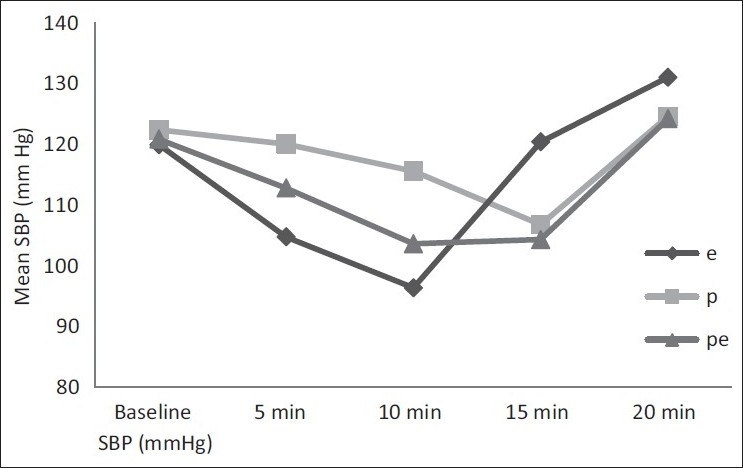
Groupwise mean systolic blood pressure (SBP) changes over time
Table 2.
Repeated measures analysis of systolic blood pressure over time intervals for different groups
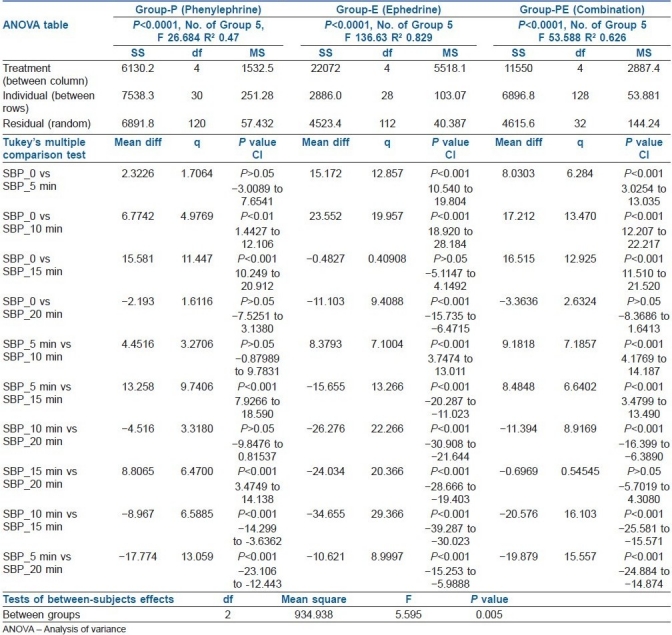
Table 3.
Incidence of hypotension
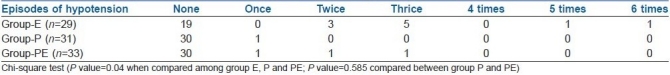
Positive outcome of this study can be represented by the NNT with 95% confidence interval (CI). The NNT in group-E compared to group-PE=3.9 (95% CI: 2.2 - 15.9), which means that four patients need to be treated in group-E compared to group-PE to prevent hypotension in one patient. NNT in group-E compared to group-P=3.2 (95% CI: 2.0 - 7.8). It signifies that three patients need to be treated in group-E to prevent hypotension in one patient. The number needed to harm (NNH) in group-PE compared to group-P is 17 (95% CI: 6 to infinity). It signifies that if we use phenylephrine–ephedrine combination instead of phenylephrine alone, then, out of 17 patients, the predelivery hypotension will not be prevented in one patient.
Apgar scores at 1 minute [median (range)] were 9 (9-10), 9 (8-9), 9 (9-9) and at 5 minutes were 10 (9-10), 10 (9-10) and 10 (9-10) in group-P, group-PE and group-E respectively. No significant difference was found.
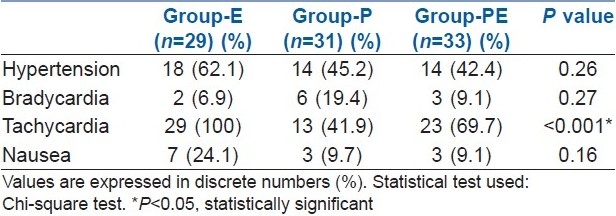
The incidences of hypertension and bradycardia seen in the three groups were comparable. Statistically significant tachycardia was seen in group-E than group-P and group-PE. In group-E, though seven patients suffered more nausea but it was statistically insignificant [Table 4].
Table 4.
Adverse events
DISCUSSION
This study reports phenylephrine as the best vasopressor among the three groups regarding prevention and control of predelivery maternal hypotension during caesarean section under spinal anaesthesia. Though, we have not designed our study to include varying combination of phenylephrine and ephedrine, we find that the combination of the half dose of phenylephrine and ephedrine works better than ephedrine alone in prevention of maternal hypotension before delivery. Calculating the NNT and NNH along with 95% confidence interval, the final impression stands that phenylephrine is superior to combination regarding the management of predelivery maternal hypotension. Better haemodynamic control can be achieved with increasing proportion of phenylephrine in the combination.[9–12]
In the present study, maternal nausea occurred despite having good systolic pressure (within 10% of baseline). In clinical practice, it is not uncommon to get a good number of patients having nausea with stable SBP. As we did not have access to monitor the cardiac output, we measured SBP and heart rate as surrogate markers of cardiac output and tissue perfusion. Appreciating that the mean arterial pressure is a better indicator of tissue perfusion, we have used SBP as a clinically useful endpoint on which our therapy was based and most of the earlier studies have used SBP as primary outcome.[2,6,7,9–12,14,15]
Incidence of hypertension in the current study is apparently more in group-E than in group-PE and group-P, but is not significant statistically. No difference in the risk of hypertension was observed by some authors[16,17] while using phenylephrine and ephedrine for prevention and treatment of maternal hypotension. In this study, block height was similar for all three groups at all of the assessment times. Gravity and posture are suggested to be the main factors for the cephalad spread of sensory block with hyperbaric bupivacaine.[9,12]
The present study observed the highest incidence of tachycardia in group-E than in group-PE and group-P. A recent study also observed a trend for tachycardia with increasing proportion of ephedrine while comparing different proportions of phenylephrine and ephedrine infusion in combinations.[11]
In the present study, the incidence of maternal bradycardia was more in group-P than in group-PE and group-E, but it was not significant when analysed. A phenylephrine–ephedrine combination produced less maternal bradycardia than phenylephrine alone group. This might be due to a higher dose of phenylephrine received by group-P than group-PE. The beta-mimetic effect of ephedrine, in addition to the lower dose of phenylephrine, might have contributed to the less incidence of maternal bradycardia in the combination group. Different studies have reported varying degrees of bradycardia from no incidence[17] to significant incidence[16] with phenylephrine than ephedrine.
The baseline HR can be better maintained by using boluses of phenylephrine or variable rate infusions with supplementary boluses of phenylephrine. With a continuous infusion of phenylephrine, the dose-dependent decline in HR is also related to corresponding curtailment of maternal cardiac output, which might not get reflected in routine monitoring, especially in the presence of a sound SBP. The infusion rate of phenylephrine is an appropriate one as long as it does not produce sinus bradycardia.[3] For the above reason, we have used infusion instead of intermittent bolus of vasopressors and the dose was modified according to a back-up plan.
The Apgar score, assessed at 1 minute and 5 minutes, were comparable in all the groups. Current evidence supports that the Apgar score is a better predictor of neonatal outcome than umbilical cord blood gas analysis.[14,18,19]
A major limitation of the current study was that a significantly large number of patients were lost to follow-up (15 in group-E, 13 in group-P and 11 in group-PE). This unforeseen dropout has underpowered the present study to predict the primary outcome. The available data could not permit us to perform an isobolographic analysis to detect the additive and synergistic effects of phenylephrine and ephedrine. Furthermore, we could not analyse umbilical cord blood gas and our sample size was not powered to measure other secondary outcomes. The current standard technique of using low-dose local anaesthetics[20] with adjuvants was not followed due to the unavailability of the preservative-free adjuvant in our institute at the time of the present study. Failure rates are reported more with low-dose bupivacaine. In another study[21] contrary to the current practice, ED95 for successful block with intrathecal hyperbaric bupivacaine coadministered with opioids for caesarean section is stated to be 11 mg. Moreover, emergency and complicated cases were not included. There is further scope of study using varying proportion of vasopressors and combination of variable rate infusion along with supplemental bolus.
CONCLUSION
Prophylactic phenylephrine infusion is superior to ephedrine infusion or combination of phenylephrine and ephedrine in the management of predelivery maternal hypotension during spinal anaesthesia for caesarean delivery. Combination does not offer any advantage over phenylephrine infusion. Combination appears to be better than ephedrine infusion alone. Phenylephrine infusion has added benefit of lower incidence of nausea, vomiting.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Riley ET, Cohen SE, Macario A, Desai JB, Ratner EF. Spinal versus epidural anesthesia for cesarean section: A comparison of time efficiency, costs, charges, and complications. Anesth Analg. 1995;80:709–12. doi: 10.1097/00000539-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 2.Saravanan S, Kocarev M, Wilson RC, Watkins E, Columb MO, Lyons G. Equivalent dose of ephedrine and phenylephrine in the prevention of post-spinal hypotension in caesarean section. Br J Anaesth. 2006;96:95–9. doi: 10.1093/bja/aei265. [DOI] [PubMed] [Google Scholar]
- 3.Stewart A, Fernando R, McDonald S, Hignett R, Jones T, Columb M. The dose-dependent effects of phenylephrine for elective cesarean delivery under spinal anesthesia. Anesth Analg. 2010;111:1230–7. doi: 10.1213/ANE.0b013e3181f2eae1. [DOI] [PubMed] [Google Scholar]
- 4.Lee A, Ngan Kee WD, Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920–6. doi: 10.1097/00000539-200204000-00028. [DOI] [PubMed] [Google Scholar]
- 5.Cyna AM, Andrew M, Emmett RS, Middleton P, Simmons SW. Techniques for preventing hypotension during spinal anaesthesia for caesarean section. Cochrane Database Syst Rev. 2006;4:CD002251. doi: 10.1002/14651858.CD002251.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Ngan Kee WD, Khaw KS, Ng FF, Lee BB. Prophylactic phenylephrine infusion for preventing hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2004;98:815–21. doi: 10.1213/01.ane.0000099782.78002.30. [DOI] [PubMed] [Google Scholar]
- 7.Ngan Kee WD, Khaw KS, Ng FF. Prevention of hypotension during spinal anesthesia for cesarean delivery: An effective technique using combination phenylephrine infusion and crystalloid cohydration. Anesthesiology. 2005;103:744–50. doi: 10.1097/00000542-200510000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Dyer RA, Reed AR. Spinal hypotension during elective cesarean delivery: Closer to a solution. Anesth Analg. 2010;111:1093–5. doi: 10.1213/ANE.0b013e3181ea5f77. [DOI] [PubMed] [Google Scholar]
- 9.Mercier FJ, Riley ET, Frederickson WL, Roger-Christoph S, Benhamou D, Cohen SE. Phenylephrine added to prophylactic ephedrine infusion during spinal anesthesia for elective cesarean section. Anesthesiology. 2001;95:668–74. doi: 10.1097/00000542-200109000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Cooper DW, Carpenter M, Mowbray P, Desira WR, Ryall DM, Kokri MS. Fetal and maternal effects of phenylephrine and ephedrine during spinal anesthesia for cesarean delivery. Anesthesiology. 2002;97:1582–90. doi: 10.1097/00000542-200212000-00034. [DOI] [PubMed] [Google Scholar]
- 11.Ngan Kee WD, Lee A, Khaw KS, Ng FF, Karmakar MK, Gin T. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: The effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295–302. doi: 10.1213/ane.0b013e31818065bc. [DOI] [PubMed] [Google Scholar]
- 12.Cooper DW, Gibb SC, Meek T, Owen S, Kokri MS, Malik AT, et al. Effect of intravenous vasopressor on spread of spinal anaesthesia and fetal acid-base equilibrium. Br J Anaesth. 2007;98:649–56. doi: 10.1093/bja/aem056. [DOI] [PubMed] [Google Scholar]
- 13.Cooper DW, Jeyaraj L, Hynd R, Thompson R, Meek T, Ryall DM, et al. Evidence that intravenous vasopressors can affect rostral spread of spinal anesthesia in pregnancy. Anesthesiology. 2004;101:28–33. doi: 10.1097/00000542-200407000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Allen TK, George RB, White WD, Muir HA, Habib AS. A double-blind, placebo-controlled trial of four fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anesthesia for cesarean delivery. Anesth Analg. 2010;111:1221–9. doi: 10.1213/ANE.0b013e3181e1db21. [DOI] [PubMed] [Google Scholar]
- 15.Ngan Kee WD, Khaw KS, Ng FF. Comparison of phenylephrine infusion regimens for maintaining maternal blood pressure during spinal anaesthesia for caesarean section. Br J Anaesth. 2004;92:469–74. doi: 10.1093/bja/aeh088. [DOI] [PubMed] [Google Scholar]
- 16.Hall PA, Bennett A, Wilkes MP, Lewis M. Spinal anaesthesia for caesarean section: Comparison of infusions of phenylephrine and ephedrine. Br J Anaesth. 1994;73:471–4. doi: 10.1093/bja/73.4.471. [DOI] [PubMed] [Google Scholar]
- 17.Ayorinde BT, Buczkowski P, Brown J, Shah J, Buggy DJ. Evaluation of pre-emptive intramuscular phenylephrine and ephedrine for reduction of spinal anesthesia-induced hypotension during caesarean section. Br J Anaesth. 2001;86:372–6. doi: 10.1093/bja/86.3.372. [DOI] [PubMed] [Google Scholar]
- 18.Casey BM, McIntire DD, Leveno KJ. The continuing value of the Apgar score for the assessment of newborn infants. N Engl J Med. 2001;344:467–71. doi: 10.1056/NEJM200102153440701. [DOI] [PubMed] [Google Scholar]
- 19.Finster M, Wood M. The Apgar score has survived the test of time. Anesthesiology. 2005;102:855–7. doi: 10.1097/00000542-200504000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Ben-David B, Miller G, Gavriel R, Gurevitch A. Low-dose bupivacaine-fentanyl spinal anesthesia for cesarean delivery. Reg Anesth Pain Med. 2000;25:235–9. [PubMed] [Google Scholar]
- 21.Ginosar Y, Mirikatani E, Drover DR, Cohen SE, Riley ET. ED50 and ED95 of intrathecal hyperbaric bupivacaine with opioids for cesarean delivery. Anesthesiology. 2004;100:676–82. doi: 10.1097/00000542-200403000-00031. [DOI] [PubMed] [Google Scholar]


