Abstract
Background:
Mixed drugs poisoning (MDP) is common in the emergency departments. Because of the limited number of intensive care unit beds, recognition of risk factors to divide the patients into different survival groups is necessary. Poisoning due to ingestion of different medications may have additive or antagonistic effects on different parameters included in the scoring systems; therefore, the aim of the study was to compare applicability of the different scoring systems in outcomes prediction of patients admitted with MDP-induced coma.
Methods:
This prospective, observational study included 93 patients with MDP-induced coma. Clinical and laboratory data conforming to the Acute Physiology and Chronic Health Evaluation (APACHE II), Modified APACHE II Score (MAS), Mainz Emergency Evaluation Scores (MEES) and Glasgow Coma Scale (GCS) were recorded for all patients on admission (time0) and 24 h later (time24). The outcome was recorded in two categories: Survived with or without complication and non-survived. Discrimination was evaluated using receiver operating characteristic (ROC) curves and area under the ROC curve (AUC).
Results:
The mortality rate was 9.7%. Mean of each scoring system was statistically significant between time0 and time24 in the survivors. However, it was not significant in non-survivors. Discrimination was excellent for GCS24 (0.90±0.05), APACHE II24 (0.89±0.01), MAS24 (0.86±0.10), and APACHE II0 (0.83±0.11) AUC.
Conclusion:
The GCS24, APACHE II24, MAS24, and APACHE II0 scoring systems seem to predict the outcome in comatose patients due to MDP more accurately. GCS and MAS may have superiority over the others in being easy to perform and not requiring laboratory data.
Keywords: Acute physiology and chronic health evaluation II, coma, glasgow coma scale, mainz emergency evaluation system, outcome, poisoning
INTRODUCTION
The most severe cases of patients who have overdosed on drugs usually require intensive care unit (ICU) admission. Knowing risk factors that can divide poisoned patients into different survival groups is necessary because of limited ICU beds. Various scoring systems have been performed as a tool for triage and ICU quality management.
The Glasgow Coma Scale (GCS) scoring has been used for outcome and recovery evaluation of patients admitted to an ICU following drug overdose[1] as a tool for the evaluation of mental status of poisoning patients,[2] the need for intubation in patients with antidepressant poisoning[3] and for predicting acute and delayed poisoning outcome.[4–9]
The Initial Acute Physiology and Chronic Health Evaluation (APACHE II) score has been used as a useful prognostic indicator in cases of organophosphate (OP) poisoning,[10,11] evaluating the severity of acute paraquat poisoning,[12,13] identifying acetaminophen-poisoned patients needing a liver transplant[14,15] and as a prognostic factor in aluminium phosphide poisoning.[16]
Comparison for effectiveness of different scoring systems has also been illustrated in studies for OP,[17–20] OP and carbamate,[21] aluminum phosphide[22] and carbamazepine poisoning.[23]
Although several severity scores have been proposed for evaluating poisoned patients on admission to the emergency department or ICUs, these have not been compared for patients with mixed drug poisoning (MDP) at different times. Because of the potential drug–drug interactions between MDPs, there could be a dominant additive or antagonistic toxidrome of various drug combinations. Therefore, there arises a need to compare the scores of individual drug combinations or various classes of drug combinations.
METHODS
The poisoning emergency department at our university hospital, Noor and Ali Asghar (PBUH) Medical Center in which this study was conducted, is the main referral centre for the central provinces of Iran, exclusively for poisoned patients. Approximately 400 patients are admitted per month and patients are managed under the supervision of an anaesthesiologist and intensive care specialist, a forensic medicine specialist, a clinical pharmacy specialist and a medical toxicologist.
This study involved prospective data collection followed by retrospective analysis, and was conducted by the Anesthesiology Research Department. The protocol of this prospective and observational study was reviewed and approved by the Institutional Ethics Committee of our university (research project number 385535). This study included consecutive hospitalizations of 93 patients with MDP-induced coma on admission. Patients whom were transferred or referred from elsewhere were not included in the study. Patients admitted after the first 24 h of ingestion were also excluded. Patients with OP, carbamate, paraquat, acetaminophen, carbamazepine and aluminium phosphide poisoning were also excluded. Gastric evacuation and activated charcoal administration occurred across patient groups in accordance with our local guidelines, which were interpreted inclusively rather than exclusively.[24]
The following data were collected by a well-trained staff physician: Demographics, APACHE II, Mainz Emergency Evaluation Scores (MEES), GCS and Modified APACHE II System (MAS) scores. APACHE II0, MAS0, MEES0 and GCS0 data were obtained on admission, whereas APACHE II24, MAS24, MEES24 and GCS24 data were obtained 24 h later in patients who stayed for 24 h or more.
To calculate the APACHE II score,[25] 12 common physiological and laboratory values (temperature, mean arterial pressure, heart rate, respiratory rate, oxygenation, arterial pH, serum sodium, serum potassium, serum creatinine, haematocrit, white blood cell count and GCS) were marked from 0 to 4, with 0 being normal and 4 being the most abnormal. The sum of these values was added to a mark adjusting for patient age and a mark adjusting for chronic health problems (severe organ insufficiency or immunocompromised patients) to arrive at the APACHE II score.
We also calculated the score of the MAS without parameters of biochemical tests [arterial oxygen tension (PaO2), arterial pH, serum sodium, serum potassium, serum creatinine, haematocrit, white blood cell count] for each patient.[18]
The GCS was determined based on three components: Eyes (4 = opens, 3 = to verbal command, 2 = to pain, 1 = none), verbal (5 = oriented, 4 = disoriented, 3 = inappropriate words, 2 = incomprehensible sounds, 1 = none) and motor (6 = obeys, 5 = localizes pain, 4 = withdrawal, 3 = abnormal flexion, 2 = abnormal extension, 1 = none).
To calculate MEES,[26,27] seven parameters (GCS, heart rate, respiratory rate, cardiac rhythm, pain, blood pressure, oxygen saturation) were marked. The scores of the APACHE II, MAS, MEE and GCS were determined by the emergency physician, formally trained in the procedures by the attending toxicologist. All other available data including toxic agent, gender and age were also recorded in a checklist. Continuous variables were compared by the standard t-test.
The binary logistic regression analysis (backward conditional stepwise method) was employed to calculate the odds ratio (OR) of different parameters of APACHE II, MAS and MEES for the occurrence of the outcomes. For simplicity, outcomes were recorded in two categories: (1) non-survived and (0) survived with or without complication. Discrimination was tested using the receiver operating characteristic (ROC) curves and by comparing areas under the curve (AUCs).[28] AUCs between 0.7 and 0.8 were classified as “acceptable” and between 0.8 and 0.9 as “excellent” discrimination.[29] For the different scoring systems tested, the sensitivity, specificity and the best cutoff point given were determined.[30] This cutoff point was also used to calculate the predicted and observed mortality. In this study, h0 hypothesis for statistical analysis was there is not any significant difference between the outcome of survivors and non-survivors in time0 (on admission) and time24 (24 h later) using APACHE II, MAS, MEES and GCS mean scores. The Chi square or Fisher's exact test was applied to compare the rate of mortality below and above the best cutoff points for the scoring systems. The data was analyzed using the Statistical Package for Social Sciences version 17.0 (SPSS Inc., Chicago, IL, USA) and Med-Calc (Med-Calc Software Inc., Mariakerke, Belgium) statistical software. A P-value less than 0.05 was considered to be statistically significant.
RESULTS
Of the 93 eligible patients, 50 (53.76%) were female and 43 (46.24%) were male. The mean age for survivors was 28.64±12.05 years and for non-survivors was 38.66±14.98 years (P value = 0.055). The most prominent drugs involved in poisoning were tricyclic antidepressant (33.3%), benzodiazepines (19.4%), anti-convulsants (11.8%), anti-psychotics (6.5%), opioids (5.4%) and others (23.6%). All the poisoning cases were intentional.
Thirty-one patients were discharged as being well during the first 24 h of hospitalization. The remaining (62 patients) were included in the other analysis. The mortality was 9.7%. Comparison of major complications between survivors and non-survivors were as follows: Intubation (83.90% and 100%), mechanical ventilation (39.30% and 66.70%) and aspiration pneumonitis (17.90% and 16.70%).
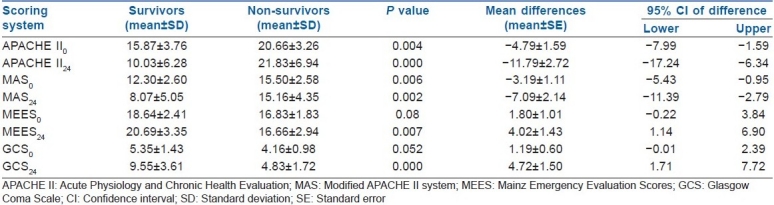
Table 1 shows that there were significant differences in APACHE II, MAS, MEES and GCS mean scores between survivors and non-survivors in time0 (on admission) and time24 (24 h later). The mean of each scoring systems was statistically significantly different between time0 and time24 in the survivors; however, it was not significant in non-survivors [Table 1].
Table 1.
Comparison of APACHE II, MAS, MEES and GCS scores between survivors and non.survivors in time0 (on admission) and time24 (24 h later)
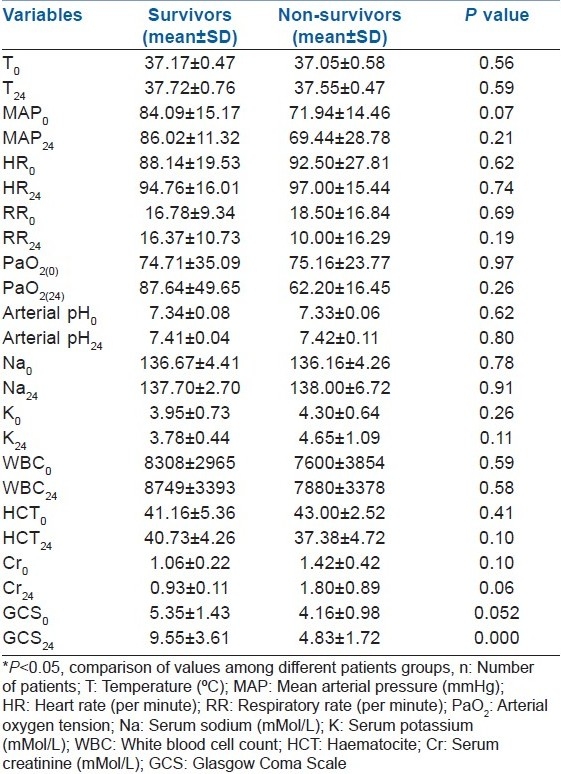
Between survivors and non-survivors, there was only a significant difference of GCS in time0 and time24 [Table 2].
Table 2.
Comparison of different parameters of scoring systems between survivors and non-survivors in time0 (on admission) and time24 (24 h later)
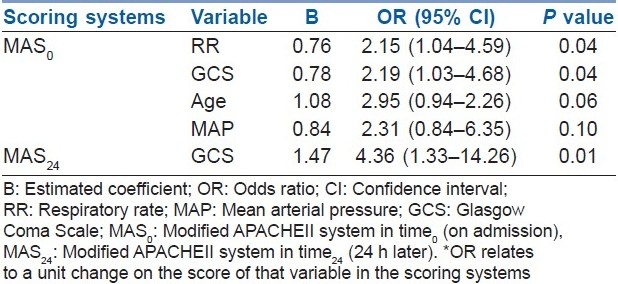
Binary logistic regression analysis was employed to calculate the OR as the estimate of the relative risk of the APACHE II, MAS and MEES determinants on time0 and time24 for the occurrence of outcomes. For simplicity, the outcomes were recorded in two categories: (1) non-survived and (0) survived with or without complication. Table 3 reports the parameters of the different scoring systems influencing outcomes in the patients. GCS, respiratory rate, age and mean arterial pressure in MAS0 and GCS in MAS24 were identified as independent risk factors for predicting outcome. There were no specific determinant parameters in APACHE II0, APACHE II24, MEES0 and MEES24 for the outcome prediction. Although the type of MDP combinations ingested and their possible proportions or dosage ingested could be a significant factor affecting the severity scores, the analysis did not support it.
Table 3.
Relative risk of the determinants for the occurrence of the outcomes
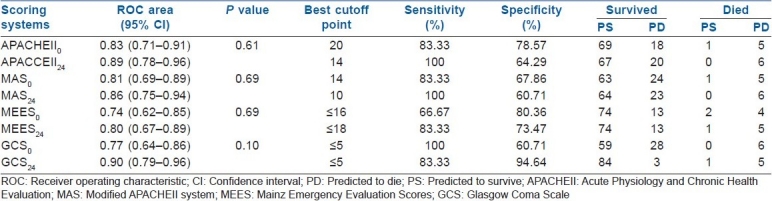
Predictive values of the various scoring systems calculated at the best cutoff point have been shown in Table 4. Discrimination was excellent for GCS24 (0.90±0.05), APACHE II24 (0.89±0.01), MAS24 (0.86±0.10) and APACHE II0 (0.83±0.11) and acceptable for MAS0 (0.81±0.11), MEES24 (0.80 ± 0.08), GCS0 (0.77±0.09) and MEES0 (0.75±0.09) AUC.
Table 4.
Classification table for the scoring systems in time0 (on admission) and time24 (24 h later)
DISCUSSION
The applicability of APACHE II, MAS, MEES and GCS were evaluated in predicting outcomes in MDP-induced coma at different times; on admission and 24 h later.
There was a significant difference in the reported mean values of each scoring system between time0 and time24 in the survivors; however, it was not significant between time0 and time24 in non-survivors. The APACHE II24 mean values were found to be higher for non-survivors than survivors on time24 (21.83±6.94 and 10.03±6.28, respectively) and time0 (20.66±3.26 and 15.87±3.76). Because one parameter of APACHE II (GCS) was significantly different between survivors and non-survivors, this might be the reason of the observed higher scores in non-survivors.
Applicability of APACHE II and GCS in different poisoning has been evaluated previously. Initial assessment of GCS may help the clinician to identify advanced grade of OP poisoning patients, which has been illustrated by Akdur et al.[20] GCS has been used for predicting delayed neuropsychological sequels of carbon monoxide (CO) poisoning.[5] GCS score equal to or less than 14 had been associated with myocardial injury in CO poisoning.[9] GCS less than eight was more associated with mortality in a study by Budhathoki et al. about the outcome of children presenting with poisoning or intoxication.[4]
Although GCS is an important factor in predicting outcome in poisoning, other variables such as the kind of toxic agent,[19,31,32] the use of an antidote[33] and the kind of intervention by different physicians[34] are also effective variables at predicting outcome. In the study by Davies et al. on acute OP poisoning, apart from admission GCS, the kind of pesticide affected the outcome.[19]
In our study, discrimination was excellent for GCS24, APACHE II24, MAS24 and APACHE II0. There is no study to compare the scoring systems at different times in MDP poisoning patients; however, our results for initial evaluation of APACHE II and GCS discrimination compare with those published in OP poisoning.[17,18] In their study, the prognostic value of APACHE II was as good as that of GCS in predicting outcome patients hospitalized for OP poisoning, although no information regarding evaluation of scoring systems at different times had not been assessed. We found that an APACHE II0 score greater than 20 and an APACHE II24 score greater than 14 predicted a poor outcome with 83.33% and 100% sensitivity and 64.29% and 78.57% specificity, respectively. In acute paraquat poisoning cases, an APACHE II score greater than 13 predicted in-hospital mortality, with 67% sensitivity and 94% specificity,[13] and in the patients with OP poisoning, the initial APACHE II score of 26 or higher had been a good predictor of mortality.[10] Difference in APACHE II score in different studies may be due to different toxic agents studied, poisoned patient population and evaluating outcomes at different times.
CONCLUSION
In conclusion, because of the potential drug–drug interactions between MDPs, there could be a dominant additive or antagonistic toxidrome of various drug combinations. Therefore, there arises a need to compare the scores of individual drug combinations or various classes of drug combinations. The results showed that the four scoring systems had an acceptable to excellent outcome prediction in patients with MDP-induced coma. The GCS24, APACHE II24, MAS24 and APACHE II0 scoring systems seem to predict the outcome in patients with MDP more accurately. GCS and MAS may have superiority over the other systems in being easy to perform and not requiring laboratory data.
Our study has some limitations:
It was performed in a referral university teaching hospital and, therefore, it may not be applicable to institutions with different patient populations.
Patients admitted after 24 h of their presentation were excluded from our study thus resulting in a mortality rate of 9.7%. It could be stated that excluding these patients may weaken our study because patients who are sicker on admission are more likely to die.
We did not make an adjustment in our results for the intensity of treatment, which may affect the rate of mortality.
We did not include the “poison severity scale” recommended by the toxic exposure surveillance system (TESS) as one of the scaling systems for outcome prediction in our study to show how the employed “physiological scales” differ or comply with the poisoning risk assessment scales.
The scores were evaluated twice within a span of 24 h and not later. Certain biochemical parameters like liver or kidney function tests may take days for recovery. We may suggest comparing the trend of scoring system each day during patients’ hospitalization for outcome prediction.
ACKNOWLEDGMENTS
This research is the result of a medical internship thesis which was financially supported by the vice-chancellery of research at the Isfahan University of Medical Sciences (research project number 385535).
We would like to thank all the staff nurses of the Poisoning Emergency Department of Noor and Ali Asghar (PBUH) Teaching Hospital for their valuable help. The help and support of Mr. Mojtaba Akbari, the academic faculty members of Anaesthesiology and Intensive Care Department and the members of the Research Committee of Isfahan University of Medical Sciences is appreciated.
Footnotes
Source of Support: Vice-chancellery of research at the Isfahan University of Medical Sciences
Conflict of Interest: None declared
REFERENCES
- 1.O’Brien BP, Murphy D, Conrick-Martin I, Marsh B. The functional outcome and recovery of patients admitted to an intensive care unit following drug overdose: A follow-up study. Anaesth Intensive Care. 2009;37:802–6. doi: 10.1177/0310057X0903700508. [DOI] [PubMed] [Google Scholar]
- 2.Heard K, Bebarta VS. Reliability of the Glasgow Coma Scale for the emergency department evaluation of poisoned patients. Hum Exp Toxicol. 2004;23:197–200. doi: 10.1191/0960327104ht436oa. [DOI] [PubMed] [Google Scholar]
- 3.Unverir P, Atilla R, Karcioglu O, Topacoglu H, Demiral Y, Tuncok Y. A retrospective analysis of antidepressant poisonings in the emergency department: 11-year experience. Hum Exp Toxicol. 2006;25:605–12. doi: 10.1177/096032706072470. [DOI] [PubMed] [Google Scholar]
- 4.Budhathoki S, Poudel P, Shah D, Bhatta NK, Dutta AK, Shah GS, et al. Clinical profile and outcome of children presenting with poisoning or intoxication: A hospital based study. Nepal Med Coll J. 2009;11:170–5. [PubMed] [Google Scholar]
- 5.Ku HL, Yang KC, Lee YC, Lee MB, Chou YH. Predictors of carbon monoxide poisoning-induced delayed neuropsychological squeal. Gen Hosp Psychiatry. 2010;32:310–4. doi: 10.1016/j.genhosppsych.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Akköse S, Türkmen N, Bulut M, Akgöz S, Işcimen R, Eren B. An analysis of carbon monoxide poisoning cases in Bursa, Turkey. East Mediterr Health J. 2010;16:101–6. [PubMed] [Google Scholar]
- 7.Christ A, Arranto CA, Schindler C, Klima T, Hunziker PR, Siegemund M, et al. Incidence, risk factors, and outcome of aspiration pneumonitis in ICU overdose patients. Intensive Care Med. 2006;32:1423–7. doi: 10.1007/s00134-006-0277-4. [DOI] [PubMed] [Google Scholar]
- 8.Eizadi-Mood N, Sabzghabee AM, Yadegarfar GH, Yaraghi A, Ramazani Chaleshtori M. Glasgow coma scale and its components on admission: Are they valuable prognostic tools in acute mixed drug poisoning. Crit Care Res Pract. 2011;2011:952956. doi: 10.1155/2011/952956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teksam O, Gumus P, Bayrakci B, Erdogan I, Kale G. Acute cardiac effects of carbon monoxide poisoning in children. Eur J Emerg Med. 2010;17:192–6. doi: 10.1097/MEJ.0b013e328320ad48. [DOI] [PubMed] [Google Scholar]
- 10.Lee P, Tai DY. Clinical features of patients with acute organophosphate poisoning requiring intensive care. Intensive Care Med. 2001;27:694–9. doi: 10.1007/s001340100895. [DOI] [PubMed] [Google Scholar]
- 11.Kang EJ, Seok SJ, Lee KH, Gil HW, Yang JO, Lee EY, et al. Factors for Determining Survival in Acute Organophosphate Poisoning. Korean J Intern Med. 2009;24:362–7. doi: 10.3904/kjim.2009.24.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang NC, Lin SL, Hung YM, Hung SY, Chung HM. Severity assessment in acute paraquat poisoning by analysis of APACHE II score. J Formos Med Assoc. 2003;102:782–7. [PubMed] [Google Scholar]
- 13.Huang NC, Hung YM, Lin SL, Wann SR, Hsu CW, Ger LP, et al. Further evidence of the usefulness of Acute Physiology and Chronic Health Evaluation II scoring system in acute paraquat poisoning. Clin Toxicol. 2006;44:99–102. doi: 10.1080/15563650500514251. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell I, Bihari D, Chang R, Wendon J, Williams R. Earlier identification of patients at risk from acetaminophen-induced acute liver failure. Crit Care Med. 1998;26:279–84. doi: 10.1097/00003246-199802000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Bailey B, Amre DK, Gaudreault P. Fulminant hepatic failure secondary to acetaminophen poisoning: A systematic review and meta-analysis of prognostic criteria determining the need for liver transplantation. Crit Care Med. 2003;31:299–305. doi: 10.1097/00003246-200301000-00048. [DOI] [PubMed] [Google Scholar]
- 16.Hajouji Idrissi M, Oualili L, Abidi K, Abouqal R, Kerkeb O, Zeggwagh AA. Severity factors of aluminium phosphide poisoning (Phostoxin) Ann Fr Anesth Reanim. 2006;25:382–5. doi: 10.1016/j.annfar.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Bilgin TE, Camdeviren H, Yapici D, Doruk N, Altunkan AA, Altunkan Z, et al. The comparison of the efficacy of scoring system in organophosphate poisoning. Toxicol Ind Health. 2005;21:141–6. doi: 10.1191/0748233705th222oa. [DOI] [PubMed] [Google Scholar]
- 18.Eizadi-Mood N, Saghaei M, Jabalameli M. Predicting outcomes in organophosphate poisoning based on APACHE II and modified APACHE II scores. Hum Exp Toxicol. 2007;26:1–6. doi: 10.1177/09603271060080076. [DOI] [PubMed] [Google Scholar]
- 19.Davies JO, Eddleston M, Buckley NA. Predicting outcome in acute organophosphorus poisoning with a poison severity score or the Glasgow coma scale. QJM. 2008;101:371–9. doi: 10.1093/qjmed/hcn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akdur O, Durukan P, Ozkan S, Avsarogullari L, Vardar A, Kavalci C, et al. Poisoning severity score, Glasgow coma scale, corrected QT interval in acute organophosphate poisoning. Hum Exp Toxicol. 2010;29:419–25. doi: 10.1177/0960327110364640. [DOI] [PubMed] [Google Scholar]
- 21.Sam KG, Kondabolu K, Pati D, Kamath A, Kumar PG, Rao PG. Poisoning severity score, APACHE II and GCS: Effective clinical indices for estimating severity and predicting outcome of acute organophosphorus and carbamate poisoning. J Forensic Leg Med. 2009;16:239–47. doi: 10.1016/j.jflm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Louriz M, Dendane T, Abidi K, Madani N, Abouqal R, Zeggwagh AA. Prognostic factors of acute aluminum phosphide poisoning. Indian J Med Sci. 2009;63:227–34. [PubMed] [Google Scholar]
- 23.Feldman R, Burda PR, Glinska-Serwin M, Kotlarska M, Szajewski J. Correlation of carbamazepine levels in blood with clinical poisoning states, evaluated with the help of the APACHE II system and the Matthew coma scale. Przegl Lek. 1997;54:410–5. [PubMed] [Google Scholar]
- 24.Eizadi-Mood N, Saghaei M, Alfred S, Zargarzadeh AH, Huynh C, Gheshlaghi F, et al. Comparative evaluation of Glasgow Coma Score and gag reflex in predicting aspiration pneumonitis in acute poisoning. J Crit Care. 2009;24:470.e9–15. doi: 10.1016/j.jcrc.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 25.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. J Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 26.Hennes HJ, Reinnhardt T, Dick W. The Mainz Emergency Evaluation Score in emergency patients. J Emerg Med. 1992;18:130–6. [Google Scholar]
- 27.Grmec S, Kupnik D. Does the Mainz Emergency Evaluation Scoring (MEES) in combination with capnometry (MEESc) help in the prognosis of outcome from cardiopulmonary resuscitation in a prehospital setting? Resuscitation. 2003;58:89–96. doi: 10.1016/s0300-9572(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 28.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 29.Hosmer DW, Lemeshow S. Assessing the Fit of the Mode. In: Hosmer DW, Lemeshow S, editors. Applied Logistic Regression. 2nd ed. New York: John Wiley and Sons; 2000. pp. 160–4. [Google Scholar]
- 30.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Kelly CA, Upex A, Bateman DN. Comparison of consciousness level assessment in the poisoned patient using the alert/verbal/painful/unresponsive scale and the Glasgow Coma Scale. Ann Emerg Med. 2004;44:108–13. doi: 10.1016/j.annemergmed.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 32.Isbister GK, O’Regan L, Sibbritt D, Whyte IM. Alprazolam is relatively more toxic than other benzodiazepines in overdose. Br J Clin Pharmacol. 2004;58:88–95. doi: 10.1111/j.1365-2125.2004.02089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poplas-Susić T, Klemenc-Ketis Z, Komericki-Grzinić M, Kersnik J. Glasgow Coma Scale in acute poisonings before and after use of antidote in patients with history of use of psychotropic agents. Srp Arh Celok Lek. 2010;138:210–3. doi: 10.2298/sarh1004210p. [DOI] [PubMed] [Google Scholar]
- 34.Donald C, Duncan R, Thakore S. Predictors of the need for rapid sequence intubation in the poisoned patient with reduced Glasgow coma score. Emerg Med J. 2009;26:510–2. doi: 10.1136/emj.2008.064998. [DOI] [PubMed] [Google Scholar]


